ABSTRACT
Background Occupational hazards are the leading cause of morbidity and mortality among abattoirs personnel and animal workers. These hazards result from direct or indirect exposure to potential infection and several distressing events during routine procedures.
Objectives To serologically investigate the potential occupational brucellosis hazard at Egyptian abattoirs. To provide an insight on the needed biosafety practices that should be implemented to mitigate the spread of occupational brucellosis among abattoir workers.
Methods Two hundred and thirty (n = 230) blood samples were collected from animals in two Egyptian abattoirs. The rose Bengal test was used to evaluate the seroprevalence of Brucella in abattoir animals. A questionnaire was distributed among abattoir personnel to address biosafety gaps and deficiencies as a cause of occupational brucellosis.
Results The overall seroprevalence of Brucella using the rose Bengal test was 75.2% in the two targeted abattoirs. It was obvious that there are gaps of malpractices and inconvenient behavior among individuals of the targeted community.
Conclusions The current findings reveal the missing role of concerned authorities and lack of written safety policy. The data highlights the need for further research, including isolation and characterization of the causative agents, and reliable epidemiological studies.
KEYWORDS: Occupational Brucellosis, abattoirs, rose Bengal, safety
Introduction
Brucellosis is a crucial zoonosis that is considered an underdiagnosed and underdocumented occupational issue, and it remains a significant public health problem in endemic countries, such as Egypt [1]. Brucellosis is a bacterial disease caused by species of the genus Brucella. Infection is usually transmitted to humans by direct or indirect contact with animals carrying the disease or their products [2,3]. Thus, brucellosis tends to be recognized as a work-related condition among individuals who are in direct contact with animals, such as abattoir workers [4].
Epidemiological information obtained lately from different countries has shown that occupational exposure to or direct contact with animals or animal material during daily activities is related to human brucellosis, documented with large variations from 18% [2] to more than 90% [5,6]. The results demonstrate that brucellosis is an occupational disease that remains a community health issue currently and potentially in the future. Clinical attributes of human brucellosis, along with its long convalescence, signify that it is a significant medical, economic, and social challenge, with community health implications at individual and population levels.
Occupational brucellosis is frequently associated with loss in the ability to work for a relatively long time, prolonged and expensive therapy, slow restoration and common relapses, and even potential severe neurological disorders [7]. Good occupational practices, control and reduction of risks, and protective measures should be implemented at any workplace with high occupational risk of infection with Brucella spp. [8]. Elimination should involve the reduction of direct or indirect exposure to infected animals or their products. However, eradication of the disease from animals is generally difficult to achieve, particularly in endemic and developing countries [9]. Consequently, the objective is always to decrease the risk associated with individual hygiene issues, increase compliance with healthy and safe working behaviors, ensure security measures at the location, and improve food sanitation [10,11]. Compliance with safe working conditions and implementation of proper practices in the work environment is a crucial approach in the prevention of the occupational hazard of brucellosis. Certain work places and working procedures with higher occupational danger of infection with Brucella spp. demand distinctive protective, shielding strategies and risk-free working practices [12,13].
In Egypt, data regarding the prevalence of the disease in animals and its transmission to humans is insufficient. Thus, there is a knowledge gap concerning the risks of the disease and its respective needed elimination approaches. In this work, the authors intend to determine the prevalence of brucellosis among animals in two focal abattoirs. The potential occupational brucellosis hazard associated with seropositive animals is investigated in the absence of good biosafety practices and a suitable safety plan.
Materials and methods
Ethical approval
The veterinary services directorate was contacted to approve the distribution of the questionnaire on abattoir workers and collection of samples from abattoir animals during slaughtering throughout the study period.
Study locations and study period
This work was conducted at two Egyptian abattoirs in two governorates, Cairo (in Al-Moneeb region) and Ismailia (in Tal Alkabeer region). The selection was based on history of acquired occupational brucellosis among abattoir workers (data not published). The survey was conducted over a period of 12 months, during the year 2016–2017.
Study group and questionnaire design
A cross-sectional study was conducted on two slaughterhouses. The study included 45 (n = 45) abattoir workers classified as follows: 29 butchers, 4 veterinarians, 4 security personnel, and 8 housekeepers. A simple “yes” or “no” questionnaire was designed to acquire the relevant information and appropriate data from the respondents. Questions were written in English first and then translated into Arabic to suit the relevant group. A structured interview method was deployed, which enabled the interviewers to clarify the questionnaire to employees with no or minimal literacy levels [14].
During the interview
Before the interviews, arrangements were done with the abattoirs’ management for acceptance to interview personnel and to facilitate the process. A representative 100% sample was taken (all workers in the abattoirs were recruited in the study), and the respondents were questioned on a one-off schedule through working time without previous notice of the interview. Before the interviewer started asking the questions, he/she presented him-/herself to the respondent, outlined the reason behind conducting the questionnaire, and assured them that the data would be handled confidentially. Moreover, the interviewer made sure that the respondent realizes the aims and the significance of the survey [15].
Data analysis of completed questionnaires
The question forms were pre-coded and a code list driven up. The questionnaires were analyzed manually using the code list and a data recording sheet [16,17].
Blood sampling collection
Two hundred and thirty (n = 230) blood samples were collected from asymptotic male calves of ages ranging from 2 to 3 years while on the slaughtering panel. Up to 153 (n = 153) blood samples were collected from Ismailia and 77 (n = 77) from Greater Cairo. Samples were processed as described by [18].
Rose Bengal test
The rose Bengal test (RBT) was conducted to serologically evaluate the potential occupational brucellosis hazard at Egyptian abattoirs. All serum samples were tested for agglutination against Brucella antigen as described by [19,20], using PrioCHECK® Brucella Rose Bengal Test Kit, Prionics-Switzerland, by a qualitative method according to the enclosed instructions.
Interventions to improve safety practices in the study locations
In order to evaluate safety knowledge and encourage good work practices, some intervention was made throughout the study period, such as providing workers with personal protective equipment (PPE) and illustrated prints, and then feedback was reported.
Statistical analysis
Data was displayed in tables with frequencies and percentages. Statistical analysis was performed with SPSS20, IBM, Armonk, NY, United States of America, GraphPad Prism and Microsoft Excel 2016. Chi-square test and one-way analysis of variance (ANOVA) test followed by multiple comparisons using Tukey’s post hoc test were applied for data analysis and calculation of significance difference.
Results
Seroprevalence by the RBT
The overall seropositivity of Brucella by RBT was n = 173 (75.2%) in the two targeted abattoirs. In Ismailia at Tal Alkabeer slaughterhouse, 106 (69.3%) from 153 samples were positive for rose Bengal agglutination test, while in Cairo at Al-Moneeb slaughterhouse 67 (87%) from 77 samples were positive. Using chi-square test for comparison between both abattoirs regarding serological test of Brucellosis, there was significant difference as (P-value <0.05), listed in Table 1 and shown in Figure 1.
Table 1.
Seroprevalence of Brucella in animals by rose Bengal test (RBT).
| Positive (RBT) |
Negative (RBT) |
||||
|---|---|---|---|---|---|
| Slaughterhouse | No. | % | No. | % | P-value |
| Tal El Kabeer | 106 | 69.3 | 47 | 30 | 0.003289** |
| El Monieb | 67 | 87 | 10 | 12.9 | |
**Significantly different.
Figure 1.
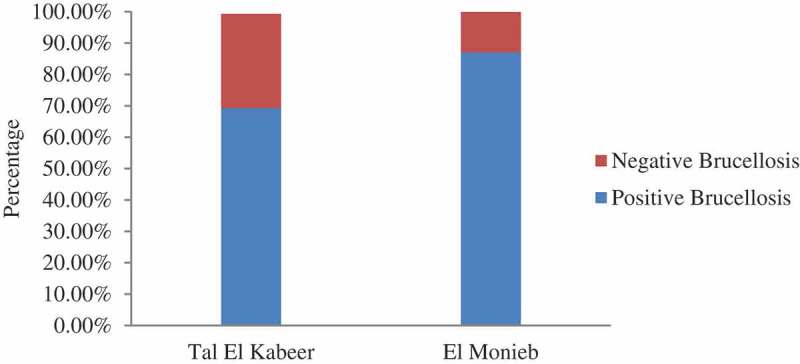
Percentages of positive and negative brucellosis by rose Bengal test regarding abattoir location.
Observations on biosafety gaps and deficiencies
Abattoirs personnel are subject to considerably loud sounds, slippery floors, and exposure to infection due to handling of potentially contaminated carcasses and sharp tools during the work time. In the undertaken questionnaire (Table 2), up to 45 workers were included; they were classified as veterinarians (4), butchers (29), housekeepers (8), and security personnel (4).
Table 2.
Questionnaire distributed to determine gaps and deficiencies within the targeted slaughterhouses.
| Variables | Frequency | Percentages | Observations |
|---|---|---|---|
|
Total number (45) (4) (29) (8) (4) |
8.8 64.4 17.7 8.8 |
Most workers are hired on a temporary basis |
(2) Age (years)
|
(5) (19) (21) |
11.1 42.2 46.6 |
|
(3) Education level
|
(13) (24) (4) (4) |
28.8 53.3 8.8 8.8 |
Most workers are not well educated |
| (4) Presence of written safety policy If yes
|
No | No | There is no written safety plan for the work place |
| (5) Is a regular medical examination conducted? If yes
|
No | No | No regulations for periodic medical examination |
| (6) History of infection with Brucella If yes
|
Three past cases Serological test after unknown fever Antibiotics therapy |
6.6 | The three cases of brucellosis were found among veterinarians and diagnosed after a period of undulant fever |
(7) Availability of PPE
|
No | No | No resources were available |
| (8) Availability of water supply If yes
|
Yes No Yes |
Only one water source was available |
|
| (9) Availability of soap If yes
|
No | No resources were available |
|
| (10) Method of carcasses transportation If yes
|
Most of the time Sometimes |
Children engage in all handling processes |
|
| (11) Clothes exchange rooms If yes
|
No | No | Only side partition for exchange |
It was observed that the majority of workers were hired on temporary basis (with no permanent contract). Most of them were not well educated; about 28.8% were illiterate, while nearly 53.3% have only completed primary school. Another finding was employment of children and minors under 18 years of age (11%); it was noticed that children participate in all abattoirs activities, such as using sharp tools, housekeeping, and carrying carcasses.
The questionnaire indicated three past human brucellosis cases (6.6%) in the targeted group. All employees claimed no regular medical examination or medical insurance, while the abattoirs’ management reported no sufficient resources for PPE and no solid safety plan.
Biosafety interventions to improve work practices
Several field visits were conducted to raise awareness about occupational zoonosis among abattoir workers. Briefs on the importance of occupational zoonotic diseases of multiple etiologies were given to abattoir workers, butchers, and animal keepers. The abattoir workers were also provided with proper PPE, including safety boots, aprons, gloves, masks, and overhead covers. The route of infection and the role of the PPE in infection prevention were instructed.
Moreover, printed posters in Arabic presented on colored waterproof material (200 cm × 120 cm) demonstrating the potential zoonotic diseases that can be transmitted from animals were also presented. The posters included short pieces of text supported with photos to give an insight on the most common diseases associated with livestock in Egypt, such as hemorrhagic colitis, leptospirosis, brucellosis, mouth–foot disease, and typhoid.
A summary of the response to all types of intervention is presented in Table 3. Most abattoir workers showed good compliance with the provided PPE; 88.8% committed to using safety shoes and 80% appreciated using aprons for protection against blood. Moreover, workers gave good response to regular hand washing and cleaning activities. Using one-way ANOVA test followed by multiple comparisons using Tukey’s post hoc test between PPE regarding compliance, there was significant difference as (P-value < 0.05 = 0.0039), shown in Figure 2. Wearing gloves and overheads showed different post hoc rank (B) as it was not prioritized by the majority of workers. Only (24.4%) and (20%) committed to wearing gloves and overheads, respectively.
Table 3.
Personal behavior regarding commitment after providing personal protective equipment
| Personal protective equipment (PPE) | Compliance |
Noncompliance |
Tukey’s post hoc rank | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Safety shoes | 40 | 88.8 | 5 | 11.11 | A |
| Aprons | 36 | 80 | 9 | 20 | A |
| Gloves | 11 | 24.4 | 34 | 75.5 | B |
| Overheads | 9 | 20 | 36 | 80 | B |
| Hand wash | 38 | 84.4 | 7 | 15.5 | A |
| Cleaning practices | 37 | 82.2 | 8 | 17.7 | A |
| P-value | 0.0039** | ||||
Same post hoc rank indicates insignificant difference and different post hoc rank indicates significant difference.
**Significantly different.
Figure 2.
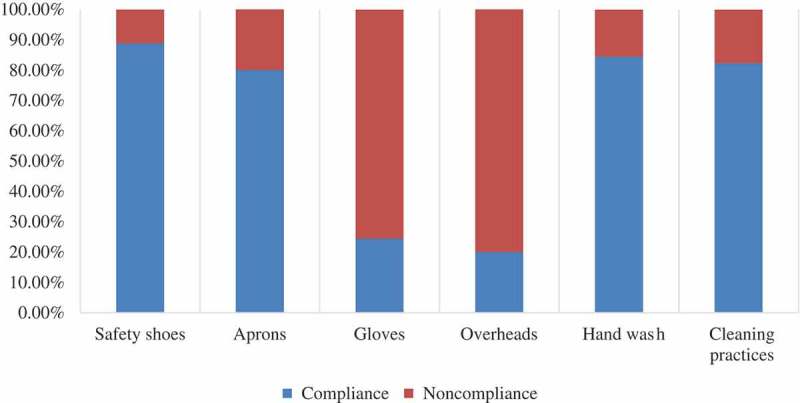
Percentages of compliance with PPE among abattoir personnel.
Discussion
Occupational diseases and infections frequently contracted by abattoir personnel could be caused by pathogenic or transmissible agents. Brucellosis is a global zoonotic disease associated with significant morbidity that can lead to increased rates of spontaneous abortions in livestock and also in humans [8,21]. In a preliminary study, we serologically investigated the potential occupational brucellosis hazard at Egyptian abattoirs. A cross-sectional study was conducted in two abattoirs, up to 230 blood samples were collected over 12 months. The overall prevalence of Brucella seropositivity among calves in the two abattoirs was about 75.2%, which is noticeably high and comes in agreement with many serological findings published elsewhere. A significance difference (= 0.003289) was determined regarding seropositivity and abattoir location. Comparatively high prevalence at El Monieb abattoir (87%) may reflect high exposure to disease and occurrence of potential risk factor in this distinct region.
Some crucial factors influencing this, such as high illiteracy, child labor, and non-permanent work contracts, have been taken into consideration during biosafety interventions. It was not feasible to constrain the working group to commit with the provided PPE. Thus, noted enhancements related to the workplace (the abattoirs) biosafety were mainly achieved through good comprehensive and friendly advice, group discussions, and illustrative colored images, instead of written material. The interventions aimed at introducing the culture of biosafety among abattoir personnel in a friendly manner and raising awareness about the impact of compliance with biosafety and protection against potential zoonotic diseases, including but not limited to brucellosis.
Abattoir workers are a limited resources manpower, and most of them (72%) are not well educated. It was reported that lack of strict application of biosafety measures, along with great shortage of proper resources, were the main challenges to fully addressing the biosafety gaps. In spite of continuous discussions and illustration of the importance of biosafety implementation to mitigate the probability of infection, it is clearly noticed that there is a gap of malpractice and inconvenient behavior among individuals of the targeted community that might be explained as a cause of the temporary work status and the resulting instability. This can be overcome by a sustainable development plan for abattoirs.
Data presented here highlights the need for further research, including isolation and characterization of the causative agents, reliable epidemiological studies, and implementing a transparency policy and effective control measures in Egypt. A clear gap of both personal and hygiene malpractices was observed in the study places. These findings might be explained by the absent role of concerned authorities and lack of written safety policy. Implementation of biosafety measures in such work places demands not only a multisectional collaboration and particular resources, but also raising awareness, powerful training, continuous follow-up, and investigative practice. The increase in infectious disease detection capacity has not necessarily been paralleled with an increase in biosafety and biosecurity capacity, particularly in low-resource countries.
Conclusion
Brucellosis is among the most common zoonotic infections globally. It has been reported as a serious public health issue with great influence on the economic accomplishments of several countries. The findings in this study conclude that brucellosis is a point of public health concern, with high seroprevalence among abattoir animals. Abattoirs workers are specifically considered to be under high risk of acquiring occupational diseases. Low-resource countries face numerous challenges that severely constrain the development, or expansion, of sustainable capacity in biosafety and biosecurity management. Overcoming these challenges requires the collaborative efforts of representatives from the highest levels of local governments and the international biosafety community to ensure continuity and compliance with safety practices.
Funding Statement
This work was supported by the American Society for Microbiology [biosafety/biosecurity research program in Egypt (2016)].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Hegazy Y, Ridler A, Guitian F.. Assessment and simulation of the implementation of brucellosis control programme in an endemic area of the Middle East. Epidemiol Infect. 2009;137:1436–1448. [DOI] [PubMed] [Google Scholar]
- [2].Al Dahouk S, Neubauer H, Hensel A, et al. Changing epidemiology of human brucellosis, Germany, 1962–2005. Emerg Infect Dis. 2007;13(12):1895–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Al-Nassir W, Lisgaris MV, Salata RA.. Brucellosis. E-medicine. Medscape. Morgan Place; 2009[cited 2010 January20] Available from: http://emedicine.medscape.com/article/213430-overview [Google Scholar]
- [4].Holt H, Eltholth M, Hegazy Y, et al. Brucella spp. infection in large ruminants in an endemic area of Egypt: cross-sectional study investigating seroprevalence, risk factors and livestock owner’s knowledge, attitudes and practices (KAPs). BMC Public Health. 2011;11:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sánchez SLP, Ordóñez BP, Díaz GMO, et al. Human and animal incidence of brucellosis declining in Spain. Euro Surveill. 2005[cited 2010 January21];10(16). Available from: http://www.eurosurveillance.org/ [DOI] [PubMed] [Google Scholar]
- [6].Minas M, Minas A, Gourgulianis K, et al. Epidemiological and clinical aspects of human brucellosis in Central Greece. J Infect Dis. 2007;60:362–366. [PubMed] [Google Scholar]
- [7].Dean AS, Crump L, Greter H, et al. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012. DOI: 10.1371/journal.pntd.0001929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Corbel MJ. Brucellosis in humans and animals. 1st ed. Geneva: World Health Organization, Food and Agriculture Organization of the United Nations, World Organisation for Animal Health; 2006. p. 22–28. [Google Scholar]
- [9].Ramalho RTR, Pinheiro JJW, De Moura SPA, et al. Epidemiological aspects of an infection by Brucella abortus in risk occupational groups in the microregion of Araguaína, Tocantins. Braz J Infect Dis. 2008;12(2):133–138. [DOI] [PubMed] [Google Scholar]
- [10].Van ZAP. Manual for the abattoir industry. 1st ed. Pretoria: Red Meat Abattior Association; 1995. [Google Scholar]
- [11].Akbarian Z, Ziay G, Schauwers W, et al. Brucellosis and Coxiella burnetii infection in householders and their animals in secure villages in Herat province, Afghanistan: a cross-sectional study. PLoS Negl Trop Dis. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gálvez VR, Rodríguez MA, Rodríguez CP, et al. Epidemiology of Brucellosis in the Granada province (I). Occupational risks. Med Clin (Barc). 1991;96(15):570–574. [PubMed] [Google Scholar]
- [13].Blasco JM, Molina-Flores B. Control and eradication of Brucella melitensis infection in sheep and goats. Veterinary Clinics of North America. Food Anim Pract. 2011;27:95–104. [DOI] [PubMed] [Google Scholar]
- [14].Coggon D. Questionnaire based exposure assessment methods. Sci Total Environ. 1995;168:175–178. [DOI] [PubMed] [Google Scholar]
- [15].Czaja R, Blair J. Designing surveys: a guide to decisions and procedures. Thousand Oaks, CA: Pine Forge Press; 1996. [Google Scholar]
- [16].Katzenellenbogen JM, Joubert G, Abdool Karim SS. Epidemiology: a manual for South Africa. Cape Town: Oxford University Press; 1997. [Google Scholar]
- [17].Varkevisser CM, Pathmanathan I, Brownlee A. Designing and conducting health systems research projects (Vol. 2, Part 1). Canada: International Development Research Centre; 1995. [Google Scholar]
- [18].Moyer NP, Holcomb LA. Brucella In: Murray PR, Baron EJ, Pfaller MA, et al, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. p. 549–555. [Google Scholar]
- [19].Altwegg M, Bohl E. Evaluation of a rapid, reliable, and inexpensive screening test for the serological diagnosis of human brucellosis. Zentralbl Bakteriol Mikrobiol Hyg [A]. 1985;260:65–70. [DOI] [PubMed] [Google Scholar]
- [20].Baum M, Zamir O, Bergman-Rios R, et al. Comparative evaluation of microagglutination test and serum agglutination test as supplementary diagnostic methods for brucellosis. J Clin Microbiol. 1995;33:2166–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bosilkovski M, Dimzova M, Grozdanovski K. Natural history of Brucellosis in an endemic region in different time periods. Acta Clin Croat. 2009;48:41–46. [PubMed] [Google Scholar]


