ABSTRACT
Seasonality represents a response of human mood, physiology, and behavior to annual variations in natural and social environment. A strong seasonal response is expected in non-native than native residents of such regions as Turkmenistan that is characterized by high air temperature in summer and Chukotka that is characterized by high-amplitude annual variation in both air temperature and day length. Seasonality was retrospectively reported by 732 residents of these regions. Self-reports on sleep-wake traits and mental and physical health were analyzed as possible confounding variables. The expectation of stronger seasonality in non-native residents was confirmed only for Chukotka samples. However, the native–non-native seasonality differences in this region paralleled the differences in several health scores, while native–non-native health difference in Turkmenistan was found to be non-significant. Given the possible role of such confounding factor as poor health in producing higher self-reported seasonality scores, caution must be taken when the conclusion is drawn from the results suggesting a reduced degree and severity of seasonality in native residents of Chukotka as compared to other native and non-native residents of the two regions.
KEYWORDS: Seasonal affective disorder, depression, health, sleep-wake pattern, aboriginals, newcomers
Introduction
Seasonality represents an individual’s tendency to respond to annual variation in the natural and social environment by changes in well-being, mood, physiology, and behavior. It is usually self-assessed retrospectively with the Seasonal Pattern Assessment Questionnaire or SPAQ [1]. The questionnaire was proposed for questionnaire surveys for winter type of Seasonal Affective Disorder (SAD). This syndrome is often characterized by recurrent episodes of fall-winter depression, fatigue, social withdrawal, oversleeping, overeating, carbohydrate craving, and weight gain. Association of this syndrome with the lack of daylight in winter was suggested to explain its spontaneous remission in spring and its beneficial response to bright light treatment [2].
In this theoretic framework of winter-type SAD research, one of the examined assumptions was that people from native populations of Arctic regions can tolerate the condition of short winter days better than newcomers. Consequently, winter-type SAD prevalence is the lowest in such populations. It seems that most of the SPAQ-based questionnaire studies failed to support this assumption. Although in Finnish Lapland, the Finns were found to be significantly more likely to exhibit SAD than the Lapps, an Arctic Indigenous group [3], no significant difference between Alaskan natives and non-natives was detected in the occurrence of syndromal and sub-syndromal SAD in Alaska [4]. Similarly, no significant difference in the prevalence of SAD was found between Greenlanders and Danes in Greenland [5]. Moreover, Haggarty et al. [6] found the increased rates of syndromal and sub-syndromal SAD in an Inuit group living in the Canadian Arctic above 70 degree north. Finally, the results of comparison of three samples, the urban aboriginal, urban non-aboriginal (Toronto), and rural aboriginals (Fort Albany) in Ontario, Canada, suggested that the former sample was at greater risk for distress and SAD, most possibly, due to a higher susceptibility to poorer mental health, whereas the last sample was least affected by seasonal changes in the environment [7,8].
Another related assumption was that, at high latitudes, prevalence of winter-type SAD is higher among newcomers as compared to native populations. This assumption was not unequivocally supported. Suhail and Cochrane [9] reported an increased prevalence of winter-type SAD among non-indigenous (Asian, e.g. Pakistani, Indian, or Bangladeshi) women in England relative to those Caucasian and Asian women who were born in England. Similarly, Low and Feissner [10] found an increased prevalence of winter-type SAD in those college students who had moved to New England from south latitudes. Williams and Schmidt [11] found that individuals with winter-type SAD in North Canada were more likely to have been born at more southern latitudes. However, African students did not differ from African American students on winter SAD in Washington, D.C. Instead, a greater percentage of African students had summer-type SAD [12]. Comparison of five immigrant groups in Norway revealed dramatic differences among them in prevalence of winter-type SAD with the lowest prevalence in Sri Lankan men and women to the highest prevalence in Iranians. Such differences were associated with country of birth, younger age, smoking, presence of mental distress, frequent visits to general practitioner or psychiatrist, self-reported poor health, and presence of chronic disorders [13]. Nilsson and collaborators [14] showed that the rate of self-reported depression on Svalbard (78 degree north) was four to five times higher in Russians living in Barentsburg, the Russian setting, than in Norwegians living in Longyearbyen, the Norwegian setting (refer also comment on this finding published by [15]).
In general, several of the mentioned above reports, that is [7,8,12–14], pointed at a possibility that only a small proportion of the variance in prevalence of SAD might be explained by the extent of acclimatization to high amplitude annual changes in natural environment, whereas a rather big proportion of the variance can be explained by other factors, such as psycho-social and health problems.
The present study aimed on comparison of native and non-native residents of Chukotka and South Turkmenistan on retrospectively reported seasonal changes in well-being, mood, and behavior.
The following hypotheses were tested:
Retrospectively reported seasonality is higher among non-native as compared to native residents of Chukotka and Turkmenistan and
the difference is persisted after accounting for differences in health self-reports.
Methods
Questionnaire data were collected in years 1989–1992 between early January and early March in Chukotka and Turkmenistan, the north-easternmost and the southernmost administrative regions of the former Soviet Union, respectively. Table 1(a) contains a brief description of variation in some of the natural factors in the regions. To recruit the participants for the questionnaire study, the researcher visited administrative buildings, such as schools and offices, during daytime hours. In total, 732 residents of Chukotka and Turkmenistan voluntarily agreed to complete the questionnaires in the presence of researcher after receiving brief information on the purposes and procedure of the questionnaire study. The residents of Chukotka lived in several small villages. Their ages ranged from 12 to 90 with mean ± Standard Deviation (SD) of 34.3 ± 12.7 years. The residents of Turkmenistan mostly lived in Ashgabat, the country’s capital. Their ages ranged from 12 to 90 with mean ± SD of 37.0 ± 12.9 years. Natives were either Turkmens or Chukchi and Eskimos in Turkmenistan and Chukotka, respectively. Non-natives or their parents migrated in these regions from different parts of Russian Federation. They were mostly of Slavic ethnicity (at least, 95%). The protocol of the questionnaire study complied with the ethical standards on human investigations of the Helsinki Declaration, and with the Ethics Committee of the Research Institute for Molecular Biology and Biophysics, Siberian Branch of the Russian Academy of Medical Sciences.
Table 1.
Description of regions and subsamples of male and female respondents.
| A. Two regions | |||||||
|---|---|---|---|---|---|---|---|
| Region | Samples |
Latitude, degree North |
Day length, h:min |
Temperature, Cº |
|||
| N | n | December | June | January | July | ||
| Chukotka | 2 | 396 | 64–66 | 3:57 | 21:23 | −14.3 | 8.5 |
| Turkmenistan | 2 | 326 | 38 | 9:31 | 14:48 | 3.5 | 31.3 |
| B. Male and female subsamples | |||||||||
| Subsample |
GSS# |
CES-D## |
SCL S## |
Health## |
|||||
| |
n |
Mean |
SEM |
Mean |
SEM |
Mean |
SEM |
Mean |
SEM |
| CNM | 111 | 3.614 | 0.423 | 13.375 | 0.55 | 3.627 | 0.445 | 3.631 | 0.077 |
| CRM | 96 | 5.281 | 0.381 | 12.034 | 0.489 | 4.923 | 0.396 | 3.384 | 0.069 |
| CNF | 85 | 4.580 | 0.365 | 12.365 | 0.470 | 3.690 | 0.380 | 3.424 | 0.066 |
| CRF | 104 | 6.076 | 0.437 | 14.466 | 0.563 | 5.058 | 0.458 | 3.334 | 0.080 |
| TNM | 89 | 6.192 | 0.516 | 16.393 | 0.716 | 5.332 | 0.491 | 3.452 | 0.081 |
| TRM | 132 | 4.748 | 0.644 | 16.469 | 0.899 | 5.452 | 0.616 | 3.249 | 0.103 |
| TNF | 62 | 5.461 | 0.448 | 16.061 | 0.623 | 4.947 | 0.426 | 3.215 | 0.071 |
| TRF | 43 | 6.638 | 0.375 | 15.648 | 0.522 | 5.791 | 0.357 | 3.300 | 0.060 |
Notes. N: Numbers of samples; n: Numbers of respondents; December and June: The shortest and longest days of the year in these months; January and July: Averaged air temperature for these months. C or T: Chukotka or Turkmenistan; R or N: Non-native (mostly Russian) or Native; M or F: Male or Female; GSS: Score on six-item Global Seasonality Scale, CES-D: Depression scored on the 20-item Center for Epidemiological Studies – Depression scale; SCL S: Score on 12-item somatization subscale of the Symptom Checklist Inventory; Health: Self-rated Health status; SEM: Standard Error of Mean; # and ##From results of ANCOVAs shown in Tables 4 and 5, respectively.
The questionnaires included the SPAQ [1], a retrospective, self-rating questionnaire for assessing seasonality of well-being, mood, and behaviors. The first part of the SPAQ inquires to determine to what degree certain characteristics change with the season. The list of such characteristics includes sleep length, social activity, mood, weight, appetite, and energy level. For each characteristic, responses can be 0 (none), 1 (mild), 2 (moderate), 3 (marked), or 4 (extreme). The sum of these six items is the Global Seasonality Score (GSS), which, therefore, can range from 0 (no seasonality) to 24 (extreme degree of seasonality). The next question asks whether seasonal changes are considered as a problem. The response possibilities for such Problem score range from 0 (no problem) to 5 (disabling problem). In epidemiological studies, for example [4], combinations of GSS and Problem score are usually used as criteria for dividing respondents into three groups: without SAD syndrome, with syndromal SAD, and with full-blown SAD. In the present analysis, GSS and Problem were analyzed separately (Table 2–4). In the total sample, Cronbach’s alpha for six-item GSS was found to be 0.84. Sub-sample-averaged GSS is reported in Table 1(b).
Table 2.
Spearman’s coefficients of correlation of seasonality scores with other variables.
| Score | Gender | Gender | Age | CES-D | SAS | SCL S | Health |
|---|---|---|---|---|---|---|---|
| GSS | Both | 0.216*** | −0.042 | 0.315*** | 0.288*** | 0.284*** | −0.189*** |
| Male | - | −0.120* | 0.266*** | 0.216*** | 0.228*** | −0.124* | |
| Female | - | 0.017 | 0.309*** | 0.276*** | 0.268*** | −0.175*** | |
| Problem | Both | 0.214*** | 0.156*** | 0.338*** | 0.327*** | 0.276*** | −0.283*** |
| Male | - | 0.137* | 0.338*** | 0.316*** | 0.242*** | −0.257*** | |
| |
Female |
- |
0.176*** |
0.290*** |
0.272*** |
0.249*** |
−0.245*** |
| Score |
Gender |
Sleep length |
S |
F |
W |
M |
E |
| GSS | Both | 0.014 | −0.134*** | −0.088* | −0.189*** | 0.170*** | −0.063 |
| Male | 0.044 | −0.025 | −0.108 | −0.142* | 0.133* | −0.006 | |
| Female | −0.031 | −0.132** | −0.024 | −0.155** | 0.186*** | −0.016 | |
| Problem | Both | −0.067 | −0.170*** | −0.081* | −0.143*** | 0.032 | −0.090* |
| Male | −0.032 | −0.186** | −0.108 | −0.096 | 0.006 | −0.076 | |
| Female | −0.108* | −0.104* | −0.009 | −0.106* | 0.033 | −0.018 |
Notes. Age: Age, years; Problem: Score for response to a question asking whether seasonal changes are considered a problem; SAS: Score on 20-item Zung Self-Rating Anxiety Scale; Sleep length: A rough estimate of mean sleep duration, hours; S, f, w, M, and E: Scores on 12-item Nighttime Sleepability scale, 4-item Anytime Sleepability and Anytime Wakeability subscales, and 12- and eight-item Morning and Evening Lateness scales of the 40-item SWPAQ. Level of significance for correlation coefficient: *** (p < 0.001), ** (p < 0.01), * (p < 0.05). Refer also notes to Table 1.
Table 4.
Two-way ANCOVAs of GSS with factors “Gender” and “Ethnicity.”
| Chukotka |
Turkmenistan |
|||||
|---|---|---|---|---|---|---|
| Region | Df | F | η2p | Df | F | η2p |
| Covariates: | ||||||
| Age | 1/380 | 7.710** | 0.020 | 1/320 | 2.834 | 0.010 |
| Problem | 1/380 | 63.231*** | 0.146 | 1/320 | 102.094*** | 0.269 |
| CES-D | 1/380 | 1.346 | 0.004 | 1/320 | 0.390 | 0.001 |
| SAS | 1/380 | 0.257 | 0.001 | 1/320 | 0.004 | 0 |
| SCL S | 1/380 | 0.018 | 0 | 1/320 | 0.001 | 0 |
| Health | 1/380 | 0.023 | 0 | 1/320 | 0.784 | 0.003 |
| Sleep length | 1/380 | 0.437 | 0.001 | 1/320 | 0.738 | 0.003 |
| SWPAQ S | 1/380 | 2.412 | 0.006 | 1/320 | 0.014 | 0 |
| SWPAQ f | 1/380 | 0.016 | 0 | 1/320 | 3.232 | 0.011 |
| SWPAQ w | 1/380 | 3.925* | 0.011 | 1/320 | 0.298 | 0.001 |
| SWPAQ M | 1/380 | 4.533* | 0.012 | 1/320 | 4.713* | 0.017 |
| SWPAQ E | 1/380 | 0.058 | 0 | 1/320 | 0.980 | 0.003 |
| Factors: | ||||||
| 1. Gender | 1/380 | 4.213* | 0.011 | 1/320 | 1.234 | 0.004 |
| 2. Ethnicity | 1/380 | 14.928*** | 0.039 | 1/320 | 0.069 | 0 |
| 1. x 2. | 1/380 | 0.048 | 0 | 1/320 | 6.918** | 0.024 |
The second part of the SPAQ requests to fill in the month or months of the year (if any) when a respondent sleeps most and least, socializes least and most, loses and gains most weight, eats least and most, feels best and worse, and most and least energetic (i.e. “Are any month(s) during the year when you …?”). Several additional questions were added to these 12 questions including four questions asking about such common symptoms of disturbed sleep as difficulties falling asleep, difficulties staying asleep, daytime sleepiness, and premature awakening. Figures 1 and 2 illustrate month-to-month variation in the rate of some of the assessed characteristics. The first 12 characteristics were transformed into six bipolar characteristics (Figures 1 and 2(a)). The responses were summed to calculate seasonality scores. One of these summing scores (Figure 3(a)) is characterized by bimodal seasonal pattern (feeling worse and best, least and most energetic, and socializing least and most). Another summing score (Figure 3(b)) is characterized by unimodal seasonal pattern (gain most and lose most weight, eat most and least, and sleep most and least). Items on disturbed sleep were also scored as a sum of retrospective reports of difficulty staying asleep, difficulty falling asleep, premature awakening, and daytime sleepiness to characterize sleeping problems in general (Figure 3(c)).
Figure 1.
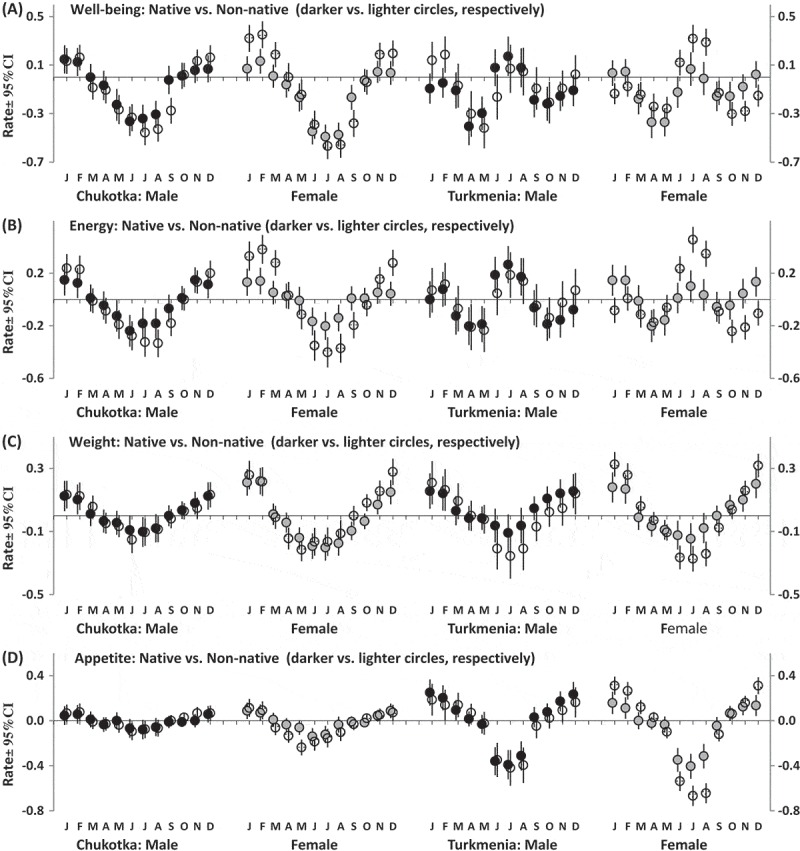
Month-to-month variation in rate of characteristics of well-being and behavior.
Estimated marginal means ± Confidence Interval (CI, vertical lines). The examples of bi- and unimodal seasonality patterns (a and b and c and d, respectively). Difference between retrospective reports of feeling worse and best (a), least and most energetic (b), gain most and lose most weight (c), and eat most and least in a particular month (d).
Figure 2.
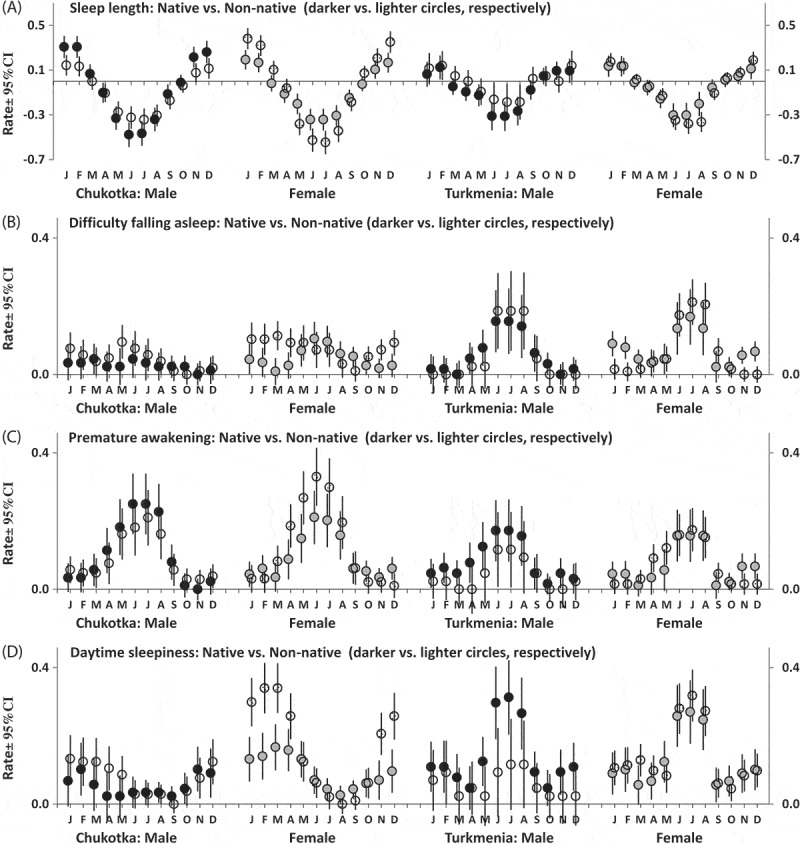
Month-to-month variation in rate of sleep characteristics.
Estimated marginal means ± Confidence Interval (CI, vertical lines). Difference between retrospective reports of sleep most and sleep least (a) and of a particular sleep problem in a particular month (b-d). Pattern for difficulty staying asleep (not shown) was very similar to that for difficulty falling asleep (b).
Figure 3.
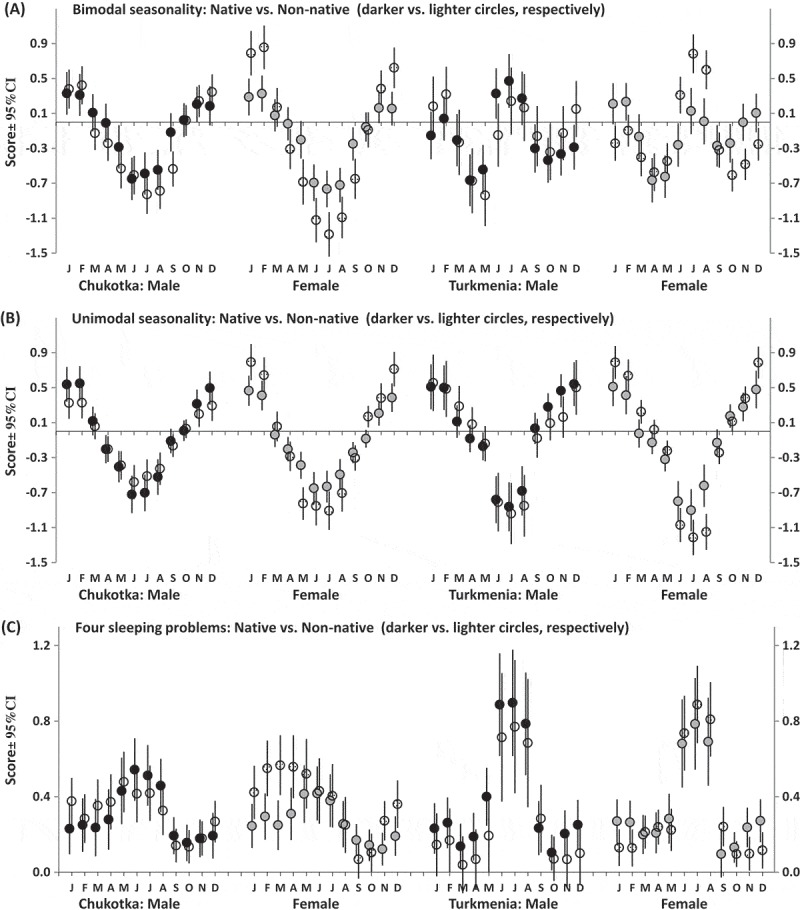
Month-to-month variation in well-being, behavior, and sleeping problems.
Estimated marginal means ± Confidence Interval (CI, vertical lines). Scores for bi- and unimodal seasonalities were calculated as a sum of differences between retrospective reports for a particular month of feeling worse and best, least and most energetic, and socializing least and most (a), gain most and lose most weight, eat most and least, and sleep most and least (b). Problems with sleep were scored as a sum of retrospective reports of difficulty staying asleep, difficulty falling asleep, premature awakening, and daytime sleepiness (c). Refer statistical results on triple interactions from rANCOVAs in Table 7.
After answering the questions about seasonality of sleep length, respondents were also asked to roughly estimate what is the maximal, minimal, and mean duration of their sleep. Mean self-reported sleep length was included in the present analysis as one of the covariates.
To self-assess the presence and severity of symptoms of depression, anxiety, and somatic dysfunction, respondents were asked to complete three questionnaire scales combined in one scale. Each question asked a respondent to indicate how often during the past week he/she had experienced a particular symptom. Responses about frequency of symptoms ranged from 0 (never or rarely) to 3 (most or all of the time) on a four-point scale. The 20-item Center for Epidemiological Studies—Depression scale or CES-D was utilized for self-assessment of symptoms of depression [16]. The 20-item Zung Self-Rating Anxiety Scale or SAS [17] was applied for self-assessment of symptoms of anxiety. Somatic symptoms were self-assessed with 12-item somatization subscale of the Symptom Checklist Inventory or SCL S [18,19]. Internal consistency assessed with Cronbach’s alpha in the total sample was 0.87, 0.85, and 0.83 for depression, anxiety, and somatic scales, respectively. Additionally, respondents answered to a simple question asking whether their current general health is very poor, poor, fair, good, or excellent. In total, four different health scores (CES-D, SAS, SCL S, and Health) were included in the present analysis (Table 1–7).
Table 7.
Results for interactions of repeated measure “Month” with independent factors from three-way rANCOVAs of month-to-month variation in three scores.
| Chukotka |
Turkmenistan |
|||||
|---|---|---|---|---|---|---|
| Region | Df | F | η2p | Df | F | η2p |
| Bimodal score: | ||||||
| x 1. Gender | 11/4172 | 2.487* | 0.007 | 11/3408 | 0.896 | 0.001 |
| x 2. Ethnicity | 11/4172 | 6.345*** | 0.017 | 11/3408 | 2.230 | 0.003 |
| x 1. x 2. | 11/4172 | 1.931 | 0.005 | 11/3408 | 17.177*** | 0.021 |
| Unimodal score: | ||||||
| x 1. Gender | 11/4172 | 2.025 | 0.005 | 11/3408 | 2.226 | 0.003 |
| x 2. Ethnicity | 11/4172 | 0.961 | 0.003 | 11/3408 | 7.451** | 0.009 |
| x 1. x 2. | 11/4172 | 5.034** | 0.013 | 11/3408 | 3.488 | 0.004 |
| Sleep score: | ||||||
| x 1. Gender | 11/4172 | 1.950 | 0.005 | 11/3408 | 0.947 | 0.002 |
| x 2. Ethnicity | 11/4172 | 3.246** | 0.009 | 11/3408 | 1.345 | 0.003 |
| x 1. x 2. | 11/4172 | 0.538 | 0.001 | 11/3408 | 0.756 | 0.002 |
Notes. Table shows interactions of repeated measure with each of two independent factors (x 1. Gender and x 2. Ethnicity) and the triple interaction (x 1. x 2.) from results of three-way rANCOVAs. The covariates were the same as in Table 4. Refer also illustrations of these three triple interactions in Figure 3.
A short (40-item) version of the Sleep Wake Pattern Assessment Questionnaire or SWPAQ [20] was developed to self-assess five individual traits of the sleep-wake cycle named morning lateness (12-item M-scale), evening lateness (eight-item E-scale), anytime wakeability (four-item w sub-scale), anytime sleepability (four-item f sub-scale), and nighttime sleepability (12-item S scale). Their Cronbach’s alpha-coefficients were 0.80, 0.77, 0.54, 0.54, and 0.80, respectively [21]. Positive score signifies either ability (w, f, and S > 0) or lateness (M and E > 0). Scores on two lateness scales were shown to predict scores on other scales for self-assessment of morning–evening preference [22], and they were also shown to predict phase positions of circadian rhythms of physiological and hormonal variables [23–25], self-reported bed and rising times [26], peaks of objective and subjective indexes of alertness-sleepiness [27,28], etc. Nighttime sleepability scale was also validated in several experimental studies, for example [26,29]. The sleep-wake self-assessments were included in the present analysis as covariates (Table 2, 4–7).
The SPSS statistical software package (IBM, Armonk, NY, USA, version 22.0) was used for all analyses. Individual scores were inter-correlated (Table 2) and subjected to linear regression analysis with GSS and problem score as independent predictors (Table 3). Residents younger than 16 (10 in total) were not included in the analyses due to lack of age-specific versions of the questionnaires. ANCOVAs were run with independent factors “Gender” and either “Ethnicity” (Table 4, 5, and 7) or “Region” (Table 6). Repeated measure ANCOVAs (rANCOVAs) were performed with independent factors “Gender” (Male/Female) and either “Ethnicity” (Native/Non-native) and repeated measure “Month” (12 months from January to December). Degrees of freedom were corrected using Huynh–Feldt correction controlling for type 1 error associated with violation of the sphericity assumption, but the original degrees of freedom are reported in Table 7.
Table 3.
Seasonality scores as predictors of age and health scores.
| Region | Age | CES-D | SAS | SCL S | Health | |
|---|---|---|---|---|---|---|
| Both | R2 | 0.039 | 0.145 | 0.140 | 0.098 | 0.074 |
| F | 14.528*** | 57.995*** | 55.316*** | 37.076*** | 28.864*** | |
| GSS | −0.166*** | 0.186*** | 0.171*** | 0.137*** | −0.055 | |
| Problem | 0.224*** | 0.249*** | 0.255*** | 0.220*** | −0.240*** | |
| Chukotka | R2 | 0.030 | 0.158 | 0.170 | 0.112 | 0.095 |
| F | 6.227** | 35.950*** | 39.283*** | 24.211*** | 20.630*** | |
| GSS | −0.192*** | 0.225*** | 0.209*** | 0.162*** | −0.042 | |
| Problem | 0.150** | 0.235*** | 0.267*** | 0.224*** | −0.285*** | |
| Turkmenistan | R2 | 0.057 | 0.107 | 0.084 | 0.070 | 0.044 |
| F | 9.678*** | 17.726*** | 13.638*** | 11.112*** | 7.378*** | |
| GSS | −0.149*** | 0.132*** | 0.114*** | 0.099 | −0.073 | |
| Problem | 0.281*** | 0.237*** | 0.213*** | 0.197** | −0.164** |
Notes. R2: R square (explained variance); F: F-ratio; GSS and Problem: Beta for each of two predictors in results of linear regression analysis. Level of significance for F-ratio (ANOVA) or t-value (beta for GSS and Problem): *** (p < 0.001), ** (p < 0.01), * (p < 0.05). Refer also notes to Tables 1 and 2.
Table 5.
Results for factors “Ethnicity” and “Gender” from two-way ANCOVAs of health and sleep-wake characteristics.
| Chukotka |
Turkmenistan |
|||||
|---|---|---|---|---|---|---|
| Region | Df | F | η2p | Df | F | η2p |
| CES-D | ||||||
| 1. Gender | 1/380 | 1.648 | 0.004 | 1/320 | 0.634 | 0.002 |
| 2. Ethnicity | 1/380 | 0.503 | 0.001 | 1/320 | 0.057 | 0 |
| 1. x 2. | 1/380 | 12.001*** | 0.032 | 1/320 | 0.122 | 0 |
| SCL S | ||||||
| 1. Gender | 1/380 | 0.049 | 0 | 1/320 | 0.002 | 0 |
| 2. Ethnicity | 1/380 | 9.633** | 0.025 | 1/320 | 1.006 | 0.004 |
| 1. x 2. | 1/380 | 0.007 | 0 | 1/320 | 0.572 | 0.002 |
| Health | ||||||
| 1. Gender | 1/380 | 2.717 | 0.100 | 1/320 | 1.279 | 0.005 |
| 2. Ethnicity | 1/380 | 5.040* | 0.025 | 1/320 | 0.542 | 0.002 |
| 1. x 2. | 1/380 | 1.216 | 0.003 | 1/320 | 3.290 | 0.012 |
| Sleep length | ||||||
| 1. Gender | 1/380 | 6.142* | 0.016 | 1/320 | 0.008 | 0 |
| 2. Ethnicity | 1/380 | 4.177* | 0.011 | 1/320 | 0.000 | 0 |
| 1. x 2. | 1/380 | 0.048 | 0 | 1/320 | 0.161 | 0.001 |
| SWPAQ S | ||||||
| 1. Gender | 1/380 | 4.540* | 0.012 | 1/320 | 0.864 | 0.003 |
| 2. Ethnicity | 1/380 | 5.226* | 0.016 | 1/320 | 2.073 | 0.007 |
| 1. x 2. | 1/380 | 4.193* | 0.011 | 1/320 | 0.039 | 0 |
Notes. CES-D, SCL S, Health, Sleep length, and SWPAQ S: Main effect of the second factor and/or interaction (1. Gender and 2. Ethnicity) were significant for these health and sleep variables. The list of covariates was the same as in Table 4 but GSS was included instead of an analyzed health or sleep variable.
Table 6.
Results for factors “Region” and “Gender” from two-way ANCOVAs of GSS and sleep-wake variables.
| Non-natives |
Natives |
|||||
|---|---|---|---|---|---|---|
| Ethnicity | Df | F | η2p | Df | F | η2p |
| GSS | ||||||
| 1. Gender | 1/353 | 7.076** | 0.021 | 1/337 | 0.006 | 0 |
| 2. Region | 1/353 | 2.753 | 0.008 | 1/337 | 2.937 | 0.009 |
| 1. x 2. | 1/353 | 1.284 | 0.004 | 1/337 | 4.256* | 0.013 |
| Sleep length | ||||||
| 1. Gender | 1/353 | 0.378 | 0.001 | 1/337 | 1.983 | 0.006 |
| 2. Region | 1/353 | 0.525 | 0.002 | 1/337 | 4.809* | 0.015 |
| 1. x 2. | 1/353 | 1.924 | 0.006 | 1/337 | 1.132 | 0.004 |
| SWPAQ S | ||||||
| 1. Gender | 1/353 | 4.941* | 0.015 | 1/337 | 0.683 | 0.002 |
| 2. Region | 1/353 | 1.928 | 0.006 | 1/337 | 4.536* | 0.014 |
| 1. x 2. | 1/353 | 0.722 | 0.002 | 1/337 | 0.367 | 0.001 |
| SWPAQ w | ||||||
| 1. Gender | 1/353 | 0.287 | 0.001 | 1/337 | 0.489 | 0.002 |
| 2. Region | 1/353 | 4.902* | 0.015 | 1/337 | 2.671 | 0.008 |
| 1. x 2. | 1/353 | 0.653 | 0.002 | 1/337 | 0.012 | 0 |
| SWPAQ M | ||||||
| 1. Gender | 1/353 | 1.081 | 0.003 | 1/337 | 0.301 | 0.001 |
| 2. Region | 1/353 | 3.877* | 0.012 | 1/337 | 2.735 | 0.008 |
| 1. x 2. | 1/353 | 0.690 | 0.002 | 1/337 | 0.467 | 0.001 |
Notes. GSS, Sleep length, SWPAQ S, w, and M: Main effect of the second factor and/or interaction (1. Gender and 2. Region) was significant for GSS and other included variables. For CSS, the list of covariates was the same as in Table 4. For other variables, GSS was included instead of an analyzed variable. Refer also notes to Table 5.
Results
Correlations of GSS and Problem score of the SPAQ with other variables are illustrated in Table 2 and some of the results of examination of a possibility to use these two scores for predicting health variables are reported in Table 3. Results presented in Table 2 (upper part) indicated that degree and severity of seasonal changes in well-being, mood, and behavior were significantly associated with health problems. Besides, results given in Table 3 suggested that seasonality scores explained from 5% to 17% of variation in these problems. Results on associations of degree and severity of seasonal changes with characteristics of sleep pattern were less consistent, but, when significant association was detected, it also suggested a link between worse health and higher seasonality (bottom part of Table 2). For example, higher seasonality scores were associated with lower night sleep quality (a lower score on Nighttime Sleepability scale, S).
Therefore, self-ratings of health and sleep characteristics were included as either covariates or dependent variables in ANCOVAs and rANCOVAs reported in Tables 4–5 and 7, respectively. The results of two-way ANCOVAs of the effects of factor “Gender” and “Ethnicity” on GSS score (Table 4) yielded highly significant main effect of factor “Ethnicity” for Chukotka sample; whereas, for Turkmenia sample only interaction of factors “Gender” and “Ethnicity” was significant. As can be seen in Table 1, the former result is explained by the reduction of degree of seasonality in native female respondents and, especially, in native male respondents from Chuckotka dataset. In contrast, degree of seasonality was very high in native male respondents and non-native female respondents in Turkmenia sample. This explained significant interaction between factors “Ethnicity” and “Gender” in this dataset (Table 4)
Results of similar analyses of some other variables are shown in Table 5. They were included in this table when, for an analyzed variable, main effect of an independent factor or interaction of independent factors was found to reach statistically significant level. These results suggested that, in Chukotka, main effect of factor “Ethnicity” was significant for several health characteristics. This implies that Chukotka natives reported not only lower degree of seasonality but also better physical health and less problematic sleep as compared to Chukotka non-natives (Table 1). In Turkmenistan, neither main effect nor interaction was significant. As can be seen in Table 1, health scores of all Turkmenia subgroups were similar and indicated a relatively high level of health problems in any subsample.
However, comparison of natives or non-natives of two regions did not yield significant main effect of factor “Region” in analysis of any of health characteristics. Table 6 shows the results indicating that significant regional differences were revealed only for some of characteristics of sleep-wake pattern. Non-natives in Turkmenistan had higher M scores and lower w scores indicating morning lateness and anytime-wake-inability, and natives in Turkmenistan had shorter sleep duration but higher nighttime sleepability (a higher S score).
Figure 1 illustrates either unimodality or bimodality of patterns of month-to-month changes in well-being, energy, weight, and appetite, and Figure 2 illustrate the patterns for all sleep characteristics including sleep problems. Month-to-month changes in scores calculated by summing responses to questions are illustrated in Figure 3, and Table 7 shows that significant interaction of factor “Month” with factor “Ethnicity” was yielded in analysis of bimodal seasonality score and sleep score in Chukotka (Figure 3(a-c)) and in analysis of unimodal seasonality score in Turkmenistan (Figure 3(b)). Such significant results for Chukotka were in agreement with the results of ANOVAs of GSS and indicated higher seasonality in Chukotka non-natives than natives (Table 4). Besides, significant triple interaction (between “Month”, “Ethnicity”, and “Gender”) was revealed in analysis of bimodal seasonality score in Turkmenistan. This result was in agreement with significant interaction between factors “Ethnicity” and “Gender” yielded by ANOVAs of GSS in this region (Table 4). The significant interactions indicated higher seasonal variation in native male respondents and non-native female respondents as compared to two other Turkmenia subgroups (Figure 3(a)).
Discussion
Seasonality represents a response of human mood, physiology, and behavior to the annual variation in natural and social environment. Such a response is expected to be stronger in such regions as Turkmenistan and Chukotka characterized by high-amplitude annual variation in air temperature and both air temperature and day length, respectively. However, people from indigenous populations might evolve to better tolerate the extreme conditions of short and cold winter days at the north and hot summer days at the south. Given evidence for positive association between seasonality and the presence of health problems, . [12, 21], it might be necessary to account for these problems in analysis of seasonality. The present results suggested that degree and severity of seasonality are significant predictors of each of analyzed health scores. The expectation of native–non-native difference in seasonality was not confirmed in analysis of data from Turkmenistan, but it was confirmed in comparison of native and non-native residents of Chukotka. However, the detected native–non-native differences were in line with the difference in health scores.
Due to the necessity to underline the possible role of such confounding factor as poor health for native–non-native difference in seasonality, caution must be taken when the drawing conclusion from the results suggesting higher seasonality in non-native residents of Chukotka. Moreover, significant interaction between gender and ethnicity in analyses of seasonality in Turkmenistan pointed on possible role of confounding factors of cultural nature.
The present results have practical relevance for preventing seasonal exacerbation of health and psychosocial problems of newcomers and native people living in the regions with high-amplitude annual variation in day length and air temperature. It seems that high seasonality scores can point at the presence of these problems.
Several limitations of the present study require acknowledgement. It is hard to generalize its results because the collected samples are rather small and do not represent the general populations of these regions. Agreement to participate in the questionnaire survey can have impact on the rates of seasonality symptoms and health scorings. For instance, people experiencing serious health problems can express their interest in filling the questionnaires more often than healthy people. The other methodological limitations of the present study include the application of cross-sectional and non-repeated measures. Moreover, respondents cannot be blind to the seasonality hypothesis when they are directly asked about perceived seasonal changes. Therefore, rates of month-to-month variation calculated from retrospective reports seem to be considerably overestimated in the present study. Similarly, health and sleep problems might be overestimated due to using self-assessments instead of medical examinations, and none of the analyzed characteristics of sleep-wake behavior was obtained by means of objective measurements in home settings. A list of covariates included in the present analysis is far from being comprehensive. Namely, possible influence of occupational, psychological, and cultural differences between native and non-native people was not accounted for. Unfortunately, a possibility of evaluation of confounding influence of other than temperature and day length environmental factors on seasonal changes in well-being was not considered in the present study. Particularly, the possible impact of outdoor light exposure, physical activity, and any of factors from workplace or residential home environment was not assessed. Not only a different lifestyle but also a different economic status of native and non-native residents may be the additional contributing factors to difference in degree and severity of seasonality. However, it has to be noted that these people lived side by side in rather egalitarian societies.
Funding Statement
The study was supported by grants from the Russian Foundation for Basic Research (number 07-06-00263-а, number 10-06-00114-а, number 13-06-00042-a, and number 16-06-00235-a) and the Russian Foundation for Humanities (number 06-06-00375-a, number 12-06-18001-e, and number 15-06-10403-a).
Acknowledgments
The author is indebted to Dr John Booker for his participation in translation and cross-validation of the applied questionnaires and to Dr Konstantin Danilenko, Dmitriy Zolotarev (Heffele), and Dr Vladislav Palchikov for their valuable help in collecting these questionnaire data.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- [1].Rosenthal NE, Bradt GH, Wehr TA.. Seasonal pattern assessment questionnaire. Bethesda, MD: National Institute of Mental Health; 1984a. [Google Scholar]
- [2].Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984b;41:72–80. [DOI] [PubMed] [Google Scholar]
- [3].Saarijarvi S, Lauerma H, Helenius H, et al. Seasonal affective disorders among rural Finns and Lapps. Acta Psychiatr Scand. 1999;99(2):95–101. [DOI] [PubMed] [Google Scholar]
- [4].Booker JM, Hellekson CJ.. Prevalence of seasonal affective disorder in Alaska. Am J Psychiatry. 1992;149(9):1176–1182. [DOI] [PubMed] [Google Scholar]
- [5].Kegel M, Dam H, Ali F, et al. The prevalence of seasonal affective disorder (SAD) in Greenland is related to latitude. Nord J Psychiatry. 2009;63(4):331–335. [DOI] [PubMed] [Google Scholar]
- [6].Haggarty JM, Cernovsky Z, Husni M, et al. Seasonal affective disorder in an Arctic community. Acta Psychiatr Scand. 2002;105(5):378–384. [DOI] [PubMed] [Google Scholar]
- [7].Tam BY, Gough WA, Edwards V, et al. Seasonal and weather-related behavioral effects among urban Aboriginal, urban non-Aboriginal, and remote Aboriginal participants in Canada. Popul Environ. 2013;35(1):45–67. [Google Scholar]
- [8].Tam B, Gough WA. Psychological distress and seasonality among urban Aboriginal participants. Pimatisiwin - A Journal of Aboriginal and Indigenous Community Health. 2014;11:457–469. [Google Scholar]
- [9].Suhail K, Cochrane R. Seasonal changes in affective state in samples of Asian and white women. Soc Psychiatry Psychiatr Epidemiol. 1997;32:149−157. [DOI] [PubMed] [Google Scholar]
- [10].Low KG, Feissner JM. Seasonal affective disorder in college students: prevalence and latitude. J Am Coll Health. 1998;47:135−137. [DOI] [PubMed] [Google Scholar]
- [11].Williams RJ, Schmidt GG. Frequency of seasonal affective disorder among individuals seeking treatment at a northern Canadian mental health center. Psychiatry Res. 1993;46:41−45. [DOI] [PubMed] [Google Scholar]
- [12].Guzman A, Rohan KJ, Yousufi SM, et al. Seasonality of mood in African college students in Washington. DC Scientific World Journal. 2007;7:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saheer TB 1, Lien L, Hauff E, et al. Ethnic differences in seasonal affective disorder and associated factors among five immigrant groups in Norway. J Affect Disord. 2013;151(1):237–242. [DOI] [PubMed] [Google Scholar]
- [14].Nilssen O, Brenn T, Høyer G, et al. Self-reported seasonal variation in depression at 78 degree north. Svalbard Study International Journal Circumpolar Health. 1999;58:14–23. [PubMed] [Google Scholar]
- [15].Schulz P, Curtin F. Confounding factors and seasonal depression. Int J Circumpolar Health. 2003;62(3):310. [DOI] [PubMed] [Google Scholar]
- [16].Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. J Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- [17].Zung WWK. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. [DOI] [PubMed] [Google Scholar]
- [18].Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: A step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–289. [DOI] [PubMed] [Google Scholar]
- [19].Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale: preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- [20].Putilov AA. A questionnaire for self-assessment of individual traits of sleep-wake cycle. Bull Siberian Branch USSR Acad Med Sci. 1990;1:22–25 [in Russian]. [Google Scholar]
- [21].Putilov AA. A questionnaire for self-assessment of individual profile and adaptability of sleep-wake cycle In: Gutenbrunner C, Hildebrandt G, Moog R, Eds. Chronobiology and chronomedicine 1991: basic research and applications. Peter Lang: Frankfurt am Main et al; 1993. p. 492–498. [Google Scholar]
- [22].Putilov AA, Putilov DA. Sleepless in Siberia and Alaska: cross-validation of factor structure of the individual adaptability of the sleep-wake cycle. Ergonomia. Int J Ergon Hum Factors. 2005;27:207–226. [Google Scholar]
- [23].Putilov AA, Russkikh GS, Danilenko KV. Phase of melatonin rhythm in winter depression. Adv Exp Med Biol. 1999;460:441–458. [DOI] [PubMed] [Google Scholar]
- [24].Putilov AA. Association of the circadian phase with two morningness-eveningness scales of an enlarged version of the sleep-wake pattern assessment questionnaire. Arbeitswissenschaft in Der Betrieblichen Praxis. 2000;17:317–322. [Google Scholar]
- [25].Danilenko KV, Putilov AA, Terman A, et al. Prediction of circadian phase and period using different chronotype questionnaires. Soc Res Biol Rhythm 2004. Abstracts, v. 9: pp. 196–196. [Google Scholar]
- [26].Putilov AA, Donskaya OG, Budkevich EV, et al. Reliability and external validity of the six scales of 72-item sleep-wake pattern assessment questionnaire (SWPAQ). Biol Rhythm Res. 2017;48:275–285. [Google Scholar]
- [27].Putilov AA, Donskaya OG, Verevkin EG. How many diurnal types are there? A search for two further “bird species”. Pers Individ Dif. 2015;72:12–15. [Google Scholar]
- [28].Putilov AA, Donskaya OG, Verevkin EG. Phase difference between chronotypes in self-reported maximum of alertness rhythm: an EEG predictor and a model-based explanation. J Psychophysiol. 2014;28:242–256. [Google Scholar]
- [29].Putilov AA. Validation of nighttime sleepability scale against objective and subjective measures of sleep quality. Sleep and Hypnosis. J Clin Neurosci Psychopathology. 2018;20:25–30. [Google Scholar]


