Abstract
Epidemiological, cross-sectional, and prospective studies have suggested that insomnia, Alzheimer’s disease (AD) and depression are mutually interacting conditions and frequently co-occur. The monoamine and amino acid neurotransmitter systems in central nervous system were involved in the examination of neurobiological processes of this symptom complex. However, few studies have reported systematic and contrastive discussion of different neurotransmitters (NTs) changing in these neurological diseases. Thus, it is necessary to establish a reliable analytical method to monitoring NTs and their metabolite levels in rat brain tissues for elucidating the differences in pathophysiology of these neurological diseases. A rapid, sensitive and reliable LC-MS/MS method was established for simultaneous determination of the NTs and their metabolites, including tryptophan (Trp), tyrosine (Tyr), serotonin (5-HT), 5-hydroxyindolacetic acid (5-HIAA), dopamine (DA), acetylcholine (ACh), norepinephrine (NE), glutamic acid (Glu), and γ-aminobutyric acid (GABA) in rat brain tissues. The mobile phase consisting of methanol and 0.01% formic acid in water was performed on an Inertsil EP C18 column, and the developed method was validated well. Results demonstrated that there were significant differences for 5-HT, DA, NE, Trp, Tyr and ACh between model and control group in all three models, and a Bayes linear discriminant function was established to distinguish these three kinds of nervous system diseases by DA, Tyr and ACh for their significant differences among control and three model groups. It could be an excellent strategy to provide perceptions into the similarity and differentia of mechanisms from the point of NTs’ changing in brain directly and a new method to distinguish insomnia, depression and AD from view of essence.
Keywords: insomnia, Alzheimer’s disease, depression, neurotransmitters, LC-MS
1. Introduction
Insomnia, depression and Alzheimer’s disease (AD) are severe diseases of the central nervous system (CNS). Neurotransmitters (NTs) are widely recognized to make the closest contact with CNS diseases. Abnormal alteration of NTs has been found to be nearly concerned to several neurological diseases including insomnia, depression, AD, Huntington’s disease and Parkinsonism etc. [1]. Serotonin (5-HT), a tryptophan-derived biogenic amine, eventually endures oxidative deamination to form 5-hydroxyindolacetic acid (5-HIAA), has been found decreasing significantly in brains of insomnia [2], depression [3] and AD [4] rats. Dopamine (DA) and norepinephrine (NE), the metabolites of tyrosine (Tyr), are involved in sleep-wake cycles, learning, memory and emotion. A number of studies [2,3,4] have shown that the levels of DA and NE decreased significantly in the brains of depression and AD rats, counter to insomnia. Acetylcholine (ACh) is recognized participant in rapid eye movement (REM) sleep [5]. The increasing release of ACh in the pontine reticular formation promoted REM sleep in murine [6,7]. Studies suggested that cholinergic insufficiency contributed to several of the most significant neuropsychiatric appearance of AD [8] and the decreasing breakdown of ACh induced symptoms of anxiety and depression [9]. γ-aminobutyric acid (GABA) and glutamic acid (Glu) are reported as major inhibitory and excitatory NTs, in CNS, respectively. The lack of transformation from Glu to GABA leading strong excitement and weak inhibition is one of causes of some neurological diseases such as insomnia [2], AD [10] and depression [11,12]. It was found that most studies have only investigated if NTs increasing or decreasing in insomnia, depression and AD, respectively, rather than revealing differences and similarities of NTs changes from an overall perspective. So, monitoring neurotransmitter and their metabolite levels in rat brain tissues is an essential tool for examining the similarities and differences in the pathophysiology of these three diseases and also a good method to study the differences in the nature of these interrelated neuropsychiatric disorders.
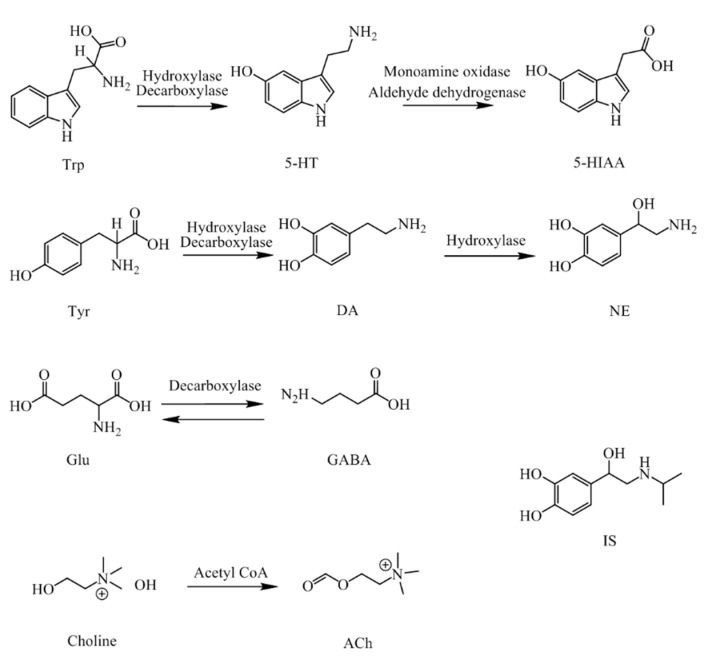
NTs and their metabolites are exist extensively in CNS and the peripheral biofluids of mammals [13,14], including neurotransmitters of amino acid, such as Trp, Tyr, ACh, Glu and GABA, and monoamine neurotransmitters, such as 5-HT, DA, and NE, and also their acidic metabolite 5-HIAA. Their molecular structures and pathways are presented in Figure 1.
Figure 1.
Structures and pathways of the neurotransmitters and their metabolites. (Trp, Tryptophan; 5-HT, Serotonin; 5-HIAA, 5-hydroxyindol acetic acid; Tyr, tyrosine; DA, dopamine; NE, norepinephrine; Glu, glutamic acid; GABA, γ-aminobutyric acid; Ach, acetylcholine; IS, isoprenaline.)
Numerous analytical methods, including high performance liquid chromatography (HPLC) or capillary electrophoresis (CE) involving a series of techniques, such as ultraviolet detection (UV), electrochemical detection (ECD), fluorescence detection (FLD), laser induced fluorescence detection (LIFD) and mass spectrometry (MS), have been engaged in the analysis of NTs in biological samples [15,16,17,18]. Whereas, except the narrow scope of application, all of the analytical procedures have several different drawbacks, such as, low sensitivity and selectivity for UV, poor repeatability and difficulty in simultaneous separation of NTs which have similar electrophoretic behavior, such as Glu and GABA for ECD, and interference after derivatization for FLD. Conventional methods of LC-MS/MS have been used to determine the concentration of NTs with more complex sample preparation, a longer assay time and fewer analytes [19,20]. Therefore, it is necessary to set up a procedure that is simpler, faster and can be used to quantify more analytes simultaneously.
Extracting important features from data is a vital procedure in the issue of pattern recognition, not just can it decrease the computational complexity, but result in better performance through eliminating data redundancy and noise, while overcoming dimension problem for a statistical classifier [21]. As to large category pattern classification, linear supervised feature extraction approach is engaged widely. Bayes linear discriminant method is widely accepted as a good way for the analysis of reliability data, which calculates the between scatter matrix and the within scatter matrix only, then figures out the problem of eigenvalue decomposition easily with efficient computation. To compare different changes of NTs in rat models of insomnia, AD and depression, in our study, a rapid and reliable LC-MS/MS method was established to determinate nine NTs and their metabolites simultaneously in rat brains with a higher sensitivity and shorter chromatographic separation time. The parameters of linearity, precision, recovery and matrix effect were completely validated and successfully applied to analyze the NTs in rat models of insomnia, AD and depression. A Bayes linear discriminant function was established to distinguish these three kinds of nervous system diseases by DA, Tyr and ACh for their significant differences among control and three model groups. The analytical method in this study can also be applied to not only brain issues but also plasma and urine samples, moreover the conclusions of this paper can be used to distinguish insomnia, depression and AD models, which can assist research of neurological diseases. Finally, the marked changes in biomarkers might be applied to clinical diagnosis.
2. Results and Discussion
2.1. Optimisation of LC-MS/MS Parameters
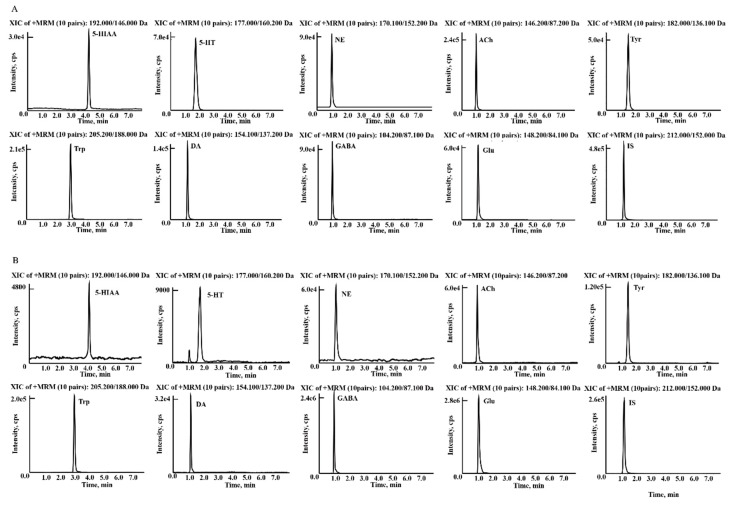
The chromatographic conditions were optimized to deliver higher ionization efficiency and resolution, as well as lower noise of the analytes. In the study, most of the NTs and their metabolites are small molecules with high polarity that show low retainability on C18 packed columns and may be co-eluted in the void volume. Therefore, an Inertsil ODS-EP column with optimized gradient elution were engaged to increase their retention and separation. The ODS-EP column was packed with silica gel with polar groups chemically bonded on an octadecyl support, to make sure the analytes cannot be eluted immediately. A signal suppression effect was also avoided, which was suitable for the retention and separation of polar compounds. The methanol-water and acetonitrile-water solvent combination as mobile phase were compared. Methanol was eventually adopted as the organic phase since it showed higher responses and lower noise than acetonitrile. The addition of formic acid could ameliorate the response and peak shapes of the analytes. 0.01% formic acid in water was employed as the aqueous phase after optimization under gradient program with the column temperature setting at 35 °C. Moreover, a tee joint was used to adjust the flow rate of 1.2 mL min−1, which could lead to a better solvent vaporization in the MS ionization process. Figure 2 exhibited the representative chromatograms of 5-HT, 5-HIAA, Glu, GABA, NE, DA, ACh, Trp, Tyr and iso-prenaline (IS) in rat brain homogenate samples from a control rat. Ion pairs and different parameters including declustering potentials, entrance potentials, collision energies and cell exit potentials with better response for each analytes were specifically investigated and the MS data were then acquired under multiple reaction monitoring (MRM) mode to obtain the highest quantitative sensitivity by triple quadrupole mass spectrometer.
Figure 2.
Typical LC-MS MRM chromatograms of (A) reference standard solution of the analytes and IS. (B) NTs and their metabolites in rat brain samples.
2.2. Sample Preparation
Endogenous metabolites such as NTs are typically small molecules with high polarity. Consequently, a few types of organic solvents were investigated as the deproteinization agent. Acetonitrile and methanol were tested in the first place. It was found that when methanol was used as deproteinization agent, not only did the recoveries give satisfactory results, but also higher response was observed. Secondly, different acids and bases were added into methanol to investigate the influence of different acid-base properties of deproteinization agents including 1% formic acid, 1% ethylic acid and 1% aqueous ammonia. The extraction recovery was closer to 100% within the acceptable range and was more stable when 1% formic acid was added than others. Finally, brain homogenate samples were extracted by methanol with 1% formic acid for protein precipitation.
2.3. Method Validation
The NTs studied are all endogenous metabolites which are all already existed in biofluids especially in the rat brain. Therefore, a pooled sample of brain homogenates from the control group (n = 8) was prepared using as blank matrix in the method validation process. In the parameters of linearity, recovery and matrix effect, the mean value of each analyte in blank matrix (n = 8) was subtracted to eliminate individual differences of rats.
2.3.1. Linearity and LLOQ
The calibration curve was constructed for each analyte by plotting the increased analyte-to-IS peak area ratio (y) between the brain tissue homogenates that spiked standard solutions and the mean ratio of blank samples versus nominal concentration (x) by 1/x2 weighted least square linear regression since the internal standard relative standard derivations (RSDs) of the linearity and precision were less than 15% during the three-day method validation test. Linear regression equations and calibration regression coefficients over concentration ranges of the analytes were shown in Table 1.
Table 1.
Linear ranges, regression equations, correlation coefficients and Lower Limit of Quantitation (LLOQ) of multicomponent in rat brain homogenates.
| Analyte | Linear Regression Equation | Linear Range (ng/mL) | Correlation Coefficient (R2) | LLOQ | |
|---|---|---|---|---|---|
| Accuracy (RE%) | Precision (RSD%) | ||||
| 5-HT | y = 3.675 × 10−4 x + 9.17 × 10−4 | 2.0~64 | 0.9979 | 10.8 | 11.5 |
| 5-HIAA | y = 1.568 × 10−4 x + 9.85 × 10−4 | 2.0~64 | 0.9920 | −5.9 | 10.2 |
| Glu | y = 1.617 × 10−1 x + 1.954 | 1.0 × 103~3.2 × 104 | 0.9928 | 3.4 | 8.1 |
| GABA | y = 2.792 × 10−1 x + 1.325 | 1.0 × 103~3.2 × 104 | 0.9935 | −6.9 | 7.8 |
| DA | y = 8.126 × 10−4 x + 1.353 × 10−3 | 20~6.4 × 102 | 0.9983 | 11.9 | 9.7 |
| ACh | y = 1.802 × 10−3 x + 1.571 × 10−2 | 20~6.4 × 102 | 0.9976 | 7.9 | 10.5 |
| Trp | y = 1.063 × 10−3 x + 1.042 × 10−1 | 20~6.4 × 102 | 0.9961 | 7.9 | 3.7 |
| Tyr | y = 6.268 × 10−4 x + 4.080 × 10−2 | 1.0 × 102~3.2 × 103 | 0.9944 | 9.1 | 6.3 |
| NE | y = 1.536 × 10−3x + 2.270 × 10−2 | 1.0 × 102~3.2 × 103 | 0.9974 | −7.4 | 5.6 |
RE, relative error; RSD, relative standard derivations.
The lower limits of quantification (LLOQ) for each NT in the brain tissue were 1.0 μg mL−1 for GABA and Glu, 2.0 ng mL−1 for 5-HT and 5-HIAA, 100 ng mL−1 for NE and Tyr, and 20 ng mL−1 for Trp, DA and ACh respectively, which were also listed in Table 1.
2.3.2. Accuracy and Precision
The accuracy and precision including intra-day precision and inter-day precision of the nine NTs in the rat brain were fully validated and the results were all acceptable (RSD%: <15%; RE%: ±15%). The results were exhibited in Table 2, which indicate an excellent accuracy and precision.
Table 2.
Summary of accuracy, precision, recovery and matrix effect of the nine analytes in rat brain (n = 8).
| Analytes | Concentration (ng mL−1) | Intra-Day RSD (%) | Inter-Day RSD (%) | Accuracy (RE%) | Recovery (%, mean ± SD) | Matrix Effect (%, mean ± SD) |
|---|---|---|---|---|---|---|
| 5-HT | 4 | 3.1 | 3.6 | −11.4 | 105.6 ± 7.4 | 98.5 ± 9.5 |
| 16 | 5.2 | 1.6 | −9.0 | 99.0 ± 9.4 | 98.0 ± 1.9 | |
| 50 | 4.8 | 7.2 | 7.3 | 102.0 ± 8.8 | 98.7 ± 2.0 | |
| 5-HIAA | 4 | 8.9 | 14.7 | 1.5 | 97.0 ± 6.7 | 92.2 ± 8.6 |
| 16 | 7.8 | 13.7 | 4.2 | 95.5 ± 6.5 | 92.2 ± 6.3 | |
| 50 | 6.7 | 10.6 | 7.0 | 97.5 ± 7.2 | 90.2 ± 5.7 | |
| Glu | 2000 | 6.5 | 2.5 | 3.0 | 102.9 ± 9.8 | 101.4 ± 8.5 |
| 8000 | 4.4 | 1.0 | 8.4 | 99.5 ± 6.4 | 95.8 ± 4.4 | |
| 25,000 | 3.9 | 4.4 | 10.9 | 98.9 ± 9.9 | 97.1 ± 5.9 | |
| GABA | 2000 | 6.9 | 6.0 | −1.7 | 102.2 ± 8.1 | 95.4 ± 10.8 |
| 8000 | 4.9 | 5.8 | 2.3 | 105.1 ± 4.7 | 98.3 ± 5.2 | |
| 25,000 | 9.4 | 7.4 | 12.9 | 92.6 ± 9.0 | 99.2 ± 4.0 | |
| DA | 40 | 5.4 | 3.2 | 8.0 | 98.1 ± 8.0 | 90.1 ± 6.8 |
| 160 | 2.9 | 2.1 | 12.0 | 95.2 ± 7.9 | 94.6 ± 3.8 | |
| 500 | 13.4 | 0.7 | 13.4 | 94.4 ± 6.8 | 99.8 ± 5.4 | |
| NE | 200 | 6.4 | 2.8 | 12.9 | 96.4 ± 9.5 | 102.2 ± 7.0 |
| 800 | 5.4 | 4.9 | −13.4 | 90.6 ± 4.3 | 104.3 ± 6.2 | |
| 2500 | 7.9 | 5.4 | 4.3 | 95.0 ± 5.2 | 97.0 ± 4.7 | |
| ACh | 40 | 5.4 | 1.2 | 4.6 | 92.3 ± 12.5 | 99.5 ± 7.7 |
| 160 | 2.4 | 3.1 | 12.6 | 95.5 ± 9.4 | 91.3 ± 3.6 | |
| 500 | 11.4 | 7.0 | 9.3 | 96.9 ± 7.8 | 103.4 ± 2.3 | |
| Trp | 40 | 9.4 | 3.5 | −5.4 | 93.2 ± 9.8 | 101.0 ± 9.7 |
| 160 | 7.2 | 2.2 | 1.7 | 101.4 ± 8.0 | 96.9 ± 6.2 | |
| 500 | 4.1 | 0.8 | 4.1 | 98.9 ± 6.0 | 101.9 ± 5.6 | |
| Tyr | 200 | 6.0 | 7.1 | −1.9 | 95.5 ± 8.9 | 102.4 ± 6.0 |
| 800 | 3.2 | 6.2 | 7.8 | 98.6 ± 7.8 | 101.4 ± 1.7 | |
| 2500 | 4.2 | 4.2 | 8.8 | 96.7 ± 6.5 | 97.3 ± 7.9 |
5-HT, Serotonin; 5-HIAA, 5-hydroxyindol acetic acid; Glu, glutamic acid; GABA, γ-aminobutyric acid; DA, dopamine; NE, norepinephrine; Ach, acetylcholine; Trp, Tryptophan; Tyr, tyrosine; RE, relative error; RSD, relative standard derivations.
2.3.3. Recovery and Matrix Effect
The mean recoveries of the nine NTs were among 85.0% and 115.0% at different concentration levels, which were also shown in Table 2. The average recovery of the IS was 94.2%, which are within acceptance. The matrix effect of the analytes ranged from 90.0% to 110.0% at three concentration levels (Table 2), while the IS was 96.0%. Results indicated that there were no significant matrix effect interferences for the analytes as well as the IS.
2.3.4. Stability
The concentration measured for the nine analytes at low and high levels deviated by 12.0%, which demonstrated that they were stable in the biosamples at room temperature for 8 h, at 4 °C in the autosampler for 8 h after preparation, 3 freeze-thaw cycles and stored at −80 °C for a month. All the data were within the acceptance and were listed in Table 3.
Table 3.
Stability of analytes at different conditions determined by LC-MS/MS (RE%, n = 3).
| Analytes | Concentration (ng mL−1) | 8 h, 4 °C | 8 h, Room Temperature | 3 Freeze-Thaw Cycles | −80 °C for 3 Months | ||||
|---|---|---|---|---|---|---|---|---|---|
| RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | ||
| 5-HT | 4 | 3.0 | 4.5 | −4.9 | 3.3 | −2.9 | 4.1 | 4.2 | 2.7 |
| 50 | 1.3 | 2.0 | −2.1 | 2.3 | 0.8 | 3.0 | −1.0 | 5.7 | |
| 5-HIAA | 4 | −9.3 | 1.6 | −9.0 | 4.5 | 2.3 | 1.0 | 1.0 | 2.2 |
| 50 | −7.4 | 7.4 | −3.7 | 5.2 | −1.1 | 6.9 | −4.6 | 2.3 | |
| Glu | 2000 | −5.0 | 3.6 | −5.9 | 6.0 | −2.0 | 1.8 | 4.1 | 6.4 |
| 25,000 | 8.5 | 5.2 | 6.6 | 3.7 | −2.0 | 6.3 | −6.6 | 3.5 | |
| GABA | 2000 | −4.0 | 2.6 | 3.8 | 2.2 | 3.1 | 1.4 | 1.5 | 1.9 |
| 25,000 | 2.7 | 4.6 | 6.7 | 1.6 | 5.9 | 6.6 | −3.1 | 1.5 | |
| DA | 40 | 5.7 | 9.5 | -9.6 | 8.8 | 1.7 | 0.7 | −2.8 | 5.8 |
| 500 | 2.3 | 7.1 | 1.9 | 6.6 | −1.2 | 5.0 | 4.5 | 4.2 | |
| NE | 200 | −3.2 | 7.2 | 2.7 | 5.0 | −2.7 | 2.8 | −2.5 | 3.6 |
| 2500 | −3.2 | 5.0 | −4.7 | 2.1 | 3.6 | 2.8 | −1.2 | 2.9 | |
| ACh | 40 | 8.7 | 4.4 | −5.8 | 2.4 | −2.9 | 1.7 | 2.2 | 1.2 |
| 500 | −6.0 | 5.6 | −1.5 | 2.5 | −2.6 | 7.7 | −5.3 | 1.6 | |
| Trp | 40 | 1.1 | 3.2 | 11.4 | 5.5 | 2.7 | 2.1 | −1.9 | 0.9 |
| 500 | −3.9 | 7.0 | 5.9 | 2.7 | 1.1 | 4.7 | −0.9 | 2.4 | |
| Tyr | 200 | 2.6 | 3.8 | 2.9 | 5.8 | 5.1 | 3.7 | −2.7 | 2.1 |
| 2500 | −7.8 | 3.5 | −5.7 | 6.0 | 2.1 | 5.4 | −1.2 | 1.3 | |
5-HT, Serotonin; 5-HIAA, 5-hydroxyindol acetic acid; Glu, glutamic acid; GABA, γ-aminobutyric acid; DA, dopamine; NE, norepinephrine; Ach, acetylcholine; Trp, Tryptophan; Tyr, tyrosine; RE, relative error; RSD, relative standard derivations.
2.4. Method Application
2.4.1. Determination of Neurotransmitters in Rat Brain
The established LC-MS/MS strategy was applied in the target quantification of the NTs in rat brain. Figure 2 shows the chromatograms of the nine analytes and the IS. For more in-depth discussion of a comparative study, the data from the three control groups were combined into one group, since there was no significant difference among the three control groups following a one-way ANOVA using SPSS shown in Table 4. The rat model of p-chlorophenylalanine (PCPA)-induced insomnia showed a significant increase of ACh, Tyr, NE and DA, as well as a marked reduction in Glu, 5-HIAA Trp and 5-HT. The levels of 5-HT, 5-HIAA, Glu, DA, NE, ACh, Trp and Tyr were significantly lower in rats with chronic unpredictable mild stress (CUMS)-induced depression. In the AD model rats, the NTs of GABA, 5-HT, DA, NE, ACh, Trp and Tyr showed a significant reduction. In CNS, amino acid and monoamine NTs were transformed into each other. Tyr was converted to Dopa by tryptophan hydroxylase, and then transformed to DA by decarboxylase. DA was converted to NE by DA hydroxylase, followed by transformation to epinephrine (Epi).
Table 4.
The concentrations of NTs and their metabolites in rat brain samples.
| Analyte (ng mg−1) | Control Group | Insomnia Group | Depression Group | AD Group |
|---|---|---|---|---|
| 5-HT | 1.551 ± 0.262 | 0.449 ± 0.113 aa | 0.940 ± 0.061 aa, bb | 0886 ± 0.087 aa,bb |
| 5-HIAA | 0.960 ± 0.263 | 0.556 ± 0.135 aa | 0.324 ± 0.121 aa | 0.766 ± 0.306 c |
| Glu | 1219.476 ± 94.828 | 948.926 ± 144.407 aa | 988.866 ± 161.352 a | 1288.012 ± 56.815 bb,cc |
| GABA | 861.936 ± 83.004 | 840.986 ± 139.038 | 896.462 ± 71.449 | 623.404 ± 140.555 a,bb,cc |
| DA | 7.225 ± 0.873 | 10.315 ± 0.824 aa | 4.022 ± 0.972 aa,bb | 5.353 ± 1.016 aa,bb,cc |
| NE | 22.616 ± 3.435 | 53.086 ± 3.267 aa | 19.771 ± 2.776 a,bb | 17.602 ± 2.372 aa,bb |
| ACh | 3.787 ± 0.506 | 5.059 ± 0.590 aa | 2.510 ± 0.273 aa,bb | 3.132 ± 0.322 aa,bb,c |
| Trp | 21.837 ± 2.311 | 19.405 ± 2.265 a | 18.135 ± 1.769 aa | 14.791 ± 1.721 aa,bb,cc |
| Tyr | 39.254 ± 5.862 | 74.728 ± 10.452 aa | 19.644 ± 2.120 aa,bb | 29.533 ± 6.896 a,bb,c |
For statistical significance a p < 0.05, aa p < 0.01 compared with control group, bb p < 0.01 compared with insomnia group, c p < 0.05, cc p < 0.01 compared with depression group.
5-OH was also converted from Trp by the effect of hydroxylase and carboxylase, and 5-HIAA was a metabolite of 5-OH and Glu was transformed into GABA by decarboxylase. Regarding the rats in the model of insomnia, the increase in DA may be caused by a significant increase in Tyr, and then more DA is transformed to NE, which is the opposite of what was found in the rat models of depression and AD. ACh, an excitatory neurotransmitter, promote the process of insomnia and reduce depression and AD process. Insomnia, depression and AD may have caused a great increase or decrease in activities of enzyme related to the synthesis and decomposition of ACh, DA and Tyr, which resulted in different changes of these compounds in rat brain. The reduction of 5-HT and 5-HIAA might be attributed to the decrease in Trp or inhibited related enzymes by the treatment methods [22]. The results distinctly indicated that the monoamine and amino acid metabolism pathways were abnormal when the rats were subjected to disposes severally. The remarkable changes of biomarker levels may provide promising understandings in clinical diagnosis of these CNS diseases.
2.4.2. The Comparation of Insomnia, Depression and AD
Except for significant changes in NTs between the control and model groups, DA, Tyr and ACh also exhibited significant differences among the control, insomnia, depression and AD groups. Data from these four groups were further analyzed by Bayes discriminant analysis. If all data were taken for discriminant analysis, only 81.2% of samples could be correctly classified and accuracy of the cross validation was only 77.1%. Low accuracy might be caused by abnormal data; therefore, the data were eliminated reasonably and effectively according to Gobla’s criterion, and accuracy of the discriminant analysis was improved successfully after data optimization. The Bayes linear discriminant function coefficients are shown in Table 5. The 95.3% group samples were correctly classified by this determining equation, and the cross-validation accuracy rate was 86.0%. Using a horizontal comparison, it was found that the absolute value of the coefficient of ACh was larger than the other coefficients in all four discriminant equations, which meant that the change in ACh concentration was most important making a greater impact on the discrimination results. In addition to different changes of NTs, similar rising or decreasing trend were also found. Subjects who have insomnia are 20 times more likely to be depressed than those who do not and, thus, insomnia and depression are interrelated [23]. Similar findings have been reported previously [24,25]. On the other hand, in the study, the correlative symptom of insomnia and depression were presumed to be resulted from commonly lower levels of 5-HT and Glu. Clearly, selective serotonin reuptake inhibitors which increase the concentration of 5-HT in the synaptic cleft are of more befit to patients with depression suffering from insomnia. Since AD is a complicated neurodegenerative disease, the treatment of most AD patients also involves receiving antipsychotic or antidepressant drugs in addition to their actual AD drug to manage the neuropsychiatric and behavioral symptoms [26,27]. The study result demonstrated that the function of DAergic and AChergic nervous systems declined in rats with depression and AD, expressed as reduced concentrations of DA, NE, ACh and Tyr. Monoamine oxidase (MAO) enzyme inhibition, which blocks the oxidation of monoamine NTs such as DA, was a key target for the management of depression and AD, and inhibitors of MAO were the most important drugs for this management. It has been reported that a normal night’s sleep may be critical for maintaining brain health in mice with neurodegenerative diseases, and, most likely, also in humans [28]. Improving sleep quality may help reduce the neurodegenerative risk in old people [29]. Above all, we can propose the following conclusion: DA, Tyr and ACh exhibited significant changes among control and three disease model groups and can be used to distinguish the three kinds of CNS diseases.
Table 5.
Bayes linear discriminant function coefficients of DA, Tyr, ACh and constant.
| Group | ||||
|---|---|---|---|---|
| Blank | Insomnia | Depression | AD | |
| DA | 7.740 | 10.723 | 4.310 | 5.692 |
| ACh | 16.614 | 21.920 | 11.152 | 13.836 |
| Tyr | 0.760 | 1.533 | 0.367 | 0.571 |
| Constant | −75.047 | −169.858 | −27.946 | −47.148 |
3. Materials and Methods
3.1. Materials and Reagents
5-HT, 5-HIAA, Glu, GABA, DA, NE, ACh, Trp and Tyr were purchased from Sigma (St. Louis, MO, USA). The internal standard isoproterenol (IS) was from the Institute for Food and Drug Control (Liaoning, China). 4-Chloro-D,L-phenylalanine (PCPA), D-galactopyranose (D-gal) and Amyloid β-Peptide Fragment 25–35 (Aβ25–35) were also supplied by Sigma-Aldrich (St. Louis, MO, USA). Distilled water was used throughout the experiments. HPLC grade methanol and acetonitrile were from Fisher Scientific (Fair Lawn, NJ, USA). HPLC grade reagents such as acetic acid, formic acid, sodium hydroxide and ammonium hydroxide were provided by Shandong Yuwang Industrial Co., Ltd. (Yucheng, China). Other reagents were all analytical grade.
3.2. Animals and Treatment
Forty-eight male wistar rats (200–220 g) were kindly provided by the Experimental Animal Center of Shenyang Pharmaceutical University and bred with unlimited access to food and water in an air-conditioned animal center at a temperature of 22 ± 2 °C and a relative humidity of 50 ± 10%, with a natural light-dark cycle. The animal study was carried out in accordance with the Guideline for Animal Experimentation of Shenyang Pharmaceutical University and the protocol was approved by the Animal Ethics Committee of the institution (Ethic approval document NO. SYPU-IACUC-C2017-1-31-203).
The animals were allowed to acclimatize to the environment for one week before the experiment. Forty-eight rats were randomly divided into six groups, with eight animals each: the insomnia control and model group, the AD control and model group, the depression control and model group. The methods of treating animals used hereinafter are recognized as scientific and effective, widely used [22,30,31,32,33].
Animal model of insomnia was induced by the chemical reagents according to precious report [34]. Briefly, PCPA was suspended in physiological saline. The animal of insomnia model group was administrated intraperitoneal injection of PCPA (350 mg kg−1), while the control group was given the same volume of saline solution once a day and last for four days, respectively. After the injection of PCPA, the model group lost their weight and circadian rhythm, and were sleepless all day which proved the success of the model.
The AD model in rats was induced by D-gal and Aβ25–35, as described previously [35]. In brief, the rats in AD model and control groups were respectively given intraperitoneal injection of D-gal (50 mg kg−1 day−1) and the same volume of saline for 6 weeks; then the AD rats were injected with 5 μL Aβ25–35 (10 μg μL−1) into each bilateral hippocampus at the co-ordinates antero-posterior, −3.5, medio lateral, +2.0, dorso-ventral, 2.8 mm according to the stereotaxic atlas on the fourth week by brain stereotaxic apparatus, individually, while the control rats were administrated the same volume of saline in the same way. After 6 weeks, all rats were trained to find a visible platform in Morris water maze testing. The result demonstrated that the rats in AD model group had a longer time to get to the platform than the control rats at the last trial (p < 0.05), which proved the success of the AD model.
The depression model was induced by chronic unpredictable mild stress (CUMS). The model rats were kept separately. Control animals were housed in a separate room and had no contact with the stressed animals. The CUMS procedure was referenced from the precious study [36]. Stressors consisted of (1) 4 h restraint; (2) 24 h wet litter; (3) 24 h food deprivation; (4) 60 s tail pinch; (5) 24 h water deprivation; (6) 5 min cage shake; (7) 12 h cage tilt (cages were tilted to 45° from the horizontal). In this respect, stressors were administrated in a semi-random manner at any time of day, and the stress sequence was changed every week in order to make the stress procedure unpredictable. After 3 weeks the autonomous behavior of rats was evaluated by the Open-field test (OFT). The result demonstrated that the vertical and horizontal bouts of rats in depression model group were lower than the control group (p < 0.05), which proved the success of the CUMS model.
3.3. Instruments and LC–MS/MS Conditions
The LC-ESI-MS/MS system was performed using an LC-20 A Prominence™ UFLC XR system consisted of a binary pump, a degasser, an autosampler and a thermostatted column compartment (Shimadzu, Kyoto, Japan); a 4000 QTRAP™ triple quadrupole-linear ion trap mass spectrometry system equipped with a turbo ion spray source (Sciex, Foster City, CA, USA). All the operations, the acquiring and analysis of data were controlled by Analyst software (version 1.6, Sciex).
Separations were accomplished on an Inertsil ODS-EP column (4.6 mm × 150 mm, 5.0 μm) (GL Sciences, Tokyo, Japan) protected by a high-pressure column pre-filter (2 mm) at 35 °C. The mobile phase consisting of 0.01% formic acid in water (A) and methanol (B) was delivered at a flow rate of 1.2 mL min−1 with one third of the eluent splitted into the inlet of mass spectrometer. The linear gradient elution program was as follows: 0–0.5 min, 15% B; 0.5–5 min, 15–85% B; 5–6 min, 85% B; 6–8 min, 15% B. The injection volume was 4 μL and the total time taken for the chromatographic run was 8.0 min. The analytes and IS were ionized by the ESI source in positive ion mode and the ion spray voltage was set at 5500 V. The curtain gas, gas 1 and gas 2 (nitrogen) were set at 20 psi, 50 psi and 50 psi with a source temperature of 500 °C. Quantitative MRM ion pairs parameters are listed in Table 6.
Table 6.
List of selected MRM parameters, declustering potential (DP), entrance potential (EP), collision energy (CE) and cell exit potential (CXP) for each analyte and IS (Isoprenaline).
| Analyte | Q1 Mass (Da) | Q3 Mass (Da) | DP | EP | CE | CXP |
|---|---|---|---|---|---|---|
| 5-HT | 177.1 | 160.2 | 38 | 10 | 10 | 7 |
| 5-HIAA | 192.0 | 146.0 | 25 | 9 | 20 | 8 |
| Glu | 148.2 | 84.1 | 41 | 10 | 24 | 15 |
| GABA | 104.2 | 87.1 | 26 | 14 | 15 | 16 |
| DA | 154.1 | 137.2 | 37 | 100 | 13 | 8 |
| NE | 170.1 | 152.2 | 33 | 5 | 11 | 9 |
| ACh | 146.2 | 87.2 | 52 | 3 | 20 | 4 |
| Trp | 205.2 | 188.0 | 40 | 4 | 14 | 12 |
| Tyr | 182.0 | 136.1 | 52 | 8 | 25 | 7 |
| IS | 212.0 | 152.0 | 46 | 8 | 20 | 9 |
3.4. Standard Solution and Quality Control Samples
Standard stock solutions of 5-HT, 5-HIAA, Glu, GABA, DA, NE, ACh, Trp, Tyr and IS were separately prepared in methanol-water (20:80, v/v). The stock solutions of the analytes were further diluted with methanol-water (20:80, v/v) to make a series of mixed working standard solutions at the desired concentrations. The brain tissue samples were homogenized in a twenty-fold volume of methanol with 1% formic acid. The brain tissue standards of analytes for each of the nine analytes were prepared as follows, GABA and Glu at concentrations of 1, 2, 4, 8, 16 and 32 μg mL−1, 5-HT and 5-HIAA at concentrations of 2, 4, 8, 16, 32 and 64 ng mL−1, NE and Tyr at concentrations of 100, 200, 400, 800, 1600 and 3200 ng mL−1, Trp, DA and ACh at concentrations of 20, 40, 80, 160, 320 and 640 ng mL−1. These standards were prepared by adding appropriate amount of standard working solutions to blank brain tissue homogenates. Quality control samples were prepared using the same method (4, 16 and 50 ng mL−1 for 5-HT and 5-HIAA; 2, 8 and 25μg mL−1 for Glu and GABA; 200, 800 and 2500 ng mL−1 for NE and Tyr; 40, 160 and 500 ng mL−1 for Trp, DA and ACh. A working solution for the IS (10 μg mL−1) was also prepared.
3.5. Sample Preparation
All rats were sacrificed by decapitation without anesthesia. The brains were rapidly removed above ice bath, frozen and stored at −80 °C until extraction. All frozen samples, calibration standards and QC samples were thawed and allowed to equilibrate at room temperature prior to analysis. The frozen brain tissue samples were dissected and homogenized in a twenty-fold volume of methanol with 1% formic acid above ice-water bath. The homogenates were centrifuged at 12,000 × g for 20 min at 4 °C. Then, 100 μL of the supernatant was transferred to a 1.5 mL centrifuge tube, spiked with 10 μL of the IS solution and 10 μL methanol, followed by vortexing for 30 s. Then 100 μL methanol was added into the supernatant, followed by vortexing for 1 min. Next, these samples were centrifuged at 12,000× g for 5 min at 4 °C. Finally, a 4 μL aliquot was injected into the LC-MS/MS system for analysis.
3.6. Method Validation
The analytical method validation procedure was in accordance with the US Food and Drug Administration Bioanalytical Method Validation Guidance for Industry [37]. Validation parameters included lower limit of quantification (LLOQ), linearity, precision, accuracy, recovery, matrix effect and stability.
Neurotransmitters are endogenous substances which already existed inherently in biosamples, especially brain homogenates. Therefore, blank value of each analyte should be subtracted from each calibration point. The calibration curve was constructed for each analyte by plotting the increased analyte-to-IS peak area ratio (y) between the brain tissue homogenates that spiked standard solutions and the mean ratio of blank samples versus nominal concentration (x) by 1/x2 weighted least square linear regression. LLOQ was defined as the lowest concentration of the calibration curve with acceptable accuracy and precision, which provided an intensity of signal-to-noise ratio above 10.
The validation of accuracy and precision and were performed with six replicates at three (low, medium and high) QC concentration levels on the same day and on three consecutive days. Precision was presented as the relative standard deviation (RSD %), while accuracy was presented as relative error (RE%).
The extraction recovery for each analyte was calculated by comparing the difference of the responses between the spiked sample before and after extraction at three QC levels, while the mean value of blank samples was removed.
The matrix effect was evaluated as follows: the analyte-to-IS peak area ratio for each analyte in the spiked sample after extraction subtracted the mean ratio of blank samples at three concentration levels of QC samples, and then the increased peak area ratio was divided by the mean ratio measured in corresponding standard solutions.
Stability tests were conducted on triplicate at low and high QC levels under the storage conditions as follows: 8 h at room temperature, 4 °C in the autosampler for 8 h after preparation, three freeze-thaw cycles and stored at −80 °C for three months.
3.7. Statistical Data Analysis
All data are illustrated as the mean ± SD. The acquired data were analyzed by ANOVA and Bayes discriminant analysis using the SPSS 20 statistical software (IBM, Armonk, NY, USA). A p value less than 0.05 was considered statistically significant for all the tests.
4. Conclusions
In this paper, a simple, fast and sensitive LC-MS/MS method using an ODS-EP column, without derivatization or ion-pairing reagents for simultaneous determination of nine NTs in rat brain was established and fully validated. Following optimization of the chromatographic conditions, the compounds were found to have symmetric peak shapes and could be assayed in 8.0 min, which was very suitable for high throughput bioanalysis. A simple sample preparation involving extraction was applied and the recoveries of all the analytes above 85.0%. In addition, we discovered changes in several NTs and their metabolites in rats with insomnia, depression and AD, and established a Bayes linear discriminant function to differentiate these three kinds of neurological diseases. Results indicated that there were significant differences for 5-HT, DA, NE, Trp, Tyr and ACh between model and control group in all three models, what is more, DA, Tyr and ACh showed significant differences among control and three model groups and were used to distinguish these three kinds of nervous system diseases successfully with a cross-validation accuracy rate 86.0%.The data obtained in this study make a promising understanding to the diagnosis and treatment of insomnia, depression and AD.
Author Contributions
Conceptualization, Q.L.; Methodology, H.X.; Validation, H.X., Z.W. and L.Z.; Formal Analysis, H.X. and Z.W.; Data Curation, W.B.; Writing-Original Draft Preparation, Z.W.; Writing-Review & Editing, H.X., Z.S., R.L., K.B. and Q.L.; Supervision, K.B.; Funding Acquisition, Q.L.
Funding
This research was funded by [Grant of National Natural Science Foundation] grant number [81473324].
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds mentioned in the manuscript are available from the authors.
References
- 1.Wei B., Li Q., Fan R., Su D., Chen X., Jia Y., Bi K. Determination of monoamine and amino acid neurotransmitters and their metabolites in rat brain samples by UFLC-MS/MS for the study of the sedative-hypnotic effects observed during treatment with S. chinensis. J. Pharm. Biomed. Anal. 2014;88:416–422. doi: 10.1016/j.jpba.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Si L., Wang Y., Wuyun G., Bao L., Agula B. The effect of Mongolian medical acupuncture on cytokines and neurotransmitters in the brain tissue of insomniac rats. Eur. J. Integr. Med. 2015;7:492–498. doi: 10.1016/j.eujim.2015.05.008. [DOI] [Google Scholar]
- 3.Strekalova T., Evans M., Chernopiatko A., Couch Y., Costa-Nunes J., Cespuglio R., Chesson L., Vignisse J., Steinbusch H.W., Anthony D.C., et al. Deuterium content of water increases depression susceptibility: The potential role of a serotonin-related mechanism. Behav. Brain Res. 2015;277:237–244. doi: 10.1016/j.bbr.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Yun H.M., Park K.R., Kim E.C., Kim S., Hong J.T. Serotonin 6 receptor controls Alzheimer’s disease and depression. Oncotarget. 2015;6:26716–26728. doi: 10.18632/oncotarget.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rada P., Hoebel B.G. Acetylcholine in the accumbens is decreased by diazepam and increased by benzodiazepine withdrawal: A possible mechanism for dependency. Eur. J. Pharm. 2005;508:131–138. doi: 10.1016/j.ejphar.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Marks G.A., Birabil C.G. Enhancement of rapid eye movement sleep in the rat by cholinergic and adenosinergic agonists infused into the pontine reticular formation. Neuroscience. 1998;86:29–37. doi: 10.1016/S0306-4522(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 7.Coleman C.G., Lydic R., Baghdoyan H.A. M2 muscarinic receptors in pontine reticular formation of C57BL/6J mouse contribute to rapid eye movement sleep generation. Neuroscience. 2004;126:821–830. doi: 10.1016/j.neuroscience.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Cummings J.L., Miller B., Hill M.A., Neshkes R. Neuropsychiatric aspects of multi-infarct dementia and dementia of the Alzheimer type. Arch. Neurol. 1987;44:389–393. doi: 10.1001/archneur.1987.00520160031010. [DOI] [PubMed] [Google Scholar]
- 9.Risch S.C., Cohen R.M., Janowsky D.S., Kalin N.H., Murphy D.L. Mood and behavioral effects of physostigmine on humans are accompanied by elevations in plasma beta-endorphin and cortisol. Science. 1980;209:1545–1546. doi: 10.1126/science.7433977. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Pi Z., Song F., Liu Z. Ginsenosides attenuate d-galactose- and AlCl3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol. 2016;194:188–195. doi: 10.1016/j.jep.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Altamura C., Maes M., Dai J., Meltzer H.Y. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol. 1995;5:71–75. doi: 10.1016/0924-977X(95)00033-L. [DOI] [PubMed] [Google Scholar]
- 12.Fekkes D., Timmerman L., Pepplinkhuizen L. Effects of clomipramine on plasma amino acids and serotonergic parameters in panic disorder and depression. Eur. Neuropsychopharmacol. 1997;7:235–239. doi: 10.1016/S0924-977X(97)00412-4. [DOI] [PubMed] [Google Scholar]
- 13.Bergquist J., Sciubisz A., Kaczor A., Silberring J. Catecholamines and methods for their identification and quantitation in biological tissues and fluids. J. Neurosci. Methods. 2002;113:1–13. doi: 10.1016/S0165-0270(01)00502-7. [DOI] [PubMed] [Google Scholar]
- 14.Bourcier S., Benoist J.F., Clerc F., Rigal O., Taghi M., Hoppilliard Y. Detection of 28 neurotransmitters and related compounds in biological fluids by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrum. RCM. 2006;20:1405–1421. doi: 10.1002/rcm.2459. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M., Fang C., Smagin G. Derivatization for the simultaneous LC/MS quantification of multiple neurotransmitters in extracellular fluid from rat brain microdialysis. J. Pharm. Biomed. Anal. 2014;100:357–364. doi: 10.1016/j.jpba.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Perry M., Li Q., Kennedy R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanli N., Tague S.E., Lunte C. Analysis of amino acid neurotransmitters from rat and mouse spinal cords by liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 2015;107:217–222. doi: 10.1016/j.jpba.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 18.Hao L., Zhong X., Greer T., Ye H., Li L. Relative quantification of amine-containing metabolites using isobaric N,N-dimethyl leucine (DiLeu) reagents via LC-ESI-MS/MS and CE-ESI-MS/MS. Analyst. 2015;140:467–475. doi: 10.1039/C4AN01582G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang F., Li J., Shi H.L., Wang T.T., Muhtar W., Du M., Zhang B.B., Wu H., Yang L., Hu Z.B., et al. Simultaneous quantification of seven hippocampal neurotransmitters in depression mice by LC-MS/MS. J. Neurosci. Methods. 2014;229:8–14. doi: 10.1016/j.jneumeth.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W., Zhu B., Liu F., Lyu C., Zhang S., Yan C., Cheng Y., Wei H. A rapid and simple method for the simultaneous determination of four endogenous monoamine neurotransmitters in rat brain using hydrophilic interaction liquid chromatography coupled with atmospheric-pressure chemical ionization tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015;1002:379–386. doi: 10.1016/j.jchromb.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Yao C., Cheng G. Approximative Bayes optimality linear discriminant analysis for Chinese handwriting character recognition. Neurocomputing. 2016;207:346–353. doi: 10.1016/j.neucom.2016.05.017. [DOI] [Google Scholar]
- 22.Wang L.E., Bai Y.J., Shi X.R., Cui X.Y., Cui S.Y., Zhang F., Zhang Q.Y., Zhao Y.Y., Zhang Y.H. Spinosin, a C-glycoside flavonoid from semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep via the serotonergic system. Pharmacol. Biochem. Behav. 2008;90:399–403. doi: 10.1016/j.pbb.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Shahar S., Hassan J., Sundar V.V., Kong A.Y., Ping Chin S., Ahmad S.A., Kuan Lee L. Determinants of depression and insomnia among institutionalized elderly people in Malaysia. Asian J. Psychiatry. 2011;4:188–195. doi: 10.1016/j.ajp.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Livingston G., Blizard B., Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Br. J. Gen. Pract. 1993;43:445–448. [PMC free article] [PubMed] [Google Scholar]
- 25.Zailinawati A., Ariff K., Nurjahan M., Teng C. Epidemiology of insomnia in Malaysian adults: A community-based survey in 4 urban areas. Asia-Pac. J. Pub. Health. 2008;20:224–233. doi: 10.1177/1010539508316975. [DOI] [PubMed] [Google Scholar]
- 26.Doraiswamy P.M., Xiong G.L. Pharmacological strategies for the prevention of Alzheimer’s disease. Expert Opin. Pharmacother. 2006;7:1–10. doi: 10.1517/14656566.7.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Farlow M.R. Utilizing combination therapy in the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2004;4:799–808. doi: 10.1586/14737175.4.5.799. [DOI] [PubMed] [Google Scholar]
- 28.Toledo J.B., Shaw L.M., Trojanowski J.Q. Plasma amyloid beta measurements-a desired but elusive Alzheimer’s disease biomarker. Alzheimer’s Res. Ther. 2013;5:8. doi: 10.1186/alzrt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benedict C., Byberg L., Cedernaes J., Hogenkamp P.S., Giedratis V., Kilander L., Lind L., Lannfelt L., Schiöth H.B. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimer’s Dementia. 2015;11:1090–1097. doi: 10.1016/j.jalz.2014.08.104. [DOI] [PubMed] [Google Scholar]
- 30.Reid I., Forbes N., Stewart C., Matthews K. Chronic mild stress and depressive disorder: A useful new model? Psychopharmacology. 1997;134:365–367. doi: 10.1007/s002130050471. [DOI] [PubMed] [Google Scholar]
- 31.De Vry J., Schreiber R. The chronic mild stress depression model: Future developments from a drug discovery perspective. Psychopharmacology. 1997;134:349–350. doi: 10.1007/s002130050464. [DOI] [PubMed] [Google Scholar]
- 32.Borbely A.A., Neuhaus H.U., Tobler I. Effect of p-chlorophenylalanine and tryptophan on sleep, EEG and motor activity in the rat. Behav. Brain Res. 1981;2:1–22. doi: 10.1016/0166-4328(81)90035-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q., Xu Z., Tang J., Sun J., Gao J., Wu T., Xiao M. Voluntary exercise counteracts Abeta25-35-induced memory impairment in mice. Behav. Brain Res. 2013;256:618–625. doi: 10.1016/j.bbr.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Huitron-Resendiz S., Rios C., Rojas P., Mexicano G., Ayala-Guerrero F. Effect of p-chlorophenylalanine (PCPA) on sleep and monoamines content in the brain of a lizard species. Brain Res. 1997;761:19–24. doi: 10.1016/S0006-8993(97)00286-2. [DOI] [PubMed] [Google Scholar]
- 35.Shen Y.X., Yang J., Wei W., Liu L.H., Xu S.Y. Induction of learning and memory dysfunction by beta-amyloid peptide fragment 25∼35 in rats. Chin. Pharmacol. Bull. 2001;17:26–29. doi: 10.1007/BF03183202. [DOI] [Google Scholar]
- 36.Willner P., Towell A., Sampson D., Sophokleous S., Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 37.Bioanalytical Method Validation Guidance for Industry. [(accessed on 3 June 2018)]; Available online: https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf.