Abstract
Background:
Asthma and COPD are complex, heterogeneous conditions comprising a wide range of phenotypes, some of which are refractory to currently available treatments. Elucidation of these phenotypes and identification of biomarkers with which to recognize them and guide appropriate treat-ment remain a priority.
Objective:
This review describes the utility of blood eosinophils as a surrogate biomarker of eosinophilic airway inflammation, a common feature of specific asthma and COPD phenotypes. The role of blood eosinophils in airway disease is described, as is their relevance in reflecting airway eosinophilia. Each disease is discussed separately as the manner in which blood eosinophils might be used as biomarkers differs. Focusing on patients with severe disease (persistent eosinophilic asthma and exacerbating COPD), we evaluate evidence examining eosinophils as biomarkers.
Results:
In asthma, the rationale for using blood eosinophils to guide treatment is clearly defined, backed by prospective, well-controlled studies. Higher eosinophil counts identify patients with more se-vere disease and poorer outcomes, patients for whom biologic therapies targeting allergic and/or eosino-philic pathways are recommended. In COPD, the evidence is less robust. High blood eosinophil counts are a modest predictor of future exacerbations, and may predict a favourable response to ICS on top of LABA/LAMA, especially in patients with a history of frequent exacerbations.
Conclusion:
Before extensive application in clinical practice, further evaluation of these findings in pro-spective clinical studies, and standardization of the appropriate thresholds of clinically relevant eosino-philia are needed, together with establishing whether single or multiple measurements are required in dif-ferent clinical settings
Keywords: Blood eosinophils, asthma, COPD, biomarkers, airway inflammation, treatment options
1. Introduction
Asthma and COPD are complex, heterogeneous conditions encompassing a wide range of phenotypes that differ in severity and natural history. This diversity is further expressed in patients with asthma–COPD overlap, where both asthma and COPD characteristics co-exist in the same individual [1, 2].
Despite the multitude of treatments and interventions available to patients with these airway diseases, many patients remain refractory, while those who do respond show marked variability in their responses. In the era of personalized medicine, the ‘one size fits all’ approach to treat airway disease could be considered outdated, and that a shift to personalized treatment is required [3, 4]. To help guide appropriate treatment decisions, elucidation of the various phenotypes specific to each airway disease is of utmost importance
[5, 6]. To this end, biomarkers specific for each phenotype are actively being sought, although in many cases, their utility in the complex environment of airways disease remains poorly understood [4, 7].
The National Institutes of Health Biomarkers Definitions Working Group defines a biomarker as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic intervention.” Several biomarkers are already available for asthma, particularly severe, difficult to treat asthma [8, 9], and in some cases, their use has been incorporated into treatment guidelines [10, 11]. None has been universally accepted for COPD [12, 13].
Eosinophilic inflammation of the airways is a common feature of certain asthma [14] and COPD phenotypes [15] and new therapies have been described that target eosinophilic or related pathways. For these to be administered appropriately, biomarkers are required that can identify and select suitable patients.
The measurement of contemporaneous eosinophilia in peripheral blood cells (either by absolute count or by percent differential of total leukocytes) has been investigated in recent years as a potential surrogate marker of bronchial and/or lung inflammation. In this review, we describe the role of eosinophils in airway disease, with a focus on patients with severe disease (persistent eosinophilic asthma and exacerbating COPD), and evaluate their utility as biomarkers of obstructive airway disease.
2. The role of eosinophils in airway inflammation
Eosinophils normally comprise less than 5% of the leukocytes in peripheral blood, but in response to type 2 helper T-cell (Th2)-mediated inflammation their production is greatly enhanced and they become pivotal effector cells in inflammatory responses. They are recruited from the bloodstream to sites of inflammation by pro-inflammatory cytokines and members of the eotaxin family of chemokines [16, 17]. In asthma, Th2-mediated eosinophilic airway inflammation is typical [18, 19] whereas in COPD, neutrophilic rather than eosinophilic inflammation predominates [20]. In each condition, however, a spectrum of phenotypes exists from asthmatics without eosinophilic inflammation to COPD patients with eosinophilic rather than neutrophilic inflammation [14, 17].
Understanding the biology of eosinophils is essential in order to appreciate how currently available treatments might affect eosinophilic inflammatory pathways. Since the pathological activity of eosinophils occurs predominantly at the tissue level, understanding the mechanisms involved in their recruitment is particularly important for the development of therapies that target eosinophils. Upon activation by pro-inflammatory cytokines such as Interleukin-3 (IL3), IL5 and granulocyte-macrophage colony-stimulating factor (GM-CSF), eosinophils are recruited into the circulation from the bone marrow, and migrate to sites of inflammation in response to chemokines such as CCL5 and CCL11 and other chemotactic factors. Extravasation into the airways is mediated by the interaction of cell surface integrins on eosinophils with adhesion molecules on the vascular endothelium, which enables transmigration across the bronchial vascular epithelium. Once within the airways, activated eosinophils release pro-inflammatory mediators, resulting in sustained inflammation and tissue damage [17, 21].
Circulating eosinophils exist in different activation states (activated, non-activated, pre-activated), with each state associated with the expression of particular cell-surface proteins, some of which correlate with asthma activity and/or respond to treatment [22]. Glucocorticoids inhibit inflammatory signaling by inducing eosinophil apoptosis, but this may depend on the eosinophil activation state. IL5-activated eosinophils have been shown to have an impaired pro-apoptotic response to glucocorticoids that are mediated by Nuclear Factor IL-3 [23]. This may represent a plausible mechanism for the efficacy of anti-IL5 treatments in patients with severe eosinophilic asthma undergoing corticosteroid treatment.
Several surrogate biomarkers have been implicated in the evaluation of airways eosinophilic inflammation and treatment response, including blood eosinophils and the fraction of exhaled nitric oxide (FeNO) [24]. In addition, eosinophil-derived neurotoxin (EDN), a granule-derived protein that is produced by activated eosinophils, is often used as a marker of eosinophil degranulation [25]. A pilot study using a point-of care diagnostic tool showed that EDN levels in capillary blood could be easily and accurately measured in asthmatic patients and demonstrated a high correlation with blood eosinophil counts [26]. As EDN release is a consequence of the inflammatory process rather than circulating eosinophils, the authors argue that EDN reflects more accurately the pathology of eosinophilic asthma than do blood eosinophils, which also comprise non-activated cells. Further investigation into the use of EDN is warranted as is the search for alternative biomarkers of eosinophilic inflammation with which to identify patients for appropriate treatment.
3. Eosinophilic pathways in asthma
Eosinophils are major contributors to the long-term inflammatory status seen in asthma [8]. Indeed, persistent eosinophilic airway inflammation is a well-defined pattern of disease in patients with severe asthma [10, 27, 28], although neutrophilic or paucigranulocytic profiles are also present [29, 30].
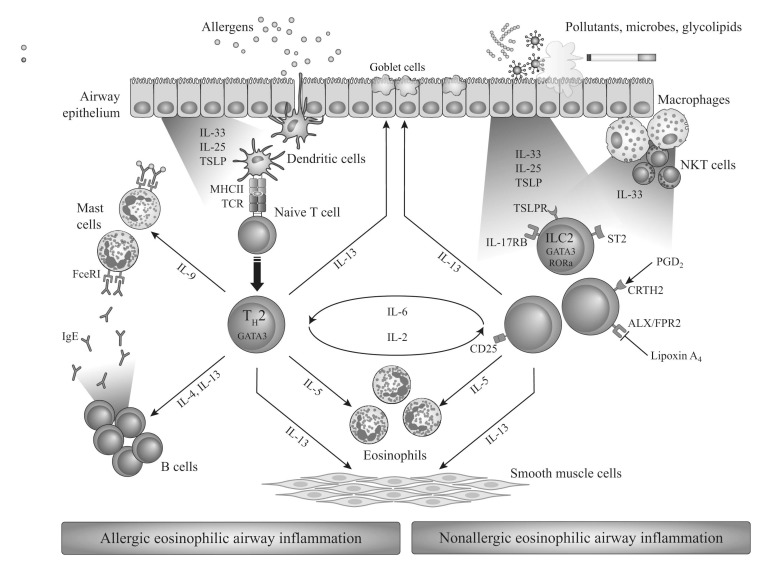
Eosinophilic asthma can occur in both allergic and non-allergic patients but the pathways for eosinophil recruitment are quite distinct (summarized in Fig. 1). Allergic eosinophilic asthma is driven by Th2 lymphocytes. Allergens, presented to naïve CD4+ T cells by dendritic cells, induce differentiation toward Th2 cells, which produce IL4, IL5 and IL13 cytokines, leading to IgE class switch in B cells, airway eosinophilia and mucous hypersecretion [31]. In nonallergic eosinophilic asthma, epithelium-derived cytokines (IL25, IL33, TSLP) are released in response to air pollutants, microbes or glycolipids. These bind to receptors on type-2 innate lymphoid cells (ILC2s), activating them to produce the Th2-associated cytokines IL5 and IL13, which lead to eosinophilia, mucous hypersecretion and airway hyper-reactivity [31].
Thus, in the majority of allergic asthma cases, the presence of eosinophils may be a secondary consequence of the allergic cascade that recruits them to the site of inflammation – in essence, they are ‘innocent bystanders’ or minor effectors in the evolution of the disease. In some patients, however, particularly those with severe, non-allergic asthma, the eosinophil may play a more central role, possibly initiating the disease; these patients are typically older women with comorbid nasal polyps, aspirin sensitivity and late-onset asthma [32].
4. Eosinophilic inflammation in COPD
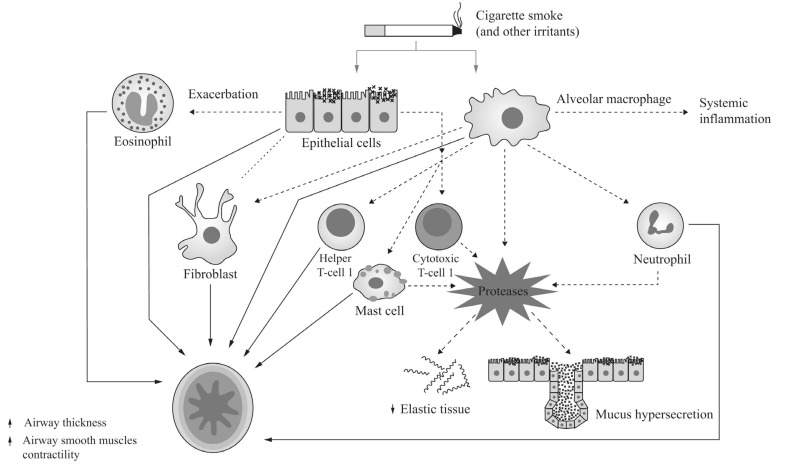
In COPD, inflammation is more commonly associated with T helper 1 lymphocyte (Th1)-mediated immunity driven by neutrophils, often in response to bacterial colonization [20] (summarized in Fig. 2). However, as with asthma, COPD presents as a number of different clinical phenotypes and, in around 10–40% of patients, a degree of eosinophilic inflammation is present during stable state [17].
Cell senescence likely plays a pathophysiological role in COPD, particularly in relation to the release of cytokines and
other factors from senescent cells, many of which are also implicated in the pathogenesis of COPD [33]. While it is likely that these factors augment the inflammatory component of the COPD lung, the precise mechanisms remain unclear and their potential implications in treatment responses are currently unknown.
As well as in the stable state, heterogeneity is also observed in COPD exacerbations. An eosinophilic-predominant phenotype was found to be associated with eosinophilia in 28% of exacerbations [34], and the degree of eosinophilia observed during exacerbations is higher than that seen in stable disease [35].
5. Measuring eosinophilic airway inflammation
Establishing that a patient has an eosinophilic-predominant inflammatory phenotype is critical for the appropriate administration of eosinophil-targeted therapy. The most accurate assessment of the degree of eosinophilic inflammation in airway disease is by examination of eosinophils recruited to the actual site of inflammation. While technically possible, however, measurement of eosinophils at the tissue level (via lung or bronchial biopsies) is invasive and can only be performed in specialist settings. An alternative means of assessing eosinophilic airway inflammation is through induced sputum samples as sputum eosinophil counts have long been known to correlate with eosinophilic airway inflammation in both asthma and COPD [36, 37]. Indeed, strategies that maintain lowered sputum eosinophils have been linked with favorable outcomes in both conditions [17, 38-40].
Although less invasive than tissue biopsy, sputum induction is also difficult to perform, requiring expertise at specialist centers, and so is impractical for large-scale clinical trials and/or community healthcare providers. Indeed, the techniques employed differ significantly between centers so it is unlikely that sputum sampling would be proposed as a routine measure unless a significant standardization effort occurs. Furthermore, sputum induction is also not without safety concerns and is often contraindicated in more severe patients, the patients more likely to require evaluation.
The potential of using blood eosinophil counts as a surrogate marker for eosinophilic airway inflammation arose from data suggesting that, in both asthma and COPD, blood eosinophil counts correlated with sputum eosinophilia, albeit with modest diagnostic performance [37, 41, 42]. How strong the correlation is between contemporaneous blood and sputum eosinophilia is debated, however, and discordances have been observed between sputum, tissue and systemic eosinophil counts [43-45].
As with sputum eosinophil counts, the relevance of blood eosinophilia as a surrogate marker has also been questioned: how can a marker derived from peripheral blood accurately represent what is happening in the lung [46]? Furthermore, the stability, and therefore reproducibility, of blood eosinophil measurements has also been questioned, as fluctuations occur over time [15, 47, 48].
Clearly, fluctuations in eosinophil stability could impact the accuracy of study findings, particularly in those studies where the degree of eosinophilia is based on a single pre-study measurement (which would be further impacted if inappropriate thresholds were used). Systemic corticosteroids have a significant impact on the levels of blood eosinophils, whereas long-term treatment with inhaled corticosteroids may have a minimal effect over time, as shown in prospective data from a COPD trial [49], and therefore the baseline treatment needs to be taken into account in the evaluation of the variability of blood eosinophils.
6. Blood eosinophils as biomarkers of eosinophilia: clinical studies and thresholds used
Many clinical studies have used blood eosinophil measurements as a surrogate biomarker to indicate eosinophilic inflammation. However, as described below, and summarized in Tables 1 and 2, the thresholds used to define blood eosinophilia have varied from study to study with no consensus as to which is the most appropriate. In an environment already subject to much variation, this lack in standardization increases confusion.
6.1. Part A: Eosinophils as Potential Biomarkers in Asthma
Inhaled corticosteroids (ICS) are the mainstay of therapy in patients with eosinophilic asthma but are often inadequate in patients with severe asthma. In recent years, a number of eosinophil-targeted biologic agents that block specific steps in eosinophil development, migration and activation have entered development, with some now approved for clinical use [16]. However, responses to these treatments are markedly heterogeneous and the discovery of markers with which to identify patients expected to achieve greater benefit has become a priority. Several studies have examined the ability of baseline blood eosinophils to predict treatment response; others have assessed their potential as predictors of asthma severity or future exacerbations (summarized in Table 1; the definitions of exacerbation used in each study are footnoted on the table).
6.1.1. Eosinophils as predictors of asthma severity and future outcomes
Eosinophilic inflammation is present in a significant proportion of patients with severe asthma and is associated with exacerbations, decreased lung function and poor pharmacological control [14]. As such, blood eosinophils can serve as a useful marker with which to assess patient risk since patients with moderate-to-severe asthma are significantly more likely to have elevated eosinophil counts pre-treatment than those with mild disease [50]. Price et al. [51] examined the relationship between blood eosinophil counts and asthma-related outcomes in a historical cohort study and found that patients with baseline blood eosinophil counts greater than 400 cells/μl experienced more severe subsequent exacerbations and had poorer asthma control; furthermore, exacerbation rate increased progressively with increasing eosinophil count.
Baseline sputum eosinophilia, although more difficult to establish due to the complex technique involved, has also been associated with improved outcomes to treatments
targeting eosinophils [52]. The prostaglandin D2 receptor has also been shown to play an important role in eosinophilic asthma and treatments targeting this receptor have been receiving much attention [53]. Fevipiprant, a potent antagonist of the receptor, was tested in patients with moderate-to-severe asthma and a ≥2% sputum eosinophil counts at screening [54]. The drug reduced eosinophilic airway inflammation and was well tolerated, providing a 4.5-fold reduction in percent sputum eosinophils versus 1.3-fold in placebo-treated patients. Improvements in lung function and asthma-related quality of life were also observed in the fevipiprant arm.
6.1.2. Eosinophils as Predictors of Response to Anti-IgE Therapy
Omalizumab is a recombinant humanized monoclonal antibody that selectively binds circulating IgE in the blood and interstitial space, thus depleting levels of circulating IgE [55]. This leads to inhibition of the release of inflammatory mediators from mast cells and diminished recruitment of eosinophils and other inflammatory cells into the airways [56]. Indicated for adults/adolescents with IgE-mediated moderate-to-severe persistent allergic asthma, omalizumab is recommended in current treatment guidelines for such patients uncontrolled on Step 4 treatment [11]. In a study examining the utility of potential biomarkers to predict omalizumab response, exacerbations were significantly reduced in the subgroup of patients with high baseline eosinophil counts (≥260 cells/μl) versus those with medium/low counts (<260 cells/μl) [57]. In another study, while omalizumab reduced exacerbations in the overall population versus placebo (although not significantly so), in patients with eosinophil counts ≥300/μl a 59% reduction in exacerbation rate was observed compared with the placebo arm (P = 0.0125). Furthermore, placebo-treated patients in the high eosinophil group had a higher exacerbation rate compared with those in the low eosinophil subgroup suggesting that, as well as guiding therapeutic benefit, eosinophil counts might also predict patients at greater risk of exacerbations [58]. The efficacy of omalizumab in patients with allergic asthma is generally associated with an acceptable safety profile [59].
6.1.3. Eosinophils as Predictors of Response to Anti-IL5 Targets
Secreted by Th2 and mast cells, IL5 is a key cytokine responsible for the proliferation, maturation, activation, and recruitment of eosinophils, making the components of the IL5 pathway attractive therapeutic targets with which to treat eosinophilic inflammation [60]. Current treatment guidelines recommend add-on anti-IL5 biologic therapy for patients with severe, uncontrolled eosinophilic asthma [11]. While no major safety concerns related to these agents have been identified in the available randomized controlled trials [61], long-term safety data are needed for the optimal understanding of their safety profile.
Mepolizumab and reslizumab are anti-IL5 MAbs that bind IL5, inhibiting eosinophilic inflammation by depleting the number of eosinophils in both sputum and blood. In a dose-ranging, efficacy and safety study (DREAM) of add-on mepolizumab in patients with severe eosinophilic asthma (≥300 cells/μl), mepolizumab significantly reduced exacerbation rate and time to first exacerbation versus placebo (P ≤ 0.0005 for all doses) [62]. Circulating blood eosinophil levels were also significantly reduced compared with placebo, and modelling analysis confirmed that baseline blood eosinophil counts predicted treatment response to mepolizumab, with efficacy intensifying as baseline blood eosinophil counts and number of prior exacerbations increased [62].
In a subsequent analysis of the DREAM study, elevated baseline blood but not sputum eosinophil counts were found to be predictive of treatment outcome. A single measurement of ≥150 cells/μl at screening predicted that 85% of placebo-treated patients would have subsequent measurements above 150 cells/μl and would likely have benefited from mepolizumab [63].
In the phase 3 MENSA study, mepolizumab administered either intravenously or subcutaneously significantly reduced asthma exacerbation rates by around one half versus placebo in severe eosinophilic asthma (>150 cells/μl at screening or ≥300 cells/μl during the previous year). Mepolizumab treatment was also associated with significantly improved forced expiratory volume in 1 second (FEV1) (P < 0.05), quality of life (QoL), as measured by the St. George's Respiratory Questionnaire (SGRQ), and asthma control, measured using the 5-item asthma control questionnaire (ACQ-5); both P < 0.001 compared with placebo [64].
In another study (SIRIUS), which included patients with severe asthma requiring maintenance with daily oral corticosteroids to control their asthma, mepolizumab significantly reduced the need for glucocorticoids by 2.39-fold versus placebo-treated patients while also maintaining asthma control [65]. Moreover, despite reduced steroid maintenance, the exacerbation rate was significantly reduced by 32% versus the placebo arm (P = 0.04).
The appropriate threshold with which to define blood eosinophilia has varied from study to study and remains a topic of debate. Ortega and colleagues [66] argued that a proposed threshold of 300 cells/μl [67] would deprive many severe asthma patients from mepolizumab benefit claiming that ‘the goal in using blood eosinophils is not to accurately predict sputum eosinophilia but to serve as a marker for response’. To further define the threshold, they conducted a post-hoc analysis on data from the DREAM/MENSA populations in which they stratified patients by various baseline eosinophil cut-offs (≥150, ≥300, ≥400, and ≥500 cells/μl). Clinically relevant reductions in exacerbations were observed above a threshold of 150 cells/μl, with these reductions becoming progressively greater with increasing baseline eosinophil count; a threshold of 150 cells/μl was proposed for the selection of patients most likely to benefit with mepolizumab treatment [68].
In reslizumab studies, the threshold used to define eosinophilia has been higher than that of mepolizumab, and in two, replicate, 52-week, phase 3 studies, patients were selected using a threshold of ≥400 cells/μl [69]. Reslizumab as add-on therapy to ICS with or without other controllers significantly reduced the frequency of asthma exacerbations versus placebo (by 50% and 59%; both P < 0.0001). In an attempt to identify the eosinophil threshold that would maximize reslizumab benefit, Corren et al. [70] examined reslizumab efficacy in a population unselected for baseline eosinophil count. No differences between reslizumab and placebo were observed with respect to the overall population, but in patients with ≥400 cells/μl clinically meaningful improvements were observed versus placebo in the primary endpoint, FEV1 at Week 16 weeks, as well as in ACQ-7, rescue medication use, and FVC. No meaningful trends were observed in patients with fewer than 400 cells/μl.
Another study examined two doses of add-on reslizumab (3.0 mg/kg vs 0.3 mg/kg) in patients with eosinophilic asthma (≥400/μl) inadequately controlled by at least a medium-dose ICS [71]. Significant and/or clinically meaningful differences versus placebo were observed for both doses for most lung function measures. Reductions in circulating eosinophils and improvements in asthma control, symptoms, and quality of life were also observed with both doses. Overall, the 3.0 mg/kg dose provided greater improvements than the 0.3 mg/kg dose.
In a re-analysis of pooled data from the two studies reported by Castro and colleagues [69], patients were stratified by late- versus early-onset asthma (<40 vs ≥40 years) [72]. Significantly greater reductions in exacerbations versus placebo were observed in patients with late-onset asthma (P = 0.0083). These patients also benefited from larger improvements in FEV1, ACQ-6 and Asthma Symptom Utility Index (ASUI) versus early-onset patients.
Another anti-IL5 antibody, benralizumab targets the alpha chain of the IL5 receptor directly and in doing so blocks the binding of IL5 and other ligands, which prevents hetero-oligomerization of the α/β subunits resulting in a loss of signal transduction [73]. As it acts directly on the IL5 receptor on eosinophils to cause apoptosis, benralizumab is arguably more effective than mepolizumab and reslizumab, which act indirectly to give incomplete depletion of eosinophils. Using a direct approach also avoids potentiation of the target cytokine through formation of cytokine/anti-cytokine immune complex, which has been reported to occur with anti-cytokine antibodies [74].
Data from 2 pivotal phase 3 trials (SIROCCO and CALIMA) evaluated the efficacy of two dosing regimens of benralizumab (30 mg administered q4w and q8w) as add-on therapy in patients with severe eosinophilic asthma (≥300 cells/μl) uncontrolled by high-dose ICS plus LABA. Significant reductions (of up to 51%; P < 0.0001) in the annual rate of exacerbations and improvements in lung function (FEV1 change up to 159 mL) were observed 4 weeks after the first dose and these were sustained throughout the treatment periods (48 and 56 weeks, respectively) [75, 76]. Improvements in asthma symptoms were also observed versus placebo for the q8w dose. In both studies, post-hoc analyses showed that greater improvements in exacerbation rate reduction, FEV1 and total asthma symptom scores were observed in patients with a history of more frequent asthma exacerbations (≥3 in the previous year).
6.1.4. Baseline Blood Eosinophils and Responsiveness to Anti-IL4/IL13 Targets
IL4 and IL13 are also key drivers of type-2 mediated inflammation. Dupilumab, a humanized anti-IL4Rα antibody that inhibits both IL4 and IL13 signaling, is currently in phase 3 development for use in patients with uncontrolled persistent moderate/severe asthma despite the use of medium-to-high-dose ICS plus LABA. An initial phase 2a study was conducted in patients selected for elevated eosinophils (≥300 cells/μl). Add-on dupilumab significantly reduced exacerbation rate versus placebo by 87% (P < 0.001). Significant improvements in most lung function measures and asthma control were also observed [77]. Reductions in the levels of some Th2-associated biomarkers were also observed although, unlike antibodies targeting IL5 pathways, there was little or no change in eosinophil levels. On the basis of these findings, a phase 2b study was conducted in order to further define the target population responsive to dupilumab and the population was expanded to include all patients with persistent moderate/severe asthma uncontrolled by ICS/LABA irrespective of eosinophil count [78]. Unlike other biologics targeting eosinophils, dupilumab efficacy was observed not only in the subgroup of patients with ≥300 cells/μl but numerical and/or significant reductions in severe exacerbations and improvements in FEV1 and patient-reported outcomes were observed in the overall population as well as in the subgroup with eosinophil counts <300 cells/μl [78].
6.2. Part B: Eosinophils as Potential Biomarkers in COPD
Eosinophilic inflammation is not found in the majority of COPD patients and, unlike in asthma where ICS is the cornerstone of therapy, in COPD they are normally used only for the treatment of exacerbations by adding on to the patient’s current LABA. In stable COPD with an eosinophilic component, whether blood eosinophil levels can predict exacerbations and/or treatment outcomes (specifically with ICS) has been the focus of much research in recent years but remains poorly understood. As is described in the following sections and summarized in Table 2, many studies have provided evidence associating eosinophilia with exacerbation risk and/or the likelihood of benefiting from ICS. However, most of the research has been retrospective and requires prospective confirmation. Furthermore, contradictory data have also been described, and recent data suggest that eosinophils may not be a useful generalizable marker with which to identify COPD phenotypes [79].
Whereas anti-IL5 therapies have shown promise in patients with eosinophilic asthma, initial proof-of-concept studies have not met with such success in COPD patients, suggesting that depletion of eosinophils may not be a valid target in COPD. An initial study using the anti-IL5R antibody, benralizumab, in COPD patients with elevated baseline sputum eosinophils (≥3%) demonstrated numerical improvements in exacerbation rates, SGRQ-C and the self-administered Chronic Respiratory Questionnaire (CRQ-SAS) scores, and FEV1; however, these improvements were not statistically significant [80]. Likewise, mepolizumab significantly reduced sputum and blood eosinophil counts compared with placebo in COPD patients with raised baseline eosinophils but, again, these differences did not translate into significant between-group differences in lung function parameters, exacerbation rates, and health-related quality of life [81]. The authors concluded that the role of eosinophils in COPD is complex and the benefits observed with ICS are likely related to their effects on cells or pathways that do not involve eosinophils. Two 1-year phase 3 studies on the efficacy of mepolizumab on exacerbation prevention in eosinophilic COPD patients were recently reported [82]. In one of the two studies (METREX) the 100 mg mepolizumab dose presented a significant reduction of moderate/severe COPD exacerbations versus placebo in a subgroup of COPD patients with an eosinophilic phenotype (eosinophil count, ≥150 cells/μL at screening or ≥300 cells/μL at any point in the previous year), but not in the overall population. The second study (METREO), which was conducted in COPD patients with a similarly-defined eosinophilic phenotype, showed no difference versus placebo for the 100 mg and 300 mg doses. In a pre-specified meta-analysis the authors were able to show that the greater effect on exacerbation prevention was observed in patients with higher blood eosinophil counts (ie, ≥300 cells/μL at screening). These results suggest a potential role for eosinophilic airway inflammation on COPD exacerbations, but also clearly underline the fact that further studies are needed in order to refine the patients who may benefit from eosinophil-targeted treatments in COPD [83].
The identification of an eosinophil-predominant phenotype in COPD exacerbations was discovered in a cluster analysis study that used putative biomarkers present during exacerbations to identify clusters of phenotypes [34]. The eosinophil-predominant phenotype could be identified in stable state from sputum or blood eosinophilia. During an exacerbation, percent peripheral blood eosinophil count was identified as the most sensitive and specific (area under ROC, 0.85 [95% CI, 0.78–0.93]) biomarker for detecting exacerbation-associated sputum eosinophilia. A cut-off of ≥2% peripheral blood eosinophil was capable of identifying a sputum eosinophilia of >3% with 90% sensitivity and 60% specificity. Since sputum eosinophilia during stable state is associated with corticosteroid responsiveness, these patients can then be appropriately treated. Indeed, a strategy using sputum eosinophil counts on top of traditional treatment guidelines to titrate corticosteroid therapy in order to minimize eosinophilic inflammation resulted in fewer COPD exacerbations than observed in patients receiving traditional treatment alone [39].
Schleich and colleagues showed that blood eosinophils could be used as a surrogate marker to predict eosinophilic inflammation in COPD (defined by a sputum eosinophilia ≥3%) albeit with modest diagnostic performance [42]. A blood eosinophil count of >162/μl (or 2.6%) identified patients with bronchial eosinophilic inflammation (sputum eosinophils ≥3%) with moderate sensitivity and high specificity (area under the ROC curve 0.75; p<0.0001, 71% sensitivity, 67% specificity). In a sub-population of patients receiving high-dose ICS the blood eosinophil cut-off for predicting a sputum eosinophil ≥3% was 215 cells/μl (AUC 0.76, p=0.001; 60% sensitivity, 93% specificity) or 2.3% (AUC 0.78, p<0.0001; 62% sensitivity, 94% specificity). The authors concluded that blood eosinophil could be useful for guiding patient selection for ICS and also to adjust dosing during recurrent exacerbations.
6.2.1. Higher Baseline Blood Eosinophils as Predictors of COPD Exacerbation Risk
In a prospective population study of COPD patients, a baseline eosinophil count of >340 cells/μl was associated with a 1.76-fold increased risk of severe exacerbations and a 1.15-fold increased risk of moderate exacerbations [84]. Patients with an eosinophil differential of >3.3% also had an increased risk of severe but not moderate exacerbations (incidence rate ratio [IRR]: 1.32 [1.19-1.48] and 0.98 [0.90-1.06], respectively) suggesting that absolute counts of blood eosinophils are more sensitive at predicting future exacerbations than percentage differential.
These findings have been confirmed recently in a historical follow-up study from another population database in which patients who had elevated blood eosinophil counts during stable disease (≥450 cells/μl) had a 13% higher exacerbation rate during the following year than patients with lower counts (P = 0.03) [85]. Interestingly, when this interaction was examined in different subgroups, highly significant differences were found between ex-smokers and current smokers, with ex-smokers with elevated eosinophil counts having the highest exacerbation rate (RR 1.30; 95% CI 1.14–1.48; P < 0.0001) versus the reference group (<450 cells/μl) while current smokers with elevated eosinophil counts had the lowest exacerbation rate (RR 0.89; 95% CI 0.73–1.08; P = 0.14). Their findings suggest that the use of elevated blood eosinophil counts for the prediction of exacerbations was modest and restricted to ex-smokers.
6.2.2. Eosinophils as Predictors of ICS Response in COPD Patients
In COPD, ICS is mainly used to reduce the risk of and treat exacerbations, their effects on symptoms and lung function being relatively small. A number of studies have shown that high eosinophil levels predict a favorable response to LABA/ICS versus LABA alone. In a post-hoc analysis of data from two randomized controlled trials, Pascoe and colleagues [86] assessed exacerbation rates in patients stratified by baseline eosinophil counts (<2% versus ≥2%) who were being treated with LABA/ICS or LABA alone (fluticasone furoate plus vilanterol vs vilanterol alone). They found that responsiveness to ICS was improved in patients with eosinophils ≥2% (or ≥150 cells/μl). Hence, patients treated with LABA/ICS had a 29% reduction in exacerbations versus those treated with LABA alone (P < 0.0001). Progressively greater reductions in exacerbations were also observed with increasing baseline percentage eosinophils. Supporting the notion that high eosinophil levels increase the risk of exacerbation, the authors also found that in the LABA-only arm the exacerbation rate was significantly higher in the patients with eosinophils ≥2% compared with those with lower counts (P = 0.0006).
In a post-hoc analysis of the FORWARD study (beclomethasone dipropionate plus formoterol fumarate vs plus formoterol fumarate alone), patients were stratified into quartiles based on baseline blood eosinophil count (0 to <110.4; 110.4 to <181.6; 181.6 to <279.8 and ≥279.8 cells/μl) [87]. LABA/ICS reduced exacerbations vs LABA alone in patients with elevated eosinophils, with progressively better responses observed with increasing baseline eosinophil count (the greatest treatment difference observed for patients in the highest quartile, ≥279.8 cells/μl). Significant improvements in FEV1 and SGRQ were also observed for LABA/ICS vs LABA particularly in the subgroup with eosinophils ≥279.8 cells/μl.
Although positive associations between blood eosinophils and subsequent treatment response have been found in some studies conducted to date, contradictory results have been found in other retrospective analyses.
Data from 3 separate studies (INSPIRE, TRISTAN, SCO30002) were reanalyzed stratifying patients by percent baseline eosinophil counts of <2% vs ≥2%. The studies were each conducted in patients with moderate-to-very severe COPD and each compared the effects of LABA/ICS (fluticasone propionate /salmeterol), LABA alone or ICS alone with a long-acting muscarinic antagonist (LAMA) (tiotropium), LABA or placebo on reducing moderate/severe exacerbation rates [88]. In INSPIRE (fluticasone propionate/salmeterol vs tiotropium), in patients with blood eosinophils ≥2% LABA/ICS was associated with significantly reduced exacerbation rates compared with LAMA (P = 0.006). In TRISTAN (fluticasone propionate /salmeterol vs salmeterol, fluticasone propionate, or placebo) patients with eosinophils ≥2% treated with LABA/ICS had significantly reduced exacerbation rates versus placebo-treated patients (P = 0.001) but not versus LABA or LAMA. Significant differences versus placebo were also observed for LABA alone and ICS alone (P = 0.02 and P = 0.005, respectively). No significant differences were observed in either study for patients with <2% eosinophils. In the third study (SCO30002), no significant treatment effects were observed between fluticasone propionate/salmeterol or fluticasone propionate and placebo. No relationship between eosinophil subgroup and FEV1 or SGRQ was observed in any of the three studies [88].
In a re-analysis of the ISOLDE study, which had compared an ICS (fluticasone propionate) with placebo, patients were also stratified using a baseline blood eosinophil threshold of ≥2% [89]. The results of this study contrasted to the earlier studies by Pascoe et al. [86] and Siddiqui et al. [87], finding that ICS significantly reduced exacerbation rates versus placebo in patients with eosinophil counts <2% (P = 0.009), with no difference versus placebo observed in the ≥2% eosinophil arm. Patients in the eosinophils ≥2% arm, however, had significantly slower FEV1 decline compared with placebo (P = 0.003), with no difference between treatments in the patients with <2%.
A few studies have investigated the usefulness of eosinophil levels to predict both treatment response and risk of future exacerbations. WISDOM was a 52-week phase 4 study comparing exacerbation risk in patients who continued triple therapy with a LAMA + LABA + ICS with those who had ICS withdrawn after 6 weeks [90]. Patients had severe/very severe COPD and a history of ≥1 exacerbation in the 12-month pre-screening period. In a post-hoc analysis of the study, patients were stratified by various eosinophil thresholds; those with higher baseline eosinophils who were withdrawn from ICS were found to have an increased risk of exacerbation versus patients with low counts [91]. A significant treatment-by-subgroup interaction was observed at a threshold of ≥4% or >300 cells/μl, with exacerbation rates increasing as absolute counts increased. More recently these data were evaluated according to exacerbation history at baseline (1 vs ≥2) and a more prominent treatment effect was found in patients with a history of ≥2 exacerbations and eosinophil counts of ≥300 or ≥400 cells/μl [92].
The FLAME study is the only prospective study that has examined differential treatment effects in COPD patients stratified by baseline eosinophils (<2% vs ≥2%) to date [93]. The study, conducted in patients with moderate-to-severe COPD and a history of ≥1 exacerbation in the previous year, compared a LABA/LAMA with a LABA/ICS (indacaterol/glycopyronnium and salmeterol/fluticasone propionate, respectively) over 52 weeks and found that LABA/LAMA significantly reduced exacerbations versus LABA/ICS by 11% in the overall population (P = 0.003: primary endpoint) but also irrespective of eosinophil counts (P = 0.004 and P = 0.01 for patients in the <2% and ≥2% eosinophil subgroups, respectively) [93]. More recently, the FLAME data were re-analysed using additional eosinophil cut-offs (<3%, <5%, < 150 cells/μl, <300 cells/μl and ≥300 cells/μl) and similar findings were evident, with statistically significant differences in favour of LABA/LAMA observed for some of the eosinophil cut-offs; at no cut-off was LABA/ICS superior [49]. The findings from these studies have implications for COPD management, in particular for patients with a history of exacerbation, and suggest that blood eosinophils may not be useful in guiding therapy choice between a LABA/LAMA and a LABA/ICS, but some evidence exists for a role of increased blood eosinophils in identifying patients who may benefit from ICS on top of LABA/LAMA treatment. Further ongoing prospective studies may clarify further the role of blood eosinophils in the identification of COPD patients who may benefit from triple therapy (LABA/ICS/LAMA).
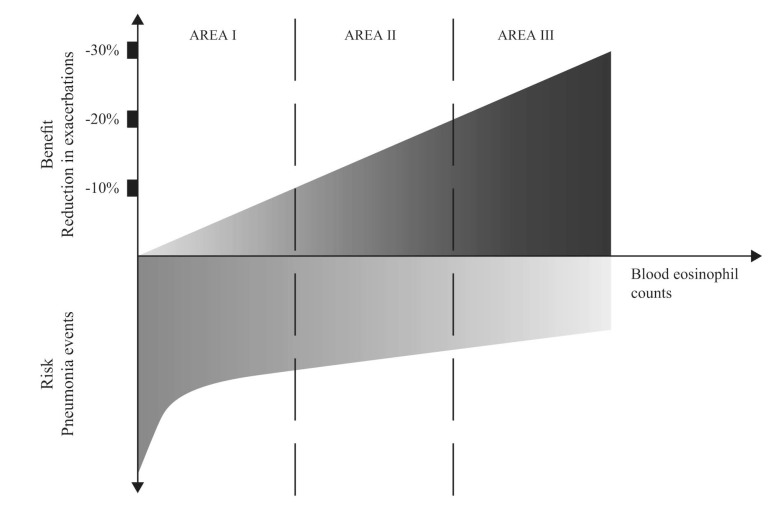
Due to the discrepancies reported in studies conducted to date, more research is needed to determine whether blood eosinophils will have utility as a biomarker with which to direct ICS use. This is particularly pertinent given that ICS use in COPD patients is clearly not without risk and has been linked with increased pneumonia incidence, as well as with other comorbidities, especially with long-term use. Indeed, a higher risk of pneumonia incidence has been observed in patients treated with ICS who have eosinophils <2% compared with those with ≥2% [94]. This aligns with the recommendation by Brusselle et al. [95-97] that ICS should be used in COPD patients in a risk-directed fashion, and that the benefit–risk ratio can be categorized into three areas, each modulated by blood eosinophil count (Fig. 3).
The positive findings by Pascoe et al. [86] and others need to be replicated and validated in independent prospective studies, including randomized trials and observational cohort studies. If confirmed, blood eosinophil count will provide a means of targeting the use of ICS to COPD patients who will benefit the most, and improving the currently modest benefit–risk ratio.
Conclusion and future directions
Both asthma and COPD are complex, heterogeneous conditions comprising a wide range of phenotypes, some of which are refractory to currently available treatments. Elucidation of these phenotypes and identification of biomarkers with which to recognize them and guide appropriate treatment remain a priority for researchers and clinicians. In recent years, the potential of blood eosinophils as surrogate markers of eosinophilic-predominant phenotypes has received much attention in this regard. In asthma, the rationale for their use as such is more clearly defined, with several well-controlled studies demonstrating that patients with higher eosinophil counts are prone to more severe disease and poorer outcomes. As a result, new biologic therapies that target specific drivers of eosinophilic pathways (whether IgE or key cytokines) have been developed to tailor treatment to these patients.
In COPD, high blood eosinophil counts may predict a favorable response to ICS on top of LABA/LAMA, especially in patients with a history of frequent exacerbations, but the exact position and the definition of clinically significant eosinophilia in this setting need to be further refined. There may also be some potential for the use of blood eosinophils for the identification of patients who may benefit from targeted treatments; however, further data are needed in order to define these patients appropriately. In contrast to asthma, where the available data from prospective studies may provide some implications for clinical practice, the use of eosinophils in clinical practice in COPD needs to be evaluated further in prospective studies before firm conclusion can be drawn.
The need for biomarkers to identify patients who may benefit from treatments in airways disease is important in order to reach informed treatment decisions for the maximal benefit of patients. Blood eosinophils present some merit, but several controversies remain. Standardization of the appropriate cut-off of clinically relevant eosinophilia and the need for single or multiple measurements in different settings are needed prior to the application in clinical practice. As more treatment options are becoming available, further research may be oriented towards the identification of novel surrogate biomarkers for certain elements or activation states of eosinophilic inflammation and/or composite indexes of different biomarkers that will support the selection of treatment options targeting specific pathways and pathology.
Fig. (1).
Allergic and non-allergic eosinophilic pathways in asthma. Adapted from [31] with the publisher’s permission.
Fig. (2).
Relationship between airway inflammation and bronchial abnormalities in COPD. Increased bronchial wall thickness, increased bronchial smooth muscle tone, mucus hypersecretion, and loss of elastic structures as a result of airway inflammation each contribute to alterations in bronchial structure and function. Reproduced/adapted from [96] and [97] with permission from the publisher.
Fig. (3).
Benefit–risk ratio of ICS in patients with COPD based on blood eosinophil level in stable disease. With increasing eosinophils counts, the use of ICS may offer increased benefit by reducing COPD exacerbations (Area III). Conversely, ICS use in patients with lower eosinophils counts is potentially associated with decreased benefit and increased risk of pneumonia (Area I). Adapted from [95] with the publisher’s permission.
Table 1.
Asthma: summary of studies.
| Study (Ref) | Design/Patients/Eosinophil cut-off | Outcomes | ||
|---|---|---|---|---|
| Higher baseline blood EOS may be associated with greater risk of exacerbation/predict future exacerbations | ||||
| Price et al. 20151 [51] Population study |
Primary care atopic asthma on standard Rx and with EOS records Stratified by baseline EOS: ≤400 or >400/μl |
Blood EOS >400 cells/μl associated with more severe exacerbations Exac rates ↑ progressively with ↑ EOS count Blood EOS >400 cells/μl associated with poorer asthma control |
||
| Busse et al. 20131 [58] | Symptomatic, atopic asthma, uncontrolled by ICS ± other controllers Add-on omalizumab or placebo Stratified by baseline EOS: ≤300 or >300/μl |
Pbo-treated pts with blood EOS ≥300/μl had higher exacerbation rates vs those with <300/μl High EOS a prognostic indicator for increased exacerbation risk |
||
| Higher baseline blood EOS predicts responsiveness to anti-IgE therapeutic targets | ||||
| Hanania et al. 20132 [57] | Severe allergic asthma for ≥ 1 year; uncontrolled despite high-dose LAB/ICS Add-on omalizumab vs placebo Baseline EOS: <260 vs ≥260 vs cells/μl |
Reductions in exacerbations significantly greater vs placebo in high EOS gps (P = 0.005) | ||
| Busse et al. 20131 [58] | Atopic asthma, symptomatic and uncontrolled by ICS +/- other controllers Add-on omalizumab or placebo Stratified by baseline EOS: ≤300 or >300/μl |
Omalizumab treatment resulted in a 59% reduction in exacerbations versus placebo (P = 0.0125) in the subgroup of pts with EOS >300/μl | ||
| Higher baseline blood EOS predicts responsiveness to anti-IL5 therapeutic targets | ||||
| Pavord et al. 20121 [62] DREAM |
Severe eosinophilic asthma (>3% or 300 cells/μl) on ICS ± other controllers; ≥2 severe exacerbation in previous year Add-on mepolizumab vs PBO (52 wks) |
Add-on mepolizumab significantly ↓ exacerbation rate and time to first exacerbation vs placebo (all doses P ≤0.0005); circulating blood EOS also significantly reduced vs placebo. Efficacy ↑ with ↑blood EOS count and exacerbation history |
||
| Katz et al. 20141 [63] DREAM |
Severe eosinophilic asthma (>3% or >300 cells/μl) on ICS ± other controllers; ≥2 severe exacerbation in previous year Placebo patients only |
Blood but NOT sputum EOS counts of ≥150/μl at screening predicted subsequent measurements being ≥150/μl in 85% of the population and predicts responsiveness to mepolizumab |
||
| Ortega et al. 20141 [64] MENSA |
Severe eosinophilic asthma (>150/μl at screening or ≥300/μl in last yr) on ICS ± other controllers; ≥2 severe exacerbation in previous year Add-on mepolizumab vs PBO (32 wks) |
Mepolizumab significantly ↓ exacerbation rate vs placebo (P < 0.001) Also associated with significantly improved FEV1 (P <0.05), and both QoL (SGRQ) and asthma control vs placebo(ACQ-5); both P <0.001) |
||
| Bel et al. 20141 [65] SIRIUS |
Severe eosinophilic asthma (>150/μl at run-in or ≥ 300/μl in last yr) and history of systemic glucocorticosteroids; on high-dose ICS + other controller Add-on mepolizumab vs PBO (20 wks) |
Mepolizumab significantly reduced need for glucocorticoids vs placebo-treated pts (2.39 x ↓ vs PBO; P = 0.007) Exacerbation rate reduced by 32% (P=0.04) vs PBO despite reduced steroid dose Significant reduction in ACQ-5 also vs placebo (P = 0.004) |
||
| Ortega et al. 20161 [68] Pooled re-analysis of DREAM/MENSA |
Severe eosinophilic asthma on ICS ± other controllers; ≥2 severe exacerbation in previous year Add-on mepolizumab vs placebo |
Exacerbation rates ↓ progressively with ↑EOS count (≥100 cells/μl = 52%↓; 500 cells/μl = 70%↓ vs PBO); EOS <150/μl predicted ↓ mepolizumab activity Further evidence showing blood EOS are associated with response to anti-IL5 therapy and serve as a robust marker for patient selection |
||
| Study (Ref) | Design/Patients/Eosinophil cut-off | Outcomes | ||
| Higher baseline blood EOS predict responsiveness to anti- IL5 therapeutic targets (continued) | ||||
| Castro et al. 20153 [69] Two phase 3 RCTs |
Inadequately controlled, moderate-to-severe eosinophilic asthma (≥400 cells/μl during screening) Add-on reslizumab vs PBO (52 wks) |
In both studies, add-on reslizumab significantly ↓ exacerbations vs PBO (by 50% and 59%; both P <0.0001) Significant improvements vs PBO were also observed for FEV1, AQLQ, ACQ-7 and ASUI, and blood EOS were significantly depleted in both studies |
||
| Corren et al. 2016 [70] | Any asthma patient poorly controlled by at least a medium-dose ICS; baseline EOS measured but not selected for Add-on reslizumab vs PBO (16 weeks) |
No differences between reslizumab and PBO in overall population (ie, any baseline EOS) In pts with ≥400/μl clinically meaningful improvements seen with reslizumab vs PBO in FEV1, ACQ-7, rescue SABA use, and FVC; no meaningful trends in pts with <400 cells/μl |
||
| Bjermer et al. 20164 [71] | Eosinophilic asthma (≥400/μl EOS at screening) inadequately controlled by at least a medium-dose ICS Add-on reslizumab (2 doses) vs PBO (16 wks) |
Significant and/or clinically meaningful differences vs PBO for both reslizumab doses for most efficacy measures; both doses also reduced EOS levels; 3.0 mg/kg IV dose provides ↑ improvements in lung function, asthma symptoms (ACQ), and quality of life (AQLQ) vs placebo than seen with 0.3 mg dose | ||
| Brusselle et al. 20173 [72] Re-analysis of Castro et al [69] Stratified by late vs early-onset asthma (<40 vs ≥40 yrs) |
Inadequately controlled, moderate-to-severe eosinophilic asthma (≥400 cells/μl during screening) Add-on reslizumab vs PBO (52 wks) |
Significantly greater exacerbation ↓ vs PBO in patients with late-onset (≥40 yrs) eosinophilic asthma versus early-onset asthma (P = 0.0083) Larger improvements in FEV1, ACQ-6 and ASUI were also observed vs early-onset patients |
||
| Bleecker et al. 20161 [75] SIROCCO |
Severe asthma uncontrolled by medium/ high-dose ICS + LABA for ≥1 year; ≥2 exacerbations in previous year Baseline stratification: EOS < 300 and ≥ 300 cells/μl Add-on benralizumab (2 doses) vs PBO (48 wks) |
Both doses of benralizumab significantly ↓ exacerbation rate vs PBO (by 45 and 51%; both P <0.0001) Both doses also significantly improved prebronchodilator FEV1 in patients at week 48 vs PBO; significant improvements in symptom control vs PBO were observed for one of the doses Improved efficacy (exacerbation ↓, FEV1 and symptom control) in pts with frequent exacerbation history (≥3 in the previous year) |
||
| Fitzgerald et al. 20161 [76] CALIMA |
Pts as above and stratified by baseline EOS: <300 and ≥300 cells/μl Add-on benralizumab (2 doses) vs PBO (56 wks) |
Both doses significantly ↓ exacerbation rate vs PBO (by 36 and 28%; both P=0.0188) Both doses also significantly improved FEV1 at Wk 48 vs PBO and significant improvement in symptom control vs PBO was also observed for one of the doses. Improved efficacy (exacerbation ↓, FEV1 and symptom control) in pts with frequent exacerbation history (≥3 in the previous year) |
||
| Baseline blood EOS and responsiveness to anti-IL4/IL13 targets | ||||
| Wenzel et al. 20135 [77] | Persistent moderate/severe eosinophilic asthma (≥300 cells per μl or 3%) not controlled by med/high dose ICS + LABA Dupilumab vs placebo after LABA/ICS withdrawal (12 wks) |
Dupilumab was associated with an 87% ↓ in exacerbations and significantly reduced time to exacerbation vs placebo (both P<0.001); Significant improvements in lung function and asthma control were also observed; dupilumab vs PBO also ↓ some Th2-associated biomarkers though not EOS levels |
||
| Wenzel et al. 20161 [78] | Persistent moderate/severe asthma uncontrolled by med/high dose ICS + LABA; all pts regardless of baseline EOS Dupilumab vs placebo after LABA/ICS withdrawal (24 wks) |
Dupilumab ↓severe exacerbations and ↑FEV1 and PROs irrespective of baseline EOS | ||
Asthma exacerbations were defined as:
1Worsening of symptoms requiring treatment with systemic corticosteroids (and/or doubling or more of the existing dose of chronic OCS) for ≥3 days, hospitalization or an emergency room visit 2Definition 1 or (for patients on OCS) an increase of ≥20 mg in daily OCS dose
3Definition 1 plus a decrease from baseline in FEV1 of ≥20% and PEF of ≥30% on 2 consecutive days 4Definition 1 plus a decrease from baseline in FEV1 of ≥20%
5Any one of Definition 1 or a decrease from baseline in PEF of ≥30% or ≥6 additional relief medication inhalations on 2 consecutive days
Abbreviations: ACQ, Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; ASUI, Asthma Symptom Utility Index; EOS, eosinophil; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; OCS, oral corticosteroid; PBO, placebo; PRO, patient-reported outcome; SABA, short-acting beta-agonist; SGRQ, St. George's Respiratory Questionnaire; Th2, Type 2 helper.
Table 2.
COPD: summary of studies.
| Study (Ref) | Design/Patients/Eosinophil cut-off | Outcomes1 / Conclusion | |
|---|---|---|---|
| Blood EOS predict EOS-predominant exacerbation phenotypes | |||
| Bafadhel et al. 2011 [34] | COPD (FEV1/FVC <70%) GOLD I-IV; ≥1 exacerbation in previous year | 28% of exacerbations associated with sputum eosinophilia of >3% ≥2% blood EOS identified sputum eosinophilia cluster with 90% sensitivity/60% specificity; AURoC 0.85 (95% CI, 0.78–0.93) |
|
| Blood EOS predicts airways eosinophilic inflammation | |||
| Schleich et al. 2016 [42] | Stable COPD (FEV1/FVC ratio <70%) CAT ≥10 in 97% of pts Consecutive clinic patients – various treatments |
Blood EOS count >162 per μl (or 2.6%) identified pts with sputum EOS ≥3% with moderate sensitivity/high specificity (P<0.0001) Blood EOS can be used to guide and adjust ICS treatment in pts with recurring exacerbations |
|
| Higher baseline blood EOS predicts risk of COPD exacerbation | |||
| Vedel-Krogh et al. 2016 [84] Prospective population analysis |
All COPD (FEV1/FVC ratio <70%) | EOS counts > 340 cells/μl (or 3.3%) at baseline associated with a 1.76x ↑ risk of severe exacerbation (1.15x ↑ risk of moderate); EOS absolute counts more accurate than % differential | |
| Kerkhof et al. 2017 [85] Historical population study |
All COPD (FEV1/FVC ratio <70%) Threshold: ≥450 cells/μl versus reference range of 50-450 cells/μl |
Pts with ≥450 cells/μl had 13% ↑ exacerbation rate during following year (P = 0.03); subgroup analysis showed ↑ rates restricted to ex-smokers with elevated counts (RR vs ref gp: 1.30; 95% CI 1.14–1.48; P <0.0001): current smokers with elevated counts had lowest rate (RR 0.89; 95% CI 0.73–1.08; P = 0.14) | |
| Higher baseline blood EOS predicts ICS responsiveness | |||
| Pascoe et al. 2015 [86] Post-hoc pooled analysis of 2 replicate studies |
Stable moderate-to-severe COPD (FEV1/FVC ratio <70%); ≥1 exacerbation in previous year FF/VI vs VI <2% vs ≥2% baseline EOS |
EOS ≥2% (or ≥150 cells/μl) improves responsiveness to LABA/ICS vs LABA alone 29% reduction in exacerbations vs pts with EOS <2%; greater Rx differences observed with ↑% EOS In VI arm (ie, no ICS) exacerbations ↑ with ↑% EOS Trough FEV1 at Wk 52 also ↑ increased in FF/VI pts with EOS ≥2% |
|
| Siddiqui et al. 2015 [87] Post-hoc analysis of FORWARD |
Severe COPD; ≥1 exacerbation in previous year BDP/FF vs FF Various EOS cut-offs: 0 to <110.4, to <181.6, <279.8 to ≥279.8 cells/μl |
LABA/ICS ↓exacerbations vs LABA alone in patients with ↑EOS counts; progressively better responses with increasing baseline EOS (greatest Rx difference in pts with ≥279.8/μl) Significant improvements in FEV1 and SGRQ also observed for LABA/ICS vs LABA particularly in EOS ≥279.8/μl group |
|
| Pavord et al. 2016 [88]: Post-hoc analyses: 3 RCTs INSPIRE TRISTAN SCO30002 |
INSPIRE: Severe/very severe COPD; exacerbation history in previous year SAL/FP vs TIO <2% vs ≥2% baseline EOS |
In pts with ≥2% EOS LABA/ICS significantly ↓ exacerbation rates vs LAMA (P=0.006); no significant differences in the <2% arm No relationship between eosinophil subgroup and FEV1 or SGRQ |
|
| TRISTAN: Moderate-to- severe COPD; ≥1 exacerbation in previous 3 years SAL/FP vs SAL, FP, or PBO <2% vs ≥2% baseline EOS |
In pts with ≥2% EOS LABA/ICS significantly ↓exacerbation rates vs PBO (P<0.001) as did LABA alone and ICS alone (P = 0.02 and P = 0.005, respectively); numerical but not significant differences observed vs LABA or LAMA; no significant differences in the <2% arm No relationship between eosinophil subgroup and FEV1 or SGRQ |
||
|
SCO30002: Moderate-to-very severe COPD with or without exacerbation history SAL/FP vs SAL, FP or PBO <2% vs ≥2% baseline EOS: |
Numerical but no significant diffs vs LABA, LAMA or PBO for LABA/ICS-treated patients with baseline EOS ≥2% No relationship between eosinophil subgroup and FEV1 or SGRQ |
||
| Study (Ref) | Design/Patients/Eosinophil cut-off | Outcomes1 / Conclusion | |
| Higher baseline blood EOS predicts ICS responsiveness (continued) | |||
| Barnes et al. 2016 [89] Posthoc analysis of ISOLDE |
Moderate-to-severe COPD (post-bronchodilator FEV1 ≥0.8 L; ≤85% predicted normal) FP vs PBO <2% vs ≥2% baseline EOS |
In pts with EOS <2%, ICS significantly ↓ exacerbation rates vs PBO (P=0.009); no significant difference vs PBO with respect to exacerbation rate in pts with ≥2% EOS Pts with EOS ≥2% and treated with FP had significantly slower FEV1 decline vs PBO (P=0.003); no difference between <2% groups Baseline EOS ≥2% identifies with slower worsening of FEV1 |
|
| Watz et al. 2016 [91] Post-hoc analysis of WISDOM |
Severe/very severe COPD (GOLD 3-4); history of ≥1 exacerbation in 12-month pre-screening period Triple therapy for 6 wks (TIO/SAL/FP) then 1:1 to ICS withdrawn or continued (52 weeks) Various EOS thresholds 2% to ≥6%, and 150 to 400 cells/μl |
Exacerbation rate more pronounced as baseline EOS rose (for both % differential or total count/μl) Baseline EOS of ≥4% (or ≥300 cells/μl) may identify patients at increased risk of exacerbations |
|
| Calverley et al. 2017 [92] Further post-hoc analysis of WISDOM |
Pts as above Data revaluated using with respect to exacerbation history (1 vs ≥2) |
Rx effect more prominent in patients with ≥2 exacerbations and higher eosinophil count (≥400) | |
| Wedzicha et al. 2016 [93] Prospective analysis |
Moderate-to-severe COPD (FEV1/FVC ratio <70%); ≥1 exacerbation in previous year IND/GLY vs SFC EOS: <2% vs ≥2% |
LABA/LAMA significantly more effective at reducing annualized exacerbation rate vs LABA/ICS irrespective of % EOS baseline stratification | |
| Roche et al. 2017 [49] Prospective and post-hoc analyses of FLAME |
Pts as above Data re-evaluated using different thresholds: <3%, or <5%; <150, <300, ≥300 cells/μl |
LABA/LAMA superior/similar to LABA/ICS irrespective of eosinophil cut points | |
1Exacerbations were generally defined as moderate if requiring treatment with systemic corticosteroids and/or antibiotics and severe if requiring hospitalization.
Abbreviations: AUROC, Area under Receiver Operating Curve; BDP, beclomethasone dipropionate; CAT, COPD Assessment Test; CI, confidence interval; EOS, eosinophil; FEV1, forced expiratory volume in 1 sec; FF, fluticasone furoate; FP, fluticasone propionate; FVC, forced vital capacity; ICS, inhaled corticosteroid; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IND/GLY, indacaterol/glycopyronnium; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; PBO, placebo; RR, rate ratio; SAL/FC, salmeterol/fluticasone; SGRQ, St. George's Respiratory Questionnaire; TIO, tiotropium; VI, vilanterol.
Acknowledgements
The authors take full responsibility for the scope, direction, content and editorial decisions relating to the manuscript, and were involved at all the stages of its development, to approve the final version and agreed to its submission to this journal. Medical writing assistance was provided by Colette O’Sullivan, PhD, of Scriva Medical Communications Ltd, funded by Novartis.
List of abbreviations
- ACQ-5
5-item Asthma Control Questionnaire
- ASUI
Asthma Symptom Utility Index
- FEV1
Forced expiratory volume in 1 second
- CI
Confidence interval
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroid
- IL
Interleukin
- ILC2
Type-2 innate lymphoid cells
- IRR
Incidence rate ratio
- GINA
Global Initiative for Asthma
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- LABA
Long-acting beta-agonist
- LAMA
Long-acting muscarinic antagonist
- RR
Rate ratio
- SGRQ
St. George's Respiratory Questionnaire
- Th2
Type 2 helper
Consent for Publication
Not applicable.
Conflict of interest
KK, CB and FF are Novartis employees and shareholders. KK has received honoraria for speeches and consulting services from AstraZeneca, Chiesi, ELPEN and Takeda, and received honoraria for speeches from Boehringer Ingelheim, outside the submitted work.
References
- 1.Gibson P.G., McDonald V.M. Asthma-COPD overlap 2015: now we are six. Thorax. 2015;70(7):683–691. doi: 10.1136/thoraxjnl-2014-206740. [DOI] [PubMed] [Google Scholar]
- 2.Barnes P.J. Asthma-COPD overlap. Chest. 2016;149(1):7–8. doi: 10.1016/j.chest.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Agusti A., Bel E., Thomas M., et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur. Respir. J. 2016;47(2):410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 4.Heaney L.G., McGarvey L.P. Personalised medicine for asthma and chronic obstructive pulmonary disease. Respiration. 2017;93(3):153–161. doi: 10.1159/000455395. [DOI] [PubMed] [Google Scholar]
- 5.Kraft M. Asthma phenotypes and interleukin-13--moving closer to personalized medicine. N. Engl. J. Med. 2011;365(12):1141–1144. doi: 10.1056/NEJMe1108666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingleton J., Travers J., Williams M., et al. Treatment responsiveness of phenotypes of symptomatic airways obstruction in adults. J. Allergy Clin. Immunol. 2015;136(3):601–609. doi: 10.1016/j.jaci.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Agusti A., Sobradillo P., Celli B. Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am. J. Respir. Crit. Care Med. 2011;183(9):1129–1137. doi: 10.1164/rccm.201009-1414PP. [DOI] [PubMed] [Google Scholar]
- 8.Wadsworth S., Sin D., Dorscheid D. Clinical update on the use of biomarkers of airway inflammation in the management of asthma. J. Asthma Allergy. 2011;4:77–86. doi: 10.2147/JAA.S15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schleich F., Demarche S., Louis R. Biomarkers in the management of difficult asthma. Curr. Top. Med. Chem. 2016;16(14):1561–1573. doi: 10.2174/1568026616666151015093406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 11.GINA 2017 http://ginasthma.org/
- 12.Kostikas K., Bakakos P., Papiris S., Stolz D., Celli B.R. Systemic biomarkers in the evaluation and management of COPD patients: are we getting closer to clinical application? Curr. Drug Targets. 2013;14(2):177–191. doi: 10.2174/1389450111314020005. [DOI] [PubMed] [Google Scholar]
- 13.GOLD 2017 http:// goldcopd.org
- 14.Fahy J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh D., Kolsum U., Brightling C.E., et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur. Respir. J. 2014;44(6):1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 16.Fulkerson P.C., Rothenberg M.E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 2013;12(2):117–129. doi: 10.1038/nrd3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George L., Brightling C.E. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis. 2016;7(1):34–51. doi: 10.1177/2040622315609251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodruff P.G., Modrek B., Choy D.F., et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson D., Humbert M., Buhl R., et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin. Exp. Allergy. 2017;47(2):161–175. doi: 10.1111/cea.12880. [DOI] [PubMed] [Google Scholar]
- 20.Barnes P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016;138(1):16–27. doi: 10.1016/j.jaci.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Tashkin D.P., Wechsler M.E. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2018;13:335–349. doi: 10.2147/COPD.S152291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson M.W. Eosinophil activation status in separate compartments and association with asthma. Front. Med. (Lausanne) 2017;4:75. doi: 10.3389/fmed.2017.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pazdrak K., Moon Y., Straub C., Stafford S., Kurosky A. Eosinophil resistance to glucocorticoid-induced apoptosis is mediated by the transcription factor NFIL3. Apoptosis. 2016;21(4):421–431. doi: 10.1007/s10495-016-1226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yancey S.W., Keene O.N., Albers F.C., et al. Biomarkers for severe eosinophilic asthma. J. Allergy Clin. Immunol. 2017;140(6):1509–1518. doi: 10.1016/j.jaci.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Cottin V., Deviller P., Tardy F., Cordier J.F. Urinary eosinophil-derived neurotoxin/protein X: a simple method for assessing eosinophil degranulation in vivo. J. Allergy Clin. Immunol. 1998;101(1 Pt 1):116–123. doi: 10.1016/S0091-6749(98)70202-7. [DOI] [PubMed] [Google Scholar]
- 26.Young S, Tigerström A. 2017.
- 27.Wenzel S.E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 28.Schleich F., Brusselle G., Louis R., et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir. Med. 2014;108(12):1723–1732. doi: 10.1016/j.rmed.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Tabatabaian F., Ledford D.K., Casale T.B. Biologic and new therapies in asthma. Immunol. Allergy Clin. North Am. 2017;37(2):329–343. doi: 10.1016/j.iac.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Ntontsi P., Loukides S., Bakakos P., et al. Clinical, functional and inflammatory characteristics in patients with paucigranulocytic stable asthma: Comparison with different sputum phenotypes. Allergy. 2017 doi: 10.1111/all.13184. [DOI] [PubMed] [Google Scholar]
- 31.Brusselle G.G., Maes T., Bracke K.R. Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma. Nat. Med. 2013;19(8):977–979. doi: 10.1038/nm.3300. [DOI] [PubMed] [Google Scholar]
- 32.de Nijs S.B., Venekamp L.N., Bel E.H. Adult-onset asthma: is it really different? Eur. Respir. Rev. 2013;22(127):44–52. doi: 10.1183/09059180.00007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuder R.M., Kern J.A., Miller Y.E. Senescence in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2012;9(2):62–63. doi: 10.1513/pats.201201-012MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bafadhel M., McKenna S., Terry S., et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 35.Saetta M., Di Stefano A., Maestrelli P., et al. Airway eosinophilia in chronic bronchitis during exacerbations. Am. J. Respir. Crit. Care Med. 1994;150(6 Pt 1):1646–1652. doi: 10.1164/ajrccm.150.6.7952628. [DOI] [PubMed] [Google Scholar]
- 36.Bafadhel M., McKenna S., Terry S., et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2012;186(1):48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagener A.H., de Nijs S.B., Lutter R., et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. doi: 10.1136/thoraxjnl-2014-205634. [DOI] [PubMed] [Google Scholar]
- 38.Green R.H., Brightling C.E., McKenna S., et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 39.Siva R., Green R.H., Brightling C.E., et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur. Respir. J. 2007;29(5):906–913. doi: 10.1183/09031936.00146306. [DOI] [PubMed] [Google Scholar]
- 40.de Groot J.C., Ten Brinke A., Bel E.H. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. 2015;1(1) doi: 10.1183/23120541.00024-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schleich F.N., Manise M., Sele J., et al. Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm. Med. 2013;13:11. doi: 10.1186/1471-2466-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schleich F., Corhay J.L., Louis R. Blood eosinophil count to predict bronchial eosinophilic inflammation in COPD. Eur. Respir. J. 2016;47(5):1562–1564. doi: 10.1183/13993003.01659-2015. [DOI] [PubMed] [Google Scholar]
- 43.Bacci E., Cianchetti S., Ruocco L., et al. Comparison between eosinophilic markers in induced sputum and blood in asthmatic patients. Clin. Exp. Allergy. 1998;28(10):1237–1243. doi: 10.1046/j.1365-2222.1998.00341.x. [DOI] [PubMed] [Google Scholar]
- 44.Schleich F.N., Chevremont A., Paulus V., et al. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur. Respir. J. 2014;44(1):97–108. doi: 10.1183/09031936.00201813. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee M., Nair P. Blood or sputum eosinophils to guide asthma therapy? Lancet Respir. Med. 2015;3(11):824–825. doi: 10.1016/S2213-2600(15)00419-1. [DOI] [PubMed] [Google Scholar]
- 46.Hastie A.T., Moore W.C., Li H., et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J. Allergy Clin. Immunol. 2013;132(1):72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGrath K.W., Icitovic N., Boushey H.A., et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am. J. Respir. Crit. Care Med. 2012;185(6):612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oshagbemi O.A., Burden A.M., Braeken D.C., et al. Stability of blood eosinophils in copd and controls and the impact of gender, age, smoking and baseline counts. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201701-0009LE. [DOI] [PubMed] [Google Scholar]
- 49.Roche N., Chapman K.R., Vogelmeier C.F., et al. Blood eosinophils and response to maintenance copd treatment: data from the flame trial. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201701-0193OC. [DOI] [PubMed] [Google Scholar]
- 50.Casciano J., Krishnan J.A., Small M.B., et al. Value of peripheral blood eosinophil markers to predict severity of asthma. BMC Pulm. Med. 2016;16(1):109. doi: 10.1186/s12890-016-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price D.B., Rigazio A., Campbell J.D., et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir. Med. 2015;3(11):849–858. doi: 10.1016/S2213-2600(15)00367-7. [DOI] [PubMed] [Google Scholar]
- 52.Jatakanon A., Lim S., Barnes P.J. Changes in sputum eosinophils predict loss of asthma control. Am. J. Respir. Crit. Care Med. 2000;161(1):64–72. doi: 10.1164/ajrccm.161.1.9809100. [DOI] [PubMed] [Google Scholar]
- 53.Brusselle G.G., Provoost S., Maes T. Prostaglandin D2 receptor antagonism: a novel therapeutic option for eosinophilic asthma? Lancet Respir. Med. 2016;4(9):676–677. doi: 10.1016/S2213-2600(16)30201-6. [DOI] [PubMed] [Google Scholar]
- 54.Gonem S., Berair R., Singapuri A., et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: a single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir. Med. 2016;4(9):699–707. doi: 10.1016/S2213-2600(16)30179-5. [DOI] [PubMed] [Google Scholar]
- 55.Busse W., Corren J., Lanier B.Q., et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 2001;108(2):184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 56.Yalcin A.D. An overview of the effects of anti-IgE therapies. Med. Sci. Monit. 2014;20:1691–1699. doi: 10.12659/MSM.890137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanania N.A., Wenzel S., Rosen K., et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013;187(8):804–811. doi: 10.1164/rccm.201208-1414OC. [DOI] [PubMed] [Google Scholar]
- 58.Busse W., Spector S., Rosen K., Wang Y., Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J. Allergy Clin. Immunol. 2013;132(2):485–6.e11. doi: 10.1016/j.jaci.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 59.Rodrigo G.J., Neffen H., Castro-Rodriguez J.A. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139(1):28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 60.Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 2011;87(8):463–485. doi: 10.2183/pjab.87.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farne H.A., Wilson A., Powell C., Bax L., Milan S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst. Rev. 2017;9:Cd010834. doi: 10.1002/14651858.CD010834.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavord I.D., Korn S., Howarth P., et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 63.Katz L.E., Gleich G.J., Hartley B.F., Yancey S.W., Ortega H.G. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann. Am. Thorac. Soc. 2014;11(4):531–536. doi: 10.1513/AnnalsATS.201310-354OC. [DOI] [PubMed] [Google Scholar]
- 64.Ortega H.G., Liu M.C., Pavord I.D., et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 65.Bel E.H., Wenzel S.E., Thompson P.J., et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014;371(13):1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 66.Ortega H., Katz L., Gunsoy N., Keene O., Yancey S. Blood eosinophil counts predict treatment response in patients with severe eosinophilic asthma. J. Allergy Clin. Immunol. 2015;136(3):825–826. doi: 10.1016/j.jaci.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 67.Fowler S.J., Tavernier G., Niven R. High blood eosinophil counts predict sputum eosinophilia in patients with severe asthma. J. Allergy Clin. Immunol. 2015;135(3):822–4.e2. doi: 10.1016/j.jaci.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 68.Ortega H.G., Yancey S.W., Mayer B., et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir. Med. 2016;4(7):549–556. doi: 10.1016/S2213-2600(16)30031-5. [DOI] [PubMed] [Google Scholar]
- 69.Castro M., Zangrilli J., Wechsler M.E., et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir. Med. 2015;3(5):355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 70.Corren J., Weinstein S., Janka L., Zangrilli J., Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150(4):799–810. doi: 10.1016/j.chest.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 71.Bjermer L., Lemiere C., Maspero J., et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150(4):789–798. doi: 10.1016/j.chest.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Brusselle G., Germinaro M., Weiss S., Zangrilli J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm. Pharmacol. Ther. 2017;43:39–45. doi: 10.1016/j.pupt.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 73.Ghazi A., Trikha A., Calhoun W.J. Benralizumab--a humanized mAb to IL-5Ralpha with enhanced antibody-dependent cell-mediated cytotoxicity--a novel approach for the treatment of asthma. Expert Opin. Biol. Ther. 2012;12(1):113–118. doi: 10.1517/14712598.2012.642359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rudulier C.D., Larche M., Moldaver D. Treatment with anti-cytokine monoclonal antibodies can potentiate the target cytokine rather than neutralize its activity. Allergy. 2016;71(3):283–285. doi: 10.1111/all.12816. [DOI] [PubMed] [Google Scholar]
- 75.Bleecker E.R., FitzGerald J.M., Chanez P., et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 76.FitzGerald J.M., Bleecker E.R., Nair P., et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 77.Wenzel S., Ford L., Pearlman D., et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 78.Wenzel S., Castro M., Corren J., et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 79.Zysman M., Deslee G., Caillaud D., et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:1819–1824. doi: 10.2147/COPD.S129787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brightling C.E., Bleecker E.R., Panettieri R.A., Jr, et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir. Med. 2014;2(11):891–901. doi: 10.1016/S2213-2600(14)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dasgupta A., Kjarsgaard M., Capaldi D., et al. A pilot randomised clinical trial of mepolizumab in COPD with eosinophilic bronchitis. Eur. Respir. J. 2017;49(3) doi: 10.1183/13993003.02486-2016. [DOI] [PubMed] [Google Scholar]
- 82.Pavord I.D., Chanez P., Criner G.J., et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N. Engl. J. Med. 2017 doi: 10.1056/NEJMoa1708208. [DOI] [PubMed] [Google Scholar]
- 83.McDonald C.F. Eosinophil biology in COPD. N. Engl. J. Med. 2017 doi: 10.1056/NEJMe1710326. [DOI] [PubMed] [Google Scholar]
- 84.Vedel-Krogh S., Nielsen S.F., Lange P., Vestbo J., Nordestgaard B.G. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. the copenhagen general population study. Am. J. Respir. Crit. Care Med. 2016;193(9):965–974. doi: 10.1164/rccm.201509-1869OC. [DOI] [PubMed] [Google Scholar]
- 85.Kerkhof M., Sonnappa S., Postma D.S., et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur. Respir. J. 2017;50(1) doi: 10.1183/13993003.00761-2017. [DOI] [PubMed] [Google Scholar]
- 86.Pascoe S., Locantore N., Dransfield M.T., Barnes N.C., Pavord I.D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 2015;3(6):435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 87.Siddiqui S.H., Guasconi A., Vestbo J., et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015;192(4):523–525. doi: 10.1164/rccm.201502-0235LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pavord I.D., Lettis S., Locantore N., et al. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax. 2016;71(2):118–125. doi: 10.1136/thoraxjnl-2015-207021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barnes N.C., Sharma R., Lettis S., Calverley P.M. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur. Respir. J. 2016;47(5):1374–1382. doi: 10.1183/13993003.01370-2015. [DOI] [PubMed] [Google Scholar]
- 90.Magnussen H., Disse B., Rodriguez-Roisin R., et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N. Engl. J. Med. 2014;371(14):1285–1294. doi: 10.1056/NEJMoa1407154. [DOI] [PubMed] [Google Scholar]
- 91.Watz H., Tetzlaff K., Wouters E.F., et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir. Med. 2016;4(5):390–398. doi: 10.1016/S2213-2600(16)00100-4. [DOI] [PubMed] [Google Scholar]
- 92.Calverley P.M., Tetzlaff K., Vogelmeier C., et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201612-2525LE. [DOI] [PubMed] [Google Scholar]
- 93.Wedzicha J.A., Banerji D., Chapman K.R., et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N. Engl. J. Med. 2016;374(23):2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 94.Pavord I.D., Lettis S., Anzueto A., Barnes N. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir. Med. 2016;4(9):731–741. doi: 10.1016/S2213-2600(16)30148-5. [DOI] [PubMed] [Google Scholar]
- 95.Brusselle G.G., Bracke K., Lahousse L. Targeted therapy with inhaled corticosteroids in COPD according to blood eosinophil counts. Lancet Respir. Med. 2015;3(6):416–417. doi: 10.1016/S2213-2600(15)00145-9. [DOI] [PubMed] [Google Scholar]
- 96.Roche N., Marthan R., Berger P., et al. Beyond corticosteroids: future prospects in the management of inflammation in COPD. Eur. Respir. Rev. 2011;20(121):175–182. doi: 10.1183/09059180.00004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barnes P.J. Emerging pharmacotherapies for COPD. Chest. 2008;134(6):1278–1286. doi: 10.1378/chest.08-1385. [DOI] [PubMed] [Google Scholar]