Abstract
Sjogren’s syndrome (SS) is a T cell-mediated autoimmune disease of the systemic exocrine glands, such as salivary and lacrimal glands. A variety of T-cell subpopulations maintain immune tolerance in the thymus and periphery through complex immune responses including cellular and humoral immunity. The T-cell subpopulations exhibiting abnormal or unique phenotypes and impaired functionality have been reported to play important roles in the cellular mechanisms of autoimmunity in SS patients and animal models of SS. In this review, we focused on follicular helper T cells related to antibody production and regulatory T cells to control immune tolerance in the pathogenesis of SS. The unique roles of these T-cell subpopulations in the process of the onset or development of SS have been demonstrated in this review of recent publications. The clinical application of these T-cell subpopulations will be helpful for the development of new techniques for diagnosis or treatment of SS in the future.
Keywords: Autoimmunity, follicular helper T cell, regulatory T cell, Sjögren’s syndrome, phenotypes, exocrine
1. INTRODUCTION
Sjögren’s Syndrome (SS) is an autoimmune disease that targets exocrine glands, such as lacrimal and salivary glands. The primary clinical symptoms are sicca syndrome, including dry eyes and mouth [1, 2]. In addition, SS often accompanies other systemic autoimmune diseases, such as rheumatoid arthritis and lupus [3, 4]. Because SS has a complex pathogenesis, fundamental treatments for this disease have not yet been established [5].
SS is generally considered to be a T cell-mediated autoimmune disorder of the salivary and lacrimal glands. Several autoantigens in SS in humans and in mouse models of SS have been reported [6-9], and the relationship between autoreactive T cells and autoantigens in SS has been described. Moreover, anti-SSA and anti-SSB autoantibodies are widely known to be clinically useful autoantibodies in SS [10]. It appears that T- and B-cell responses during the onset or development of SS are differentially and intricately regulated by the interaction and communication of a variety of immune cells.
Follicular helper T (Tfh) cells are specialized providers of T cell help to B cells and are essential for Germinal Center (GC) formation, affinity maturation, and production of high-affinity antibodies [11-13]. Many studies have demonstrated that Tfh cells influence various immune responses, including autoimmunity and infection, in addition to other T-helper cell subsets [14, 15]. Tfh cells are increased in the peripheral blood and target organs in SS patients, together with enhanced memory B and GCB cells [16-19]. Therefore, Tfh cells have been highlighted for understanding the pathogenesis of SS.
Regulatory T (Treg) cells are one of the central players in complex immune responses required to maintain immune homeostasis [20-22]. In particular, increasing evidence suggests that Treg cells can suppress and control the autoimmune response to protect the body from autoimmune diseases in humans and in animal models [23-25]. It is also well known that Treg cells play potent roles in the onset and development of SS [26, 27].
In this review focusing on the pathogenesis of SS, the significant involvement of Tfh and Treg cells is highlighted to understand the precise molecular mechanisms of the pathogenesis of this disease.
2. ABNORMAL T-HELPER CELL SUBSETS IN SS
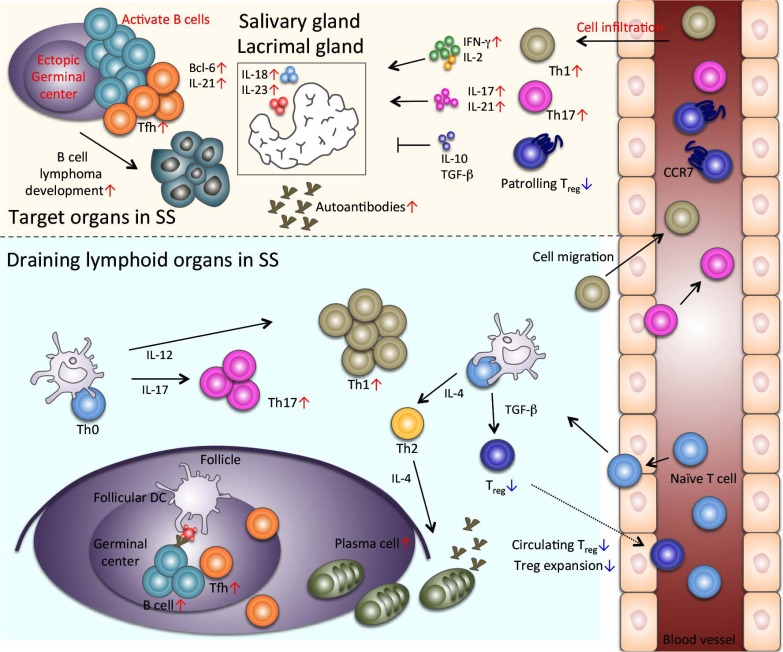
CD4+ T helper cells play a crucial role in the pathogenesis of SS (Fig. 1). A large population of CD4+ T cells and small numbers of B cells, CD8+ T cells, macrophages, and dendritic cells infiltrate the target organs, such as salivary and lacrimal glands, during the early stages of SS [28, 29]. With aging and disease progression, the infiltration of B cells or plasma cells increases in the autoimmune lesions. The majority of infiltrating CD4+ T cells exhibits a memory and/or activated phenotype in the salivary glands of SS patients [30-32]. The pathogenesis of SS is mediated by the Th1 derived responses [33, 34]. Moreover, elevated numbers of IFN-γ positive CD4+ T cells are detected in the salivary glands of SS patients, and intracellular cytokine analysis demonstrated the polarization of a Th1 phenotype [35]. Furthermore, interleukin (IL)-2 and IFN-γ are consistently detected in the target tissues form SS patients [36], whereas IL-4 and IL-5 are only detected in patients with high levels of B-cell accumulation in the salivary glands [37]. Several studies have evaluated cytokine profiles produced by a variety of cell types in SS patients. Among them, IL-10, IL-6, and Transforming Growth Factor beta (TGF-β) are also consistently detected in all patients, whereas IL-12 mRNA is only detected in some of the patients [38, 39]. Further, many reports demonstrated that the pathogenesis of SS in animal models is associated with Th1 cytokine-producing cells [38, 39].
Fig. (1).
Tfh cells and Treg cells in SS. Tfh cells are found in ectopic GC of autoimmune lesions in SS. Increased infiltrates of Th1 and Th17 cells are detected in the target tissues of SS. Patrolling Treg cells are significantly decreased in the target organs. In the draining lymphoid tissues of SS, Th1 and Th2 cells are increased. Expansion of Treg cells is impaired, and circulating Treg cells are reduced in SS.
Th17 cells are characterized by production of the proinflammatory cytokine, IL-17 and have been implicated in various immune responses, such as autoimmunity [40-44]. Th17 cells are observed in the autoimmune lesions of the salivary gland tissues of SS patients [27, 45]. In addition, IL-17 levels are significantly elevated in the sera of SS patients compared with that of the controls [46, 47]. Because Th17 cells also can produce IFN-γ, Th17 cells seem to play a critical role in the IFN-γ-mediated pathogenesis of SS. Furthermore, IL-18 and IL-23 produced by salivary epithelial cells also contribute to the pathogenesis of SS, activating or regulating Th17 cells [45].
3. TFH CELLS IN SS
Recently, Tfh cells have been identified as a CD4+ T-cell subset capable of activating B cells in the lymphoid organs [11-14]. Tfh cells play a crucial role in the formation and maintenance of the GCs of secondary lymphoid organs and the regulation of B-cell differentiation of memory B cells and plasma cells [11-14]. Tfh cells may also contribute to B-cell activation, as a hallmark of SS is the frequent association of Tfh cells with autoantibody secretion (Fig. 1). In addition, GC formation may be of critical importance for further clarification of the disease pathogenesis (Fig. 1).
Tfh cells highly express the CXC Chemokine Receptor 5 (CXCR5), which is critical for homing and signaling [14, 48]. Moreover, the phenotype of Tfh cells includes the expression of the surface receptor inducible T cell co-stimulator and programmed cell death protein 1 (PD-1) as well as the nuclear transcriptional repressor B-cell lymphoma 6 (Bcl-6) [14, 49]. In addition, IL-21 is another key cytokine produced by Tfh cells [50]. IL-21 plays an important role in B-cell differentiation and antibody production, in combination with other cytokines [14].
T cell-mediated autoimmune diseases have been well studied and demonstrated using several SS animal models and patients [33, 51-53]. However, it is unclear whether the B cell-dependent mechanisms contribute to the onset of autoimmunity. Thus, there is little evidence for understanding the pathogenesis of autoimmune diseases via B cell-mediated mechanisms. However, autoantibodies from different autoimmune diseases are probably related to the severity or symptoms of the disease [54, 55]. In this context, Tfh cells play an important role in the B-cell autoimmune responses. The presence of peripheral Tfh cells is one of the biomarkers of autoimmune diseases, such as myasthenia gravis, autoimmune thyroiditis, rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, type 1 diabetes, inflammatory bowel disease, and SS in both humans and animal models [17, 56-63].
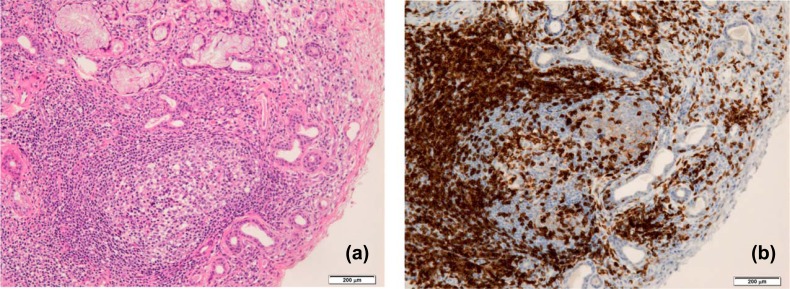
The ectopic GC formation is observed in the salivary gland tissues of SS patients by histological analysis (Fig. 2a). CD3+ T cells including Tfh cells infiltrate within GC in addition to the accumulation out side GC in salivary gland tissue from SS patients (Fig. 2b). Ectopic GC formation has been associated with development and outcome of B cell lymphoma [64-66]. In addition, a previous study demonstrated that a large number of Tfh cells were detected in the peripheral blood of SS patients at the time of disease onset, with aberrations of serum anti-Ro/SSA and anti-La/SSB levels. Moreover, CD4+CXCR5+Tfh cells are significantly elevated in the salivary gland tissues and peripheral blood of SS patients, together with aberrant B cells and plasma cells. This suggests that CD4+CXCR5+Tfh cells contribute to the pathogenesis of SS by promoting the maturation of B cells [61].
Fig. (2).
Ectopic GC formation in the salivary gland tissue from SS patients. (a) Inflammatory lesions including CG in the lip biopsy tissue from a SS patient is shown by histological staining with hematoxylin and eosin. A lot of lymphocytes infiltrate extensively in the salivary gland tissue with destruction of acinar cells. (b) CD3+ T cells in lip biopsy tissue from a SS patient are shown by immunohistochemistry. Scale bar: 200 μm.
IL-21 is a key regulator of B-cell activation and is primarily secreted by Tfh cells. Previous reports have indicated that the number of Tfh cells is significantly increased in the peripheral blood and that the expression of the IL-21/IL-21 receptor is elevated in the salivary glands of SS patients [17, 67]. Other studies have also suggested that IL-21 plays a pathogenic role in the onset or development of other autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis [68-70]. On the other hand, salivary gland epithelial cells are capable of promoting Tfh-cell differentiation and IL-21 secretion through the production of IL-6 and inducible T cell co-stimulator ligand expression [71]. Increased serum IL-21 levels in SS patients are associated with systemic disease activity [72]. Furthermore, IL-21 and IL-21 receptor gene polymorphisms are associated with an increased susceptibility to several autoimmune diseases [73-76].
Bcl-6 expression in T cells has been reported to be essential for the formation of Tfh and GC B cells [14, 49]. Recent studies have described the mRNA expression levels of Bcl-6 to be significantly higher in ectopic GC of the salivary gland tissues from SS patients [77]. In addition to CXCR5, CD84 and PD-1 expression were also detected on infiltrating lymphocytes in the salivary gland tissues of SS patients [77].
4. TREG CELLS IN SS
Treg cells are a unique subset of T cells that play an important role in the maintenance of immune tolerance [78, 79]. The expression of the transcription factor forkhead box p3 (Foxp3) is the genetic hallmark of Treg cells [80, 81]. Moreover, naturally occuring Treg (nTreg) cells arise as a discrete and largely stable lineage in the thymus [21, 82]. The nTreg subset exhibits a T-cell Receptor (TCR) repertoire that is distinct from those of Foxp3−conventional T cells and induced Treg (iTreg) cells [83]. In contrast to nTreg cells, iTreg cells can be formed from naïve CD4+ T cells in the presence of TGF-β and IL-2 outside the thymus [79, 84]. Studies using animal models have demonstrated that the adoptive transfer of iTreg cells generated from naïve T cells can prevent the onset of autoimmune diseases [85-87]. Thus, the number and function of Treg cells, including nTreg and iTreg cells, are maintained in our body to prevent and control the breakdown of immunological tolerance (Fig. 1).
A simple animal model of Inflammatory Bowel Disease (IBD) has been well characterized by the adoptive transfer of CD25− naïve T cells into lymphopenic mice, such as recombination-activating gene−/−, severe combined immunodeficiency, or irradiated mice [88, 89]. Considerable evidence suggests that an altered balance between Treg cells and T effector cells in the intestinal microenvironment contributes to the onset or development of IBD [90, 91]. In addition, several studies have shown that Treg cells are also present within non-lymphoid sites in the periphery, including autoimmune lesions, infectious sites, and tumor tissues [92-94]. Depletion of Treg cells from normal mice by injecting anti-CD25 antibodies can induce the spontaneous development of various autoimmune lesions [95-97]. Tissue-resident Treg cells in non-lymphoid sites other than peripherally circulating Treg cells also contribute to the maintenance of local immune tolerance.
The numbers of peripheral Treg cells in SS patients is significantly reduced compared with healthy controls [98]. In contrast, it was reported that CD4+CD25high Treg cells are not impaired in primary SS patients based on experiments involving an in vitro suppression assay [99]. In addition, the frequency of Foxp3+ Treg cells in the salivary gland tissues from SS patients correlates with the inflammation grade and certain risk factors for the development of lymphoma [26].
We previously established a murine model for SS in NFS/sld mutant mice that were thymectomized 3 days after birth [6, 100]. Neonatal thymectomy in certain strains of mice results in the spontaneous development of inflammatory lesions resembling human autoimmune diseases in the thyroid gland, ovaries, kidneys, testes, and stomach [101-106]. The ratio of Treg cells to effector T cells in the SS model was significantly lower than that of control mice [107]. In addition, the in vitro induction of iTreg cells by TGF-β using naïve T cells from the SS mouse model was severely impaired [107]. Moreover, Treg cells derived from mice with SS exhibited an IFN-γ-producing Th1-like phenotype [107]. An in vivo transfer of Treg cells from the SS model was not sufficient to provide protection from the onset of autoimmune lesions in the murine model of SS [107]. These findings suggest that the abnormal expansion, differentiation, and inflammatory cytokines producing by Treg cells contribute to the pathogenesis of SS (Fig. 1).
C-C-chemokine receptor 7 (CCR7)-deficient mice have been well characterized as one of the models of SS [108]. Autoimmune lesions are observed in the lacrimal and salivary glands in CCR7−/− mice, similar to that observed in SS [108]. The enhanced immune response observed in CCR7−/− mice is caused by the defective lymph node positioning of Treg cells and consequent suppressor function impairment of the Treg cells [109]. In addition, our study demonstrated that CCR7 particularly controls the patrolling functions of Treg cells by regulating their migration into target organs [93]. Furthermore, we found that the migratory function of CCRKO Treg cells was impaired in response to sphingosine 1-phosphate (S1P), suggesting that CCR7 participates in the molecular mechanism underlying the migratory function of peripheral Treg cells through S1P and one of its receptors, S1P1 [110].
The impaired migratory response of CCR7−/− Treg cells in response to S1P occurs because of a defective association between S1P1 and a G-coupled protein [110]. In addition, TCR- and S1P1-mediated Ras-related C3 botulinum toxin substrate 1, extracellular signal-related kinase, and c-Jun phosphorylation required for activator protein 1 (AP-1) transcriptional activity were significantly impaired in CCR7−/− Treg cells [110]. We also detected an abnormal nuclear localization of Foxp3 following the abrogation of c-Jun and Foxp3 interaction in the nucleus of CCR7KO Treg cells [110]. These results indicate that CCR7 controls the migratory function of Treg cells through S1P1-mediated AP-1 signaling. This pathway is regulated through the interaction of CCR7 with Foxp3 in the nucleus, thereby protecting the body from autoimmunity. Moreover, the histopathological findings of the salivary gland tissues from SS patients revealed that the number of CCR7+Foxp3+ patrolling Treg cells in the healthy control samples was significantly increased compared with SS patients [93]. This finding indicates that CCR7+ Treg cells patrol within the target organs of the salivary and lacrimal glands to protect against autoimmune lesions.
Recently, it has been reported that an IL-17-producing CD161+CD25−CD4+ T-cell subpopulation as effector cells and a CD161+CD25+CD4+ T-cell subpopulation as regulatory cells in peripheral blood mononuclear cells (PBMCs) from SS patients are related to the clinical severity of the pathogenesis of SS [111]. Compared with healthy controls, a significant increase in the number of CD161+CD25+CD4+ T cells was also observed in PBMCs from SS patients [111]. In addition, the function of this unique regulatory cell population in SS patients is more impaired than that of CD161−CD25+CD4+ Treg cells [111].
5. RELATIONSHIP BETWEEN TFH AND TREG CELLS
Although IL-2 inhibits the differentiation and development of Tfh cells, the differentiation, maintenance, and function of Treg cells are promoted by IL-2 signaling [112, 113]. However, CXCR5-expressing T follicular regulatory cells control Tfh and GC B cell responses [114-116]. In addition, the depletion of Treg cells impairs the differentiation of influenza virus-specific Tfh cells [117]. Treg cells may promote antigen-specific GC responses by controlling excessive IL-2 signaling, which inhibits the differentiation of Tfh cells. However, direct evidence of the relationship between Tfh and Treg cells remains to be defined. In particular, it is unclear whether the direct or indirect interaction between Tfh and Treg cells influences the onset or development of autoimmune diseases, such as SS.
CONCLUSION
SS is caused by multiple factors via the complex interaction between the immune system and the target organs. Although the precise mechanisms of the onset or development of SS remain unclear, the phenotypes or functions of Tfh and Treg cells in human SS patients and animal models of SS are abnormal or unique (Fig. 1). As these cells contribute to the regulation of SS pathogenesis, the clinical defection or manipulation of their cells would be expected to aid in the development of new diagnosis techniques or treatment strategies for SS.
Consent for Publication
Not applicable.
Acknowledgements
This work was supported by JSPS KAKENHI (Grant Numbers. 15K15676, 16H02690).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Fox R.I. Sjögren’s syndrome. Lancet. 2005;366(9482):321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Seror R., Theander E., Bootsma H., et al. Outcome measures for primary Sjögren’s syndrome: a comprehensive review. J. Autoimmun. 2014;51:51–56. doi: 10.1016/j.jaut.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-Casals M., Brito-Zerón P., Sisó-Almirall A., Bosch X., Tzioufas A.G. Topical and systemic medications for the treatment of primary Sjögren’s syndrome. Nat. Rev. Rheumatol. 2012;8(7):399–411. doi: 10.1038/nrrheum.2012.53. [DOI] [PubMed] [Google Scholar]
- 4.Voulgarelis M., Tzioufas A.G. Pathogenetic mechanisms in the initiation and perpetuation of Sjögren’s syndrome. Nat. Rev. Rheumatol. 2010;6(9):529–537. doi: 10.1038/nrrheum.2010.118. [DOI] [PubMed] [Google Scholar]
- 5.Romme Christensen J., Börnsen L., Ratzer R., et al. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8(3):e57820. doi: 10.1371/journal.pone.0057820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haneji N., Nakamura T., Takio K., et al. Identification of alpha-fodrin as a candidate autoantigen in primary Sjögren’s syndrome. Science. 1997;276(5312):604–607. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen C.Q., Cha S.R., Peck A.B. Sjögren’s syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Front. Biosci. 2007;12:1767–1789. doi: 10.2741/2187. [DOI] [PubMed] [Google Scholar]
- 8.Sumida T., Tsuboi H., Iizuka M., Nakamura Y., Matsumoto I. Functional role of M3 muscarinic acetylcholine receptor (M3R) reactive T cells and anti-M3R autoantibodies in patients with Sjögren’s syndrome. Autoimmun. Rev. 2010;9(9):615–617. doi: 10.1016/j.autrev.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Lin D.F., Yan S.M., Zhao Y., et al. Clinical and prognostic characteristics of 573 cases of primary Sjögren’s syndrome. Chin. Med. J. (Engl.) 2010;123(22):3252–3257. [PubMed] [Google Scholar]
- 10.Vitali C., Bombardieri S., Jonsson R., et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnston R.J., Poholek A.C., DiToro D., et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurieva R.I., Chung Y., Martinez G.J., et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu D., Rao S., Tsai L.M., et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suh W.K. Life of T follicular helper cells. Mol. Cells. 2015;38(3):195–201. doi: 10.14348/molcells.2015.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maehara T., Moriyama M., Hayashida J.N., et al. Selective localization of T helper subsets in labial salivary glands from primary Sjögren’s syndrome patients. Clin. Exp. Immunol. 2012;169(2):89–99. doi: 10.1111/j.1365-2249.2012.04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo K., Papp G., Barath S., Gyimesi E., Szanto A., Zeher M. Follicular helper T cells may play an important role in the severity of primary Sjögren’s syndrome. Clin. Immunol. 2013;147(2):95–104. doi: 10.1016/j.clim.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Jin L., Yu D., Li X., et al. CD4+CXCR5+ follicular helper T cells in salivary gland promote B cells maturation in patients with primary Sjogren’s syndrome. Int. J. Clin. Exp. Pathol. 2014;7(5):1988–1996. [PMC free article] [PubMed] [Google Scholar]
- 19.Szabó K., Papp G., Szántó A., Tarr T., Zeher M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren’s syndrome and systemic lupus erythematosus. Clin. Exp. Immunol. 2016;183(1):76–89. doi: 10.1111/cei.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S., Miyara M., Costantino C.M., Hafler D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 21.Liston A., Gray D.H. Homeostatic control of regulatory T cell diversity. Nat. Rev. Immunol. 2014;14(3):154–165. doi: 10.1038/nri3605. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblum M.D., Way S.S., Abbas A.K. Regulatory T cell memory. Nat. Rev. Immunol. 2016;16(2):90–101. doi: 10.1038/nri.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckner J.H. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat. Rev. Immunol. 2010;10(12):849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Boehmer H., Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat. Immunol. 2010;11(1):14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 25.Miyara M., Ito Y., Sakaguchi S. TREG-cell therapies for autoimmune rheumatic diseases. Nat. Rev. Rheumatol. 2014;10(9):543–551. doi: 10.1038/nrrheum.2014.105. [DOI] [PubMed] [Google Scholar]
- 26.Christodoulou M.I., Kapsogeorgou E.K., Moutsopoulos N.M., Moutsopoulos H.M. Foxp3+ T-regulatory cells in Sjogren’s syndrome: correlation with the grade of the autoimmune lesion and certain adverse prognostic factors. Am. J. Pathol. 2008;173(5):1389–1396. doi: 10.2353/ajpath.2008.080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alunno A., Carubbi F., Bistoni O., et al. T Regulatory and T Helper 17 Cells in Primary Sjögren’s Syndrome: Facts and Perspectives. Mediators Inflamm. 2015;2015:243723. doi: 10.1155/2015/243723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamson T.C., III, Fox R.I., Frisman D.M., Howell F.V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren’s syndrome using monoclonal antibodies. J. Immunol. 1983;130(1):203–208. [PubMed] [Google Scholar]
- 29.Christodoulou M.I., Kapsogeorgou E.K., Moutsopoulos H.M. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J. Autoimmun. 2010;34(4):400–407. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Xanthou G., Tapinos N.I., Polihronis M., Nezis I.P., Margaritis L.H., Moutsopoulos H.M. CD4 cytotoxic and dendritic cells in the immunopathologic lesion of Sjögren’s syndrome. Clin. Exp. Immunol. 1999;118(1):154–163. doi: 10.1046/j.1365-2249.1999.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsifis G.E., Moutsopoulos N.M., Wahl S.M. T lymphocytes in Sjögren’s syndrome: contributors to and regulators of pathophysiology. Clin. Rev. Allergy Immunol. 2007;32(3):252–264. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- 32.Karabiyik A., Peck A.B., Nguyen C.Q. The important role of T cells and receptor expression in Sjögren’s syndrome. Scand. J. Immunol. 2013;78(2):157–166. doi: 10.1111/sji.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosmann T.R., Coffman R.L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 34.Mitsias D.I., Tzioufas A.G., Veiopoulou C., et al. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren’s syndrome. Clin. Exp. Immunol. 2002;128(3):562–568. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozaki Y., Amakawa R., Ito T., et al. Alteration of peripheral blood dendritic cells in patients with primary Sjögren’s syndrome. Arthritis Rheum. 2001;44(2):419–431. doi: 10.1002/1529-0131(200102)44:2<419::AID-ANR61>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Moriyama M., Tanaka A., Maehara T., Furukawa S., Nakashima H., Nakamura S. T helper subsets in Sjögren’s syndrome and IgG4-related dacryoadenitis and sialoadenitis: a critical review. J. Autoimmun. 2014;51:81–88. doi: 10.1016/j.jaut.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Ohyama Y., Nakamura S., Matsuzaki G., et al. Cytokine messenger RNA expression in the labial salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 1996;39(8):1376–1384. doi: 10.1002/art.1780390816. [DOI] [PubMed] [Google Scholar]
- 38.Delaleu N., Nguyen C.Q., Peck A.B., Jonsson R. Sjögren’s syndrome: studying the disease in mice. Arthritis Res. Ther. 2011;13(3):217. doi: 10.1186/ar3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donate A., Voigt A., Nguyen C.Q. The value of animal models to study immunopathology of primary human Sjögren’s syndrome symptoms. Expert Rev. Clin. Immunol. 2014;10(4):469–481. doi: 10.1586/1744666X.2014.883920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrington L.E., Hatton R.D., Mangan P.R., et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 41.Park H., Li Z., Yang X.O., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettelli E., Korn T., Kuchroo V.K. Th17: the third member of the effector T cell trilogy. Curr. Opin. Immunol. 2007;19(6):652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weaver C.T., Hatton R.D. Interplay between the TH17 and TReg cell lineages: a (co-)evolutionary perspective. Nat. Rev. Immunol. 2009;9(12):883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 44.Miossec P., Kolls J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012;11(10):763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 45.Sakai A., Sugawara Y., Kuroishi T., Sasano T., Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J. Immunol. 2008;181(4):2898–2906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen C.Q., Hu M.H., Li Y., Stewart C., Peck A.B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: findings in humans and mice. Arthritis Rheum. 2008;58(3):734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fei Y., Zhang W., Lin D., et al. Clinical parameter and Th17 related to lymphocytes infiltrating degree of labial salivary gland in primary Sjögren’s syndrome. Clin. Rheumatol. 2014;33(4):523–529. doi: 10.1007/s10067-013-2476-z. [DOI] [PubMed] [Google Scholar]
- 48.Chevalier N., Jarrossay D., Ho E., et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J. Immunol. 2011;186(10):5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 49.Tangye S.G., Ma C.S., Brink R., Deenick E.K. The good, the bad and the ugly - TFH cells in human health and disease. Nat. Rev. Immunol. 2013;13(6):412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 50.Eto D., Lao C., DiToro D., et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6(3):e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 52.Zygmunt B., Veldhoen M. T helper cell differentiation more than just cytokines. Adv. Immunol. 2011;109:159–196. doi: 10.1016/B978-0-12-387664-5.00005-4. [DOI] [PubMed] [Google Scholar]
- 53.Ivanova E.A., Orekhov A.N. T Helper Lymphocyte Subsets and Plasticity in Autoimmunity and Cancer: An Overview. BioMed Res. Int. 2015;2015:327470. doi: 10.1155/2015/327470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards J.C., Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat. Rev. Immunol. 2006;6(5):394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 55.Elkon K., Casali P. Nature and functions of autoantibodies. Nat. Clin. Pract. Rheumatol. 2008;4(9):491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutloff A., Büchner K., Reiter K., et al. Involvement of inducible costimulator in the exaggerated memory B cell and plasma cell generation in systemic lupus erythematosus. Arthritis Rheum. 2004;50(10):3211–3220. doi: 10.1002/art.20519. [DOI] [PubMed] [Google Scholar]
- 57.Saito R., Onodera H., Tago H., et al. Altered expression of chemokine receptor CXCR5 on T cells of myasthenia gravis patients. J. Neuroimmunol. 2005;170(1-2):172–178. doi: 10.1016/j.jneuroim.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Bubier J.A., Sproule T.J., Foreman O., et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl. Acad. Sci. USA. 2009;106(5):1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Platt A.M., Gibson V.B., Patakas A., et al. Abatacept limits breach of self-tolerance in a murine model of arthritis via effects on the generation of T follicular helper cells. J. Immunol. 2010;185(3):1558–1567. doi: 10.4049/jimmunol.1001311. [DOI] [PubMed] [Google Scholar]
- 60.Ma J., Zhu C., Ma B., et al. Increased frequency of circulating follicular helper T cells in patients with rheumatoid arthritis. Clin. Dev. Immunol. 2012;2012:827480. doi: 10.1155/2012/827480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X.Y., Wu Z.B., Ding J., et al. Role of the frequency of blood CD4(+) CXCR5(+) CCR6(+) T cells in autoimmunity in patients with Sjögren’s syndrome. Biochem. Biophys. Res. Commun. 2012;422(2):238–244. doi: 10.1016/j.bbrc.2012.04.133. [DOI] [PubMed] [Google Scholar]
- 62.Le Coz C., Joublin A., Pasquali J.L., Korganow A.S., Dumortier H., Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One. 2013;8(9):e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu X., Shi Y., Cai Y., et al. Inhibition of increased circulating Tfh cell by anti-CD20 monoclonal antibody in patients with type 1 diabetes. PLoS One. 2013;8(11):e79858. doi: 10.1371/journal.pone.0079858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papageorgiou A., Voulgarelis M., Tzioufas A.G. Clinical picture, outcome and predictive factors of lymphoma in Sjӧgren syndrome. Autoimmun. Rev. 2015;14(7):641–649. doi: 10.1016/j.autrev.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 65.Quartuccio L., Isola M., Baldini C., et al. Biomarkers of lymphoma in Sjögren’s syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J. Autoimmun. 2014;51:75–80. doi: 10.1016/j.jaut.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Johnsen S.J., Gudlaugsson E., Skaland I., et al. Low Protein A20 in Minor Salivary Glands is Associated with Lymphoma in Primary Sjögren’s Syndrome. Scand. J. Immunol. 2016;83(3):181–187. doi: 10.1111/sji.12405. [DOI] [PubMed] [Google Scholar]
- 67.Kang K.Y., Kim H.O., Kwok S.K., et al. Impact of interleukin-21 in the pathogenesis of primary Sjögren’s syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res. Ther. 2011;13(5):R179. doi: 10.1186/ar3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Odegard J.M., Marks B.R., DiPlacido L.D., et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J. Exp. Med. 2008;205(12):2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong W., Zhu P., Wang Y., Wang Z. Follicular helper T cells in systemic lupus erythematosus: A potential therapeutic target. Autoimmun. Rev. 2011;10(6):299–304. doi: 10.1016/j.autrev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Patakas A., Platt A.M., Butcher J.P., et al. Putative existence of reciprocal dialogue between Tfh and B cells and its impact on infectious and autoimmune disease. Immunol. Lett. 2011;138(1):38–46. doi: 10.1016/j.imlet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Gong Y.Z., Nititham J., Taylor K., et al. Differentiation of follicular helper T cells by salivary gland epithelial cells in primary Sjögren’s syndrome. J. Autoimmun. 2014;51:57–66. doi: 10.1016/j.jaut.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Yuan S.L., Jiang L., Zhang X.L., Li S.F., Duan H.M., Wang X.F. Serum IL-21 level in patients with primary Sjogren’s syndrome and clinical significance of IL-21. Xibao Yu Fenzi Mianyixue Zazhi. 2007;23(2):124–126. [PubMed] [Google Scholar]
- 73.Zhang J., Zeng H., Ren M., et al. Interleukin-21 is associated with disease activity in patients with Graves’ disease. Endocrine. 2014;46(3):539–548. doi: 10.1007/s12020-013-0105-x. [DOI] [PubMed] [Google Scholar]
- 74.Adamovic S., Amundsen S.S., Lie B.A., et al. Association study of IL2/IL21 and FcgRIIa: significant association with the IL2/IL21 region in Scandinavian coeliac disease families. Genes Immun. 2008;9(4):364–367. doi: 10.1038/gene.2008.27. [DOI] [PubMed] [Google Scholar]
- 75.Asano K., Ikegami H., Fujisawa T., et al. Molecular scanning of interleukin-21 gene and genetic susceptibility to type 1 diabetes. Hum. Immunol. 2007;68(5):384–391. doi: 10.1016/j.humimm.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 76.Webb R., Merrill J.T., Kelly J.A., et al. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009;60(8):2402–2407. doi: 10.1002/art.24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szabo K., Papp G., Dezso B., Zeher M. The histopathology of labial salivary glands in primary Sjögren’s syndrome: focusing on follicular helper T cells in the inflammatory infiltrates. Mediators Inflamm. 2014;2014:631787. doi: 10.1155/2014/631787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 79.Zheng S.G., Wang J., Wang P., Gray J.D., Horwitz D.A. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178(4):2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 80.Sakaguchi S., Ono M., Setoguchi R., et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 81.Wan Y.Y., Flavell R.A. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 82.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 83.Chen W., Jin W., Hardegen N., et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng S.G., Gray J.D., Ohtsuka K., Yamagiwa S., Horwitz D.A. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J. Immunol. 2002;169(8):4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 85.Tang Q., Bluestone J.A. Regulatory T-cell physiology and application to treat autoimmunity. Immunol. Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 86.O’Connor R.A., Anderton S.M. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J. Neuroimmunol. 2008;193(1-2):1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 87.Miyara M., Wing K., Sakaguchi S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J. Allergy Clin. Immunol. 2009;123:749–755. doi: 10.1016/j.jaci.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Mizoguchi A. Animal models of inflammatory bowel disease. Prog. Mol. Biol. Transl. Sci. 2012;105:263–320. doi: 10.1016/B978-0-12-394596-9.00009-3. [DOI] [PubMed] [Google Scholar]
- 89.Yamada A., Arakaki R., Saito M., Tsunematsu T., Kudo Y., Ishimaru N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2016;22(7):2195–2205. doi: 10.3748/wjg.v22.i7.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hovhannisyan Z., Treatman J., Littman D.R., Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140(3):957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mayne C.G., Williams C.B. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 2013;19(8):1772–1788. doi: 10.1097/MIB.0b013e318281f5a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sather B.D., Treuting P., Perdue N., et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204(6):1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ishimaru N., Nitta T., Arakaki R., et al. In situ patrolling of regulatory T cells is essential for protecting autoimmune exocrinopathy. PLoS One. 2010;5(1):e8588. doi: 10.1371/journal.pone.0008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Burzyn D., Benoist C., Mathis D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 2013;14(10):1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shevach E.M., McHugh R.S., Piccirillo C.A., Thornton A.M. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 96.Reddy J., Illes Z., Zhang X., et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2004;101(43):15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kohm A.P., McMahon J.S., Podojil J.R., et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol. 2006;176(6):3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 98.Li X., Li X., Qian L., et al. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjögren’s syndrome. J. Rheumatol. 2007;34(12):2438–2445. [PubMed] [Google Scholar]
- 99.Gottenberg J.E., Lavie F., Abbed K., et al. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjögren’s syndrome. J. Autoimmun. 2005;24(3):235–242. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 100.Haneji N., Hamano H., Yanagi K., Hayashi Y. A new animal model for primary Sjögren’s syndrome in NFS/sld mutant mice. J. Immunol. 1994;153(6):2769–2777. [PubMed] [Google Scholar]
- 101.Yunis E.J., Hong R., Grewe M.A., Martinez C., Cornelius E., Good R.A. Postthymectomy wasting associated with autoimmune phenomena. I. Antiglobulin-positive anemia in A and C57BL-6 Ks mice. J. Exp. Med. 1967;125(5):947–966. doi: 10.1084/jem.125.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kojima A., Tanaka-Kojima Y., Sakakura T., Nishizuka Y. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab. Invest. 1976;34(6):550–557. [PubMed] [Google Scholar]
- 103.Taguchi O., Nishizuka Y., Sakakura T., Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin. Exp. Immunol. 1980;40(3):540–553. [PMC free article] [PubMed] [Google Scholar]
- 104.Kojima A., Prehn R.T. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14(1-2):15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 105.Tung K.S., Smith S., Matzner P., et al. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Autoantibodies. Am. J. Pathol. 1987;126(2):303–314. [PMC free article] [PubMed] [Google Scholar]
- 106.Tung K.S., Smith S., Teuscher C., Cook C., Anderson R.E. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Immunopathology. Am. J. Pathol. 1987;126(2):293–302. [PMC free article] [PubMed] [Google Scholar]
- 107.Yamada A., Ushio A., Arakaki R., et al. Impaired expansion of regulatory T cells in a neonatal thymectomy-induced autoimmune mouse model. Am. J. Pathol. 2015;185(11):2886–2897. doi: 10.1016/j.ajpath.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Kurobe H., Liu C., Ueno T., et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24(2):165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 109.Schneider M.A., Meingassner J.G., Lipp M., Moore H.D., Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J. Exp. Med. 2007;204(4):735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ishimaru N., Yamada A., Nitta T., et al. CCR7 with S1P1 signaling through AP-1 for migration of Foxp3+ regulatory T-cells controls autoimmune exocrinopathy. Am. J. Pathol. 2012;180(1):199–208. doi: 10.1016/j.ajpath.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 111.Li L., He J., Zhu L., et al. The Clinical Relevance of IL-17-Producing CD4+CD161+ Cell and Its Subpopulations in Primary Sjögren’s Syndrome. J. Immunol. Res. 2015;2015:307453. doi: 10.1155/2015/307453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malek T.R., Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33(2):153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Campbell D.J., Koch M.A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lim H.W., Hillsamer P., Banham A.H., Kim C.H. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J. Immunol. 2005;175(7):4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 115.Linterman M.A., Pierson W., Lee S.K., et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011;17(8):975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chung Y., Tanaka S., Chu F., et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.León B., Bradley J.E., Lund F.E., Randall T.D., Ballesteros-Tato A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat. Commun. 2014;5:3495. doi: 10.1038/ncomms4495. [DOI] [PMC free article] [PubMed] [Google Scholar]