Abstract
The pancreas, the salivary glands and the dental enamel producing ameloblasts have marked developmental, structural and functional similarities. One of the most striking similarities is their bicarbonate-rich secretory product, serving acid neutralization. An important difference between them is that while pancreatic juice and saliva are delivered into a lumen where they can be collected and analyzed, ameloblasts produce locally precipitating hydroxyapatite which cannot be easily studied. Interestingly, the ion and protein secretion by the pancreas, the salivary glands, and maturation ameloblasts are all two-step processes, of course with significant differences too. As they all have to defend against acid exposure by producing extremely large quantities of bicarbonate, the failure of this function leads to deteriorating consequences. The aim of the present review is to describe and characterize the defense mechanisms of the pancreas, the salivary glands and enamel-producing ameloblasts against acid exposure and to compare their functional capabilities to do this by producing bicarbonate.
Keywords: Salivary gland, pancreas, acinar cell, ductal cell, dental enamel, ameloblast, bicarbonate, acid, defense
1. Introduction
The pancreas, the salivary glands and the teeth all originate from the foregut and, as a consequence, they have great develop-mental, structural and functional similarities. Additionally, the pancreas, the salivary glands and perhaps surprisingly, the dental enamel-forming ameloblasts have similar functions producing a bicarbonate-rich product containing certain digestive enzymes besides other minor components. However, a major difference between them is that while pancreatic juice and saliva are delivered into the gut, ameloblasts produce locally precipitating hydroxy-apatite. Ion and protein secretion by the pancreas, the salivary glands, and maturation ameloblasts is a two-step process [1-4]. In the first step, pancreatic and salivary acinar cells secrete an isotonic, protein-rich fluid with NaCl being the major salt component. Subsequently, pancreatic and salivary ducts modify the secreted juice in a different manner. In the pancreas, ductal cells absorb Cl- and secrete bicarbonate driving a large volume of water into the gut. Salivary ducts, however, are water impermeable under physiological conditions, so they cannot modify the volume of water produced by the acini. Instead, these ducts reabsorb the electrolytes from the primary acinar secretion that leads to highly hypotonic fluid reaching the oral cavity. In amelogenesis, the same maturation ameloblast cell cycles between two stages multiple times. Ruffle-ended ameloblasts are thought to secrete large quantities of calcium and phosphate ions, and contribute to luminal acidification to promote hydroxyapatite formation. Subsequently, the same cells convert to smooth-ended phenotype, and this is accompanied by the neutralization of the luminal space most probably by bicarbonate ion delivery. This alkalization then promotes the precipitation of calcium and phosphate forming hydroxyapatite crystals.
In the tissues of our interest, protein secretion is also quite different on the one hand, but also has great similarities on the other hand. Pancreatic acini produce and secrete a wide range of digestive enzymes, while the main salivary digestive product is parotid amylase, and other salivary acini secrete mucin [3]. Pancreatic and salivary ductal cells also secrete mucin, and antimicrobial proteins [1, 5]. Maturation ameloblasts secrete two important digestive enzymes, matrix metalloproteinase-20 and kallikrein-4, both of which have important functions in breaking down the previously secreted structural protein amelogenin, a process which is absolutely necessary for the full mineralization of enamel, filling up the space of removed proteins by hydroxyapatite crystals [6]. Disturbance of epithelial secretions in these organs can lead to serious diseases such as cystic fibrosis, chronic pancreatitis, autoimmune pancreatitis, Sjögren’s syndrome, as well as enamel hypomineralization defects and amelogenesis imperfecta [7-13].
During embryonic development, the pancreas, the salivary glands and ameloblasts originate from the foregut, and share great formation similarities which may explain their intriguing resemblances [3, 14, 15]. These tissues start to develop during embryonic life through the proliferation of a group of epithelial cells into the underlying mesenchyme. Subsequently, in the pancreas and the salivary glands, a branching process will form grape-like arrangements of the secretory lobes while enamel surface formation depends on the type of the tooth. The tissues of our interest develop from different embryonic origins. Ameloblasts and the parotid are derived from the ectoderm, whereas the submandibular and sublingual glands and also the pancreas are endodermal. Their morphogenetic development is driven by overlapping cytokines, growth factors and extracellular matrix components. Ultimately, they all develop by a well-defined sequence of epithelial-mesenchymal interactions. The surrounding mesenchyme is absolutely required for tissue formation [3, 14, 15].
All the above-described similarities can underline the relevance for comparing of the pancreas, salivary glands and enamel producing ameloblasts. Furthermore, there is one common striking feature of these organs. They all have to defend against acid exposure by producing extremely large quantities of bicarbonate. The pancreas and the salivary glands discharge bicarbonate to neutralize acidity in the oral and intestinal lumen [1], while ameloblasts secrete it to buffer protons, liberated during hydroxyapatite formation [10, 16-21]. The failure of this function leads to deteriorating consequences in each case. Unlike studying pancreatic and salivary luminal spaces, it is not possible to directly investigate the luminal secretion of ameloblasts into the enamel space by standard physiological methods. However, ameloblasts must have similar molecular machinery for bicarbonate transport to the two other exocrine glands of interest.
The main purpose of the present review is to summarize defense mechanisms of the pancreas, the salivary glands and enamel-producing ameloblasts against acid exposure, and to compare their functional capabilities of doing this by producing bicarbonate in order to understand their similarities and differences.
2. Beneficial defense properties of bicarbonate
The human body has numerous buffer systems which protect the intracellular and extracellular spaces from drastic pH changes due to acid or base exposure. The three major buffer systems acting against acids and therefore regulating pH in the blood and tissues are bicarbonate, phosphate and proteins. Because of its high total buffering capacity, and also because of its direct coupling to the respiratory system, bicarbonate seems to be the most important one amongst these [22]. Bicarbonate is a base, which is able to pick up a proton, forming water and CO2, a process that is highly facilitated by carbonic anhydrases. Bicarbonate does not move freely through cellular and intracellular membranes, but its transport across membranes strongly and immediately affects compartmental pH. The increase of bicarbonate concentration elevates the pH by alkalinization, while its decrease reduces the pH by achieving acidification [22].
Besides being a buffering electrolyte, bicarbonate bears additional properties that could also be important when considering its importance in epithelial secretory processes. Recent evidence suggests that bicarbonate is able to regulate intracellular cyclic AMP (cAMP) level by controlling soluble adenylate cyclase (sAC) activity inside epithelial cells [23]. cAMP is an intracellular second messenger, which regulates a number of cellular functions. Bicarbonate directly regulates sAC by relieving substrate inhibition and elevating the activity of sAC [24]. As sAC is regulated by bicarbonate, it may function as a cellular pH sensor, modulated by local bicarbonate concentration.
Another potentially important function of bicarbonate is the mucin molecule unwrapping [25, 26]. Mucin is stored in intracellular vesicles, having high calcium and proton concentrations. To remain well packed intravesicularly, an acidic environment and high level of intravesicular Ca2+ are necessary to shield its negative charges. When mucin is released from the vesicles during exocytosis, bicarbonate binds to Ca2+ and protons to form H2CO3 and Ca(HCO3)2. As a consequence, negative charges become unshielded and mucin molecules open up to become very well soluble and movable extracellularly [27], a process which could be critical for both pancreatic and salivary secretion. By analogy, during enamel formation, amelogenins are also well soluble in acidic conditions and might utilize similar mechanisms that maintain solubility intracellularly. When secreted into the luminal surface, they may develop hydrophobic properties that could be critical for organizing crystal growth in the secretory stage of amelogenesis [4].
The antimicrobial properties of bicarbonate have also been described in various epithelial surfaces where microbes are able to colonize in physiological or pathological conditions [28, 29]. This action could be due to bicarbonate, as its buffering effect may restore the activity of lysozyme, lactoferrin, and other antimicrobial peptides [29]. Additionally, a direct anti-biofilm activity has been postulated [30, 31]. Bicarbonate most probably works by inhibiting enzyme activities, which are required for quorum sensing, a bacterial communication form to build biofilm communities cooperatively [31-34]. The antimicrobial effects of bicarbonate may be added to its wide scale of physiological effects during pancreatic and salivary secretion delivered to the gut, but certainly not during enamel formation, since the latter process happens in a microbe-free environment, the enamel space.
3. Molecular physiology methods to study the functional processes leading to acid neutralization – examples demonstrated on HAT-7 cells
Special cellular models are needed to continue functional studies on pH regulation in pancreatic and salivary cells and ameloblasts. To seek for the exact molecular mechanisms conducting epithelial ion transport processes, conventional in vivo physiological methods are not suitable. Cellular models may include freshly isolated cells, primary cultures and cell lines. There are two major disadvantages of freshly isolated cell and primary culture studies. These cells and structures sooner or later disintegrate, and thus the reproducibility of studies is often doubtful. The other problem is that it is difficult or impossible to organize them into polarized monolayers to investigate their vectorial transport processes. Therefore, cell lines, which are spontaneously immortalized, mutated or artificially modified to become immortal, such as cancer cell lines which retain the most important characteristics of the original phenotype, are most suitable for modelling. For the pancreas, such cellular models are readily available, and very extensive studies have been conducted using them in the past [2, 35, 36]. Although to a lesser extent, salivary cellular models are also available to model acinar and ductal transport processes [3, 37, 38], while practically no functional model has been available to investigate the molecular transport mechanisms in amelogenesis. Therefore, we have recently developed a novel ameloblast monolayer model [39, 40] using HAT-7, a spontaneously immortalized dental epithelial cell line, originating from the rat incisor cervical loop [41]. HAT-7 cells show ameloblast characteristics such as expression of enamel proteins ameloblastin and amelogenin [41] and maturation-stage ameloblast marker enzymes like kallikrein-4 [42-45]. We observed that HAT-7 cells are suitable for functional pH regulatory studies and vectorial ion transport assessments [39]. These cells form confluent, polarized monolayers or bilayers on permeable supports, and their time-dependent tight junction formation is paralleled with increased transepithelial electrical resistance. In fact, this is a required for vectorial electrolyte secretion, as these structures prevent uncontrolled transepithelial electrolyte movements permitting only selected ions between the cells [2, 46, 47]. Then we used similar experimental strategies as previously described in molecular physiological studies characterizing pancreatic and salivary bicarbonate and proton transport processes [3, 35, 36]. We applied microfluorometry to measure intracellular pH changes, after preloading the cells with the pH sensitive dye BCECF-AM [48]. Afterward, we followed pH changes within the cell, while changing the components of the superfusion media and using various ion channel/transporter inhibitors.
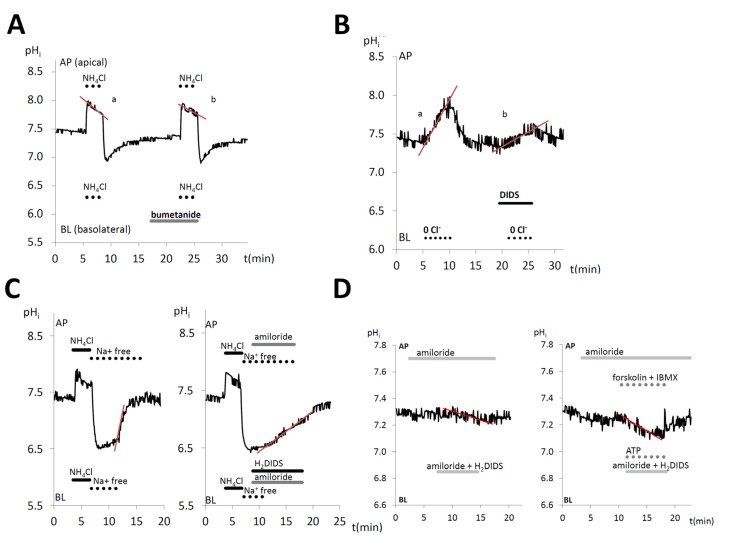
As examples, here we show the functional identification of four pH-related ion transport processes in polarized HAT-7 cells. Chloride ions has been available to be coupled to HCO3− transport. No information has been available about Cl- accumulation in ameloblasts, although the cells must take up Cl- to secrete it apically. Na+/K+/Cl− cotransporters (NKCC), electroneutral symporters transport Na+, K+ and Cl− to the intracellular space doing secondary active transport by a Na+/K+-ATPase driven mechanism. NKCCs are assumed to be involved in amelogenesis without any functional evidence [49]. To prove this, we used bumetanide, an NKCC1 inhibitor. During NH4Cl exposure after the initial alkalization, NKCC1 is activated to remove NH4+ from the cell. When bumetanide was applied, this compensatory response was greatly diminished suggesting the presence of NKCC in functional HAT-7 cells [40] (Fig. 1A). This property is quite similar to other secretory epithelia including the pancreatic HPAF and Capan-1 ductal cell line models [50, 51] and salivary acinar cells, where Cl- transport is strongly dependent on NKCC1 activity at the basolateral side [47, 49]. We also observed anion exchanger activity in HAT-7 cells by Cl- substitution experiments. Fig. 1B shows that when the anion exchanger inhibitor 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS) was used, the exchange activity led to intracellular alkalization as bicarbonate entering into the cell was substantially inhibited [40]. AE2 expression has been shown to be ubiquitous in most epithelial cells at the basolateral membrane [52]. This could be an important mechanism for intracellular Cl- accumulation against electrochemical concentration difference [47, 53]. HAT-7 cells are similar to maturation ameloblasts both having AE2 expression at the basolateral side [54, 55].
In HAT-7 cells, after the acidification phase of NH4Cl exposure no recovery in pHi was observed in the absence of extracellular Na+ (Fig. 1C). Also, simultaneous inhibition of the basolateral Na+-dependent, amiloride-sensitive proton extruding activity together with the H2DIDS-sensitive basolateral Na+-HCO3- cotransporter (NBC) greatly suppressed intracellular pH (pH1) changes [40] indicating the presence of both Na+/H+ exchanger (NHE) and NBC activities at this membrane [40]. This is in line with the presence of NHE activity at the basolateral side in most epithelia [56]. Also, the basolateral appearance of NBC activity in these cells is similar to that found in pancreatic and salivary secretory epithelia [57, 58]. To investigate whether HAT-7 cells achieve vectorial HCO3- secretion, we used the pH-drop method (Fig. 1D). In order to maintain electroneutrality in epithelial cells, HCO3- uptake at the basolateral membrane is coupled to HCO3- efflux at the luminal side and proton exit at the basolateral side [50, 51, 59, 60]. Therefore, during HCO3- entry blockade by bicarbonate transport inhibitor and proton exit blockade by NHE inhibitor, the sustaining bicarbonate efflux at the apical side results in a fall in pHi. Thus, the initial fall of pHi can be used to measure bicarbonate secretion at the apical membrane. Indeed, H2DIDS and amiloride inhibited most of the basolateral bicarbonate uptake via electrogenic NBC 1 (NBCe1) and NHE1 in HAT-7 cells, demonstrating the presence of transcellular bicarbonate transport in HAT-7 cells [39, 40]. Thus, such novel functional approaches suggest that there are great similarities in bicarbonate regulation in ameloblasts to that of pancreatic and salivary secretory processes.
4. Pancreatic bicarbonate secretion to neutralize acids
The pancreas has two main functions. The exocrine tissue is composed of acinar cells secreting digestive enzymes in a chloride-rich isotonic fluid, and of the ductal system that modifies the primary fluid and drains a bicarbonate-rich secretion to the intestine. The endocrine part is arranged in discrete islets of Langerhans, its distinct cell types secreting glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide into the bloodstream. All of these cells with fundamentally different functions develop from the same epithelial progenitors in a process of well controlled epithelial-mesenchymal interactions, interplaying with the underlying mesenchyme [61].
The human exocrine pancreas produces approximately two liters of pancreatic juice in a day, an alkaline isotonic fluid containing high concentrations of bicarbonate, digestive enzymes and some other proteins. Acinar and ductal cells form the exocrine functional unit, meaning that these cell types work together. Digestive enzymes are produced by the acinar cells which also release protons during exocytosis causing significant luminal acidosis. Pancreatic ductal cells secrete an alkaline fluid that may contain up to 150 mM bicarbonate [2, 35, 36]. This product primarily serves the neutralization of acidic environment caused by the acid secretion of acini, then in the duodenum where gastric content is delivered. Importantly, under stimulated conditions, bicarbonate concentration in the pancreatic juice is 5-6 times higher than that in the blood [2, 35, 36].
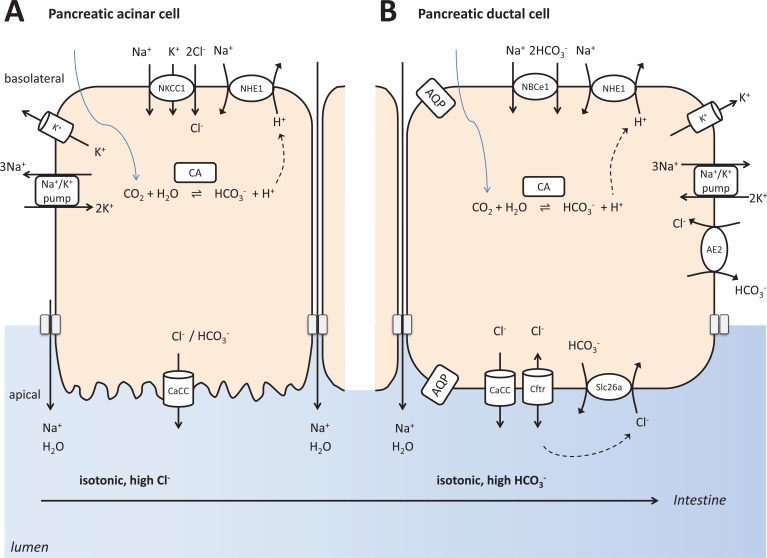
Pancreatic ductal bicarbonate secretion can be separated into two interrelated steps (Fig. 2). First, bicarbonate is loaded into the ductal cells at the basolateral membrane. This is achieved by a direct mechanism through NBCs. A parallel mechanism is the indirect, passive diffusion of CO2 into the cells through the basolateral membrane followed by its carbonic anhydrase catalyzed conversion. CO2 and H2O will form HCO3- and H+ by this enzyme, and excess protons are removed from the cell by basolateral transport activity by NHEs and perhaps vacuolar H+-ATPase although the activity of the latter one is still under debate. The intracellular accumulation of bicarbonate then permits its secretion through apical membrane ion channels and transporters such as cystic fibrosis transmembrane conductance regulator (CFTR) and coupled SLC26 anion exchangers [35, 36]. When CFTR function is defective, such as in the human CFPAC-1 pancreatic cell line, cells could still transport bicarbonate at a much lower degree utilizing their calcium-activated chloride channels [62, 63]. It is still under debate how such a high HCO3- concentration in the pancreatic juice is achieved. One possibility is that bicarbonate is secreted by the electroneutral Cl-/HCO3- exchanger until apical concentration reaches 70 mM, then a further increase to 140 mM is produced by CFTR. Another possibility is that the electrogenic SLC26 anion exchangers mediate HCO3- secretion at different sites of the ductal tree while CFTR serves to sequentially activate the exchangers and conducts Cl- into the lumen that is necessary for anion exchange to occur [35, 36].
Bicarbonate secretion by the pancreas is regulated by multiple cellular signaling pathways, but most prominently by cytosolic cyclic adenosine monophosphate (cAMP) and Ca2+ signaling, amongst them the first one being dominant. CFTR is the most critical player in pancreatic ductal bicarbonate secretion, and its mutations cause serious loss of function, cystic fibrosis [64, 65]. It is activated by cAMP, which opens this channel for Cl- and to a smaller extent for bicarbonate. Additionally its ATP-binding cassette motif utilizes the energy of ATP binding and hydrolysis to carry out various biological processes having multiple binding sites [64, 65]. The primary signaling system for bicarbonate secretion is the cAMP/protein kinase A pathway, but the intracellular Ca2+-mediated pathways also play a significant role here. The interaction and crosstalk between these pathways regulate the activity at multiple levels within ductal cells [66].
It is worth noting that CFTR can not only transport Cl-, but also bicarbonate, although it is about 4 times more selective for Cl- over bicarbonate [65]. The Cl-/HCO3- selectivity of CFTR is modulated by external Cl-. In case of physiological Cl- concentrations in proximal pancreatic ductal lumen, CFTR functions as a Cl- channel. However, when luminal Cl- is low at the distal part of the ducts, CFTR secretes bicarbonate rather than Cl- through the apical membrane [67, 68]. Additionally, CFTR regulates both the expression and the activities of apically located Cl-/HCO3- exchangers and other transporters primarily via its protein-binding domain [63, 65, 69-71]. All these modulations will result in very high bicarbonate concentration in the juice reaching the intestine.
Exocrine pancreatic secretion is primarily driven by food intake. It can be divided into cephalic, gastric, and intestinal phases. The control by the latter is the most important, which is in contrast with salivary secretion, where direct neuronal regulation dominates. Resting secretion is only a small fraction of the stimulated volume. Food intake stimulates both pancreatic acinar and duct cells to secrete enzymes and fluid in a special neurohumoral action, mediated by a number of gut hormones and parasympathetic muscarinic cholinergic nerves. The main stimulants of acinar secretion are muscarinic activation by acetylcholine and cholecystokinin, the effect by the latter one is mediated, at least in part, by vago-vagal reflexes. Secretin and vasoactive intestinal peptide are the major stimulants of ductal activity. The fine tuning and modulation of these secretory functions is mediated by a number of additional bioactive compounds, such as the stimulatory bombesin and gastrin-releasing peptide and the inhibitory 5-hydroxytryptamine (serotonin), galanin, substance P and arginine vasopressin [51, 72-76]. Activation of apically located Ca2+-sensing receptors in the pancreas also stimulates bicarbonate and fluid secretion by intracellular Ca2+ concentration increase [77]. Multiple types of purinergic receptors have been identified in the pancreatic duct [78]. ATP seems to differentially affect secretory function depending on the side, stimulating apically and inhibiting at the basolateral membrane [51, 79, 80]. Obviously, all this pancreatic ductal bicarbonate secreting function serves two major purposes. First, to neutralize the acid produced by pancreatic acinar cells [35], second to neutralize the extreme quantity of gastric acid arriving into the small intestine [36].
5. Salivary glands and their bicarbonate secretion
The three pairs of major salivary glands are the submandibular, sublingual and parotid glands, secreting approximately 95% of salivary fluid. A large number of minor glands, located in the palatal, lingual, labial and buccal regions, produce the remaining few percent [81]. Salivary glands consist of three types of cells: acinar, ductal and myoepithelial cells. The parotid mainly contains serous acini secreting watery saliva. The sublingual glands consist of mucous cells, discharging thick saliva, while the submandibular glands contain only few mucous, but mainly serous acini producing mixed saliva. Acini formed by acinar cells secrete into the ductal system consisting of intercalated, striated and excretory ducts. Contractile myoepithelial cells, surrounding acinar and intercalated ductal cells, can contract during stimulation to support secretory function. The ducts are arranged as branching network, the large excretory ducts serve to lead saliva to the oral cavity [82, 83]. The function of this complex fluid is the dilution of food and drink arriving to the oral cavity, buffering the ingested acid, starting the digestion of carbohydrates, support mastication and oral clearance, and also to water the mouth during speech and exhalation [82, 83].
Saliva is about 98% water, and it contains both organic and inorganic (chloride, sodium, bicarbonate, potassium, phosphate and calcium) components [84]. Salivary ion and water secretion is a two step process. Acini secrete an isotonic fluid during the first stage. Then primary saliva becomes highly hypotonic as it passes through the ducts [82, 85]. Passive acinar water transport is driven by active anion transport through polarized acinar cells. Transcellular water movement occurs through aquaporin water channels and paracellular water passage through tight junctions [86], and results in isotonic primary secretion in the acinar lumen [87-89].
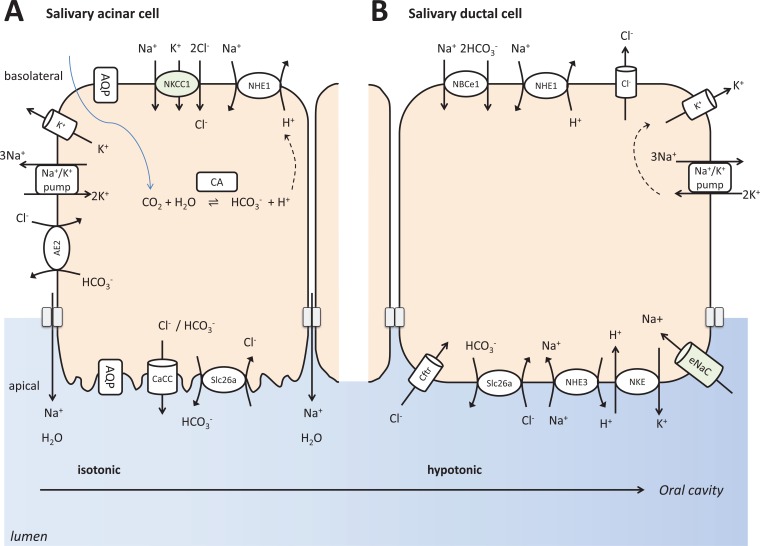
The main event during acinar ion secretion is transcellular Cl- transport, which is partly coupled to bicarbonate transport (Fig. 3). The Na+/K+ ATPase creates the driving force for this, providing high Na+ concentration difference, Na+ being very low intracellularly, and very high extracellularly [90, 91]. The Na+/K+/2Cl- cotransporter (NKCC1) brings Na+, K+ and Cl- into the cell [91, 92]. M3 muscarinic receptor activation releases Ca2+, an intracellular second messenger [1, 93]. Then Ca2+-activated chloride channel opening apically allows Cl- and to a much lower level, bicarbonate to be secreted to the apical surface [91]. Apical Cl-/HCO3- exchangers may also contribute to bicarbonate secretion by exchanging intracellular bicarbonate to the already secreted extracellular Cl- [94]. As acinar cell tight junctions have high sodium permeability, luminal accumulation of Cl- and bicarbonate drives Na+ also into the lumen from the interstitium, to compensate electrochemical potential difference. Luminal electrolyte accumulation creates substantial osmotic gradient, driving water across acinar cells [90]. Additional Cl- and bicarbonate uptake mechanisms of the acinar cells include CO2 diffusion into the cells through the basolateral membrane and the basolateral activities of NHEs and Cl-/HCO3- exchangers located at this membrane [95].
The secondary changes of the primary fluid are achieved by the ductal modification (Fig. 3). Essentially, ions are reabsorbed through ductal cells by ENaC and CFTR, both of which serve to passively equilibrate gradient differences between the two sides of cell membranes, as long as they are open [96]. Again, the driving force for this is the Na+/K+ ATPase pump creating an extreme Na+ concentration gradient between the intracellular and extracellular compartments. An additional mechanism to enrich luminal bicarbonate concentration is an anion exchanger here, which can move Cl- into the cell coupled to bicarbonate out of the cell, according to gradient differences [3]. These processes are not accompanied by water movement since ducts are impermeable to water. Thus, secondary changes by the ducts result in highly hypotonic saliva delivered to the mouth [1]. This bicarbonate-rich fluid then serves to neutralize eventual acid exposure in the oral cavity and esophagus. When this function is diminished because of certain causes, the incidence of caries, periodontitis and even esophagitis is increased.
Salivary secretory function is controlled mainly by the nervous system [3, 37, 38]. This is driven by the smell and taste of food and drinks, primarily the acidic gustatoric stimulation, resulting in watery saliva. Stress and anxiety also induce salivary flow, but the result is a very thick, mucin-rich saliva. In the first type of secretion, primarily parasympathetic activation is involved, while the latter type is induced by sympathetic components. Parasympathetic innervation is provided by the trigeminal nerve [37]. Sympathetic centers can be found in the spinal cord at upper thoracic segments [37]. Postganglionic sympathetic fibers from the superior cervical ganglion reach the glands through the external plexus of carotid artery [37]. Parasympathetic activation by cholinergic muscarinic receptors results in watery saliva. On the other hand, sympathetic activation by β-adrenergic receptors results in protein-rich saliva [38]. Muscarinic Gq/11 subtype G protein-coupled receptor activation by acetylcholine induces hydrolysis of phosphatidylinositol 4,5 bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5, trisphosphate (IP3) mediated by phospholipase C. The water-soluble IP3 initiates Ca2+ release from endoplasmic reticulum, and cell membrane Ca2+ channel openings [97]. This way, increase of intracellular Ca2+ concentration induces the opening of Ca2+-activated apical Cl- and basolateral K+ channels. Luminal Cl- and bicarbonate discharge induces paracellular Na+ passage, and also transcellular and paracellular water movement. Adrenaline and noradrenaline bind to β-adrenoceptors activating Gs subtype G proteins, and induce the activation of intracellular adenylate cyclase (AC), resulting in the production of cAMP production [97]. As a result, protein kinase A activation leads to exocytosis of zymogen-stored proteins. In addition to major neurotransmitters, other regulators such as ATP and nitric oxide [94, 98], and several gastrointestinal regulatory peptides are also involved in the regulation of fluid and protein secretion to a lower extent [3, 37, 38]. The parasympathetic and sympathetic signaling mechanisms have strong interactions [38]. Muscarinic activation not only increases ion and water secretion, but also protein discharge to a certain level, for example amylase secretion in response to food [3]. Sympathetic stimulation primarily affects protein secretion, but also evokes moderate fluid secretion that is also necessary to deliver the sticky saliva to the mouth.
Clearly, bicarbonate secretion of the salivary glands serves two very important functions. First is to neutralize acidic food and drink components consumed, second is to neutralize acidity produced inside the body, either by oral bacteria during their fermentation process, or parietal cells produce acid, which eventually arrive to the oral cavity from the stomach by reflux.
6. Ameloblasts and their bicarbonate secretion
Dental enamel is the hardest material in mammalians as a result of its 96-98% mineral concentration. During development, mineral formation can be greatly impaired due for genetic and environmental conditions [99, 100]. In the already developed tooth, dental erosion and caries are the most frequent causes of enamel loss, both of which are actually caused by local acid exposure [101]. Fully formed enamel cannot be regenerated by cells as ameloblasts die in an apopotopic process when teeth erupt. However, better understanding of the mechanisms of enamel formation may lead to the development of nanotechnological methods for achieving its reconstruction.
Unlike luminal spaces of the salivary glands and pancreas, the enamel space is not accessible to direct sample collection and analysis. However, many studies have been published on the matter using fine structural hard and soft tissue examinations, histological studies, also applying genetic knock-out of genes and studies investigating the chemical composition of enamel. Based on these works, four recent reviews have been published that nicely cover our present knowledge on amelogenesis [4, 102-104].
Enamel secretion by ameloblasts is a two-stage process, and the latter stage can also be split into two parts (Fig. 4). First, a 20-30% mineralized matrix structure is formed by secretory ameloblasts, and then the restructuring of this matrix is conducted by maturation ameloblasts to reach extremely high, 96-98% of mineralization. Ameloblasts, just like salivary epithelial cells, originate from the oral epithelium. These epithelial cells form the well-structured enamel organ, in which the one-cell layer inner enamel epithelium become the layer of ameloblasts. These cells go through various morphological forms during the different functional states of amelogenesis [105]. Importantly, just like in salivary and pancreatic epithelia, ameloblast tight junctions completely seal the two sides of the cells allowing extreme concentration differences between the apical and the basolateral sides. Calcium, phosphate, chloride and bicarbonate ions are thought to be actively transported into the mineralization space through the cells.
Specific ion channels and transporters have been described in the ameloblast membrane by immunohistochemistry and by PCR methods [4, 102-104], but their role in crystal formation is not well understood in detail, because of the lack of functional evidence about how these transporters work [105, 106]. In amelogenesis, during the secretory phase, the whole thickness of the enamel is produced, but at this early stage mineralization is only at the level of 20-30%, and the intercrystal space is filled up with amelogenin. In the maturation stage, ameloblast morphology changes substantially. To achieve mineralization and the removal of temporarily built amelogenin structure, ameloblasts cyclically change their phenotype between ruffle-ended and smooth-ended states. This cyclical modulation is explained by their dual function. On the one hand, ameloblasts secrete phosphate and calcium, and also buffer the liberated hydrogen ions during hydroxyapatite formation. On the other hand, they absorb amelogenin that is broken down by kallikrein-4 and matrix metalloproteinase-20 (MMP-20) [105, 107]. Crystal expansion and protein degradation and removal result in a practically impermeable, completely sealing crystalline structure [105, 107].
The tight regulation of acid/base balance both at the extracellular space and inside ameloblasts is required for hydroxyapatite formation. Crystal growth strongly depends on the ionic secretion and the pH control of the extracellular fluid by the cells [108]. Each mol of hydroxyapatite formation generates an eight molar equivalents of protons. Therefore, proton neutralization is required to sustain crystal growth, otherwise local acidic conditions prevent the further precipitation of calcium and phosphate and mineralization stops [102, 103, 106]. As it was also confirmed in our recently described functional molecular physiology experiments, bicarbonate is secreted into the enamel space to buffer the hydrogen ions liberated during mineralization [4, 39, 102, 103, 106]. This is further supported by mouse studies, where maturation-stage enamel of the incisors that was stained with pH indicators exhibited wide acidic bands and narrow neutral stripes [18, 109]. These zones correspond to smooth-ended ameloblasts at the neutral regions and ruffle-ended ameloblasts having high-surface apical membrane over an acidic enamel [18, 21]. This process has a wavelike pattern along the enamel resulting in transverse bands, cycling with about an eight-hour cycle between pH 6.2 and 7.2 extracellular regions over ruffle-ended and smooth-ended cells, respectively (Fig. 4) [109].
Previously published papers on acid/base regulation during amelogenesis are contradictory, but it was obvious that ameloblasts should have an appropriate molecular machinery to produce bicarbonate ions in order to buffer the large quantity of acid liberated during hydroxyapatite buildup. The channels and transporters thought to modulate extracellular pH and pHi include NBCe1, AE2, CFTR, NHE1, SLC26A4, SLC26A3, SLC26A6, and carbonic anhydrases (CA) [4, 102-104]. However, our understanding of ameloblast electrolyte transport is based almost exclusively on immunohistochemistry, expression and chemical composition analysis data from wild-type and knockout mouse models.
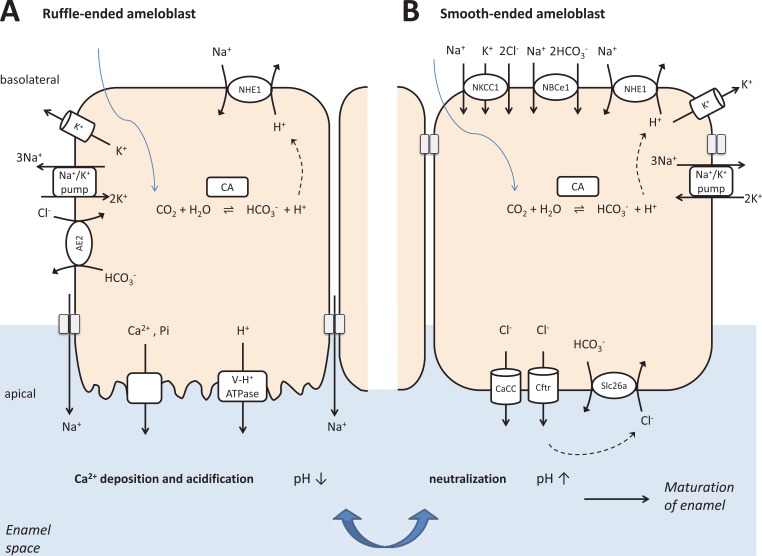
Our present hypothetical model [4, 106] on maturation ameloblasts cycling between ruffle-ended and smooth-ended states in order to secrete bicarbonate and electrolytes is based on molecular physiology studies combined with the previously accumulated knowledge (Fig. 4). Ruffle-ended ameloblast cells secrete proteins as well as calcium and phosphate ions into the enamel space. Ca2+ enters the cells primarily by the store-operated calcium entry pathway (SOCE) mediated by the ORAI1 and STIM1 proteins basolaterally and leaves the cells at the apical pole via NCKX4 and NCX. Phosphate import probably occurs via Na+-dependent phosphate (Pi) transporters, while their basolateral exit pathway is unknown. The pH slowly decreases during mineralization because a large number of protons are liberated during the formation of hydroxyapatite crystals, and also probably by an active transport through apical V-type H+ATPases [106].
Smooth-ended ameloblast cells reorganize tight junctions to neutralize acidity in the enamel space by bicarbonate [39, 40]. Intracellular bicarbonate accumulation is facilitated by the basolateral NBCe1 and also supported by a carbonic anhydrase (CA2) catalyzed process supplemented with proton extrusion by NHE1. Cl- can be uploaded into the cells in exchange to bicarbonate by AE2 and also by NKCC1. Apically Cl- exits the cells via CFTR and by calcium-activated chloride channels. Bicarbonate is exchanged to Cl- at the luminal side by SLC26A transporters and also by Cl- channels. The Na+/ K+ ATPase powers all of these processes. The opening of basolateral K+ channels is necessary to compensate for the created electrochemical differences between extracellular and intracellular spaces. The cyclical changes from ruffle-ended to smooth-ended cell morphology and the ability to modulate pH and transport functions ultimately allow the continuous expansion of hydroxyapatite crystals to reach an extreme level of mineralization [39, 40]. This hypothetical model strongly supports the assumptions that bicarbonate transporting mechanisms in ameloblasts are very similar to that found in salivary glands and in the pancreas [4].
Differently from the pancreas and salivary glands, the possible neuronal and hormonal regulation of ameloblast function is practically unknown. One exception is about steroid hormones, which are suggested to be involved in the regulation of maturation-stage ameloblasts [110, 111]. But even in this case, many unanswered questions remain about the role of steroid receptors. Additionally, steroid hormones are not able to regulate short term, 8-hour cycle in maturation ameloblasts [18, 21, 109], because their action primarily affects functional protein expression, but not their activity. The fast-acting regulators are most likely intracellular and extracellular pH sensors and G-protein coupled receptors, which have been well characterized in other epithelia, such as the pancreas and the salivary glands.
GPR68, a G-protein-coupled receptor that senses protons, is a very strong candidate for being master pH sensor in ameloblasts. Its pH sensitive range is similar to enamel matrix pH changes during amelogenesis as being completely inactive at pH 7.8, and fully active at pH 6.8 [112]. It is activated by IP3 formation and by an increase in intracellular calcium concentration [112]. Furthermore, amelogenesis imperfecta was connected to homozygous GPR68 mutations [11], and GPR68 overexpression resulted in increased barrier formation in Caco-2 cells [113]. In addition, the expression of NHE and H+-ATPase transporter systems was shown to be regulated by them in epithelial cells [114]. These data together indicate that GPR68 may be an important proton sensor during amelogenesis. Another candidate for pH sensing is the sAC. Forskolin, by intracellular cAMP liberation, potentiates the ATP-induced vectorial bicarbonate secretion in HAT-7 ameloblasts [39]. sAC is also considered as a pH sensor [24]. Direct functional studies are needed to see if such a pH sensory mechanism exists in enamel formation.
One most promising G-protein coupled receptor-related candidate regulator is extracellular ATP, acting on purinergic receptors to elevate intracellular Ca2+ concentration [115]. ATP-stimulated purinergic receptors increase cytosolic Ca2+ concentration, which in turn activates Ca2+-dependent Cl- channels, that are well-known players in salivary bicarbonate secretion. Indeed, similar to other secretory epithelia, luminal, but not basolateral ATP stimulates bicarbonate transport in HAT-7 ameloblast cells [39, 50, 51]. These findings suggest that ATP acting via calcium-activated chloride channels could be a key extracellular regulator of ameloblast function [116] just like it is in the salivary glands and the pancreas [51, 115, 117]. The extracellular calcium sensing receptor (CaR), which has previously been described in the pancreas [77, 118], can be another critical component regulator in ameloblasts since it has been found to be expressed in these cells [119]. Furthermore, in ameloblast originated PABSo-E cells, CaR was demonstrated to increase extracellular Ca2+ concentration when activated [119]. However, no direct evidence has been provided about its ion secretory role. Even less is available about the potential involvement of other G-protein receptor ligands, although cholecystokinin has been shown to have a great increase in its expression between early and late-maturation ameloblasts, as evaluated by real-time PCR [120]. The expression of PACAP, another neuropeptide, was also shown in amelogenesis and PACAP-deficient mice demonstrated serious structural changes in both the dentin and the enamel [121, 122]. Similarly, the expression of neuropeptide Y (NPY), was also detected in ameloblasts [120]. Other regulatory peptides such as somatostatin, vasoactive intestinal peptide, calcitonin-gene related peptide, peptide YY, galanin, and substance P, are also potential candidates as functional regulators in amelogenesis [123]. However, none of these regulatory peptides acting through G-protein coupled receptors have been studied in enamel formation, primarily because of the paucity of available functional cellular models.
Conclusion
Overall, novel functional molecular physiology approaches together with previously generated in vitro and in vivo results suggest that there are great similarities in the regulation of bicarbonate transport in ameloblasts to that of pancreatic and salivary secretory processes. The common function in each case is to transport bicarbonate luminally, and to neutralize liberated or consumed acid. In amelogenesis, this is necessary for the progress of mineralization leading to the hardest tissue of our body. Salivary glands produce the most watery, most hypotonic fluid of the organism to buffer consumed acidic food and drinks. Finally, the pancreas delivers a great volume of the highest concentrated bicarbonate containing juice into the intestine to buffer gastric acidity. All of them work for the sake of buffering and neutralization. Therefore, lessons learned about bicarbonate transport mechanisms in one of these organs should be very useful for understanding similar processes in the other organs.
Consent for Publication
Not applicable.
Fig. (1).
Exemplary records of intracellular pH changes to demonstrate the applicability of molecular physiological microfluorometry for evaluating cellular pH regulatory and bicarbonate transport functions. All experiments shown here were performed in the presence of bicarbonate.
(A) Compensation of pHi changes in HAT-7 cells exposed bilaterally to NH4Cl (alkali load). A partial pHi compensation (a) can be observed. Inhibition of pHi compensation can be seen (b) in the presence of basolateral (BL) bumetanide, a selective blocker of the Na+/K+/Cl− cotransporter. (B) Chloride withdrawal experiment to investigate anion exchangers. An increase in pHi (a) can be seen in HAT-7 cells upon basolateral Cl− withdrawal, most probably as a result of HCO3− influx. After the restoration of BL Cl−, pHi returns to the baseline. A smaller pHi increase (b) can be seen when the specific inhibitor, DIDS is administered basolaterally before a second Cl− withdrawal, suggesting the presence of a HCO3−/Cl− exchanger. (C) Sodium withdrawal experiment to investigate bicarbonate/proton transporters. HAT-7 cells are exposed bilaterally to NH4Cl followed by withdrawal of NH4Cl and Na+ (acid load). A fast recovery of pHi can be seen (a) following basolateral restoration of extracellular Na+. A slower pHi recovery (b) can be observed following the restoration of Na+ in the presence of basolateral (BL) amiloride and H2DIDS to block NHE1 exchanger and NBCe1 cotransporter, respectively. (D) The effect of HCO3− secretion can be perceived in the form of a slow intracellular acidification (pHi drop). Basolateral HCO3− uptake in HAT-7 cells was inhibited by simultaneous application of H2DIDS and amiloride. Amiloride was also included in the apical (AP) perfusate to inhibit any apical Na+/H+ exchanger activity. Typical pHi traces obtained in unstimulated control conditions (a), and in cells that were stimulated with ATP, forskolin, and 3-isobutyl-1-methylxanthine (IBMX) (b).
Fig. (2).
Simplified model depicting pancreatic ductal and acinar fluid, bicarbonate and electrolyte secretion. The bottom of the figure shows that pancreas secretes bicarbonate to neutralize acidity in the intestinal lumen.
(A) Pancreatic acinar cells secrete numerous digestive proteins. In addition, these cells secrete an isotonic fluid, rich in NaCl. The main mechanism behind is the transcellular, vectorial transport of Cl− for which the Na+/K+/Cl− cotransporter mediates the basolateral Cl− uptake in the expense of the Na+/K+ ATPase generated Na+ gradient. Apical membrane Cl− channels contribute to the luminal secretion of Cl− down its electrochemical gradient driven by the intracellular Cl− accumulation produced by basolateral Cl− uptake. Opening of basolateral K+ channels serves to keep electrochemical neutrality when Cl− leaves the cell apically. Apical Cl- transport also results in paracellular Na+ passage through tight junctions toward the lumen. Water follows NaCl passively also through tight junctions.
(B) Pancreatic duct cells secrete an isotonic, fluid having very high bicarbonate concentration. The basolateral bicarbonate uptake is achieved by the inward Na+-HCO3- cotransporter and the activity of carbonic anhydrase supported by proton extrusion through Na+/H+ exchangers. Apical bicarbonate secretion is mediated by cystic fibrosis transmembrane conductance regulator (CFTR) and by HCO3−/Cl− exchangers of the SLC26 family. CFTR conducts not only Cl− but also bicarbonate at a lesser extent. The necessary Cl− recycling is facilitated by direct interactions between CFTR and SLC26 exchangers. Importantly, there is no or very little Na+/K+/Cl− cotransporter activity in pancreatic ducts, therefore, intracellular Cl− concentration is low. As a result, pancreatic juice is rich in bicarbonate, its concentration could be up to 150 mMol to neutralize the acid released by the process of acinar exocytosis, and to buffer the large quantity of gastric acid that enters the duodenum from the stomach.
Fig. (3).
Simplified model depicting pancreatic ductal and acinar fluid, bicarbonate and electrolyte secretion. The bottom of the figure shows that salivary glands secrete bicarbonate to neutralize acidity in the oral lumen.
(A) Salivary acinar cells secrete an isotonic fluid that also contain various proteins: serous acinar cells excrete α-amylase, while mucous acini produce mucin. The other major function of salivary acinar cells is electrolyte and fluid secretion, creating an isotonic fluid. Similar to the pancreatic acinar cells, basolateral uptake of Cl− through Na+ /K+/Cl− cotransporters is supported by Na+/ K+ ATPase provided gradients. Subsequently, apical secretion of Cl− is mediated by calcium-activated chloride channels (CaCC). Parallel opening of basolateral K+ channels is necessary to keep intracellular electroneutrality. Cl− is followed by Na+ via the paracellular pathway. Then water follows passively through aquaporin water channels and also paracellularly into the lumen. Salivary acinar cells also secrete a modest level of bicarbonate, primarily through apical exchange to already secreted Cl− to intracellularly accumulated bicarbonate via SLC26 anion exchangers.
(B) Salivary duct cells make a hypotonic fluid that is poor in Na+ and Cl− but relatively rich in K+ and HCO3− by absorbing Na+ and Cl−, and to a lower degree secreting K+ and bicarbonate. Na+ reabsorption is achieved by apical epithelial Na+ channels (ENaC) allowing Na+ reuptake from the lumen. Then the Na+/ K+ ATPase extrudes the accumulated Na+ basolaterally. Cl− is also reabsorbed by a transcellular process. Apical Cl− entry into the cell is primarily facilitated by CFTR. Ductal cells effectively absorb Na+ and Cl−, but practically impermeable for water as it lacks aquaporin water channels and their tight junctions are also resistant to water passage. The outcome is a highly hypotonic saliva, reaching the oral cavity at basal secretion. During parasympathetic stimulation, the highly accelerated flow rate does not permit complete Na+ and Cl− reuptake resulting less hypotonic salivary juice, relatively high in bicarbonate. This fluid serves to buffer oral acidification by food and drink, or gastric reflux.
Fig. (4).
Simplified model depicting bicarbonate and electrolyte secretion in ruffle-ended and smooth-ended ameloblast cells. The bottom of the figure shows that ameloblasts secrete bicarbonate to neutralize acidity in the mineralizing enamel space.
(A) Ruffle-ended ameloblast cells secrete Ca2+ and phosphate ions into the enamel space. Ca2+ is mostly taken up by the store-operated calcium entry pathway basolaterally and transported out of the cells at the apical pole by NCKX4 and NCX exchangers. Phosphate transport probably occurs via Na+-dependent phosphate (Pi) transporters. The pH is slowly decreasing during mineralization because a great quantity of protons liberated during the formation of hydroxyapatite crystals and also, probably by an active process, the apical activity of V-type H+ ATPases.
(B) Smooth-ended ameloblast cells reorganize the tight junctions to neutralize luminal acidity in the enamel space by bicarbonate. Intracellular bicarbonate accumulation is facilitated by the basolateral electrogenic Na+/ HCO3− cotransport and by carbonic anhydrase-supported by proton extrusion through Na+/H+ exchangers. The main mechanism of intracellular Cl− accumulation is probably the activity of Na+/K+/Cl− cotransporter driven by the Na+/K+ ATPase generated Na+ gradient. Apically, Cl− and to a lesser extent bicarbonate leave the ameloblasts via both cAMP activated CFTR and Ca2+-activated chloride-channels. Bicarbonate can also be exchanged to already secreted Cl− at the apical side by SLC26A exchangers. The cyclical changes from ruffle-ended to smooth-ended cell morphology and the ability to modulate pH in the enamel space ultimately allow the continuous expansion of hydroxyapatite crystal formation, to reach an extremely high level of mineralization.
Acknowledgements
This work was supported by the Hungarian National Research, Development and Innovation Fund (K-125161) and by the Hungarian Human Resources Development Operational Programme (EFOP-3.6.2-16-2017-00006), and also by the Higher Education Excellence Program of Hungarian Ministry of Human Capacities to Semmelweis University, Therapy Research Modul.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Lee M.G., Ohana E., Park H.W., Yang D., Muallem S. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol. Rev. 2012;92(1):39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steward M.C., Ishiguro H., Case R.M. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu. Rev. Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 3.Varga G. Physiology of the salivary glands. Surgery. 2015;33:581–586. [Google Scholar]
- 4.Varga G., DenBesten P., Rácz R., Zsembery Á. Importance of bicarbonate transport in pH control during amelogenesis - need for functional studies. Oral Dis. 2017 doi: 10.1111/odi.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahuja M., Schwartz D.M., Tandon M., et al. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab. 2017;25(3):635–646. doi: 10.1016/j.cmet.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y., Papagerakis P., Yamakoshi Y., Hu J.C., Bartlett J.D., Simmer J.P. Functions of KLK4 and MMP-20 in dental enamel formation. Biol. Chem. 2008;389(6):695–700. doi: 10.1515/BC.2008.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakonczay Z., Jr, Vág J., Földes A., et al. Chronic inflammation in the pancreas and salivary glands--lessons from similarities and differences in pathophysiology and treatment modalities. Curr. Pharm. Des. 2014;20(7):1104–1120. doi: 10.2174/13816128113199990415. [DOI] [PubMed] [Google Scholar]
- 8.Parry D.A., Poulter J.A., Logan C.V., et al. Identification of mutations in SLC24A4, encoding a potassium-dependent sodium/calcium exchanger, as a cause of amelogenesis imperfecta. Am. J. Hum. Genet. 2013;92(2):307–312. doi: 10.1016/j.ajhg.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright J.T., Carrion I.A., Morris C. The molecular basis of hereditary enamel defects in humans. J. Dent. Res. 2015;94(1):52–61. doi: 10.1177/0022034514556708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sui W., Boyd C., Wright J.T. Altered pH regulation during enamel development in the cystic fibrosis mouse incisor. J. Dent. Res. 2003;82(5):388–392. doi: 10.1177/154405910308200512. [DOI] [PubMed] [Google Scholar]
- 11.Parry D.A., Smith C.E., El-Sayed W., et al. Mutations in the pH-sensing g-protein-coupled receptor GPR68 cause amelogenesis imperfecta. Am. J. Hum. Genet. 2016;99(4):984–990. doi: 10.1016/j.ajhg.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Márton K., Boros I., Varga G., et al. Evaluation of palatal saliva flow rate and oral manifestations in patients with Sjögren’s syndrome. Oral Dis. 2006;12(5):480–486. doi: 10.1111/j.1601-0825.2005.01224.x. [DOI] [PubMed] [Google Scholar]
- 13.Márton K., Madléna M., Bánóczy J., et al. Unstimulated whole saliva flow rate in relation to sicca symptoms in Hungary. Oral Dis. 2008;14(5):472–477. doi: 10.1111/j.1601-0825.2007.01404.x. [DOI] [PubMed] [Google Scholar]
- 14.Cleveland M.H., Sawyer J.M., Afelik S., Jensen J., Leach S.D. Exocrine ontogenies: on the development of pancreatic acinar, ductal and centroacinar cells. Semin. Cell Dev. Biol. 2012;23(6):711–719. doi: 10.1016/j.semcdb.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harunaga J., Hsu J.C., Yamada K.M. Dynamics of salivary gland morphogenesis. J. Dent. Res. 2011;90(9):1070–1077. doi: 10.1177/0022034511405330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronckers A.L. Ion transport by ameloblasts during amelogenesis. J. Dent. Res. 2017;96(3):243–253. doi: 10.1177/0022034516681768. [DOI] [PubMed] [Google Scholar]
- 17.Bronckers A.L., Lyaruu D.M., Jalali R., DenBesten P.K. Buffering of protons released by mineral formation during amelogenesis in mice. Eur. J. Oral Sci. 2016;124(5):415–425. doi: 10.1111/eos.12287. [DOI] [PubMed] [Google Scholar]
- 18.Josephsen K., Takano Y., Frische S., et al. Ion transporters in secretory and cyclically modulating ameloblasts: A new hypothesis for cellular control of preeruptive enamel maturation. Am. J. Physiol. Cell Physiol. 2010;299(6):C1299–C1307. doi: 10.1152/ajpcell.00218.2010. [DOI] [PubMed] [Google Scholar]
- 19.Lacruz R.S., Nanci A., Kurtz I., Wright J.T., Paine M.L. Regulation of pH During Amelogenesis. Calcif. Tissue Int. 2010;86(2):91–103. doi: 10.1007/s00223-009-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paine M.L., Snead M.L., Wang H.J., et al. Role of NBCe1 and AE2 in secretory ameloblasts. J. Dent. Res. 2008;87(4):391–395. doi: 10.1177/154405910808700415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith C.E. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 1998;9(2):128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- 22.Alka K., Casey J.R. Bicarbonate transport in health and disease. IUBMB Life. 2014;66(9):596–615. doi: 10.1002/iub.1315. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Cann M.J., Litvin T.N., et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289(5479):625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 24.Litvin T.N., Kamenetsky M., Zarifyan A., Buck J., Levin L.R. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem. 2003;278(18):15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 25.Chen E.Y., Yang N., Quinton P.M., Chin W.C. A new role for bicarbonate in mucus formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299(4):L542–L549. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinton P.M. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372(9636):415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 27.Yang N., Garcia M.A., Quinton P.M. Normal mucus formation requires cAMP-dependent HCO3- secretion and Ca2+-mediated mucin exocytosis. J. Physiol. 2013;591(18):4581–4593. doi: 10.1113/jphysiol.2013.257436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myneni S.R. Effect of baking soda in dentifrices on plaque removal. J. Am. Dent. Assoc. 2017;148(11S):S4–S9. doi: 10.1016/j.adaj.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Pezzulo A.A., Tang X.X., Hoegger M.J., et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487(7405):109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gawande P.V., LoVetri K., Yakandawala N., et al. Antibiofilm activity of sodium bicarbonate, sodium metaperiodate and SDS combination against dental unit waterline-associated bacteria and yeast. J. Appl. Microbiol. 2008;105(4):986–992. doi: 10.1111/j.1365-2672.2008.03823.x. [DOI] [PubMed] [Google Scholar]
- 31.Koestler B.J., Waters C.M. Bile acids and bicarbonate inversely regulate intracellular cyclic di-GMP in Vibrio cholerae. Infect. Immun. 2014;82(7):3002–3014. doi: 10.1128/IAI.01664-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rémy B., Mion S., Plener L., Elias M., Chabrière E., Daudé D. Interference in bacterial quorum sensing: a biopharmaceutical perspective. Front. Pharmacol. 2018;9:203. doi: 10.3389/fphar.2018.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciofu O., Tolker-Nielsen T., Jensen P.O., Wang H., Høiby N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015;85:7–23. doi: 10.1016/j.addr.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Wu H., Moser C., Wang H.Z., Høiby N., Song Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015;7(1):1–7. doi: 10.1038/ijos.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegyi P., Maléth J., Venglovecz V., Rakonczay Z., Jr Pancreatic ductal bicarbonate secretion: challenge of the acinar Acid load. Front. Physiol. 2011;2:36. doi: 10.3389/fphys.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pallagi P., Hegyi P., Rakonczay Z., Jr The physiology and pathophysiology of pancreatic ductal secretion: The background for clinicians. Pancreas. 2015;44(8):1211–1233. doi: 10.1097/MPA.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 37.Ferreira J.N., Hoffman M.P. Interactions between developing nerves and salivary glands. Organogenesis. 2013;9(3):199–205. doi: 10.4161/org.25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proctor G.B., Carpenter G.H. Salivary secretion: mechanism and neural regulation. Monogr. Oral Sci. 2014;24:14–29. doi: 10.1159/000358781. [DOI] [PubMed] [Google Scholar]
- 39.Bori E., Guo J., Rácz R., et al. Evidence for bicarbonate secretion by ameloblasts in a novel cellular model. J. Dent. Res. 2016;95(5):588–596. doi: 10.1177/0022034515625939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rácz R., Földes A., Bori E., et al. No Change in bicarbonate transport but tight-junction formation is delayed by fluoride in a novel ameloblast model. Front. Physiol. 2017;8:940. doi: 10.3389/fphys.2017.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawano S., Morotomi T., Toyono T., et al. Establishment of dental epithelial cell line (HAT-7) and the cell differentiation dependent on Notch signaling pathway. Connect. Tissue Res. 2002;43(2-3):409–412. doi: 10.1080/03008200290000637. [DOI] [PubMed] [Google Scholar]
- 42.Harada H., Ichimori Y., Yokohama-Tamaki T., et al. Stratum intermedium lineage diverges from ameloblast lineage via Notch signaling. Biochem. Biophys. Res. Commun. 2006;340(2):611–616. doi: 10.1016/j.bbrc.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto A., Harada H., Saito M., Taniguchi A. Induction of enamel matrix protein expression in an ameloblast cell line co-cultured with a mesenchymal cell line in vitro. In Vitro Cell. Dev. Biol. Anim. 2011;47(1):39–44. doi: 10.1007/s11626-010-9362-7. [DOI] [PubMed] [Google Scholar]
- 44.Yoshizaki K., Yamamoto S., Yamada A., et al. Neurotrophic factor neurotrophin-4 regulates ameloblastin expression via full-length TrkB. J. Biol. Chem. 2008;283(6):3385–3391. doi: 10.1074/jbc.M704913200. [DOI] [PubMed] [Google Scholar]
- 45.Zheng L.W., Linthicum L., DenBesten P.K., Zhang Y. The similarity between human embryonic stem cell-derived epithelial cells and ameloblast-lineage cells. Int. J. Oral Sci. 2013;5(1):1–6. doi: 10.1038/ijos.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou J. The kidney tight junction. Int. J. Mol. Med. 2014;34(6):1451–1457. doi: 10.3892/ijmm.2014.1955. [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melvin J.E., Yule D., Shuttleworth T., Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu. Rev. Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 48.Paulais M., Turner R.J. Beta-adrenergic upregulation of the Na(+)-K(+)-2Cl- cotransporter in rat parotid acinar cells. J. Clin. Invest. 1992;89(4):1142–1147. doi: 10.1172/JCI115695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bronckers A.L., Lyaruu D., Jalali R., Medina J.F., Zandieh-Doulabi B., DenBesten P.K. Ameloblast modulation and transport of Cl−, Na+, and K+ during amelogenesis. J. Dent. Res. 2015;94(12):1740–1747. doi: 10.1177/0022034515606900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demeter I., Hegyesi O., Nagy A.K., et al. Bicarbonate transport by the human pancreatic ductal cell line HPAF. Pancreas. 2009;38(8):913–920. doi: 10.1097/MPA.0b013e3181b32c08. [DOI] [PubMed] [Google Scholar]
- 51.Szucs A., Demeter I., Burghardt B., et al. Vectorial bicarbonate transport by Capan-1 cells: A model for human pancreatic ductal secretion. Cell. Physiol. Biochem. 2006;18(4-5):253–264. doi: 10.1159/000097672. [DOI] [PubMed] [Google Scholar]
- 52.Romero M.F., Fulton C.M., Boron W.F. The SLC4 family of HCO 3 - transporters. Pflugers Arch. 2004;447(5):495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- 53.Demeter I., Szucs A., Hegyesi O., et al. Vectorial bicarbonate transport by Par-C10 salivary cells. J. Physiol. Pharmacol. 2009;60(Suppl. 7):197–204. [PubMed] [Google Scholar]
- 54.Lyaruu D.M., Bronckers A.L., Mulder L., et al. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol. 2008;27(2):119–127. doi: 10.1016/j.matbio.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyaruu D.M., Medina J.F., Sarvide S., et al. Barrier formation: potential molecular mechanism of enamel fluorosis. J. Dent. Res. 2014;93(1):96–102. doi: 10.1177/0022034513510944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiela P.R., Xu H., Ghishan F.K. Apical NA+/H+ exchangers in the mammalian gastrointestinal tract. J. Physiol. Pharmacol. 2006;57(Suppl. 7):51–79. [PubMed] [Google Scholar]
- 57.Ishiguro H., Naruse S., Kitagawa M., et al. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J. Physiol. 2000;528(Pt 2):305–315. doi: 10.1111/j.1469-7793.2000.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gresz V., Kwon T.H., Vorum H., et al. Immunolocalization of electroneutral Na(+)-HCO cotransporters in human and rat salivary glands. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283(2):G473–G480. doi: 10.1152/ajpgi.00421.2001. [DOI] [PubMed] [Google Scholar]
- 59.Hegyi P., Gray M.A., Argent B.E. Substance P inhibits bicarbonate secretion from guinea pig pancreatic ducts by modulating an anion exchanger. Am. J. Physiol. Cell Physiol. 2003;285(2):C268–C276. doi: 10.1152/ajpcell.00574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishiguro H., Steward M.C., Lindsay A.R., Case R.M. Accumulation of intracellular HCO3- by Na(+)-HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J. Physiol. 1996;495(Pt 1):169–178. doi: 10.1113/jphysiol.1996.sp021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jennings R.E., Berry A.A., Strutt J.P., Gerrard D.T., Hanley N.A. Human pancreas development. Development. 2015;142(18):3126–3137. doi: 10.1242/dev.120063. [DOI] [PubMed] [Google Scholar]
- 62.Rakonczay Z., Jr, Fearn A., Hegyi P., Boros I., Gray M.A., Argent B.E. Characterization of H+ and HCO3- transporters in CFPAC-1 human pancreatic duct cells. World J. Gastroenterol. 2006;12(6):885–895. doi: 10.3748/wjg.v12.i6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rakonczay Z., Jr, Hegyi P., Hasegawa M., et al. CFTR gene transfer to human cystic fibrosis pancreatic duct cells using a Sendai virus vector. J. Cell. Physiol. 2008;214(2):442–455. doi: 10.1002/jcp.21220. [DOI] [PubMed] [Google Scholar]
- 64.Hegyi P., Wilschanski M., Muallem S., et al. CFTR: A new horizon in the pathomechanism and treatment of pancreatitis. Rev. Physiol. Biochem. Pharmacol. 2016;170:37–66. doi: 10.1007/112_2015_5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saint-Criq V., Gray M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017;74(1):93–115. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahuja M., Jha A., Maléth J., Park S., Muallem S. cAMP and Ca2+ signaling in secretory epithelia: crosstalk and synergism. Cell Calcium. 2014;55(6):385–393. doi: 10.1016/j.ceca.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishiguro H., Steward M.C., Naruse S., et al. CFTR functions as a bicarbonate channel in pancreatic duct cells. J. Gen. Physiol. 2009;133(3):315–326. doi: 10.1085/jgp.200810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park H.W., Nam J.H., Kim J.Y., et al. Dynamic regulation of CFTR bicarbonate permeability by [Cl-]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139(2):620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Choi J.Y., Muallem D., Kiselyov K., Lee M.G., Thomas P.J., Muallem S. Aberrant CFTR-dependent HCO3- transport in mutations associated with cystic fibrosis. Nature. 2001;410(6824):94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guggino W.B. The cystic fibrosis transmembrane regulator forms macromolecular complexes with PDZ domain scaffold proteins. Proc. Am. Thorac. Soc. 2004;1(1):28–32. doi: 10.1513/pats.2306011. [DOI] [PubMed] [Google Scholar]
- 71.Ko S.B., Shcheynikov N., Choi J.Y., et al. A molecular mechanism for aberrant CFTR-dependent HCO(3)(-) transport in cystic fibrosis. EMBO J. 2002;21(21):5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Argent BE, Gray MA, Steward MC, Case RM. Cell Physiology of Pancreatic Ducts. 2012.
- 73.Burghardt B., Wenger C., Barabás K., et al. GRP-receptor-mediated signal transduction, gene expression and DNA synthesis in the human pancreatic adenocarcinoma cell line HPAF. Peptides. 2001;22(7):1119–1128. doi: 10.1016/s0196-9781(01)00433-8. [DOI] [PubMed] [Google Scholar]
- 74.Hegyi P., Rakonczay Z., Jr, Tiszlavicz L., et al. Protein kinase C mediates the inhibitory effect of substance P on HCO3- secretion from guinea pig pancreatic ducts. Am. J. Physiol. Cell Physiol. 2005;288(5):C1030–C1041. doi: 10.1152/ajpcell.00430.2003. [DOI] [PubMed] [Google Scholar]
- 75.Kisfalvi I., Jr, Burghardt B., Bálint A., Zelles T., Vizi E.S., Varga G. Antisecretory effects of galanin and its putative antagonists M15, M35 and C7 in the rat stomach. J. Physiol. Paris. 2000;94(1):37–42. doi: 10.1016/s0928-4257(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 76.Varga G., Campbell D.R., Bussjaeger L.J., Solomon T.E. Role of gastrin and cholecystokinin receptors in regulation of peptone-stimulated gastric acid secretion in conscious rats. Eur. J. Pharmacol. 1993;250(1):37–42. doi: 10.1016/0014-2999(93)90618-r. [DOI] [PubMed] [Google Scholar]
- 77.Rácz G.Z., Kittel A., Riccardi D., Case R.M., Elliott A.C., Varga G. Extracellular calcium sensing receptor in human pancreatic cells. Gut. 2002;51(5):705–711. doi: 10.1136/gut.51.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4(3):237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishiguro H., Naruse S., Kitagawa M., Hayakawa T., Case R.M., Steward M.C. Luminal ATP stimulates fluid and HCO3- secretion in guinea-pig pancreatic duct. J. Physiol. 1999;519(Pt 2):551–558. doi: 10.1111/j.1469-7793.1999.0551m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kittel A., Garrido M., Varga G. Localization of NTPDase1/CD39 in normal and transformed human pancreas. J. Histochem. Cytochem. 2002;50(4):549–556. doi: 10.1177/002215540205000412. [DOI] [PubMed] [Google Scholar]
- 81.Eliasson L., Carlén A. An update on minor salivary gland secretions. Eur. J. Oral Sci. 2010;118(5):435–442. doi: 10.1111/j.1600-0722.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 82.Bardow A., Lynge Pedersen A.M., Nauntofte B. Saliva Quintessence Publishing Co. 2004. [Google Scholar]
- 83.Dawes C., Pedersen A.M., Villa A., et al. The functions of human saliva: A review sponsored by the world workshop on oral medicine VI. Arch. Oral Biol. 2015;60(6):863–874. doi: 10.1016/j.archoralbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Humphrey S.P., Williamson R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001;85(2):162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 85.Carpenter G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013;4:267–276. doi: 10.1146/annurev-food-030212-182700. [DOI] [PubMed] [Google Scholar]
- 86.Zhang G.H., Wu L.L., Yu G.Y. Tight junctions and paracellular fluid and ion transport in salivary glands. Chin. J. Dent. Res. 2013;16(1):13–46. [PubMed] [Google Scholar]
- 87.Hosoi K. Physiological role of aquaporin 5 in salivary glands. Pflugers Arch. 2016;468(4):519–539. doi: 10.1007/s00424-015-1749-6. [DOI] [PubMed] [Google Scholar]
- 88.Matsuzaki T., Susa T., Shimizu K., et al. Function of the membrane water channel aquaporin-5 in the salivary gland. Acta Histochem. Cytochem. 2012;45(5):251–259. doi: 10.1267/ahc.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gresz V., Kwon T.H., Hurley P.T., et al. Identification and localization of aquaporin water channels in human salivary glands. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281(1):G247–G254. doi: 10.1152/ajpgi.2001.281.1.G247. [DOI] [PubMed] [Google Scholar]
- 90.Morth J.P., Pedersen B.P., Buch-Pedersen M.J., et al. A structural overview of the plasma membrane Na+,K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 2011;12(1):60–70. doi: 10.1038/nrm3031. [DOI] [PubMed] [Google Scholar]
- 91.Hong J.H., Park S., Shcheynikov N., Muallem S. Mechanism and synergism in epithelial fluid and electrolyte secretion. Pflugers Arch. 2014;466(8):1487–1499. doi: 10.1007/s00424-013-1390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Evans R.L., Park K., Turner R.J., et al. Severe impairment of salivation in Na+/K+/2Cl- cotransporter (NKCC1)-deficient mice. J. Biol. Chem. 2000;275(35):26720–26726. doi: 10.1074/jbc.M003753200. [DOI] [PubMed] [Google Scholar]
- 93.Gautam D., Heard T.S., Cui Y., Miller G., Bloodworth L., Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol. Pharmacol. 2004;66(2):260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- 94.Hegyesi O., Földes A., Bori E., et al. Evidence for active electrolyte transport by two-dimensional monolayers of human salivary epithelial cells. Tissue Eng. Part C Methods. 2015;21(12):1226–1236. doi: 10.1089/ten.TEC.2014.0614. [DOI] [PubMed] [Google Scholar]
- 95.Luo X., Choi J.Y., Ko S.B., et al. HCO3- salvage mechanisms in the submandibular gland acinar and duct cells. J. Biol. Chem. 2001;276(13):9808–9816. doi: 10.1074/jbc.M008548200. [DOI] [PubMed] [Google Scholar]
- 96.Catalán M.A., Nakamoto T., Gonzalez-Begne M., et al. Cftr and ENaC ion channels mediate NaCl absorption in the mouse submandibular gland. J. Physiol. 2010;588(Pt 4):713–724. doi: 10.1113/jphysiol.2009.183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ambudkar I.S. Calcium signalling in salivary gland physiology and dysfunction. J. Physiol. 2015 doi: 10.1113/JP271143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lohinai Z., Burghardt B., Zelles T., Varga G. Nitric oxide modulates salivary amylase and fluid, but not epidermal growth factor secretion in conscious rats. Life Sci. 1999;64(11):953–963. doi: 10.1016/s0024-3205(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 99.Aoba T., Fejerskov O. Dental fluorosis: chemistry and biology. Crit. Rev. Oral Biol. Med. 2002;13(2):155–170. doi: 10.1177/154411130201300206. [DOI] [PubMed] [Google Scholar]
- 100.Denbesten P., Li W. Chronic fluoride toxicity: dental fluorosis. Monogr. Oral Sci. 2011;22:81–96. doi: 10.1159/000327028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.West N.X., Joiner A. Enamel mineral loss. J. Dent. 2014;42(Suppl. 1):S2–S11. doi: 10.1016/S0300-5712(14)50002-4. [DOI] [PubMed] [Google Scholar]
- 102.Lacruz R.S. Enamel: Molecular identity of its transepithelial ion transport system. Cell Calcium. 2017;65:1–7. doi: 10.1016/j.ceca.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lacruz R.S., Habelitz S., Wright J.T., Paine M.L. Dental enamel formation and implications for oral health and disease. Physiol. Rev. 2017;97(3):939–993. doi: 10.1152/physrev.00030.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yin K., Paine M.L. Bicarbonate transport during enamel maturation. Calcif. Tissue Int. 2017;101(5):457–464. doi: 10.1007/s00223-017-0311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robinson C. Enamel maturation: A brief background with implications for some enamel dysplasias. Front. Physiol. 2014;5:388. doi: 10.3389/fphys.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Varga G., Kerémi B., Bori E., Földes A. Function and repair of dental enamel - Potential role of epithelial transport processes of ameloblasts. Pancreatology. 2015;15(4) Suppl.:S55–S60. doi: 10.1016/j.pan.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 107.Zhu L., Liu H., Witkowska H.E., Huang Y., Tanimoto K., Li W. Preferential and selective degradation and removal of amelogenin adsorbed on hydroxyapatites by MMP20 and KLK4 in vitro. Front. Physiol. 2014;5:268. doi: 10.3389/fphys.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takagi T., Ogasawara T., Tagami J., et al. pH and carbonate levels in developing enamel. Connect. Tissue Res. 1998;38(1-4):181–187. doi: 10.3109/03008209809017035. [DOI] [PubMed] [Google Scholar]
- 109.Damkier H.H., Josephsen K., Takano Y., Zahn D., Fejerskov O., Frische S. Fluctuations in surface pH of maturing rat incisor enamel are a result of cycles of H(+)-secretion by ameloblasts and variations in enamel buffer characteristics. Bone. 2014;60:227–234. doi: 10.1016/j.bone.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 110.Houari S., Loiodice S., Jedeon K., Berdal A., Babajko S. Expression of steroid receptors in ameloblasts during amelogenesis in rat incisors. Front. Physiol. 2016;7:503. doi: 10.3389/fphys.2016.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Babajko S., Jedeon K., Houari S., Loiodice S., Berdal A. Disruption of steroid axis, a new paradigm for molar incisor hypomineralization (MIH). Front. Physiol. 2017;8:343. doi: 10.3389/fphys.2017.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ludwig M.G., Vanek M., Guerini D., et al. Proton-sensing G-protein-coupled receptors. Nature. 2003;425(6953):93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 113.de Vallière C., Vidal S., Clay I., et al. The pH-sensing receptor OGR1 improves barrier function of epithelial cells and inhibits migration in an acidic environment. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309(6):G475–G490. doi: 10.1152/ajpgi.00408.2014. [DOI] [PubMed] [Google Scholar]
- 114.Mohebbi N., Benabbas C., Vidal S., et al. The proton-activated G protein coupled receptor OGR1 acutely regulates the activity of epithelial proton transport proteins. Cell. Physiol. Biochem. 2012;29(3-4):313–324. doi: 10.1159/000338486. [DOI] [PubMed] [Google Scholar]
- 115.Novak I. Purinergic signalling in epithelial ion transport: Regulation of secretion and absorption. Acta Physiol. (Oxf.) 2011;202(3):501–522. doi: 10.1111/j.1748-1716.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 116.Lacruz R.S., Smith C.E., Bringas P., Jr, et al. Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J. Cell. Physiol. 2012;227(5):2264–2275. doi: 10.1002/jcp.22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kordás K.S., Sperlágh B., Tihanyi T., et al. ATP and ATPase secretion by exocrine pancreas in rat, guinea pig, and human. Pancreas. 2004;29(1):53–60. doi: 10.1097/00006676-200407000-00056. [DOI] [PubMed] [Google Scholar]
- 118.Riccardi D., Valenti G. Localization and function of the renal calcium-sensing receptor. Nat. Rev. Nephrol. 2016;12(7):414–425. doi: 10.1038/nrneph.2016.59. [DOI] [PubMed] [Google Scholar]
- 119.Mathias R.S., Mathews C.H., Machule C., Gao D., Li W., Denbesten P.K. Identification of the calcium-sensing receptor in the developing tooth organ. J. Bone Miner. Res. 2001;16(12):2238–2244. doi: 10.1359/jbmr.2001.16.12.2238. [DOI] [PubMed] [Google Scholar]
- 120.Lacruz R.S., Smith C.E., Chen Y.B., Hubbard M.J., Hacia J.G., Paine M.L. Gene-expression analysis of early- and late-maturation-stage rat enamel organ. Eur. J. Oral Sci. 2011;119(Suppl. 1):149–157. doi: 10.1111/j.1600-0722.2011.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sandor B., Fintor K., Felszeghy S., et al. Structural and morphometric comparison of the molar teeth in pre-eruptive developmental stage of PACAP-deficient and wild-type mice. J. Mol. Neurosci. 2014;54(3):331–341. doi: 10.1007/s12031-014-0392-6. [DOI] [PubMed] [Google Scholar]
- 122.Sandor B., Fintor K., Reglodi D., et al. Structural and morphometric comparison of lower incisors in PACAP-deficient and wild-type mice. J. Mol. Neurosci. 2016;59(2):300–308. doi: 10.1007/s12031-016-0765-0. [DOI] [PubMed] [Google Scholar]
- 123.Monteiro MP, Batterham RL. The Importance of the Gastrointestinal Tract in Controlling Food Intake and Regulating Energy Balance Gastroenterology. 2017. [DOI] [PubMed]