ABSTRACT
Schistosomiasis is a parasitic infection that has evolved together with the humankind. Evidence in ancient Egyptian medical papyri or Assyrian medical texts reported signs and symptoms that could resemble schistosomiasis; similarly, some biblical passages describe an epidemic (depicted as a ‘curse’) that has been hypothesized to be associated with schistosomiasis’ spread in Mesopotamia. In the modern era, Theodor Maximilian Bilharz and Patrick Manson (the ‘father of tropical medicine’) gave an impetus to the knowledge about the parasite and its spread until the present time, when immunoassays and molecular biology on mummies allowed retracing important milestones regarding schistosomiasis’ evolution. Schistosomiasis affects more than 200 millions of people worldwide and it is an emblem of how hard it is to prevent, control and treat neglected tropical diseases. Our work reviews the history of schistosomiasis with regard to human infections.
KEYWORDS: Human Schistosomiasis, history, evolution, Egyptian mummies, molecular biology
Introduction
Schistosomiasis (also known as bilharziasis) is a parasitic infection caused by flatworms (flukes) of the genus Schistosoma that can cause acute and chronic disease. The most important species for human pathology are S. haematobium (responsible for urogenital disease), S. mansoni, and S. japonicum (both responsible for intestinal/hepatic disease). S. haematobium is endemic in Africa and Middle East; S. mansoni in Africa, Middle East, Central and South America and S. japonicum in eastern Asia [1–3]. Apart from the above-mentioned, there are at least other 5 species pathogenic to humans: S. intercalatum (Central Africa), S. guineensis (West Africa), S. mattheei (Southern Africa), S. mekongi (Southeast Asia) and S. malayensis (Peninsular Malaysia) [3].
Currently, with more than 700 million people living in endemic areas and increasing migratory flux to non-endemic areas, 200 million people worldwide are at risk for urogenital and intestinal/hepatic schistosomiasis [4,5]. Despite medical progress, liver fibrosis and bladder cancer remain oppressive complications of the chronic infection sustained by Schistosoma spp [1,2].
Etymologically the word ‘schistosomiasis’ comes from the union of two Greek words: ‘schistos’ that means ‘split’ and ‘soma’ that means ‘body’. The name was proposed by David Friedrich Weinland in 1858 because of the male worms’ morphology and was adopted by the International Commission on Zoological Nomenclature [6]. Originally, schistosomiasis was possibly a disease of animals that, subsequently, evolved as zoonosis; in fact, its life cycle includes both animals and humans as reservoirs; however, up to date this has been definitely proven only for S. japonicum and S. mekongi. For further historical and paleopathological details, a very comprehensive review is available in the literature, that has also served as a valuable reference for some aspects of the present work [2].
Going more into details, the actual life-cycle of schistosomiasis includes a mammalian definitive host and an intermediate host consisting of species of aquatic snails: Biomphalaria for S. mansoni, Bulinus for S. haematobium and Oncomelania for S. japonicum [1,7]. Larval stages, miracidia, emerge from the eggs when they reach water and bore into the snails. Hence, schistosomes multiply asexually within the snail, and when conditions are suitable, free-swimming larval forms, cercariae, escape into the water, penetrate the skin of the definitive host and after passing through the lungs, migrate to the venules of the pelvis (only Schistosoma haematobium) or mesentery, where they mature in situ and lay eggs intravascularly, possibly causing both acute and chronic disease and symptoms. The lifespan of an adult schistosome is about 3–5 years, but they can live up to 40 years in the definitive hosts [8,9]. Since 1970, the treatment of choice for both genitourinary and intestinal/hepatic schistosomiasis is praziquantel [10].
Milestones
The german parasitologists Theodor Maximilian Bilharz and Carl Theodor Ernst first discovered a schistosoma in 1851 during an autopsy performed at Kasr-El-Ainy Hospital in Cairo [11]. Bilharz at that time was the chief of the surgery at the Kasr-el-Ainy Hospital and schistosoma was firstly named Distomum haematobium [12,13]. Bilharz also described the emergence of embryos from the eggs in the bladder and their subsequent passage in urine to fresh water. He also hypothesized the existence of a link between the parasite and the clinical symptoms historically attributed to the disease (dysentery and haematuria). Bilharz personal life was strongly connected with infectious diseases and he died in 1862 (at just 38 years of age) because of complications of typhoid fever [14].
In 1859, three years before Bilharz died, Cobbold (the person who named ‘Bilharzia’ as a generic term for the parasite) showed that schistosomes were not confined to man when he discovered ‘Bilharzia magna’ in a West African monkey [15]. Later on, definitive hosts of schistosomes were found in other primates, ruminants, rodents and cattle.
The existence of an intermediate host for the life cycle of schistosomiasis was first considered only in 1902. In fact Patrick Manson, the ‘Father of Tropical Medicine’, discovered the spread of schistosomiasis in Central America and wrote: ‘Possibly our zoologists may be able to point to some mollusc or arthropod which the West Indies and Africa have in common, and thereby indicate the long-sought-for, but hitherto undiscovered, intermediate host of bilharzia haematobia’ [16].
Eventually, sixty-four years after the discovery of Bilharz, Robert Thomson Leiper understood the complete cycle of Schistosoma spp., with the recognition of aquatic snails as intermediate hosts of these trematodes. Leiper was a Scottish physician later on named the ‘Father of modern helminthology’; apart from schistosomiasis, he made other considerable discoveries on life cycles and mode of transmission of other helminths, namely Dracunculus medinensis and Loa loa. In 1915, when he was a member of the Royal Army Medical Corps, he also distinguished between S. mansoni and S. haematobium by their morphology, egg type, and snail host [17].
Hepatic involvement in schistosomiasis was first described in 1904, when Fujiro Katsurada, a Japanese professor of pathology, examined a cat in Yamanashi Prefecture and found 32 parasites, including five pairs, in the portal vein. He wrote a scientific paper describing the parasite with figures and named it as a new species: S. japonicum [18].
Almost 60 years earlier (1847), another Japanese physician, Dr. Yoshinao Fujii reported signs of a ‘new’ disease in Hiroshima Prefecture, resembling cutaneous manifestation of schistosomiasis: ‘During the past 2 or 3 years, farmers have had small eruptions on their legs when they entered the water to cultivate the rice field. The eruptions are unendurably painful and itchy. Cows and horses also show the same symptoms. Most of the residents suffer from this disease and they consider that the symptoms are due to the lacquer spread out in this area in ancient times’ [2].
Regarding Katayama syndrome (a possible clinical manifestation of schistosomiasis in naive patients characterized by fever, cough, myalgia, headache and abdominal tenderness [19]), descriptions fitting its clinical manifestations can be found in ancient books of traditional Chinese medicine referring to more than 2400 years ago [20]. However, Yoshinao Fujii, firstly, properly recorded Katayama syndrome in Japan, in the Kwanami district, only in 1847, in a report that did not become available until 1909 [21].
Milestones in schistosoma discoveries are summarized in Figure 1.
Figure 1.
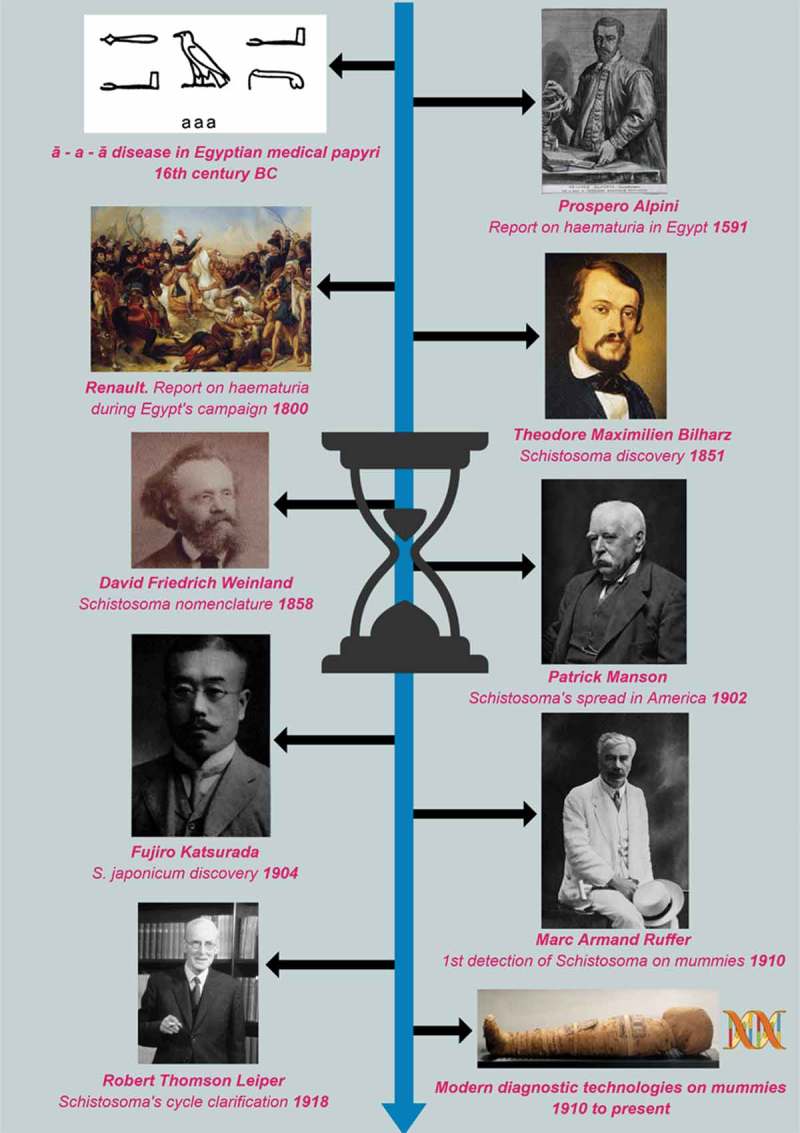
Milestones in schistosoma discoveries. legend. All the images not representing authors’ creations have been included unaltered in the figure, and are either in the public domain or from https://commons.wikimedia.org. In the latter case, the original materials have been distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/). The figure for the Egypt’s campaign is the painting by Antoine-Jean Gros (1810) – The battle of Pyramids. Château de Versailles, France. The figure for modern diagnostic technologies on mummies is Nesi mummy (dynasty XX), Biblioteca Museu Víctor Balaguer, Vilanova i la Geltrú, Spain.
Evidence of schistosomiasis in ancient times
Schistosomiasis is an ancient disease, but still nowadays, due to its peculiar life-cycle, it is considered ‘the most important water-based disease from a global public-health perspective’ reflecting the fundamental role of water for its spread [22]. Water and life have always been interconnected, therefore it does not sound strange that schistosomiasis have accompanied the history of ancient civilizations over the millennia.
The most ancient evidence of schistosomiasis dates back to more than 6000 years ago. In fact, studies conducted on human skeletal found in the area of Tell Zeidan, an early settlement of farmers in northern Syria (5800–4000 years before Christ [BC]), demonstrated the evidence of a terminal spined schistosome from the pelvic sediment of skeletal remains [23]. Nonetheless, even though Tell Zeidan is in Syria, it has been suggested that the ‘cradle’ of schistosomes lies in the region of African great lakes, an area in which both the parasites and their intermediate hosts are in an active state of evolution [24]. Subsequently, it is believed that schistosomiasis have spread to Egypt as a result of the importation of monkeys and slaves during reign of the fifth dynasty of pharaohs (~ 2494–2345 BC) [7,25].
The ‘ā – a – ā disease’, a possible ancient name for schistosomiasis, is mentioned in several Egyptian medical papyri from as early as 1500 years BC, and described a disease characterized by discharge from penis. However, there is no certainty that it was truly schistosomiasis, given the multiple possible differential diagnosis (e.g. kidney stones, genitourinary neoplasms, or gonorrhea). Taken in account this limitation, it is nonetheless interesting to notice that to stop ‘ā – a – ā disease’, the ancient Egyptians were encouraged to avoid polluted water, and fishermen, farmers and others in regular contact with the river were advised to wear penile sheaths made of linen, facts which resemble the course of schistosomiasis. The ā – a – ā disease is mentioned 22 times in the medical papyri, suggesting that it was a common condition [26].
Symptoms resembling schistosomiasis, such as hematuria, urethral discharge and bladder discomfort can also be found in the Assyrian medicine: ‘mūsu’ is the term used to identify any abnormal-looking material coming from the urethra. It is a noun probably derived from a verb (wasû) which means to ‘come out’. Interestingly, it was only applied to urine, and only to urine of unusual color or consistency, thus possibly indicating to the ancient physician the presence of a discharge [27].
In the Bible there are passages ascribable to a ‘curse’ that could resemble schistosomiasis. Jericho (actually belonging to Palestinian Territories) existed as a walled city during pre-pottery Neolithic times (~ 7000 BC) and is one of the oldest towns in the world. Its name probably means ‘city of palms’ [28]. In a biblical passage, Joshua ordered to kill each inhabitant a part from the prostitute Rahab and her family, and he cursed the city. According to the Bible, it seems there was a strong local belief that any community living in Jericho would produce fewer children than normal. This ‘deficit’ was believed to be associated with infected well water (in line with the fact that S. haematobium may obstruct Fallopian tubes). Evidence from excavations suggests that Jericho was suddenly abandoned. With excavations, some possible intermediate host of S. haematobium, more precisely several Melanopsis praemorsa and one Bulinus truncatus, have been found in Jericho (likely derived from the water used to make the mud bricks). Similarly, Zakaria examined mud bricks at Tel ‘Aqeir (4000–2500 BC), the ziggurat at ‘Aqar Quf (1350 BC), the summer palace at Babylon (~ 625 BC), and the Bismaya (third to sixth centuries AD) and found shells of Bulinus truncatus at each site [29]. A plausible hypothesis is that at the time of Joshua, schistosomiasis had been spread in Mesopotamia from Egypt, thus possibly representing the ‘curse’ and the cause for reduced fertility of Jericho’s inhabitants.
It is worth noting that history of man and the epidemiology of schistosomiasis share a parallel path. In fact, incidence and prevalence of schistosomiasis dramatically increased with the shift from a basin irrigation system to the introduction of perennial irrigation by means of artificial canals [30]. In the 1920s, approximately 70% of the Egyptian male population was infected with S. haematobium [31].
Evidence of schistosomiasis in the ‘modern era’
Prospero Alpini (1553–1616) was an Italian physician and botanist from the Republic of Venice. In 1580, he was appointed to be the physician of Giorgio Emo, the consul of Venice in Cairo. Alpini worked in Egypt from March 1581 to October 1584 and wrote ‘De Medicina Aegyptiorum’ (On the Medicine of Egyptians). Alpini noticed an oddly high incidence of hematuria in Egypt 200 years earlier than other European colleagues [32,33].
In 1798, Renault, a French physician with Napoleon’s campaign, described Egypt as the only country where men menstruate [31]. He described an obstinate haematuria affecting numbers of the French troops in Egypt, and particularly the cavalry. The unknown disease presented with pain in the bladder, extending along the urethra to the extremity of the glans penis, and with frequent urgency to urinate. The last drops excreted were frequently of pure blood. Renault had opportunities of examining bladders from the soldiers, founding inflamed inner membrane of the bladder, compatible with chronic infection [34].
In 1902, Sir Patrick Manson reported a case of intestinal schistosomiasis (‘bilharzia… lateral spined’) in stool of a ‘38 year-old Englishman’ who have lived in Lesser Antilles (Caribbean region) thus, through the help of a microscope, demonstrating the presence of S. mansoni in America [16]. Probably, S. mansoni was transported along with African slaves to the New World. Manson was the first to understand that there were different species of Schistosoma spp., basing his judgement on the different distribution and morphology of the vesical (terminal spined eggs) and intestinal (lateral spined eggs) forms of the disease [35].
As discussed in the previous paragraph, the upgrade to a ‘modern’ type of irrigation was like a flywheel for the spread of schistosomiasis [36]. Indeed, with the development of agriculture and the institution of perennial irrigation, its prevalence increased. Notably, while the prevalence of schistosomiasis was approximately 5% in areas under basin irrigation (naturally inundated by the Nile flood and cultivating one crop a year), after 1821 it rose to 60% and more in areas where perennial irrigation was introduced by means of artificial canals, with cultivation all the year round (two or three crops yearly) [30]. With the extension of agriculture to feed an increasing population, the problem of schistosomiasis assumed tremendous proportions and became a very oppressive health problem worldwide.
Lessons from mummies
The word ‘mummy’ derives from the Persian or Arabic word ‘mumia’, which means pitch or bitumen which issued from the ‘Mummy Mountain’. In the last two decades, thanks to molecular biology technology, which enable the research of specific fragments of DNA, some novel insights upon historical events have been provided [37]. This is obviously interesting when looking at ancient diseases like schistosomiasis.
Before molecular techniques were developed, in 1910, Marc Armand Ruffer, professor of bacteriology, president of the Sanitary, Maritime, and Quarantine Council of Egypt (Alexandria), published a paper on The British Medical Journal, reporting his studies on Egyptian mummies of the twentieth dynasty (1250–1000 B.C.): ‘in the kidneys of the two mummies of the twentieth dynasty I have demonstrated in microscopic sections a large number of calcified eggs of Bilharzia haematobia, situated, for the most part, among the straight tubules.’ [38] This paper is considered a milestone as the beginning of the sub-discipline of paleo-parasitology. In 1973, the Manchester Egyptian Mummy Project was started and information about mummies held in collections worldwide began to be recorded [39].
In 1990, Deelder et al. using an enzyme-linked immunosorbent assay (ELISA) detected a schistosome circulating anodic antigen in cheek, gut and shin of Egyptian mummies known to be infected with S. haematobium, giving the chance to indirectly detect schistosomiasis in mummies’ fragments when viscera are not properly preserved or inaccessible for directly detecting parasites [40]. A similar study was published two years later (1992), that used the same technique in samples of desiccated skin and brain from 23 mummies recovered from Sudanese Nubia (350–550 AD). As many as 15/23 mummies (65%) had schistosomiasis [41]. In 1995, the Manchester Egyptian Mummy Project and Medical Service Corporation International of Arlington (US) began a joint study on the epidemiology of schistosomiasis in ancient and modern Egypt [42].
More recently, in 2014, molecular biology techniques started to be used to confirm schistosomiasis in mummies. PCR primers suitable for direct detection of little fragmented of ancient DNA, specific for either S. mansoni or S. haematobium were developed. Scientist found S. mansoni and S. haematobium DNA from the liver of Nekht-Ankh mummy (~ 3900 BP) and S. haematobium DNA from intestinal samples from the Khnum-Nakht mummy [43].
All the discoveries made by molecular biology techniques, peculiar for each schistosoma spp, allowed highlighting the endless coexistence between schistosomiasis and human beings through human evolution.
Conclusions
Schistosomiasis is an excellent example of an infectious disease that evolves and spreads in parallel with human evolution and human migratory flows. The expansion of schistosomiasis is still ongoing, as recently demonstrated by new infections carried by human migration in previously unaffected areas such as Cavu river in Corsica island (France) [44]. Studying how and when it spread in the past, apart from being fascinating for itself, could help us in understanding the current (and maybe in foreseeing the future) diffusion of the disease.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Colley DG, Bustinduy AL, Secor WE, et al. Human schistosomiasis. Lancet. 2014;383:2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bergguist R, Kloos H, Adugna A.. Schistosomiasis: paleopathological perspectives and historical notes In: Jamison B, ed. Schistosoma: biology, pathology and control. Baton Rouge: CRC Press; 2017. p. 8–33. [Google Scholar]
- [3].Standley CJ, Mugisha L, Dobson AP, et al. Zoonotic schistosomiasis in non-human primates: past, present and future activities at the human-wildlife interface in Africa. J Helminthol. 2012June;86(2):131–140. [DOI] [PubMed] [Google Scholar]
- [4].Lai YS, Biedermann P, Ekpo UF, et al. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect Dis. 2015August;15(8):927–940. [DOI] [PubMed] [Google Scholar]
- [5].Riccardi N, Nosenzo F, Peraldo F, et al. Increasing prevalence of genitourinary schistosomiasis in Europe in the migrant era: neglected no more? PLoS Negl Trop Dis. 2017March16;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bilharz T. Fernere Beobachtungen über das die Pfortader des Menschen bewohnende Distomum haematobium und sein Verhältniss zu gewissen pathologischen Bildungen (aus brieflichen Mittheilungen an Professor v. Siehold vom 29. März 1852). Zeitschrift Für Wissenschaftliche Zoologie. 1852;4(1):72–76. [Google Scholar]
- [7].Adamson PB. Schistosomiasis in antiquity. Med Hist. 1976;20:176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chabasse D, Bertrand G, Leroux JP, et al. Developmental bilharziasis caused by Schistosoma mansoni discovered 37 years after infestation. Bull Soc Pathol Exot Filiales. 1985;78:643–647. [PubMed] [Google Scholar]
- [9].Warren KS, Mahmoud AA, Cummings P, et al. Schistosomiasis mansoni in Yemeni in California: duration of infection, presence of disease, therapeutic management. Am J Trop Med Hyg. 1974;23:902–909. [DOI] [PubMed] [Google Scholar]
- [10].WHO Schistosomiasis strategy; Available at: http://www.who.int/schistosomiasis/strategy/en/
- [11].Bilharz T, Siebold CT. Ein Beitrag zur Helminhographia humana, aus brieflichen Mitteilungen des Dr. Bilharz in Cairo, nenst Bermerkungen von Prof. C. Th. von Siebold in Breslau. Z Wiss Zool. 1852–1853;4:53–76. [Google Scholar]
- [12].Tan SY, Ahana A. Theodor Bilharz (1825–1862): discoverer of schistosomiasis. Singapore Med J. 2007;48:184–185. [PubMed] [Google Scholar]
- [13].Bilharz T. Fernere mittheilungen über Distomum haematobium. Z Wiss Zool. 1853;4:454–456. [Google Scholar]
- [14].Jamieson BGM. Schistosoma: biology, pathology and control. Brisbane (Australia): CRC Press; 2016. [Google Scholar]
- [15].Cobbold TS. On some new forms of Entozoa. Trans Linnean Soc London. 1859;22:363–366. [Google Scholar]
- [16].Manson P. Report of a Case of Bilharzia from the West Indies. Br Med J. 1902;2:1894–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Leiper RT. Report on the results of the Bilharzia mission in Egypt, 1915. J R Army Med Corps. 1918;30:235–260. [Google Scholar]
- [18].Katsurada F. Schistosoma japonicum, ein neuer menshlicher Parasit durch welchen eine endemisch Krankheit in vershiedenen Genenden Japans versucht wird. Annot Zool Japan. 1904;5:147–160. [Google Scholar]
- [19].Mao SP, Shao BR. Schistosomiasis control in the people’s Republic of China. Am J Trop Med Hyg. 1982January;31(1):92–99. [PubMed] [Google Scholar]
- [20].Mao SP, Shao BR. Schistosomiasis control in the People’s Republic of China. Am J Trop Med Hyg. 1982;31:92–99. [PubMed] [Google Scholar]
- [21].Fujinami A, Nakamura A. The mode of transmission of katayama disease of Hiroshima prefecture. Japanese schistosomiasis, the development of the causative worm and the disease in animals caused by it. Hiroshima Iji Geppo. 1909;132:324–341. [Google Scholar]
- [22].Steinmann P, Keiser J, Bos R, et al. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. [DOI] [PubMed] [Google Scholar]
- [23].Anastasiou E, Lorentz KO, Stein GJ, et al. Prehistoric schistosomiasis parasite found in the Middle East. Lancet. 2014;14:553–554. [DOI] [PubMed] [Google Scholar]
- [24].Nelson GS, Teesdale C, Highton RB. The role of animals as reservoirs of bilharziasis in Africa. Bilharziasis: Ciba Foundation Symposium; 1962. [Google Scholar]
- [25].Breasted JH. Ancient records of Egypt, vols I-IV. Chicago (IL): University of Chicago Press; 1906. [Google Scholar]
- [26].Leake CD. The Old Egyptian medical papyri. Lawrence: University of Kansas; 1952. [Google Scholar]
- [27].Scurlock J, Andersen BR. Diagnoses in Assyrian and Babylonian medicine. Champaign: University of Illinois Press; 2005. [Google Scholar]
- [28].Hulse EV. Joshua’s curse and the abandonment of ancient Jericho: schistosomiasis as a possible medical explanation. Med Hist. 1971;15:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zakaria H. Historical study of Schistosoma haematobium and its intermediate host, Bulinus truncatus, in central Iraq. J Fac Med Baghdad. 1959;1:2–10. [Google Scholar]
- [30].El Halawani AA. Bilharziasis control as an integral part of rural health services. Bilharziasis: Ciba Foundation Symposium; 1962. [Google Scholar]
- [31].Sarant L. Egypt: the flatworm’s revenge. Nature. 2017;551:S46–47. [DOI] [PubMed] [Google Scholar]
- [32].Alpini P. De Medicina Aegyptiorum Libri IV. Venice: Franciscum de Franciscis; 1591. [DOI] [PubMed] [Google Scholar]
- [33].De Santo N, Aliotta G, Bisaccia C, et al. De Medicina Aegyptiorum by Prospero Alipini (Venice, Franciscus de Franciscis, 1591). J Nephrol. 2013;26:S117–S123. [DOI] [PubMed] [Google Scholar]
- [34].Renault AJ. Notice sur l’hématurie qu’éprouvent les Européens dans la haute Egypte et la Nubie. Journal Général De Médecine, De Chirurgie Et De Pharmacie. 1808;17:366–370. [Google Scholar]
- [35].Manson P. ed. Tropical diseases: a manual, new and rev. London: Cassell; 1903. [Google Scholar]
- [36].Farooq M. Progress in bilharziasis control. The situation in Egypt. WHO Chron. 1967;21:175–184. [PubMed] [Google Scholar]
- [37].Raoult D, Dutour O, Houhamdi L, et al. Evidence for louse-transmitted diseases in soldiers of Napoleon’s Grand Army in Vilnius. J Infect Dis. 2006;193:112–120. [DOI] [PubMed] [Google Scholar]
- [38].Ruffer MA. Note on the presence of “Bilharzia haematobia” in Egyptian mummies of the Twentieth dynasty. BMJ. 1910;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].David AR. The Manchester Museum Mummy Project: multidisciplinary research on ancient Egyptian mummified remains. Manchester: Manchester Museum; 1979. [Google Scholar]
- [40].Deelder AM, Miller RL, De Jonge N, et al. Detection of schistosome antigen in mummies. Lancet. 1990;335:724–725. [DOI] [PubMed] [Google Scholar]
- [41].Miller RL, Armelagos GJ, Ikram S, et al. Palaeoepidemiology of Schistosoma infection in mummies. BMJ. 1992;304:555–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Contis G, David AR. The epidemiology of Bilharzia in Ancient Egypt: 5000 years of Schistosomiasis. Parasitology Today. 1996;6:253–255. [Google Scholar]
- [43].Matheson CD, David R, Spigelman M, et al. Molecular confirmation of Schistosoma and family relationship in two ancient Egyptian mummies. Yearbook of Mummy Studies. 2014;2:39–47. [Google Scholar]
- [44].Berry A, Fillaux J, Martin-Blondel G, et al. Evidence for a permanent presence of schistosomiasis in Corsica, France, 2015. Euro Surveill. 2016;21:1. [DOI] [PubMed] [Google Scholar]


