Abstract
Background:
The objective of this study is to evaluate the value of neutrophil gelatinase-associated lipocalin (NGAL) for becoming a good endogenous marker of renal function in asphyxial preterm babies.
Materials and Methods:
This is a two-center retrospective study. Between October 2016 and October 2017, 71 asphyxial preterm infants were included in asphyxia group. Seventy babies were randomly included in control group. Samples were tested at 24, 48, and 96 h after birth. Quantitative data were compared by independent sample t-test or repeated measures ANOVA. For qualitative data, Pearson's Chi-squared test was performed. Draw ROC and compare the area under the curve (AUC), 95% confidence interval for AUC, specificity (Spe), sensitivity (Sen), and Youden index (Sen+Spe-1) at 24-h, 48-h, and 96-h time points.
Results:
(1) There are no significant differences concerning on baseline data. However, blood gas, Apgar score, and resuscitation showed a significant difference (P < 0.05). (2) In 24-h samples, only uNGAL and estimated glomerular filtration rate (eGFR) showed differences between the two groups (P < 0.05). In 48-h samples, significant differences could be found in uKIM-1, uNGAL, blood urea nitrogen, and eGFR (P < 0.05). In 96-h samples, almost all indicators have significant differences except urine output and eGFR (P < 0.05). (3) All biomarkers showed statistical difference in the three time points (P < 0.05), but only uNGAL showed a downward trend after the increase of expression. (4) uNGAL has better Sen and Spe than other indicators (24-h AUC 0.870, Youden index 0.606; 48-h AUC 0.879, Youden index 0.692; and 96-h AUC 0.806, Youden index 0.606).
Conclusion:
uNGAL has a better distinguishability in asphyxial neonates compared with other indicators. Certainly, a larger sample, prospective study is still needed.
Keywords: Asphyxia, kidney injury, neonate, neutrophil gelatinase-associated lipocalin
INTRODUCTION
After preterm babies were born, kidney development is still unfinished. Kidney function and structure are immature and fragile accordingly. These newborns are often affected by renal immaturity and potential injury during the early postnatal stage.[1,2] For example, asphyxia is a common and important cause of disability and death (such as periventricular leukomalacia and cerebral palsy) for preterm babies. In Neonatal Intensive Care Unit (NICU), in spite of great advances in neonatal resuscitation, there are still a significant amount of preterm infants suffering from asphyxia, and it is often related to acute kidney injury (AKI).[3] Furthermore, renal function is routinely monitored to ensure safe dosing of medicine and to assess hydration status as well as observe renal effects of various pathologies.[4] Therefore, early and accurate identifying deterioration of renal function induced by asphyxia would be beneficial for better preterm outcome.
So far, in clinical practice, the most commonly used endogenous glomerular filtration markers have still been serum creatinine (sCr) and blood urea nitrogen (BUN), especially in the developing countries like China. However, sCr and BUN concentrations are insensitive to moderate reductions in glomerular filtration rate (GFR).[5] Moreover, sCr and BUN are also affected by extrarenal factors such as protein, muscle mass, and intake of nitrogen,[6,7] which is increased with the amount of milk formula and other parenteral nutrition preparations.[8] Furthermore, sCr and BUN have a lot to do with age, gender, and body weight. More importantly, accurate evaluation of actual GFR remains difficult in the newborn period. Clinically, it can only be expressed by an approximate value – estimated GFR (eGFR).
Compared with the above classical biomarkers, neutrophil gelatinase-associated lipocalin (NGAL) has emerged as a promising indicator of kidney injury.[9] It was demonstrated that oxidative stress, infection, ischemia, cytokines, intoxication, and other conditions leading to cellular necrosis, death, and apoptosis all could induce the upregulation of NGAL synthesis in kinds of tissues.[10,11] Mishra et al.[12] once evaluated NGAL as a sensitive biomarker for AKI. Seventy-one children underwent cardiothoracic surgery, which represents a typical model of renal ischemia-reperfusion. Moreover, NGAL was found 34 h earlier than sCr to detect AKI. Furthermore, in newborns, Song et al. found that area under the curve (AUC) of NGAL in the group of asphyxial renal damage was significantly higher than kidney injury molecule-1 (KIM-1) and cystatin C (Cys-C). Consequently, the diagnosis of renal injury in asphyxiated newborns using NGAL seems more efficiently compared with other indicators.[13]
In previous researches related to NGAL, there have been few studies being studied and reported in asphyxiated newborns, especially in preterm infants.[14,15] Moreover, in the above studies, the number of sample cases seems few. In view of this situation, we aimed to compare the values of NGAL with classical indicators (such as sCr, BUN, KIM-1, eGFR, and urine output) in asphyxiated infants (≤34 weeks) from two centers of China. Through this study, we hope to evaluate an efficient and accurate marker (NGAL) for identifying AKI in preterm infants.
MATERIALS AND METHODS
Study design
This is a two-center retrospective study. This study was performed on consecutive infants, born in the First Affiliated Hospital of Nanjing Medical University or Children's Hospital of Nanjing Medical University, between October 2016 and October 2017. The above two centers are both the largest NICUs (Level III) with 150/200 neonatal intensive care beds in Jiangsu Province of Eastern China. Written informed consent was given by patients’ parents, and the local ethics committee approved this study (NO. NJMU201610134).
Patients
Asphyxia group: After birth, preterm infants (≤34 weeks) comply with asphyxia standard (adopt standard made by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists)[16] were all included in the group. The asphyxia standard includes (a) profound metabolic or mixed acidemia (pH <7.00) on an umbilical arterial blood sample, if obtained, (b) an Apgar score of 0–3 for longer than 5 min, (c) neurologic manifestation, and (d) evidence of multiorgan dysfunction. However, severe infection (such as necrotizing enterocolitis and sepsis), shock, congenital anomaly, hyperbilirubinemia, and inherited metabolic diseases were excluded from our study. At last, 71 preterm infants were enrolled in asphyxia group. Control group: 70 preterm infants (≤34 weeks) were randomly included in the group (by odd and even numbers to avoid selection bias) during the same period. These participants contained 22 cases of neonatal swallowing syndrome, 21 cases of neonatal intracranial hemorrhage (≤ Grade II), 19 cases of ABO hemolysis, and 8 cases of neonatal wet lung syndrome. Exclusion criteria are as follows: Perinatal asphyxia, severe infection (such as necrotizing enterocolitis and sepsis), shock, congenital anomaly, hyperbilirubinemia, and inherited metabolic disease.
Samples and medical history collection
Peripheral venous blood (2 ml) and urine (8 ml) samples were obtained at 24, 48, and 96 h after birth. Enzyme-linked immunosorbent assay (ELISA) (using Quantikine ELISA kit, United States) was used to detect NGAL (urine, ng/ml) and KIM-1 levels (urine, ng/ml). The eGFR was calculated by Schwartz equation (eGFR ml/[min*1.73 m2] = K*height[cm]/sCr[μmol/l]).[17] Fully automatic chemistry analyzer was used to detect sCr (umol/l) and BUN (mmol/l) levels by AU 5800 (Beckman Coulter, United States). Urine output was collected and calculated by nurses from NICU, presented as ml/kg/h. Moreover, general clinical data (including birth weight, gender, gestational age, mode of delivery, mother's age, Apgar score, and blood gas at birth) were also collected and checked.
Statistical methods
Statistical analysis was performed using SPSS 13.0 software. Quantitative data obeying normal distribution (by Kolmogorov–Smirnov test) were showed as mean ± standard deviation. Data between the two groups were compared by independent sample t-test, and data among three time points were compared using repeated measures ANOVA (pairwise comparison was performed with LSD test) through general linear model in SPSS software. For qualitative data, Pearson's Chi-squared test was performed. Draw ROC and compare the AUC, 95% confidence interval for AUC, specificity (Spe), sensitivity (Sen), and Youden index (Sen+Spe-1) at 24-h, 48-h and 96-h time points. Use Excel 2007 to draw line chart. P < 0.05 was considered statistically significant.
RESULTS
Comparisons of baseline data between asphyxia group and control group
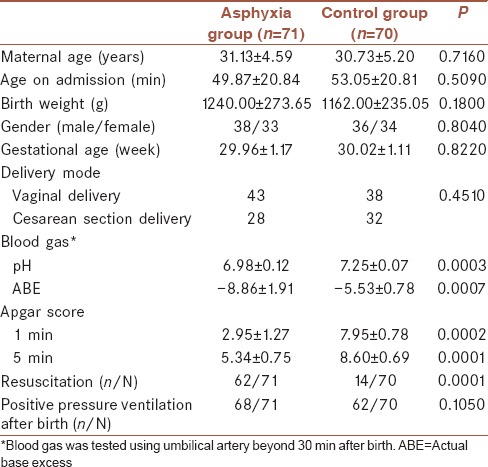
We performed comparisons of the baseline data between asphyxia group and control group. As for the demographic characteristics, there are no significant differences on mother's age, age on admission, birth weight, gender, gestational age, and delivery mode. However, blood gas, Apgar score, and resuscitation all showed statistical differences between the two groups (P < 0.05) [Table 1].
Table 1.
Comparisons of clinical data between asphyxia group and control group
Comparisons of biomarkers between asphyxia group and control group
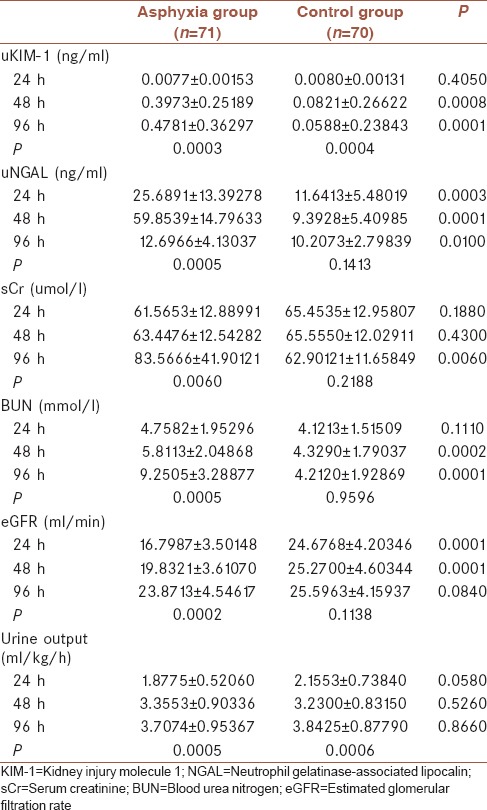
In 24-h samples, only uNGAL and eGFR showed significant differences between asphyxia and control groups (P < 0.05). At 48-h stage, significant differences could be gradually found in uKIM-1, uNGAL, BUN, and eGFR between asphyxia and control groups (P < 0.05). In 96-h samples, almost all indicators have significant differences between asphyxia and control groups except urine output and eGFR [Table 2].
Table 2.
Comparisons of biomarkers between asphyxia group and control group (horizontal comparison) and comparisons of biomarkers among 24, 48, and 96 h in asphyxia group (vertical comparison)
Comparisons of biomarkers during 24, 48, and 96 h in asphyxia group
We performed repeated measures ANOVA analysis along with time (24, 48, and 96 h) in asphyxia group. It was found that all biomarkers showed statistical difference in the three time points (P < 0.05) [Table 2]. However, there are differences in the trend of changes in various indicators. Moreover, only uNGAL showed a downward trend after the increase of expression [Supplementary Material 1 (224.2KB, tif) ].
Comparisons of trends of biomarkers during 24, 48, and 96 h in asphyxia group
Analysis of biomarkers by receiver operating characteristic curve in 24-, 48-, and 96-h samples
Compared with other indicators, uNGAL has better Sen and Spe. At the time point of 24-h, AUC of uNGAL is 0.870, Sen is 0.895, Spe is 0.711, and Youden index is 0.606; at the time point of 48-h, AUC is 0.879, Sen is 0.842, Spe is 0.850, and Youden index 0.692; at the time point of 96-h, AUC is 0.806, Sen is 0.632, Spe is 0.974, and Youden index 0.606 [Table 3 and Figures 1–3].
Table 3.
Data of indicators in 24-, 48-, and 96-h samples by receiver operating characteristic curve analysis
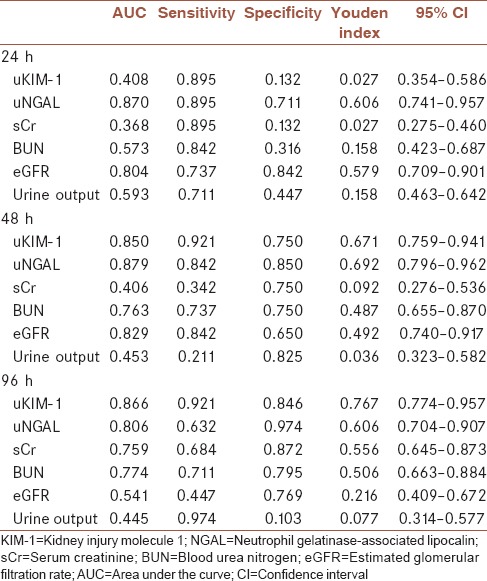
Figure 1.
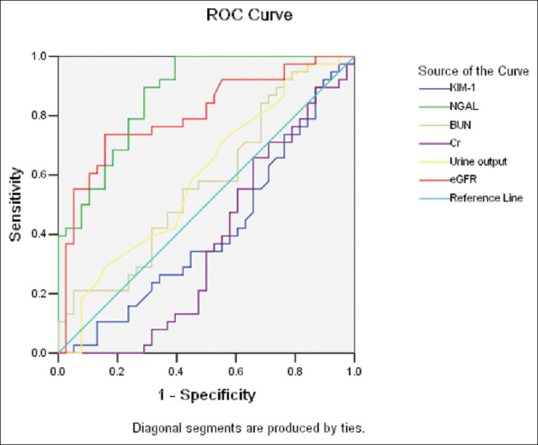
Receiver operating characteristic analysis of indicators in 24-h sample between asphyxia group and control group
Figure 3.
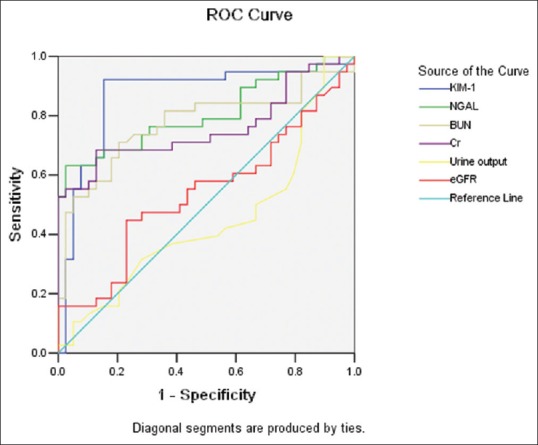
Receiver operating characteristic analysis of indicators in 96-h sample between asphyxia group and control group
Figure 2.
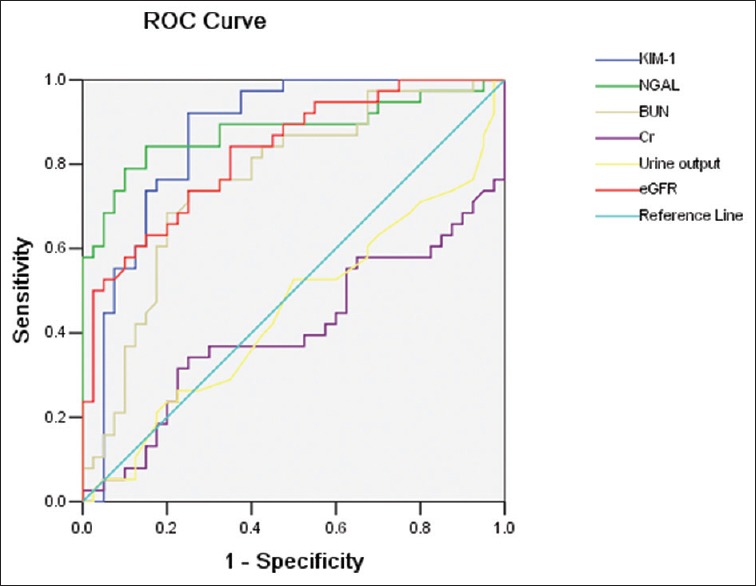
Receiver operating characteristic analysis of indicators in 48-h sample between asphyxia group and control group
DISCUSSION
Generally speaking, a low Apgar score is an important risk factor for impaired renal function in preterm infants.[18,19] Including AKI, asphyxia could cause multiple organ damages.[20] Liu et al. once performed a retrospective study and found that the incidence of renal damage was about 66% in asphyxiated newborns of China.[21] Therefore, looking for sensitive indicator of renal damage becomes an urgent issue.
The traditional diagnosis of asphyxia-induced AKI depends on a biomarker of steady-state kidney function, muscle-derived sCr. However, neonatal period is often marked by a nonsteady-state condition, and even kidney injury itself represents an unstable pathological state. Hence, sCr becomes an insensitive, retrospective, and sometimes deceptive biomarker of renal damage.[22,23,24,25] BUN is widely applied for evaluating kidney function in routine clinical practice too. Unfortunately, similar to sCr, BUN is not a reliable and specific marker of AKI because different factors could affect the concentration. Sometimes, an increase in BUN concentration could be found under the condition of no tubular injury.[25] Moreover, BUN rises with the increase of serum urea synthesis concomitantly as occurs with endogenous (such as bleeding in gastrointestinal tract) or exogenous (such as protein supplementation) protein increase.[26]
In healthy people, circulating NGAL is filtered through the glomerulus and then captured by megalin within the proximal tubule, where it traffics to lysosomes and degrades to a 14-kDa fragment being not recycled.[27,28] AKI could induce a rapid upregulation of NGAL mRNA within the thick ascending limb of Henle's loop and the collecting ducts. Then, the accumulation of NGAL in the distal nephron causes an increase in urine NGAL level. Zhang et al.[29] once studied and concluded that NGAL is better than plasma Cr and has a satisfactory early predictive value for AKI. Moreover, NGAL has emerged as a high potential biomarker of renal damage because of its rapid response. NGAL could increase rapidly (within a few minutes) in both serum and urine after kidney tissue damage (up to 1000-fold).[25,30,31] It is also demonstrated that massive quantities of NGAL are expressed and excreted by kidney epithelia just within 30 min into urine when stressed by nephrotoxins, infection, chronic progressive changes, and ischemia-reperfusion injury.[30,31] Therefore, it has been widely evaluated for the early precise diagnosis, monitoring, and risk stratification of AKI in various clinical studies. These findings have been demonstrated in different studies in newborns, children, and adults.[32,33]
Although positive results have been reported in different researches, the usefulness of urinary NGAL as a useful biomarker of AKI in preterm infants still remains controversial to some extent. Gubhaju et al.[14] found that neonates with pathological proteinuria did not show high urinary NGAL level. Between NGAL levels, they did not found associations at time points before or at the time of proteinuria onset, which seems to suggest that NGAL maybe not predictive of renal damage. Suchojad et al. also did not find increased NGAL values in infants with perinatal asphyxia.[15] Contrary to the findings made by the above authors, Song et al. found that the AUC of NGAL was significantly higher than KIM-1 and Cys-C in the group of asphyxiant renal injury (NGAL susceptibility: 87.5%; Spe: 84.3%). Diagnosis of renal injury in asphyxiated newborns using NGAL seems more efficiently compared with other methods.[13] From our study, we found that only NGAL and eGFR showed statistical differences between asphyxia group and control group in the first 24 h after birth. Moreover, urinary NGAL concentration is well correlated with other inflammatory markers, such as KIM-1 and BUN. Furthermore, uNGAL is the only indicator that reflects difference during full-time course of kidney injury (for 24 h–96 h after birth). The AUC and Youden index of NGAL were also markedly higher than other indicators during ROC analysis. This contradictory result may be explained by different test time points and different sample sizes among various researches. As for time points, it is concluded that the effect of perinatal asphyxia on the release of NGAL is declining with time. As found in our results, the level of NGAL rapidly increases and then gradually decreases after birth [Supplementary Material 1 (224.2KB, tif) ]. In fact, this sensitively reflects the change of asphyxia and the improvement of kidney function.
In the newborns, uNGAL could be detectable at birth and shows a wide range of variability in premature babies (0.51–2815.7 μg/L); the average of our measurements is 11.64 ± 5.48 ng/ml. This difference is possible because NGAL plays an important role in proliferating nephrons of the kidney in premature infants.[34] Different birth weights and gestational ages all have different degrees of impact on the results. Certainly, consistent with previous reports, a significant inverse correlation was found between gestational age and urinary NGAL (data not showed). These findings may be relevant to the immature kidney and the clinical instability of the younger neonates.
CONCLUSION
uNGAL has a better distinguishability in asphyxiated neonates compared with other indicators. Moreover, it showed a rapid decline following the restoration of glomerular function. Of course, further studies with large samples are required to assess whether uNGAL could replace sCr and BUN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We sincerely appreciate the patients’ parents for their understanding and support of our study. The local ethics committee approved the study (NO. NJMU201610134).
REFERENCES
- 1.Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011;22:1365–74. doi: 10.1681/ASN.2010121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: Implications for long-term renal health. Reprod Sci. 2011;18:322–33. doi: 10.1177/1933719111401659. [DOI] [PubMed] [Google Scholar]
- 3.Neonatal Learning Group. Chinese society of Pediatrics: Epidemiological survey of hospitalized neonates in China. Zhong Guo Dang Dai Er Ke Za Zhi. 2009;1:15–20. [PubMed] [Google Scholar]
- 4.Yakut I, Tayman C, Oztekin O, Namuslu M, Karaca F, Kosus A, et al. Ischemia-modified albumin may be a novel marker for the diagnosis and follow-up of necrotizing enterocolitis. J Clin Lab Anal. 2014;28:170–7. doi: 10.1002/jcla.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schurink M, Kooi EM, Hulzebos CV, Kox RG, Groen H, Heineman E, et al. Intestinal fatty acid-binding protein as a diagnostic marker for complicated and uncomplicated necrotizing enterocolitis: A prospective cohort study. PLoS One. 2015;10:e0121336. doi: 10.1371/journal.pone.0121336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465–90. doi: 10.1146/annurev.me.39.020188.002341. [DOI] [PubMed] [Google Scholar]
- 7.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem. 1992;38:1933–53. [PubMed] [Google Scholar]
- 8.Premji SS, Fenton TR, Sauve RS. Higher versus lower protein intake in formula-fed low birth weight infants. Cochrane Database Syst Rev. 2006;25:CD003959. doi: 10.1002/14651858.CD003959.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 10.Kjeldsen L, Bainton DF, Sengeløv H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83:799–807. [PubMed] [Google Scholar]
- 11.Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31:433–41. doi: 10.1023/a:1003708808934. [DOI] [PubMed] [Google Scholar]
- 12.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Sun S, Yu Y, Li G, Song J, Zhang H, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin for renal injury in asphyxiated preterm infants. Exp Ther Med. 2017;13:1245–8. doi: 10.3892/etm.2017.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A, et al. Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Renal Physiol. 2014;307:F149–58. doi: 10.1152/ajprenal.00439.2013. [DOI] [PubMed] [Google Scholar]
- 15.Suchojad A, Tarko A, Smertka M, Majcherczyk M, Brzozowska A, Wroblewska J, et al. Factors limiting usefulness of serum and urinary NGAL as a marker of acute kidney injury in preterm newborns. Ren Fail. 2015;37:439–45. doi: 10.3109/0886022X.2014.996109. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for Perinatal Care. 5th ed. Elk Grove Village (IL): American Academy of Pediatrics; 2002. American Academy of Pediatrics and American College of Obstetricians and Gynecologists, Care of the Neonate, Gilstrap LC, Oh W; pp. 196–7. [Google Scholar]
- 17.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–90. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 18.Deis JN, Creech CB, Estrada CM, Abramo TJ. Procalcitonin as a marker of severe bacterial infection in children in the emergency department. Pediatr Emerg Care. 2010;26:51–60. doi: 10.1097/PEC.0b013e3181c399df. [DOI] [PubMed] [Google Scholar]
- 19.Li BQ, Chen XQ. Discussion of reasons for low C-reactive protein level combined with high count of white blood cells of children with early infectious diseases. Mod J Integr Chin Trad West Med. 2007;16:2571–2. [Google Scholar]
- 20.Yang Y, Wu Y, Pan JJ, Cheng R. Change of cystatin C values in preterm infants with asphyxia-from two centers of china. J Clin Lab Anal. 2017;31:1–5. doi: 10.1002/jcla.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Zhang YF, Lu HZ, Fan QH, Liu M. The value of uKIM-1 in diagnosis of AKI in asphyxiated newborns. Guangdong Medical Journal. 2013;34:386–388. [Google Scholar]
- 22.Chiou WL, Hsu FH. Pharmacokinetics of creatinine in man and its implications in the monitoring of renal function and in dosage regimen modifications in patients with renal insufficiency. J Clin Pharmacol. 1975;15:427–34. doi: 10.1002/j.1552-4604.1975.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 23.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985;27:928–37. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 24.Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:1217–21. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mussap M, Noto A, Fanos V, Van Den Anker JN. Emerging biomarkers and metabolomics for assessing toxic nephropathy and acute kidney injury (AKI) in neonatology. Biomed Res Int 2014. 2014:602526. doi: 10.1155/2014/602526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachoin JS, Daher R, Moussallem C, Milcarek B, Hunter K, Schorr C, et al. The fallacy of the BUN: Creatinine ratio in critically ill patients. Nephrol Dial Transplant. 2012;27:2248–54. doi: 10.1093/ndt/gfr705. [DOI] [PubMed] [Google Scholar]
- 27.Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N, et al. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–7. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Langelueddecke C, Roussa E, Fenton RA, Wolff NA, Lee WK, Thévenod F, et al. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J Biol Chem. 2012;287:159–69. doi: 10.1074/jbc.M111.308296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Han J, Liu J, Liang B, Wang X, Wang C, et al. Clinical significance of novel biomarker NGAL in early diagnosis of acute renal injury. Exp Ther Med. 2017;14:5017–21. doi: 10.3892/etm.2017.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 31.Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P, et al. Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–15. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 32.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavery AP, Meinzen-Derr JK, Anderson E, Ma Q, Bennett MR, Devarajan P, et al. Urinary NGAL in premature infants. Pediatr Res. 2008;64:423–8. doi: 10.1203/PDR.0b013e318181b3b2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparisons of trends of biomarkers during 24, 48, and 96 h in asphyxia group


