ABSTRACT
Background: Foodborne nematodiasis are caused by the ingestion of food contaminated by helminths. In Europe, these diseases are present in all countries.
Objectives: To review the available data on epidemiology and management of foodborne nematodiasis in the European Union, detect any trends and determine the possible causes of the observed changes.
Methods: A review of available literature published between 2000 and 2016 was conducted.
Results: Out of 1523 cases described in the literature, 1493 cases were autochthonous and 30 cases were imported. The detected parasites were Toxocara spp (34.7%), Ascaris lumbricoides (27.1%), Trichinella spp (21.9%), Anisakis spp (15.5%) and Angiostrongylus cantonensis (0.8%).
Conclusions: Foodborne nematodiasis remains a public health challenge for the European Union. Autochthonous cases of nematodiasis present the greatest health risk within the European Union. Foodborne nematodes due to lack of hygiene in food processing are diseases that can be avoided by increasing
KEYWORDS: Foodborne, nematodiasis, Europe, zoonosis
Introduction
Foodborne nematodiasis infections affect millions of people each year and are a major global concern [1]. Globalization has introduced new eating habits resulting in new fresh foods being consumed. Therefore, the import of foods is now necessary to satisfy consumer demands for exotic meats, vegetables, and fruits. Transportation and new methods of cooking are a risk to public health. Asymptomatic carriers and the zoonotic potential of some parasites contribute to the spread of pathogens and misdiagnoses of parasitic infections are very common [2].
Foodborne nematodiasis are infections acquired following the consumption of raw or improperly cooked food, such as meat, snails, seafood, freshwater fish and aquatic invertebrates containing various nematodes in an infective stage [3]. Contaminated water and various kinds of aquatic vegetation may also contain nematodes [1]. The consumption of raw or undercooked cooked food is commonplace in many parts of the developing world, while in developed countries the consumption of such foods has steadily increased [1].
There are concerns about these infections in the European Union, due to possible cases of imported infections, local infections that may have been acquired from tainted imported foodstuff and infections acquired within the European Union due to the presence of infected intermediate hosts imported from endemic areas [4].
The purpose of this review is to provide information on imported and local cases of foodborne nematodiasis in the European Union between 2000-2016: number of detected cases, country of diagnosis, main symptoms, date of publication, first author field of activity, diagnosis, treatment and case diagnosis certainty.
Nematodes are probably one of the most abundant and widespread of all animal groups, occurring in the sea, freshwater, and soil and as parasites of vertebrates, invertebrates and plants [5]. The chosen organisms for this study are various species nematodes in the genera Anisakis (Dujardin, 1845), Trichinella (Raillet 1895), Angiostrongylus (Kamensky 1905), Toxocara (Werner 1782) and Ascaris (Linnaeus 1758).
Anisakis spp
Anisakiasis is caused by the ingestion of larval nematodes of the Anisakidae family. Humans acquire the infection by eating raw, salted, marinated or undercooked seafood. Human infection is accidental and humans are not suitable hosts for these parasites. No multiplication occurs in human [6].
Avoiding the consumption of raw or undercooked fish can prevent Anisakiasis. Sushi, ceviche, and sashimi should be consumed from fish that has undergone inspection for infective larvae. Cooking fish to an internal temperature of 60ºC for 1 minute or 65ºC for 30 seconds or freezing at −20ºC or below for at least 60 hours can destroy this larva [7].
Trichinella spp
Trichinella spp is one of the most important parasites of this study; it is associated with the ingestion of game meats and sausages that are not properly cooked.
Trichinellosis is both a public health and economic problem. Trichinella may live in a very wide variety of host species however, only humans are clinically affected [8]. Apart from its effects on human health, it seriously affects the porcine agricultural industry due to the fact that the domestic pig is the most important source of human infection worldwide. In recent outbreaks in Europe, horses and wild boars have also played significant roles as sources of infection [9]. The spread of Trichinellosis may be avoided by eliminating the feeding of pigs using infected or possibly infected meats. The increased movement of both people and livestock to appease the currents demands of globalization exacerbates the risk of disease dissemination [9].
According to the Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015, this disease can be prevented by the thorough cooking of meats to an internal temperature of 60ªC or higher. Freezing times and temperatures depend on the thickness of the product. The temperature of freezing must never be less than −15ºC for 20 days when the thickness of the piece is less than 15cm or −15ºC for 30 days if the thickness is between 15 and 50 cm.
Angiostrongylus cantonensis
Angiostrongylus cantonensis can normally be found in rat lungs. Both rats and humans may become infected the same way, by eating contaminated foods such as snails or uncooked vegetables [10].
Diagnosis is difficult and involves a history of exposure in an endemic area. An elevated cerebrospinal fluid (CSF) eosinophilia along with serologic testing will confirm infection.
Avoidance of uncooked or undercooked snails and transport hosts is the best way to prevent infection, as well as washing or cooking all food items that may have been contaminated by slug secretions [2].
Toxocara spp
Toxocariasis caused by either Toxocara canis or Toxocara catis can be found worldwide. The most common way for humans to become infected is by consuming contaminated plants or invertebrates that live in contaminated soils [2]. The normal source of contamination is dog or cat feces. The consumption of infected chickens, cattle and sheep may also lead to infection but this transmission is much less frequent [2].
Children are the main infected group. Behaviors common in children such as geophagia or poor personal hygiene as well as close contact with young dogs increases the risk of infection [11].
Ascaris lumbricoides
Ascaris lumbricoides has a direct life-cycle, inhabiting the intestinal tract as adults and producing eggs that are voided to the external environment with the feces. It is transmitted passively within the egg, being ingested by the host as a result of fecal contamination of food or lack of hygiene. The occurrence of Ascaris lumbricoides usually peaks in childhood or early adolescence. Infection may produce some degree of impaired cognitive function in children [12].
Methods
A review of the indexed literature on foodborne nematodiasis observed in the European Union and published during the period 2000–2016 was conducted.
This study follows a methodology similar to previous studies [4] but our study covers a larger number of parasites and clinical cases. A literature search was performed first to identify articles possibly apt for inclusion. This was followed by a screening process to assure selected articles met all inclusion criteria. Data was then extracted from the articles for analysis and discussion.
In addition, this paper informs about the current situation of the recent epidemiological data of foodborne nematodes in the European Union.
The investigation covered the 28 current member states of the European Union, namely Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and United Kingdom.
Search
The following search strategy was adopted in PubMed, SciELO, Google Scholar and PLOS: (Angiostrongyl* OR Anisakis* OR Ascaris* OR Toxocar* OR Trichinell* OR Nematod*) AND (Austria OR Belgium OR Bosnia OR Bulgaria OR Croatia OR Cyprus OR Czech Republic OR Denmark OR Estonia OR Finland OR France OR Germany OR Greece OR Hungary OR Ireland OR Italy OR Latvia OR Lithuania OR Luxembourg OR Malta OR Netherlands OR Poland OR Portugal OR Romania OR Slovakia OR Slovenia OR Spain OR Sweden OR United Kingdom OR Europe) AND (Humans[Mesh] AND (‘‘2000/01/01ʹ’[PDAT]: ‘‘2016/10/31ʹ’[PDAT])). Figure 1 shows the Search Strategy.
Figure 1.

Flow diagram showing the search strategy of the study.
Selection
The authors screened articles found by electronic search and evaluated their appropriateness based on title and abstract according to the established exclusion criteria. Exclusion criteria were: 1) studies concerning the wrong agent (for example helminthiasis but not nematodiasis or not the selected nematodes); 2) reviews, letters or editorials without original data; 3) studies concerning animals only; 5) studies of European investigator outside the continent; 6) duplicated data.
Extraction
Data from epidemiological studies were summarized, while data from case reports and case series were extracted using a standardized electronic form in which the main characteristics of study, clinical and epidemiological features of subjects with foodborne nematodiasis were recorded. The form included all the information needed to ascertain whether cases satisfied the definition of definitive, probable or possible case (reported below).
Cases of food-borne nematodiasis retrieved by the search were classified according to the following definitions:
Definitive or probable case of food-borne nematodiasis. Definitive cases were defined as those where the clinical manifestations of the patient fit with nematodiasis and nematodes were observed in a biological sample taken from the patient. Probable cases were defined as those were nematodes were not able to be observed in biological samples but were diagnosed as nematodiasis due to the clinical presentation of the patient.
Autochthonous case (case diagnosed in a subject without a history of travel to or living in high endemic regions) or imported case (case diagnosed in a subject with a history of travel or long stay in an endemic region (Latin America, Asia or Africa)).
Results
The flow diagram in Figure 2 shows the number of papers identified in each database and the review process. Out of 134-screened documents, 94 were included. Out of 1523 cases described in the 94 papers included in the analysis, 1493 cases were autochthonous and 30 cases were imported.
Figure 2.
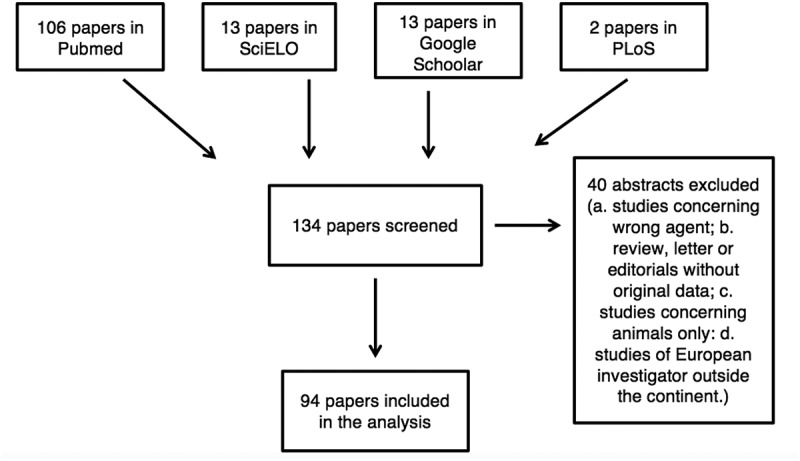
Flow diagram showing the number of papers identified in each database and the review process.
Table 1 shows the cases detected in this study of the nematodiasis in the European Union. The main countries involved in this parasitosis are Estonia, Romania, Poland and Spain.
Table 1.
Autochthonous and imported cases of Foodborne nematodiasis in the European Union by country of diagnosis (2000-2016).
| Autochthonous |
Imported |
|
|---|---|---|
| Number of cases | 1493/1523 (98.03%) | 30/1523 (1.97%) |
| Country of diagnosis | ||
| Austria | 3 | - |
| Belgium | 28 | 1 |
| Bulgaria | 16 | - |
| Croatia | 2 | - |
| Cyprus | 1 | - |
| Czech Republic | 8 | - |
| Denmark | 79 | 3 |
| Estonia | 299 | - |
| Finland | 1 | - |
| France | 11 | 5 |
| Germany | 19 | 4 |
| Greece | - | 2 |
| Hungary | 57 | - |
| Ireland | - | 1 |
| Italy | 71 | 2 |
| Latvia | 45 | - |
| Lithuania | 1 | - |
| Luxembourg | 84 | - |
| Malta | - | - |
| Netherlands | - | 2 |
| Poland | 211 | - |
| Portugal | 8 | - |
| Romania | 260 | - |
| Slovakia | 1 | - |
| Slovenia | 67 | - |
| Spain | 212 | - |
| Sweden | - | 9 |
| United Kingdom | 9 | 1 |
Angiostrongylus cantonensis
Information on angiostrongyliasis originated from 5 papers. No cases were reported in the period between 2000 and 2005. All detected cases [12] in the European Union were imported, either from Cuba, the Philippines, Thailand or Samoa. Five (41.6%) cases were diagnosed only by the clinical history meaning a probable diagnostic [13]; the rest were diagnosed by serology (41.67%) [14,15] and Polymerase Chain Reaction (PCR) (16.66%) [16]. Clinical manifestations develop between 2 to 35 days after the ingestion of larvae [14]. Patients may present neurologic symptoms as photophobia or diffuse paraesthesia in the arms and legs [13,16]. Table 2 shows the results for this parasite.
Table 2.
Selected studies reporting autochthonous and imported cases of angiostrongyliasis in the European Union (2000–2016, [13,14,15,16, 55]).
| Origin of the infection | - Undercooked snails or slugs - Vegetables contaminated - Paratenic host (freshwater shrimp and crab) |
|---|---|
| Number of cases | 12 |
| Autochthonous/Imported | Imported 12/12 (100%) |
| Date of publication | |
| 2000-2005 | -/12 (0%) |
| 2006-2011 | 7/12 (58.33%) |
| 2011-2016 | 5/12 (41.67%) |
| First author field of activity | |
| Tropical Medicine | 5/12 (41.66%) |
| Microbiology | 3/12 (25%) |
| Neurology | 2/12 (16.67%) |
| Travel Medicine | 2/12 (16.67%) |
| Case diagnostic certainty | |
| Definitive | 7/12 (58.33%) |
| Probable | 5/12 (41.67%) |
| Main symptoms | |
| Headache | 10/28 (35.71%) |
| Paresis | 8/28 (28.57%) |
| Photophobia | 6/28 (21.43%) |
| Fever | 4/28 (14.29%) |
| Diagnosis | |
| Serology | 5/12 (41.67%) |
| Medical History | 5/12 (41.67%) |
| PCR | 2/12 (16.66%) |
| Treatment | |
| Anthelminthic | 12/12 (100%) |
Anisakis spp
A total of 236 cases were detected, all of them autochthonous and definitive diagnostic.
In 62 patients the authors explicitly specified that the infection was produced by ingesting anchovy (Engraulis encrasicolus). In the rest of the cases, this data was not available. The country with the highest number of cases was Spain with 158 (6.9%) [17,18] and the second was Italy with 67 (28.4%) cases [19,20].
One of the outbreaks was originated by the consumption of Peruvian food ‘ceviche’ [21]. Serology is the most common diagnosis method (86.44%) but some patients are diagnosed by exploratory laparotomy (2.97%) [22,23] or gastroscopy (10.17%) [24,25]. Table 3 shows the results for this parasite.
Table 3.
Selected studies reporting autochthonous and imported cases of anisakiasis in the European Union (2000–2016, [17,18,19,20,21,22,23,24,25,54,60,62,63,64,65,66,67,68, 69,70,71,72,73,74,75,76,77,78,79,80,81]).
| Origin of the infection | Raw or undercooked fish |
|---|---|
| Number of cases | 236 |
| Autochthonous/Imported | Autochthonous 236/236 (100%) |
| Date of publication | |
| 2000-2005 | 65/236 (27.54%) |
| 2006-2011 | 151/236 (63.98%) |
| 2012-2016 | 20/236 (8.48%) |
| First author field of activity | |
| Parasitology | 86/236 (36.44%) |
| Surgery | 60/236 (25.42%) |
| Dermatology | 49/236 (20.76%) |
| Alergology | 10/236 (4.24%) |
| Infectious Diseases | 10/236 (4.24%) |
| Gastroenterology and Endoscopy | 10/236 (4.24%) |
| Family Medicine | 4/236 (1.71%) |
| Epidemiology | 3/236 (1.27%) |
| Cardiology | 2/236 (0.84%) |
| Patology | 1/236 (0.42%) |
| Radiodiagnosis | 1/236 (0.42%) |
| Case diagnostic certainty | |
| Definitive/Probable | Definitive 236/236 |
| Main symptoms | |
| Pruritus | 92/250 (36.8%) |
| Abdominal pain | 83/250 (33.2%) |
| Cutaneous | 59/250 (23.6%) |
| Vomit | 7/250 (2.8%) |
| Diarrhea | 5/250 (2%) |
| Chest pain | 2/250 (0.80%) |
| Fever | 2/250 (0.80%) |
| Diagnosis | |
| Serology | 204/236 (86.44%) |
| Gastroscopy | 24/236 (10.17%) |
| Exploratory laparatomy | 7/236 (2.97%) |
| PCR | 1/236 (0.42%) |
| Treatment | |
| Symptomatic | 131/236 (55.50%) |
| Surgical | 103/236 (43.65%) |
| Surgical+Symptomatic | 2/236 (0.85%) |
Ascaris lumbricoides
Only six (1.46%) imported cases were detected of ascaridiasis [26,29] compared to 406 autochthonous cases (98.54%). Patients may present gastrointestinal bleeding [30] and intestinal necrosis [31]. The main symptom is abdominal pain (43.47%) [28,32,35]. The coprological examination is the most important tool for diagnosis (66.5%).
The countries with more cases detected were Romania (41.01%) [36] and Luxembourg (20.38%) [37]. Table 4 shows the results for this parasite.
Table 4.
Selected studies reporting autochthonous and imported cases of ascariasis in the European Union (2000–2016, [26,27,28,29,30,31,32,33,34,35,36,37,57,58,82,83,84,85,86,87,88,89]).
| Origin of the infection | Food or water contaminated with fecal matter containing eggs |
|---|---|
| Number of cases | 412 |
| Autochthonous | 406/412 (98.54%) |
| Imported | 6/412 (1.46%) |
| Date of publication | |
| 2000-2005 | 6/412 (1.45%) |
| 2006-2011 | 8/412 (1.95%) |
| 2012-2016 | 398/412 (96.6%) |
| First author field of activity | |
| Pharmacy | 169/412 (41.02%) |
| Veterinary | 129/412 (31.31%) |
| Microbiology | 84/412 (20.39%) |
| Tropical Medicine | 13/412 (3.16%) |
| Family Medicine | 7/412 (1.7%) |
| Surgery | 4/412 (0.97%) |
| Gastroenterology and Endoscopy | 3/412 (0.73%) |
| Radiodiagnosis | 2/412 (0.48%) |
| Infectious Diseases | 1/412 (0.24%) |
| Case diagnostic certainty | |
| Definitive/Probable | 410/412 (99.51%) |
| Probable | 2/412 (0.49%) |
| Main symptoms | |
| Abdominal pain | 10/23 (43.47%) |
| Vomit | 4/23 (17.39%) |
| Asymptomatic | 3/23 (13.04%) |
| Weight loss | 2/23 (8.7%) |
| Fever | 2/23 (8.7%) |
| Diarrhea | 2/23 (8.7%) |
| Diagnosis | |
| Coprological examination | 274/412 (66.50%) |
| Serology | 127/412 (30.82%) |
| Gastroscopy | 3/412 (0.73%) |
| Exploratory Laparatomy | 3/412 (0.73%) |
| CT findings | 3/412 (0.73%) |
| Colonoscopy | 2/412 (0.49%) |
| Treatment | |
| Anthelminthic | 412/412 (100%) |
Toxocara spp
The species included in this nematodiasis are not clarified, in the 55.5% of the papers the infection was caused by Toxocara canis, in the rest of the papers (44.5%), the authors did not specify the species of Toxocara.
All the cases detected were autochthonous. The country with the highest prevalence of the parasite infection was Poland with 190 (35.9%) cases detected [38], followed by Denmark (14.9%) [39] and Slovenia (12.6%) [40]. Table 5 shows the results for this parasite.
Table 5.
Selected studies reporting autochthonous and imported cases of toxocariasis in the European Union (2000–2016, [11,38,39,40,90,91,92,93,94,95,96,97,98,99,100, 101,102]).
| Origin of the infection | Food or water contaminated with fecal matter containing eggs |
|---|---|
| Number of cases | 529 |
| Autochthonous/Imported | Autochthonous 529/529 (100%) |
| Date of publication | |
| 2000-2005 | 76/529 (14.36%) |
| 2006-2011 | 87/529 (16.45%) |
| 2012-2016 | 366/529 (69.19%) |
| First author field of activity | |
| Parasitology | 154/529 (29.11%) |
| Veterinary | 145/529(27.41%) |
| Pediatrics | 103/529 (19.47%) |
| Infectious Diseases | 84/529 (15.88%) |
| Tropical Medicine | 28/529 (5.29%) |
| Microbiology | 8/529 (1.51%) |
| Oftalmology | 3/529 (0.57%) |
| Surgery | 2/529 (0.38%) |
| Neurology | 1/529 (0.19%) |
| Family Medicine | 1/529 (0.19%) |
| Case diagnostic certainty | |
| Definitive/Probable | Definitive 529/529 (100%) |
| Main symptoms | |
| Eye injurie | 79/171 (46.2%) |
| Abdominal pain | 33/171 (19.3%) |
| Atopy | 23/171 (13.45%) |
| Headache | 13/171 (7.6%) |
| Fever | 12/171 (7.02%) |
| Asymptomatic | 5/171 (2.93%) |
| Vomit | 2/171 (1.17%) |
| Cough | 2/171 (1.17%) |
| Weight loss | 1/171 (0.58%) |
| Diarrhea | 1/171 (0.58%) |
| Diagnosis | |
| Serology | 528/529 (99.81%) |
| Endoscopy | 1/529 (0.19%) |
| Treatment | |
| Anthelminthic | 529/529 (100%) |
Trichinella spp
The species of Trichinella analyzed were Trichinella britovi in 17.9% of cases, Trichinella spiralis in 7.8% and Trichinella pseudospiralis in 1,2%. For the rest of the cases (73.1%), the authors did not specify the species of Trichinella.
Twelve (3.6%) cases were imported from: Spain (eight cases) [41], Montenegro (two cases) [42] Poland (one case) [43] and Bosnia and Herzegovina (one case) [44]. The rest of cases were autochthonous (96.4%).
The autochthonous cases (96.4%) were distributed among twelve countries. The main countries were Romania with 91 (28.3%) cases[45], Hungary with 57 (17.7%) cases[46], Spain with 46 (14.3%) cases [47], Germany with 17 (5.3%) cases[48] and Bulgaria with 16 (5%) cases[49]. 7,4% of cases were asymptomatic[47]
In 35 (10.48%) patients the diagnostic included a PCR of food sample [41,48,50,52]. Table 6 shows the results for this parasite.
Table 6.
Selected studies reporting autochthonous and imported cases of trichinellosis in the European Union (2000–2016, [41,42,43,44,45,46,48,49,50,51,52,61,103,104, 105,106,107,108]).
| Origin of the infection | Raw or undercooked meat |
|---|---|
| Number of cases | 334 |
| Autochthonous | 322/334 (96.4%) |
| Imported | 12/334 (3.6%) |
| Date of publication | |
| 2000-2005 | 67/334 (20.06%) |
| 2006-2011 | 238/334 (71.26%) |
| 2012-2016 | 29/334 (8.68%) |
| First author field of activity | |
| Parasitology | 171/334 (51.2%) |
| Infectious Diseases | 53/334 (15.87%) |
| Family Medicine | 51/334 (15.27%) |
| Veterinary | 27/334 (8.08%) |
| Epidemiology | 17/334 (5.09%) |
| Patology | 8/334 (2.39%) |
| Neurology | 4/334 (1.2%) |
| Microbiology | 2/334 (0.6%) |
| Surgery | 1/334 (0.3%) |
| Case diagnostic certainty | |
| Definitive/Probable | Definitive 334/334 (100%) |
| Main symptoms | |
| Fever | 164/620 (26.45%) |
| Myalgia | 137/620 (22.1%) |
| Oedema | 126/620 (20.32%) |
| Asthenia | 117/620 (18.87%) |
| Asymptomatic | 46/620 (7.42%) |
| Diarrhea | 24/620 (3.87%) |
| Headache | 5/620 (0.81%) |
| Abdominal pain | 1/620 (0.16%) |
| Diagnosis | |
| Serology | 299/334 (89.52%) |
| Serology + PCR of food sample | 35/334 (10.48%) |
| Treatment | |
| Anthelminthic | 334/334 (100%) |
Discussion
This review has more cases of parasitism detected than other previous similar studies[4] and contains current information.
Also, we have detected more cases of parasitism in the last years in Spain than previous studies in larger countries such as the USA in the same period of time [1].
Advances in the diagnosis of these diseases could explain the increase in cases detected [53,54] although we also suggest that the globalization of exotic food and changes in eating habits may have an important effect [21].
We have found the results of our findings of the epidemiology of foodborne nematodiasis in Europe suggest that these diseases are high risk and have an important impact on public health. Migration and travel do not appear to be particular risk factors for most of the investigated diseases as most cases found were autochthonous. However, all cases of nematodiasis by Angiostrongylus cantonensis were imported so therefore for this particular parasitosis, migration and travel do seem to play an important role.
Angiostrongylus cantonensis is endemic in Southeast Asia and the Caribbean. In Europe, this disease does not appear unless it is imported. Globalization has increased the number of European tourists attracted by distant and exotic countries [16] where parasites, such as A. cantonensis are endemic. This is why we suggest that the number of cases imported into Europe of this parasite has increased. When the infection appears in children, it is usually associated with the child eating or playing with the intermediate host [13].
This helminth is responsible for the majority of eosinophilic meningitis cases worldwide [55]. A. cantonensis meningitis may be suspected if the patients has consumed possibly contaminated foods and by the patient´s clinical presentation [14]. Eosinophilic pleocytosis of the cerebrospinal fluid can also aid in the diagnosis [14].
Toxocara spp is the parasite with the highest number of cases in our study, with the highest geographical prevalence in Poland [11,38]. More than half of the cases are in children and the infection of this parasite is attributed to lack of hygiene along with contact with the intermediary hosts. The diagnosis of human toxocariasis is confirmed through the assessment of clinical symptoms and the detection of blood eosinophilia and circulating immunoglobulin G (IgG) antibodies to Toxocara excretory–secretory antigens [56].
Ascaris lumbricoides infection affects 25% of world population [57]. In the case of Ascaris lumbricoides, more than 40% of the cases occur in children [36,58] and it is a parasite which exhibits monoxenous development and in which the infection occurs only due to lack of hygiene. Diagnoses are made by coprological identification, serology, CT findings and clinical history. Incidental diagnosis occurs when the host passes a worm in the stool or vomit [29].
Because of the worldwide popularization of exotic food, the traditional Asian fish dishes are served in restaurants and prepared by consumers. These recipes include raw fish and are a risk of infection. Anchovies are a fish that produces many infections [59]. Also, consumption of marinated anchovies in the European Union should be cautioned, in Italy, is the main source of anisakiasis [60].
Trichinella spp is the most important parasite of this study from a public health point of view. It is a parasite that causes a very aggressive clinical picture and can cause the death of the host. Its transmission would be avoidable if the current European legislation regarding meat processing was adhered to. Outbreaks of this parasitic infection occur when meat from hunted animals is consumed [41] or when corresponding laws are not complied with [61]. As shown in Table 6, trichinellosis does not decrease over the years and the detected cases oscillated; therefore, it is not a controlled disease. To avoid the presence of Trichinella in meat intended for human consumption, areas, where wildlife and livestock holdings coexist, should be subject to a strict monitoring program.
When analyzing the results of this study, the limitations present must be taken into consideration. First, it is possible to not have included all available relevant literature that met the inclusion criteria in the study, mainly due to a lack of accessibility given that the search was restricted to studies published in English or Spanish available through PubMed, SciELO, Google Scholar and PLOS. Publication bias may also be one of the limitations of this study since it is believed that studies with positive results are more widely distributed than those without significant results or negative ones.
Conclusion
Considering both autochthonous and imported cases, parasitologists, pharmacists, veterinary and podiatrist were the most often involved professionals in the treatment and care of people with foodborne nematodiasis in Europe. The large number of different specialists involved suggests the need for knowledge in different settings in order to properly manage foodborne nematodiasis.
Travel Medicine is specialized in the detection of these cases. It is of great importance the clinical history in the diagnosis of non-endemic parasitosis since without it the symptomatology can lead us to a wrong diagnosis.
The data collected in this study show the set of number of cases of foodborne nematodiasis. Changes in eating habits and the effect of globalization may increase infected patients. For good management of these nematodiasis infections it is necessary to increase the control of food processing and to respect the laws that control food. Foodborne nematodes due to lack of hygiene in food processing are diseases that can be avoided by increasing the information to consumers. The high number of cases collected and the description of medical cases are an excellent tool for clinicians and doctors responsible for the diagnosis and treatment of these currently underdiagnosed diseases.
The methods used for this study could be adapted to estimate the proportion of illnesses attributable to other helminthiasis and the estimates from this study can be used to help direct policy and interventions and conduct other analyses.
References
- 1.Fried B, Abruzzi A.. Food-borne trematode infections of humans in the United States of America. Parasitol Res. 2010May;106(6):1263–1280. [DOI] [PubMed] [Google Scholar]
- 2.Ortega Y. Foodborne Parasites. 1st ed. New York: Springer; 2006. [Google Scholar]
- 3.Newell D, Koopmans M, Verhoef L, et al. Food-borne diseases - the challenges of 20 years ago still persists while new ones continue to emerge. Inf Food Microbiol. 2010;28(145):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zammarchi L, Strohmeyer M, Bartalesi F, et al. COHEMI Project Study Group. Epidemiology and management of cysticercosis and taenia solium taeniasis in europe, systematic review 1990-2011. PLoS One. 2013July29;8(7):e69537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller R. Worms and human disease. 2nd ed. New York: CABI Publishing; 2001. [Google Scholar]
- 6.Sakanari JA, McKerrow JH. Anisakiasis. Clin Microbiol Rev. 1989July;2(3):278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jay JM, Loessner MJ, Golden DA. Modern Food Microbiology. 6th ed. New York: Springer; 2006. [Google Scholar]
- 8.Bruschi F, Murrell KD. New aspects of human trichinellosis: The impact of new trichinella species. Postgrad Med J. 2002January;78(915):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottstein B, Pozio E, Nockler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009January;22(1):127,45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pien FD, Pien BC. Angiostrongylus cantonensis eosinophilic meningitis. Int J Infect Dis. Spring1999;3(3):161–163. [DOI] [PubMed] [Google Scholar]
- 11.Mazur-Melewska K, Mania A, Figlerowicz M, et al. The influence of age on a clinical presentation of toxocara spp. infection in children. Ann Agric Environ Med. 2012;19(2):233–236. [PubMed] [Google Scholar]
- 12.Bradley JE, Jackson JA. Immunity, immunoregulation and the ecology of trichuriasis and ascariasis. Parasite Immunol. 2004Nov-Dec;26(1112):429–441. [DOI] [PubMed] [Google Scholar]
- 13.Malvy D, Ezzedine K, Receveur MC, et al. Cluster of eosinophilic meningitis attributable to angiostrongylus cantonensis infection in french policemen troop returning from the pacific islands. Travel Med Infect Dis. 2008September;6(5):301–304. [DOI] [PubMed] [Google Scholar]
- 14.Luessi F, Sollors J, Torzewski M, et al. Eosinophilic meningitis due to angiostrongylus cantonensis in germany. J Travel Med. 2009Jul-Aug;16(4):292–294. [DOI] [PubMed] [Google Scholar]
- 15.Padilla-Docal B, Dorta-Contreras AJ, Bu-Coifiu-Fanego R, et al. Mannose-binding lectin deficiency with eosinophilic meningoencephalitis due to angiostrongylus cantonensis in children: A case series. J Med Case Rep. 2011July;28(5):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lammers A, Goorhuis A, Beek D, et al. Eosinophilia à deux: A brain nagging souvenir from the philippines. J Inf. 2015;43:615–617. [DOI] [PubMed] [Google Scholar]
- 17.Daschner A, Cuellar C, Valls A. Towards a differential definition of atopy: Anisakis simplex and the relationship between parasites and arthropods in respiratory allergy. Parasite Immunol. 2008August;30(8):417–424. [DOI] [PubMed] [Google Scholar]
- 18.Del Rey-Moreno A, Valero-Lopez A, Gomez-Pozo B, et al. Use of anamnesis and immunological techniques in the diagnosis of anisakidosis in patients with acute abdomen. Rev Esp Enferm Dig. 2008March;100(3):146–152. [PubMed] [Google Scholar]
- 19.Foti C, Nettis E, Cassano N, et al. Acute allergic reactions to anisakis simplex after ingestion of anchovies. Acta Derm Venereol. 2002;82(2):121–123. [DOI] [PubMed] [Google Scholar]
- 20.Mattiucci S, Fazii P, De Rosa A, et al. Anisakiasis and gastroallergic reactions associated with anisakis pegreffii infection, italy. Emerg Infect Dis. 2013March;19(3):496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabrera R. Anisakiasis outbreak by anisakis simplex larvae associated to peruvian food in spain. Rev Esp Enferm Dig. 2010October;102(10):610–611. [DOI] [PubMed] [Google Scholar]
- 22.Caramello P, Vitali A, Canta F, et al. Intestinal localization of anisakiasis manifested as acute abdomen. Clin Microbiol Infect. 2003July;9(7):734–737. [DOI] [PubMed] [Google Scholar]
- 23.Moschella CM, Mattiucci S, Mingazzini P, et al. Intestinal anisakiasis in italy: A case treated by emergency surgery. G Chir. 2005May;26(5):201–205. [PubMed] [Google Scholar]
- 24.Bao F, Alvarez M, Marti C. Anisakis simplex on an ulcer in a billroth II patient. Rev Esp Enferm Dig. 2005July;97(7):532–533. [DOI] [PubMed] [Google Scholar]
- 25.García J, Romero M. Dolor torácico anginoso como manifestación inicial de anisakiasis gástrica. An Med Interna. 2004;21:185–186. [PubMed] [Google Scholar]
- 26.Deprez FC, Pauls C, Puttemans T. CT imaging of ascaris lumbricoides. JBR-BTR. 2010Jul-Aug;93(4):227. [DOI] [PubMed] [Google Scholar]
- 27.Papazahariadou M, Papadopoulos E, Frydas S, et al. Prevalence of gastrointestinal parasites in the greek population: Local people and refugees. Ann Gastroenterol. 2004;17:194–198. [Google Scholar]
- 28.Salamone G, Atzeni J, Agrusa A, et al. A rare case of abdominal cocoon. Ann Ital Chir. 2013October5;84(ePub):S2239253X13021531. [PubMed] [Google Scholar]
- 29.Scarlata F, Giordano S, Infurnari L, et al. Un caso di addome acuto da infestazione masiva da ascaridi. Infez Med. 2008;1:37–39. [PubMed] [Google Scholar]
- 30.Walter BM, Born P, Winker J. Ascaris lumbricoides causing obscure gastrointestinal bleeding detected by double-balloon enteroscopy. Endoscopy. 2015;47(Suppl 1 UCTN):E354–5. [DOI] [PubMed] [Google Scholar]
- 31.Lhasong K, Dey S, Singh V, et al. Infarkte an mesenterischen lymphknoten und intestinale gangran durch ascaris lumbricoides bei einem kind. GMS. 2011;9:1–6. [Google Scholar]
- 32.Albal A, Segura J, Gómez S, et al. Pancreatitis aguda recidivante por ascaris lumbricoides. Rev Clin Med Fam. 2008;2:186–187. [Google Scholar]
- 33.Ananthakrishnan G, Flinn J. An unusual case of abdominal pain. Br J Radiol. 2010;83:628–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnedo G, Flor X, Jimenez Y, et al. Ascaris lumbricoides y coprocultivo negativo: Vencer o ignorar. Aten Primaria. 2003;32:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casado-Maestre MD, Alamo-Martinez JM, Segura-Sampedro JJ, et al. Ascaris lumbricoides as etiologic factor for pancreas inflammatory tumor. Rev Esp Enferm Dig. 2011November;103(11):592–593. [DOI] [PubMed] [Google Scholar]
- 36.Singer C, Stancu P, Cosoveanu S, et al. Study on the frequency and values of sanguine eosinophilia in children admitted with parasitary diseases. Curr Health Sci J. 2013April;39(2):93–96. [PMC free article] [PubMed] [Google Scholar]
- 37.Weleba M, Sangaré D, Diarra D. Prevalence and evolution of intestinal parasites and urinary in hospital luxembourg: Endemicity risk of ascaris lumbricoides and entamoeba histolytica in the district of bamako. Sci J Microbiology. 2015;4:3. [Google Scholar]
- 38.Wisniewska-Ligier M, Wozniakowska-Gesicka T, Sobolewska-Dryjanska J, et al. Analysis of the course and treatment of toxocariasis in children-a long-term observation. Parasitol Res. 2012June;110(6):2363–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stensvold CR, Skov J, Moller LN, et al. Seroprevalence of human toxocariasis in denmark. Clin Vaccine Immunol. 2009September;16(9):1372–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logar J, Soba B, Kraut A, et al. Seroprevalence of toxocara antibodies among patients suspected of ocular toxocariasis in slovenia. Korean J Parasitol. 2004September;42(3):137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallardo MT, Mateos L, Artieda J, et al. Outbreak of trichinellosis in spain and sweden due to consumption of wild boar meat contaminated with trichinella britovi. Euro Surveill. 2007March15;12(3):E070315.1. [DOI] [PubMed] [Google Scholar]
- 42.Pinelli E, Mommers M, Homan W, et al. Imported human trichinellosis: Sequential IgG4 antibody response to trichinella spiralis. Eur J Clin Microbiol Infect Dis. 2004January;23(1):57–60. [DOI] [PubMed] [Google Scholar]
- 43.McHugh G, Kiely D, Low J, et al. Importation of polish trichinellosis cases to ireland, june 2007. Euro Surveill. 2007July19;12(7):E070719.3. [DOI] [PubMed] [Google Scholar]
- 44.Lozano B, Gurtner V, Pozio EB. E. Trichinellosis in immigrants in switzerland. J Travel Med. 2012;19(3):195–197. [DOI] [PubMed] [Google Scholar]
- 45.Neghina R, Neghina AM, Marincu I. Trichinellosis in hospitalized patients from a romanian endemic area, 2007-2009. Clin Microbiol Infect. 2012January;18(1):86–90. [DOI] [PubMed] [Google Scholar]
- 46.Glatz K, Danka J, Kucsera I, et al. Human trichinellosis in hungary from 1965 to 2009. Parasite. 2010September;17(3):193–198. [DOI] [PubMed] [Google Scholar]
- 47.Arevalo A, Bringas MJ, Rodriguez R. Description of a trichinosis outbreak in the province of salamanca. Rev Esp Quimioter. 2009June;22(2):115–116. [PubMed] [Google Scholar]
- 48.Littman M, Nockler K, Hallauer J. Cluster of trichinellosis cases in mecklenburg-vorpommern, germany. Euro Surveill. 2006May18;11(5):E060518.1. [DOI] [PubMed] [Google Scholar]
- 49.Kurdova-Mintcheva R, Jordanova D, Ivanova M. Human trichinellosis in bulgaria–epidemiological situation and trends. Vet Parasitol. 2009February23;159(3–4):316–319. [DOI] [PubMed] [Google Scholar]
- 50.Gari-Toussaint M, Tieulie N, Baldin J, et al. Human trichinellosis due to trichinella britovi in southern france after consumption of frozen wild boar meat. Euro Surveill. 2005June;10(6):117–118. [PubMed] [Google Scholar]
- 51.Milne LM, Bhagani S, Bannister BA, et al. Trichinellosis acquired in the united kingdom. Epidemiol Infect. 2001October;127(2):359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ranque S, Faugere B, Pozio E, et al. Trichinella pseudospiralis outbreak in france. Emerg Infect Dis. 2000Sep-Oct;6(5):543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno M, Canut S, Vila V, et al. Anisakiasis, ¿la tenemos presente en los diagnósticos?. Aten Primaria. 2004April15;33(6):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bucci C, Gallotta S, Morra I, et al. Anisakis, just think about it in an emergency! Int J Infect Dis. 2013November;17(11):e1071–e1072. [DOI] [PubMed] [Google Scholar]
- 55.Jones M, Rajiv M, Sandip S. Eosinophilic meningitis due to angiostrongylus cantonensis: First reported case in the UK. ACNR. 2007;6:20–21. [Google Scholar]
- 56.Van Den Broucke S, Kanobana K, Polman K, et al. Toxocariasis diagnosed in international travelers at the institute of tropical medicine, antwerp, belgium, from 2000 to 2013 In PLoS Negl Trop Dis. 2015;9(3):e0003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tortajada-Laureiro L, Olveira-Martin A, Marin-Serrano E, et al. Biliary parasite (ascaris) as a cause of acute pancreatitis. ultrasound diagnosis. Rev Esp Enferm Dig. 2012July;104(7):389–390. [DOI] [PubMed] [Google Scholar]
- 58.Manganelli L, Berrilli F, Di Cave D, et al. Intestinal parasite infections in immigrant children in the city of rome, related risk factors and possible impact on nutritional status. Parasit Vectors. 2012November20;5:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bao M, Pierce GJ, Pascual S, et al. Assessing the risk of an emerging zoonosis of worldwide concern: Anisakiasis. Sci Rep. 2017March;13(7):43699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattiucci S, Paoletti M, Borrini F, et al. First molecular identification of the zoonotic parasite anisakis pegreffii (nematoda: Anisakidae) in a paraffin-embedded granuloma taken from a case of human intestinal anisakiasis in italy. BMC Infect Dis. 2011March31;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perevoscikovs J, Jansone I, Jansone S, et al. Trichinellosis outbreak in latvia linked to bacon bought at a market, january-march 2005. Euro Surveill. 2005May12;10(5):E050512.2. [DOI] [PubMed] [Google Scholar]
- 62.Amo M, Muñoz C, Martínez P, et al. Anisakiasis multiple. Rev Esp Enferm Dig. 2008;100:581–582. [DOI] [PubMed] [Google Scholar]
- 63.Aneiros-Fernández J, Caba M, Rios R, et al. Intestinal eosinophilic: Anisakiasis. J Med Cases. 2010;1(3):84–86. [Google Scholar]
- 64.Brieau B, Rahmi G, Benosman H, et al. Acute dysphagia and odynophagia revealing an unusual case of oesophageal anisakiasis. Dig Liver Dis. 2015;47(12)e21. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez-Delgado FJ, Martinez-Castillo R, Lasanta-Melero B, et al. Anisakis infection with atypical presentation: Report of a case. Semergen. 2015April;41(3):176–177. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez Salazar LI, Guantes De Vigo B, Herreros Rodriguez J, et al. Another multiple gastric anisakiasis case. Rev Esp Enferm Dig. 2010January;102(1):60–61. [DOI] [PubMed] [Google Scholar]
- 67.Herranz-Bachiller MT, Atienza-Sanchez R, Barrio-Andres J, et al. Colonic polyp secondary to anisakis simplex. Rev Esp Enferm Dig. 2012Oct-Nov;104(10):554–555. [DOI] [PubMed] [Google Scholar]
- 68.Jurado-Palomo J, Lopez-Serrano MC, Moneo I. Multiple acute parasitization by anisakis simplex. J Investig Allergol Clin Immunol. 2010;20(5):437–441. [PubMed] [Google Scholar]
- 69.Juric I, Pogorelic Z, Despot R, et al. Unusual cause of small intestine obstruction in a child: Small intestine anisakiasis: Report of a case. Scott Med J. 2013February;58(1):e32–e36. [DOI] [PubMed] [Google Scholar]
- 70.López-González R, Marquez-Moreno A, Casals-Sánchez J, et al. Anisakiasis intestinal diagnosticada por enteroclisis. Semergen. 2010;36:44–46. [Google Scholar]
- 71.Mariano E, Fioranelli M, Grazia M, et al. Anectodal report of acute gastric anisakiasis and severe chest discomfort. J Integr Cardiol. 2015;1:210–212. [Google Scholar]
- 72.Martínez A, Sánchez A, Egea J, et al. Suboclusión intestinal por anisakis. Rev Esp Enferm Dig. 2009;101:813–819. [DOI] [PubMed] [Google Scholar]
- 73.Menendez P, Pardo R, Delgado M, et al. Mesenteric tumor due to chronic anisakiasis. Rev Esp Enferm Dig. 2015September;107(9):570–572. [PubMed] [Google Scholar]
- 74.Monroy C, Santamaría A, Clemente I, et al. Gastritis aguda por anisakis. Rev Clín Med Fam. 2014;7:56–58. [Google Scholar]
- 75.Nicola P, Napolitano L, Bartolomeo N, et al. Su di un caso di anisachiasi con perforazione del cieco. G Chir. 2005;26:375–377. [PubMed] [Google Scholar]
- 76.Nunes C, Ladeira S, Mergulhao A. Allergy to anisakis simplex in the portuguese population. Allergy Clin Immunol. 2003;11:30–40. [Google Scholar]
- 77.Ponferrada A, Matilla A, Borrego G, et al. Hemoperitoneo espontáneo secundario a yeyunoileítis por anisakis. Rev Esp Enferm Dig. 2005;97:292–293. [DOI] [PubMed] [Google Scholar]
- 78.Pontone S, Leonetti G, Guaitoli E, et al. Should the host reaction to anisakiasis influence the treatment? different clinical presentations in two cases. Rev Esp Enferm Dig. 2012December;104(11):607–610. [DOI] [PubMed] [Google Scholar]
- 79.Valle J, Lopera E, Sánchez M, et al. Spontaneus splenic ruptura and anisakis apendicitis presenting as abdominal pain: A case report. J Med Cases. 2012;6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vallejo M, Valero E, Charro M, et al. Dolor abdominal recidivante: Afectación gástrica e ileal por anisakis. An Med Interna. 2006;23:556–557. [DOI] [PubMed] [Google Scholar]
- 81.Zullo A, Hassan C, Scaccianoce G, et al. Gastric anisakiasis: Do not forget the clinical history! J Gastrointestin Liver Dis. 2010December;19(4):359. [PubMed] [Google Scholar]
- 82.Joki-Erkkila M. Vomiting in an immigrant during early pregnancy. Duodecim. 2001;117(24):2573–2575. [PubMed] [Google Scholar]
- 83.Julian-Gomez L, Barrio J, De La Serna C, et al. Intestinal and biliary infection with ascaris lumbricoides in gastrointestinal endoscopy. Rev Esp Enferm Dig. 2009June;101(6):427,8,429. [DOI] [PubMed] [Google Scholar]
- 84.Jung O, Ditting T, Grone HJ, et al. Acute interstitial nephritis in a case of ascaris lumbricoides infection. Nephrol Dial Transplant. 2004June;19(6):1625–1628. [DOI] [PubMed] [Google Scholar]
- 85.Kilimovskij M, Dukskas A, Kraulyte Z, et al. Ascariasis of the pancreatic duct. BMJ Case Rep. 2015September15;2015:2014–79 doi: 10.1136/bcr-2014-207936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koreniewski K, Augustynowicz A, Lass A. Intestinal parasites in polish community on the example of military environment. Int Marit Health. 2014;65:216–222. [DOI] [PubMed] [Google Scholar]
- 87.Lassen B, Janson M, Viltrop A, et al. Serological evidence of exposure to globally relevant zoonotic parasites in the estonian population. PLoS One. 2016October10;11(10):e0164142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomaso H, Dierich MP, Allerberger F. Helminthic infestations in the tyrol, austria. Clin Microbiol Infect. 2001November;7(11):639–641. [DOI] [PubMed] [Google Scholar]
- 89.Zukiewicz-Sobczak W, Zwolinski J, Chmielewska-Badora J, et al. Prevalence of antibodies against selected zoonotic agents in forestry workers from eastern and southern poland. Ann Agric Environ Med. 2014;21(4):767–770. [DOI] [PubMed] [Google Scholar]
- 90.Bellanger AP, Runge M, Wendling D, et al. Lumbar myositis associated with toxocara spp. infection. Reumatol Clin. 2014Jan-Feb;10(1):54–55. [DOI] [PubMed] [Google Scholar]
- 91.Broucke S, Kanobana K, Polman K, et al. Toxocariasis diagnosed in international travelers at the institute of tropical medicine, antwerp, belgium, from 2000 to 2013. PLoS Negl Trop Dis. 2015;9(3):e0003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fellrath JM, Magnaval JF. Toxocariasis after slug ingestion characterized by severe neurologic, ocular, and pulmonary involvement. Open Forum Infect Dis. 2014August11;1(2):ofu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Georgiou C, Efstathiades Y, Dimitriou N, et al. An unusual case of toxocara canis of the ascending colon. Eur J Gastroenterol Hepatol. 2007December;19(12):1149–1153. [DOI] [PubMed] [Google Scholar]
- 94.Gómez L, Rueda T, Pulido C, et al. Toxocariasis ocular: A propósito de un caso. Arch Soc Esp Oftalmol. 2007;83:49–52. [DOI] [PubMed] [Google Scholar]
- 95.Helbok R, Brenneis C, Engelhardt K, et al. A rare case of toxocara canis cerebral valculitis. Eur J Neurol. 2007;14:49. [DOI] [PubMed] [Google Scholar]
- 96.Magnaval JF, Berry A, Fabre R, et al. Eosinophil cationic protein as a possible marker of active human toxocara infection. Allergy. 2001November;56(11):1096–1099. [DOI] [PubMed] [Google Scholar]
- 97.Paul M, Stefaniak J, Twardosz-Pawlik H, et al. The co-occurrence of toxocara ocular and visceral larva migrans syndrome: A case series. Cases J. 2009May11;2:6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez-Salvador E, Díez-Feijóo E, García-Gallardo M, et al. Granuloma posterior como manifestación de toxocariasis ocular. Rev Mex Oftalmol. 2011;85:201–204. [Google Scholar]
- 99.Sicbaldi V, Bellodi A, Molinari E, et al. Human toxocariasis presenting with fever and coleastic hepatitis: An underestimated but current zoonosis. IJCM. 2012;3:595–597. [Google Scholar]
- 100.Uhlikova M, Hubner J, Leissova M. The ocular form of larval toxocariasis in the czech republic. Cesk Slov Oftalmol. 2002April;58(2):75–83. [PubMed] [Google Scholar]
- 101.Verallo O, Fragiotta S, Verboschi F, et al. Diagnostic aspects and retinal imaging in ocular toxocariasis: A case report from italy. Case Rep Med. 2012;2012:984512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sereinigg M, Stiegler P, Schaffellner S, et al. First documented case of toxocara associated ascites after pancreas-kidney transplantation.: 493. Transplantation. 2010;90:657. [DOI] [PubMed] [Google Scholar]
- 103.Kristek J, Marjanovic K, Dmitrovic B, et al. Trichinella spiralis and breast carcinoma–a case report. Coll Antropol. 2005December;29(2):775–777. [PubMed] [Google Scholar]
- 104.Pozio E, Rossi P. Dipartimento di Malattie Infettive Parassitarie e Immunomediate, Laboratorio Comunitario di Riferimento per i Parassiti, Istituto Superiore di Sanita. Guidelines for the identification and development of sampling methods and design of suitable protocols for monitoring of trichinella infection in indicator species. Ann Ist Super Sanita. 2008;44(2):200–204. [PubMed] [Google Scholar]
- 105.Tint D, Cocuz ME, Ortan OF, et al. Cardiac involvement in trichinellosis: A case of left ventricular thrombosis. Am J Trop Med Hyg. 2009August;81(2):313–316. [PubMed] [Google Scholar]
- 106.Velasco A, Bringas M, Rodriguez R, et al. Descripción de un brote de triquinosis en la provincia de salamanca. Rev Esp Quimioter. 2009;22(2):115–116. [PubMed] [Google Scholar]
- 107.Dubinsky P, Boor A, Kincekova J, et al. Congenital trichinellosis? case report. Parasite. 2001June;8(2 Suppl):S180–2. [DOI] [PubMed] [Google Scholar]
- 108.Romano F, Motta A, Melino M, et al. Investigation on a focus of human trichinellosis revealed by an atypical clinical case: After wild-boar (sus scrofa) pork consumption in northern italy. Parasite. 2011February;18(1):85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


