Abstract
Purpose
Cancer-related cognitive impairment (CRCI) is an important clinical problem in patients with breast cancer receiving chemotherapy. Nationwide longitudinal studies are needed to understand the trajectory and severity of CRCI in specific cognitive domains.
Patients and Methods
The overall objective of this nationwide, prospective, observational study conducted within the National Cancer Institute Community Clinical Oncology Research Program was to assess trajectories in specific cognitive domains in patients with breast cancer (stage I-IIIC) receiving chemotherapy, from pre- (A1) to postchemotherapy (A2) and from prechemotherapy to 6 months postchemotherapy (A3); controls were assessed at the same time-equivalent points. The primary aim assessed visual memory using the Cambridge Neuropsychological Test Automated Battery Delayed Match to Sample test by longitudinal mixed models including A1, A2, and A3 and adjusting for age, education, race, cognitive reserve score, and baseline anxiety and depressive symptoms. We also assessed trajectories of CRCI in other aspects of memory as well as in attention and executive function with computerized, paper-based, and telephone-based cognitive tests.
Results
In total, 580 patients with breast cancer (mean age, 53.4 years) and 363 controls (mean age, 52.6 years) were assessed. On the Delayed Match to Sample test, the longitudinal mixed model results revealed a significant group-by-time effect (P < .005); patients declined over time from prechemotherapy (A1) to 6 months postchemotherapy (A3; P = .005), but controls did not change (P = .426). The group difference between patients and controls was also significant, revealing declines in patients but not controls (P = .017). Several other models of computerized, standard, and telephone tests indicated significantly worse performance by patients compared with controls from pre- to postchemotherapy and from prechemotherapy to 6 months postchemotherapy.
Conclusion
This nationwide study showed CRCI in patients with breast cancer affects multiple cognitive domains for at least 6 months postchemotherapy.
INTRODUCTION
Cancer-related cognitive impairment (CRCI) in patients with breast cancer has become a growing area of clinical concern. Research suggests that CRCI occurs in up to 25% of patients with cancer before chemotherapy and in up to 75% of patients during chemotherapy.1-17 CRCI can remain a significant problem post-treatment in up to 35% of survivors18-22 and negatively affects quality of life.4,8,23,24
A key contribution to understanding CRCI has been the use of a prechemotherapy assessment, because patients sometimes perform within normative ranges during treatment but show a significant decline from their prechemotherapy baselines.4 Larger studies with prechemotherapy baselines are needed to confirm results of previous studies. Assessment of longitudinal changes of specific cognitive domain scores has been recommended by the International Cancer and Cognition Task Force.25,26 Additional limitations of previous research include enrollment of heterogeneous disease groups; conduct at academic medical centers, which limits generalizability; and failure to include age- and sex-matched controls to adequately control for practice and aging effects.7,9-15
Recently, the National Cancer Institute emphasized the need for the study and validation of cognitive neuroscience–based measures of cognitive function in specific domains to assess the impact of chemotherapy and other treatments on cognitive functioning. Additionally, more modern computerized cognitive assessments may enable clinicians and researchers to assess more mild cognitive impairments in specific domains.
The objective of this study was to assess the trajectory of CRCI in specific cognitive domains, with the primary aim of assessing longitudinal changes in visual memory in patients with breast cancer from pre- (A1) to postchemotherapy (A2; ie, within 1 month after completion of chemotherapy) and from A1 to 6 months postchemotherapy (A3) compared with controls assessed at the same time intervals using the Delayed Match to Sample (DMS) test in the computerized Cambridge Neuropsychological Test Battery (CANTAB).27-31 The DMS test largely involves function of the prefrontal cortex and hippocampus; both areas are important in neuroimaging studies of patients receiving chemotherapy.32 Additionally, our preclinical model of chemotherapy-related cognitive impairments identified impairments in delayed spatial alternation, which has some analogous features to the DMS,33 and DMS has been used in clinical research studies evaluating neurotoxicants.34,35 Visual-related memory deficits have been implicated in CRCI in previous research,36-39 and visual-related memory encompasses a domain in which patients complain of deficit, representing an important cognitive domain for further study. Here, we were interested in measuring the ability to assess and remember a complex pattern even after varying delay times, thus also incorporating a short-term working memory component.
To help guide clinicians on factors that may increase susceptibility to CRCI, we investigated the impact of factors that may influence cognitive impairment, including age, race, education,40 cognitive reserve,5 chemotherapy type (anthracycline v nonanthracycline,41,42 adjuvant v neoadjuvant), hormonal or radiation therapy after chemotherapy, and baseline menopausal status, anxiety, and symptoms of depression.17,20-22,42 We hypothesized that patients with breast cancer would experience visual memory declines over time compared with age-matched controls assessed at the same time points.
PATIENTS AND METHODS
Participants
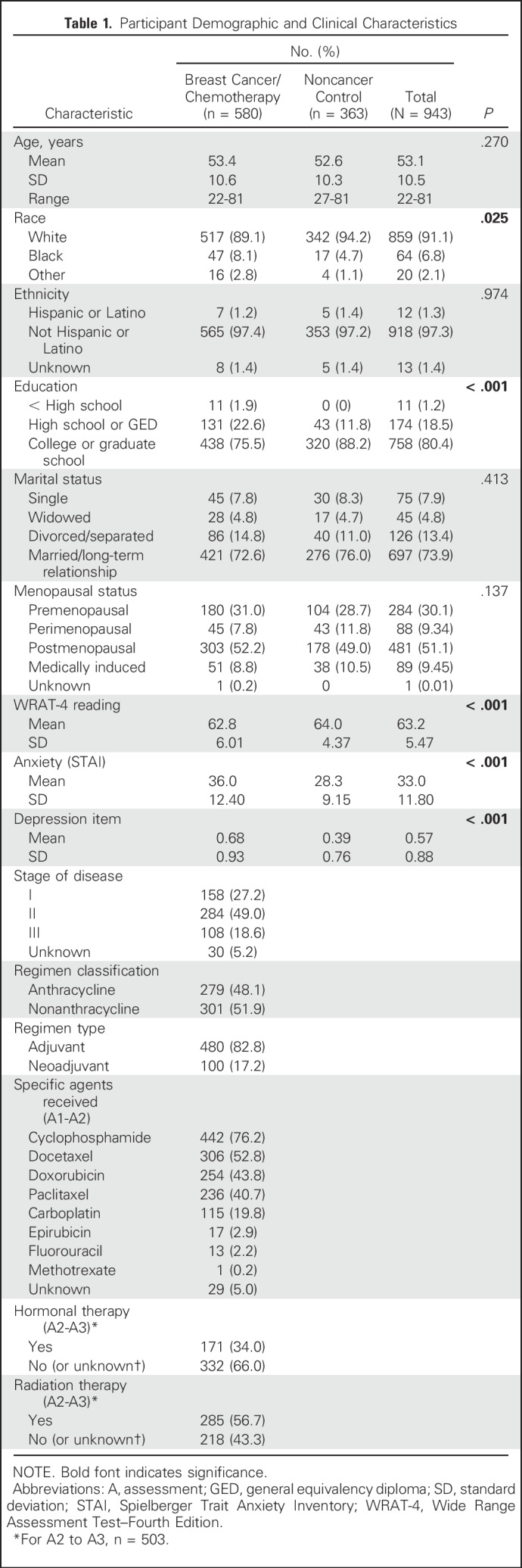
Patients with breast cancer and healthy noncancer controls were recruited from 22 National Cancer Institute Community Oncology Research Program (NCORP) locations nationwide from 2011 to 2013. Eligibility for patients with breast cancer included: woman with stage I to IIIC disease, scheduled for a standard course of chemotherapy, chemotherapy naïve, age ≥ 21 years, no CNS disease, no neurodegenerative disease, no recent major psychiatric illness leading to hospitalization, and no plan to receive concurrent radiation therapy from pre- to postchemotherapy. Control participants were the same age (within 5 years) as the paired patients with breast cancer and met all eligibility criteria except the first two. This study was approved by the institutional review board of each NCORP and the University of Rochester Cancer Center NCORP Research Base; all participants provided informed consent. Table 1 details participant characteristics at baseline.
Table 1.
Participant Demographic and Clinical Characteristics
Cognitive Assessments and Covariates
All cognitive assessments were conducted at the following time points for patients: prechemotherapy baseline assessment within 7 days before the first chemotherapy administration (A1), postchemotherapy assessment within 1 month of the last chemotherapy administration (A2), and 6-month follow-up at 6 months after A2, inclusive of a 1-month range (A3). Controls also completed the same assessments at the same time intervals as patients.
All clinical research coordinators were formally trained. A standardized cognitive assessment manual was used to administer the computerized testing first, followed by paper-based testing and then self-report items. The telephone-based measures were administered after the in-person assessments; we did allow A1 telephone assessments to occur after chemotherapy infusion because of scheduling conflicts, which represented < 5% of the data. Alternate versions of the computerized tests were preprogrammed into computers. Alternate forms were not used for other measures to minimize administration errors; we felt these were not necessary, because longitudinal changes were compared with a control group to account for practice effects.
Computerized neuropsychological assessments.
Additional details are provided in the Appendix (online only). CANTAB eclipse software was used in this study (Cambridge Cognition, Cambridge, UK). The CANTAB DMS test evaluated visual memory. A priori, we chose percent correct at the 12-second delay on the DMS test for the primary analysis. The Verbal Recognition Memory (VRM) test assessed immediate recall and delayed recognition memory. The Rapid Visual Processing (RVP) test evaluated sustained attention, and the One Touch Stockings of Cambridge assessed executive function.29,30
Paper-based neuropsychological assessments.
Paper-based assessments included the Hopkins Verbal Learning and Memory Test–Revised,43,44,60 the Trail Making Test (TMT) A (Comprehensive TMT 1) and B (Comprehensive TMT 5),45,46,61 and the Controlled Oral Word Association (COWA) test.47,62
Telephone-based cognitive assessments.
The Brief Test of Adult Cognition by Telephone included the Rey Auditory Verbal Learning Test (RAVLT), digits backward, category fluency, and backward counting.48
Single-item self-report assessments.
On a Likert scale (0-10), participants rated their level of difficulty over 7 days on three single items in specific cognitive domains (eg, remembering things, paying attention, and multitasking) as part of a modified MD Anderson Symptom Inventory.49
Covariate measures.
Participants self-identified race and ethnicity. Medical information was abstracted from medical records. Chemotherapy was dichotomized into anthracycline- versus non–anthracycline-containing treatment as well as adjuvant versus neoadjuvant treatment. Baseline reading ability, a proxy for cognitive reserve, was assessed with the Wide Range Assessment Test–Fourth Edition (WRAT-4) reading subscale.50 Anxiety was assessed with the Spielberger Trait Anxiety Inventory,51 and depression was measured by an item from the Multidimensional Fatigue Symptom Inventory.52
Statistical Analyses
For comparison of baseline characteristics for the patients and controls, t tests were used for continuous variables, and χ2 tests were used for categorical variables. Means and standard errors were tabulated for all cognitive measures at each assessment.
The primary aim of this study was to assess trajectories of change in the DMS test from A1 to A2 and from A1 to A3 using longitudinal linear mixed modeling (LMM), controlling for important a priori baseline covariates. Additionally, per protocol, we proposed to also conduct Welch two-sample t tests. More patients were accrued compared with controls to address if there were cognitive differences in those patients receiving anthracycline versus nonanthracycline regimens. Using a two-sample t test for the power analysis, with 200 evaluable patients receiving anthracycline treatment and 200 receiving nonanthracycline treatment, we had 80% power to detect an effect size (ES) of 0.3 (approximately 5% longitudinal change in 400 evaluable patients compared with 200 controls) on the DMS 12-second delay. We estimated a 25% dropout rate, aiming to accrue 267 participants per group (534 total patients and 267 controls). Statistical computations were performed using R software (version 3; www.r-project.org) and SAS software (version 9.4; SAS Institute, Cary, NC). For the primary aim, a two-sided P < .05 was considered significant for overall group differences, and a two-sided P ≤ .025 was considered statistically significant for assessments of anthracycline versus nonanthracycline regimens each compared with controls. For secondary outcomes, P < .05 was considered significant.
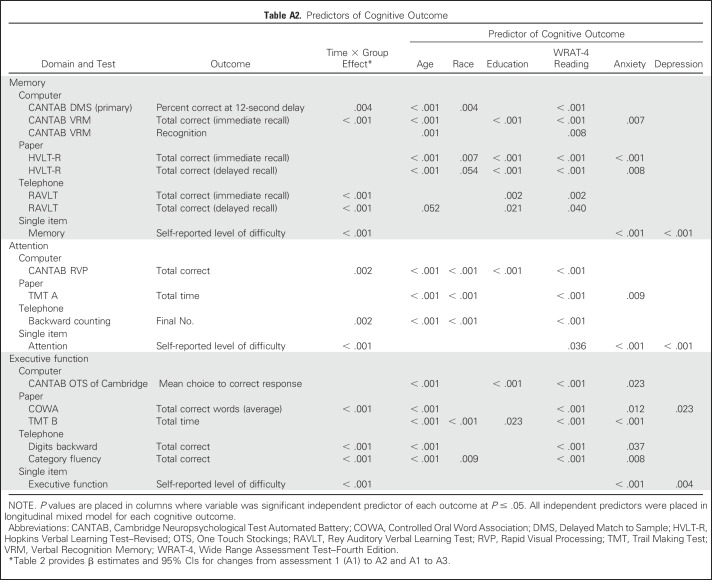
For LMM analyses, the LMM fixed effects were time (A1, A2, and A3 treated as nominal), group (patient or control), group-by-time interaction, and baseline covariates: age, education (less than high school, high school or general equivalency diploma, college or graduate), race (black, white, ther), and A1 cognitive reserve, anxiety, and depressive symptoms. Subject-specific mean cognitive function score was the random effect, independent of residual error. Estimation was performed using the restricted maximum likelihood method, and inferences were performed using the Kenward-Roger procedure.53 Marginal adjusted means were used to quantify the changes from A1 to A2 and A1 to A3 (in addition to means for each time) by group.
For the primary aim, we also conducted an LMM that added anthracycline versus nonanthracycline treatment as another covariate, as well as additional models that included anthracycline versus nonanthracycline treatment, with menopausal status at baseline and radiation and hormonal therapies from A2 to A3.
For all other cognitive outcomes, we conducted the main LMM analysis as stated. The distribution of the TMT was skewed, and values were log transformed. The VRM recognition distribution was also skewed; we dichotomized this test into perfect versus not perfect and used a generalized LMM with residual profile likelihood estimation and the Kenward-Roger test procedure.53 For all cognitive outcomes, we also adjusted for multiple comparisons with the false discovery rate; Adjusted P values are listed in Table 2.
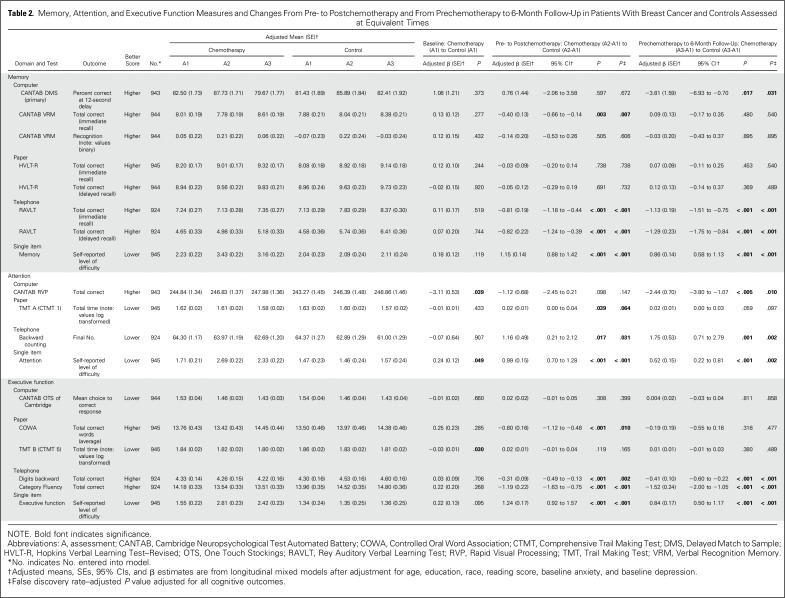
Table 2.
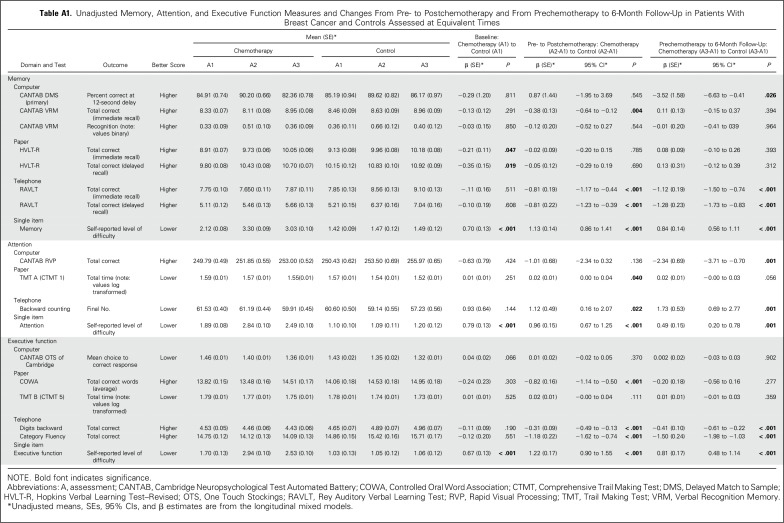
Memory, Attention, and Executive Function Measures and Changes From Pre- to Postchemotherapy and From Prechemotherapy to 6-Month Follow-Up in Patients With Breast Cancer and Controls Assessed at Equivalent Times
For each cognitive outcome, we also used exploratory analyses of variance to determine if there were differences by adjuvant versus neoadjuvant treatment. We also generated ES estimates for changes from A1 to A2 and A1 to A3.
All analyses contained all available data. Missing data were not common, except with the Brief Test of Adult Cognition by Telephone, as a result of inability to contact participants by telephone, where we assumed they were missing at random.54 Three participants developed metastatic disease from A2 to A3. For the primary aim, we conducted analyses with and without these participants. The results were not affected, and we retained the participants in the analyses.
RESULTS
Baseline Characteristics
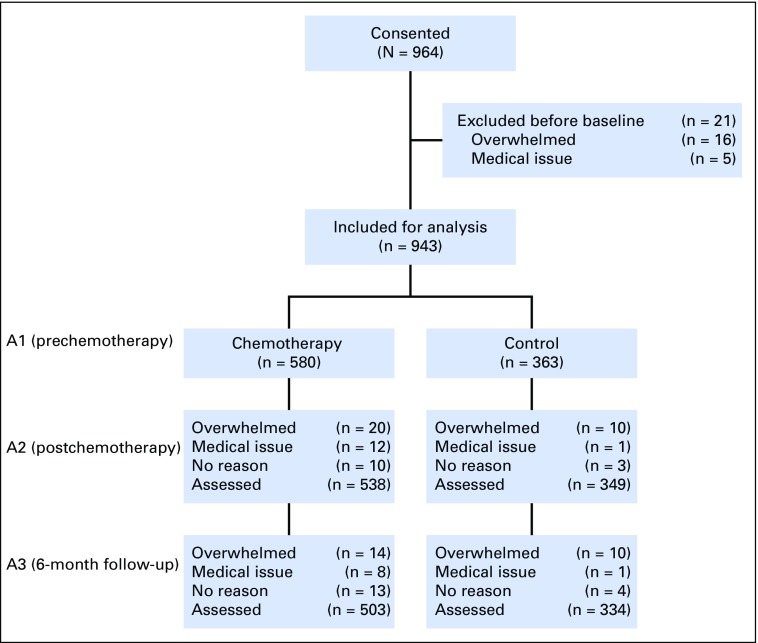
In total, 964 participants consented to the study. Of those, 943 completed the DMS test at A1, including 580 patients with breast cancer scheduled to receive chemotherapy (82.8% adjuvant) and 363 noncancer controls. There were 503 patients and 334 controls who completed all three time points (Fig 1). The groups were balanced with respect to age, ethnicity, and marital status (Table 1). Participants were fairly balanced with respect to education, except there were more high school–educated patients compared with controls (P ≤ .001). There were more black participants in the breast cancer group than in the noncancer control group (P = .025). Controls had higher reading scores (P < .001). Retention was 86.7% in the breast cancer group and 92.0% in the control group.
Fig 1.
CONSORT diagram. A, assessment.
Baseline Cognitive Function
At A1, before adjustment, only HVLT-R and the single-item questions of memory, attention, and executive function showed a significant difference in patients compared with controls, with patients reporting higher difficulty (Appendix Table A1). After adjustment, the single-item attention question and the RVP and TMT tests showed significant baseline differences, with patients performing worse than controls (P < .05; Table 2).
Memory
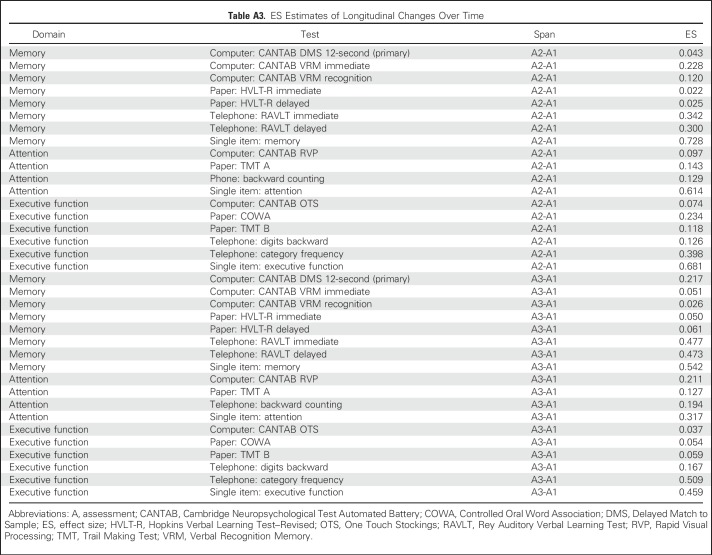
Compared with controls assessed at the same time intervals, the LMM revealed that patients with breast cancer showed a significant decline on DMS memory score at the 12-second delay from A1 to A3 (P = .017); patients significantly declined (P = .005), whereas controls did not change significantly, even after adjustment for all covariates (Table 2; Appendix Table A1). The LMM indicated a significant group-by-time interaction (P < .005; Table 2; Appendix Table A2) with patients performing worse than controls at A3. As part of the LMM, covariates that were significantly related to poorer DMS score included older age, lower WRAT-4 reading score, and black race (all P < .005; Appendix Table A2; online only). The two-sample t test analyses were consistent with the LMM, and the DMS ES was 0.217 (Figs 2 and 3; Appendix Table A3; online only).
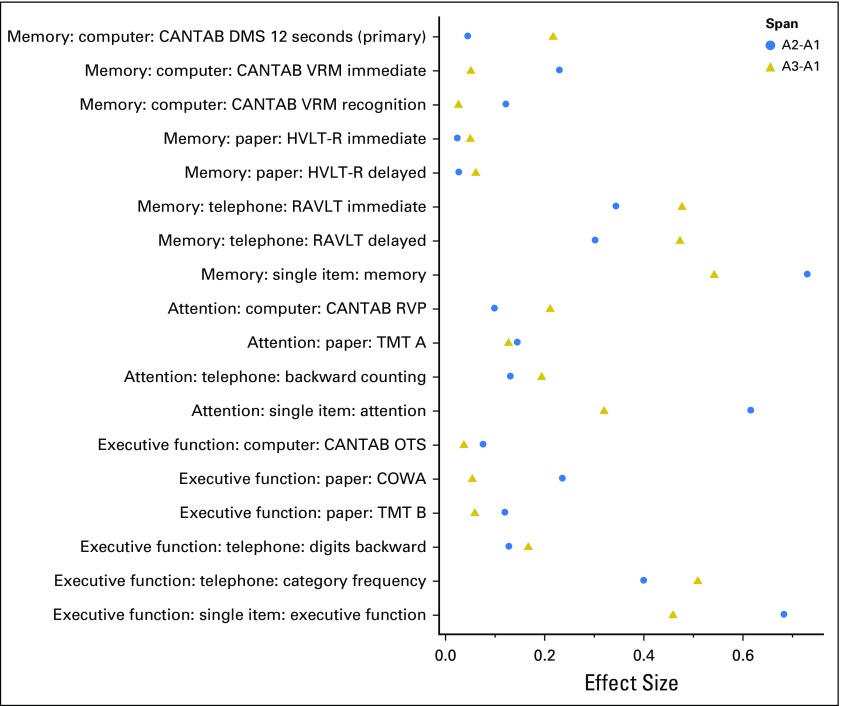
Fig 2.
Effect sizes for changes on cognitive measures. A, assessment; CANTAB, Cambridge Neuropsychological Test Automated Battery; COWA, Controlled Oral Word Association; DMS, Delayed Match to Sample; HVLT-R, Hopkins Verbal Learning Test–Revised; OTS, One Touch Stockings; RAVLT, Rey Auditory Verbal Learning Test; RVP, Rapid Visual Processing; TMT, Trail Making Test; VRM, Verbal Recognition Memory.
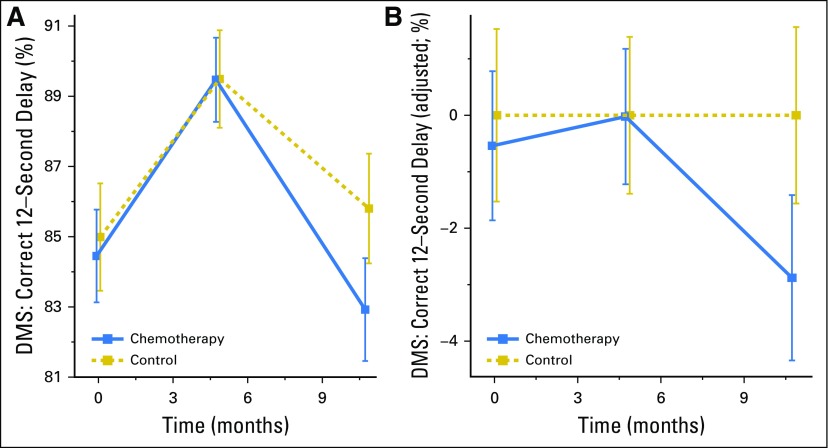
Fig 3.
Memory scores in patients with breast cancer and controls prechemotherapy (assessment 1 [A1]), postchemotherapy (A2), and 6 months after chemotherapy (A3; or time equivalent). Smaller values imply greater cognitive deficit. (A) Mean scores on the Delayed Match to Sample (DMS) test at the 12-second delay and corresponding 95% CIs are shown for A1, A2, and A3; (B) control-adjusted values, where the group control mean is subtracted from the patient group at each time point.
In another LMM model with anthracycline versus nonanthracycline treatment, regimen was not significantly related to lower cognitive scores. Of note, in a third LMM model where we added hormonal therapy to our main LMM model, we observed significantly greater decline in those not receiving hormone therapy from A2 to A3 (P = .020) compared with those receiving hormonal therapy, although both groups showed declines.
Other assessments that revealed significantly lower scores over time in patients compared with controls were the CANTAB VRM test (immediate recall), the telephone-based RAVLT immediate and delayed recall, and the single-item memory question; the latter three revealed significant changes both from A1 to A2 and from A1 to A3 (Table 2; Appendix Table A1). ES estimates revealed the largest effects on the telephone-based RAVLT and the single-item question (Fig 2; Appendix Table A3).
Attention
Assessments that revealed significantly lower scores over time in patients compared with controls from A1 to A2 after adjustment for covariates were the TMT A, backward counting, and the single-item question. From A1 to A3, the RVP, backward counting, and the single-item question were significantly different across groups, with patients performing worse than controls (Table 2). ES estimates were the largest for the CANTAB RVP and single-item question (Fig 2; Appendix Table A3).
Executive Function
From A1 to A2, assessments that significantly showed lower scores over time in patients compared with controls after adjustment were the COWA, telephone-based digits backward and category fluency, and the single-item executive function question. All remained significant except the COWA from A1 to A3 (Table 2). Effect estimates were the largest for telephone-based category fluency and the single-item question (Fig 2; Appendix Table A3).
Predictors of Cognitive Decline and Chemotherapy Regimen Effects for Secondary Outcomes
In LMMs, older age, black race (compared with white), lower education level (compared with more than high school), WRAT-4 reading score, higher baseline anxiety score, and higher baseline depression score were all significant predictors of cognitive decline, but variations existed from test to test (all P < .05; Appendix Table A2). In exploratory unadjusted analyses of variance for each cognitive test, we saw no consistent pattern for decline based on anthracycline versus nonanthracycline treatment or adjuvant versus neoadjuvant therapy.
DISCUSSION
Using several well-validated and novel measures of cognitive function, we found that CRCI existed in multiple cognitive domains for at least 6 months postchemotherapy compared with noncancer controls. Our primary aim analysis revealed that patients with breast cancer exhibited a significant decline in visual memory from A1 to A3, even after adjustment for relevant covariates; this decline was not seen from A1 to A2, and therefore, the effect was delayed and subtle on the basis of an ES of 0.21 for the DMS test (Figs 2 and 3; Table 2).
Although several assessments of memory, attention, and executive function identified significantly lower scores over time in patients compared with controls from A1 to A2, the persistent decline or delayed decline effect at A3 was most pronounced in the computerized, telephone-based, and single-item measures. Some paper-based neuropsychological tests identified significant effects from A1 to A2. The computerized tests that were largely based in cognitive neuroscience, DMS and RVP, showed a significant decline at A3, indicating that these precise measures may be able to detect more subtle and persistent declines and supporting the idea that domain-specific computerized tests may be critical for detecting long-term CRCI in a subset of patients. Our results with objective assessments showed similar overall patterns to our previous report, which assessed CRCI with the Functional Assessment of Cancer Therapy–Cognitive (FACT-Cog)17; however, some of the objective tests did not reveal significant changes from A1 to A2, and some did not reveal changes from A1 to A3. Overall, the paper-based neuropsychological results were most likely to be influenced by anxiety and depression compared with the computerized and telephone-based measures in domains of memory and attention. Similar to the FACT-Cog study, in this study, single-item self-report measures in specific cognitive domains revealed the largest effects (Fig 2), with similar patterns to the FACT-Cog study.17
Ahles et al,2,5,19 Root et al,55,56 and others have shown that attentional processes are disrupted in patients with cancer receiving chemotherapy and that these deficits may also subsequently affect memory and executive function. The finding that the telephone-based measures unanimously showed significant deficits in patients, with larger effect sizes, may support this hypothesis, because telephone-based measures require high attentional demand. Additionally, the most significant baseline deficits on the subjective and objective tests were observed in attention. In fact, not until A2 and A3 did patients report difficulties in memory and executive function as well as attention.
The use of a study-specific control group was critical, because some of the measures had marked practice effects, and the differences between patients and controls were essential to identifying changes over time between groups. For example, on the primary aim, both groups improved from A1 to A2, although the slopes of their changes were not different, indicating an equivalent practice effect in both groups. The group differences were not revealed until A3 (Fig 3).
We did not find consistent results for specific group effects comparing anthracycline- versus non–anthracycline-based regimens. These results are similar to our published study using the FACT-Cog in this cohort, as well as studies conducted by others.17,57 Future research needs to address whether subgroups receiving specific chemotherapies are most vulnerable and determine interactions between specific chemotherapies with host factors.42
The strengths of this study include a large, homogeneous, nationwide longitudinal sample within the NCORP network, which increases generalizability over current research, and the use of multiple cognitive outcomes in specific cognitive domains at pre- and postchemotherapy time points. Additionally, age-matched controls of the same sex were measured at the same times as controls. This study had excellent retention.
There are also limitations to this work, as well as opportunities for future research. The enrollment of minority populations was low, despite being a nationwide study. This study focused on patients with breast cancer, and our results only extended to 6 months postchemotherapy. We are currently accruing a lymphoma cohort with a similar study design that will be used to compare findings between men and women and between tumor types.27,58,59
In summary, we have conducted a nationwide study that identified declines in memory, attention, and executive function in patients with breast cancer up to 6 months after completion of chemotherapy, revealing persistent, mild-to-moderate effects. These data shed light on the trajectory of CRCI, as well as CRCI risk factors and possible tests that may best identify CRCI. Interventions need to be developed that target specific domains of CRCI.
ACKNOWLEDGMENT
We thank the participants of this study and all staff at the University of Rochester Cancer Center National Cancer Institute (NCI) Community Oncology Research Program (NCORP) Research Base and our NCORP affiliate sites that recruited and observed participants. We thank the NCI Community Oncology Research Program and NCORP programs for their funding and support of this project. Karen Schmidt was an author for the 2017 American Society of Clinical Oncology abstract related to this study, and we acknowledge her efforts as a study leader while she was at the Metro Minnesota NCORP. We also thank the staff of the Cancer Control Behavioral Medicine Research Unit, the Cancer Control and Psychoneuroimmunology Lab, and the Physical Exercise, Activity and Kinesiology Lab for help with setting up this study, and Susan Rosenthal, MD, and Amber Kleckner, PhD, for their critical review of this manuscript.
Appendix
Study Participation
The following Community Clinical Oncology Programs/National Cancer Institute Community Oncology Research Programs participated in this study: Central Illinois, Columbus, CRCWM, Dayton, Delaware, Grand Rapids, Greenville, Hematology/Oncology Associates of Central New York, Kalamazoo, Kansas City, Marshfield, Metro Minnesota, Nevada, North Shore, PCRC, SCCC, SCOR, Upstate Carolina, Virginia Mason, Wichita, WiNCORP, and WORC
Cognitive Methods
Computerized Neuropsychological Assessments
Initially, participants completed a motor function screen to become familiar with the computer by pressing the center of a flashing X. Then, the Delayed Match to Sample test evaluated visual working memory. The participant was shown a visual image consisting of four patterns, each of unique shape and color, and then asked to identify the complex pattern either simultaneously or after a 0-, 4-, or 12-second delay (from memory). The delivery of the delay was random. A priori, we chose the 12-second delay of the Delayed Match to Sample test for the primary analysis, because this is when memory is most taxed. The Verbal Recognition Memory test assessed immediate recall of a list of 12 words that the participant read aloud. After all other computerized tasks, a delayed recognition memory test was delivered where the participant recalled “yes” or “no” to previously reading a word aloud. The Rapid Visual Information Processing test evaluated visual sustained attention and processing speed through recognition of a set of number series of three numbers. Executive function was examined using the One Touch Stockings of Cambridge test, which assesses spatial planning by arranging colored balls into the correct spatial orientation within three pockets.
Paper-Based Neuropsychological Assessments
Short-term memory was assessed by the Hopkins Verbal Learning and Memory Test–Revised, a word list test of immediate and delayed recall (form 1). Attention/scanning, speed/sequencing, and executive function were assessed by the Trail Making Test (Comprehensive Trail Making Test Trails 1 and 5, named A and B herein) in which the participant had to connect numbers or alternating numbers and letters, respectively. Verbal fluency/executive function was assessed by the Controlled Oral Word Association test and included the recall of as many words as possible beginning with C, F, and L within a 60-second timeframe per letter.
Telephone-Based Cognitive Assessments
The Brief Test of Adult Cognition by Telephone, developed at the Lifespan Developmental Psychology Lab (http://www.brandeis.edu/projects/lifespn) included the Rey Auditory Verbal Learning Test, digits backward (of number series with varying lengths), category fluency (number of animals correctly identified within 30 seconds), and backward counting (from 100).
Table A1.
Unadjusted Memory, Attention, and Executive Function Measures and Changes From Pre- to Postchemotherapy and From Prechemotherapy to 6-Month Follow-Up in Patients With Breast Cancer and Controls Assessed at Equivalent Times
Table A2.
Predictors of Cognitive Outcome
Table A3.
ES Estimates of Longitudinal Changes Over Time
Footnotes
Supported by National Cancer Institute (NCI) Grant Supplement No. U10CA037420S and NCI Grants No. UG1CA189961, DP2195765, K07CA168886, and R25CA102618.
Presented at the Oral Poster Discussion Session, Patient and Survivor Care Session, of the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2-6, 2017.
Clinical trial information: NCT01382082.
AUTHOR CONTRIBUTIONS
Conception and design: Michelle C. Janelsins, Charles E. Heckler, Luke J. Peppone, Gary R. Morrow
Financial support: Michelle C. Janelsins, Gary R. Morrow
Administrative support: Michelle C. Janelsins
Provision of study materials or patients: Michelle C. Janelsins, Jodi Geer, Shaker R. Dakhil, Judith O. Hopkins
Collection and assembly of data: Michelle C. Janelsins, Charles E. Heckler, Jodi Geer, Shaker R. Dakhil, Judith O. Hopkins
Data analysis and interpretation: Michelle C. Janelsins, Charles E. Heckler, Luke J. Peppone, Tim A. Ahles, Supriya G. Mohile, Karen M. Mustian, Oxana Palesh, Ann M. O’Mara, Lori M. Minasian, Annalynn M. Williams, Allison Magnuson, Gary R. Morrow
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Longitudinal Trajectory and Characterization of Cancer-Related Cognitive Impairment in a Nationwide Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Michelle C. Janelsins
No relationship to disclose
Charles E. Heckler
No relationship to disclose
Luke J. Peppone
No relationship to disclose
Tim A. Ahles
No relationship to disclose
Supriya G. Mohile
Consulting or Advisory Role: Seattle Genetics
Karen M. Mustian
No relationship to disclose
Oxana Palesh
Travel, Accommodations, Expenses: Ad Salutem
Ann M. O’Mara
Stock or Other Ownership: Pfizer
Lori M. Minasian
No relationship to disclose
Annalynn M. Williams
No relationship to disclose
Allison Magnuson
No relationship to disclose
Jodi Geer
No relationship to disclose
Shaker R. Dakhil
No relationship to disclose
Judith O. Hopkins
Consulting or Advisory Role: AIM Specialty Health
Gary R. Morrow
No relationship to disclose
REFERENCES
- 1.Brezden CB, Phillips KA, Abdolell M, et al. : Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695-2701, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ahles TA, Saykin AJ, Furstenberg CT, et al. : Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol 20:485-493, 2002 [DOI] [PubMed] [Google Scholar]
- 3.van Dam FS, Schagen SB, Muller MJ, et al. : Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210-218, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Wefel JS, Lenzi R, Theriault RL, et al. : The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer 100:2292-2299, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Ahles TA, Saykin AJ, McDonald BC, et al: Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol 28:4434-4440, 2010. [DOI] [PMC free article] [PubMed]
- 6.Jansen CE, Dodd MJ, Miaskowski CA, et al. : Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology 17:1189-1195, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Hermelink K, Untch M, Lux MP, et al. : Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer 109:1905-1913, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Hurria A, Hurria A, Zuckerman E, et al. : A prospective, longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy. J Am Geriatr Soc 54:1119-1124, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Schagen SB, Muller MJ, Boogerd W, et al. : Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J Natl Cancer Inst 98:1742-1745, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Hurria A, Rosen C, Hudis C, et al. : Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc 54:925-931, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Collins B, Mackenzie J, Stewart A, et al. : Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology 18:134-143, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Jenkins V, Shilling V, Deutsch G, et al. : A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 94:828-834, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahles TA, Saykin AJ, McDonald BC, et al. : Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat 110:143-152, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart A, Collins B, Mackenzie J, et al. : The cognitive effects of adjuvant chemotherapy in early stage breast cancer: A prospective study. Psychooncology 17:122-130, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Ouimet LA, Stewart A, Collins B, et al. : Measuring neuropsychological change following breast cancer treatment: An analysis of statistical models. J Clin Exp Neuropsychol 31:73-89, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Jansen CE, Cooper BA, Dodd MJ, et al. : A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer 19:1647-1656, 2011 [DOI] [PubMed] [Google Scholar]
- 17. doi: 10.1200/JCO.2016.68.5826. Janelsins MC, Heckler CE, Peppone LJ, et al: Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol 35:506-514, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janelsins MC, Kesler SR, Ahles TA, et al. : Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 26:102-113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahles TA, Root JC, Ryan EL: Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol 30:3675-3686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merriman JD, Sereika SM, Brufsky AM, et al. : Trajectories of self-reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psychooncology 26:44-52, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bender CM, Merriman JD, Gentry AL, et al. : Patterns of change in cognitive function with anastrozole therapy. Cancer 121:2627-2636, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganz PA, Petersen L, Castellon SA, et al. : Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: An observational cohort study. J Clin Oncol 32:3559-3567, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid-Arndt SA, Yee A, Perry MC, et al. : Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. J Psychosoc Oncol 27:415-434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley CJ, Neumark D, Bednarek HL, et al. : Short-term effects of breast cancer on labor market attachment: Results from a longitudinal study. J Health Econ 24:137-160, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Joly F, Giffard B, Rigal O, et al. : Impact of cancer and its treatments on cognitive function: Advances in research from the Paris International Cognition and Cancer Task Force Symposium and update since 2012. J Pain Symptom Manage 50:830-841, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Wefel JS, Vardy J, Ahles T, et al. : International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 12:703-708, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Vardy JL, Dhillon HM, Pond GR, et al. : Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J Clin Oncol 33:4085-4092, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russo S, Nielen MM, Boon JC, et al. : Neuropsychological investigation into the carcinoid syndrome. Psychopharmacology (Berl) 168:324-328, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Fray PJ, Robbins TW: CANTAB battery: Proposed utility in neurotoxicology. Neurotoxicol Teratol 18:499-504, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Robbins TW, James M, Owen AM, et al. : Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia 5:266-281, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Sahakian BJ, Owen AM: Computerized assessment in neuropsychiatry using CANTAB: Discussion paper. J R Soc Med 85:399-402, 1992 [PMC free article] [PubMed] [Google Scholar]
- 32.Deprez S, Kesler SR, Saykin AJ, et al. : International Cognition and Cancer Task Force recommendations for neuroimaging methods in the study of cognitive impairment in non-CNS cancer patients. J Natl Cancer Inst 110:223-231, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janelsins MC, Heckler CE, Thompson BD, et al. : A clinically relevant dose of cyclophosphamide chemotherapy impairs memory performance on the delayed spatial alternation task that is sustained over time as mice age. Neurotoxicology 56:287-293, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins TW, Semple J, Kumar R, et al. : Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: Comparison with diazepam and implications for dementia. Psychopharmacology (Berl) 134:95-106, 1997 [DOI] [PubMed] [Google Scholar]
- 35.van Wijngaarden E, Winters PC, Cory-Slechta DA: Blood lead levels in relation to cognitive function in older U.S. adults. Neurotoxicology 32:110-115, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Jim HS, Phillips KM, Chait S, et al. : Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol 30:3578-3587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wieneke MH, Dienst ER: Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology 4:61-66, 1995 [Google Scholar]
- 38.Schagen SB, van Dam FS, Muller MJ, et al. : Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85:640-650, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Ahles TA, Saykin AJ, Noll WW, et al. : The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 12:612-619, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Matthews F, Marioni R, Brayne C: Examining the influence of gender, education, social class and birth cohort on MMSE tracking over time: A population-based prospective cohort study. BMC Geriatr 12:45, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janelsins MC, Mustian KM, Palesh OG, et al. : Differential expression of cytokines in breast cancer patients receiving different chemotherapies: Implications for cognitive impairment research. Support Care Cancer 20:831-839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesler SR, Blayney DW: Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol 2:185-192, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapiro AM, Benedict RH, Schretlen D, et al. : Construct and concurrent validity of the Hopkins Verbal Learning Test-Revised. Clin Neuropsychol 13:348-358, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Rasmusson DX, Bylsma FW, Brandt J: Stability of performance on the Hopkins Verbal Learning Test. Arch Clin Neuropsychol 10:21-26, 1995 [PubMed] [Google Scholar]
- 45.Gaudino EA, Geisler MW, Squires NK: Construct validity in the Trail Making Test: What makes Part B harder? J Clin Exp Neuropsychol 17:529-535, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Goul WR, Brown M: Effects of age and intelligence on trail making test performance and validity. Percept Mot Skills 30:319-326, 1970 [DOI] [PubMed] [Google Scholar]
- 47. Lezak M, Howieson DB, Loring DW, et al. Neuropsychological Assessment (ed 4). Oxford, United Kingdom, Oxford University Press, 2004. [Google Scholar]
- 48.Tun PA, Lachman ME: Telephone assessment of cognitive function in adulthood: The Brief Test of Adult Cognition by Telephone. Age Ageing 35:629-632, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Cleeland CS, Mendoza TR, Wang XS, et al. : Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer 89:1634-1646, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Wilkinson GS, Robertson GJ: Wide Range Achievement Test 4 (WRAT4) professional manual. Lutz, FL, Psychological Assessment Resources, 2006.
- 51. Spielberger CD, Sydeman SJ, Owen AE, et al: Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI), in Maruish ME (ed): The Use of Psychological Testing for Treatment Planning and Outcomes Assessment (ed 2). Mahwah, NJ, Lawrence Erlbaum Associates, 1999, pp 993-1021. [Google Scholar]
- 52.Mendoza TR, Wang XS, Cleeland CS, et al. : The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer 85:1186-1196, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Kenward MG, Roger JH: Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983-997, 1997 [PubMed] [Google Scholar]
- 54.Little R, Rubin DB: Statistical Analysis with Missing Data. Hoboken, NJ, Wiley, 2002 [Google Scholar]
- 55.Root JC, Ryan E, Barnett G, et al. : Learning and memory performance in a cohort of clinically referred breast cancer survivors: The role of attention versus forgetting in patient-reported memory complaints. Psychooncology 24:548-555, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Root JC, Andreotti C, Tsu L, et al. : Learning and memory performance in breast cancer survivors 2 to 6 years post-treatment: The role of encoding versus forgetting. J Cancer Surviv 10:593-599, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Dyk K, Petersen L, Ganz PA: Comparison of neurocognitive function after anthracycline-based chemotherapy vs nonanthracycline-based chemotherapy. JAMA Oncol 2:964-965, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandelblatt JS, Jacobsen PB, Ahles T: Cognitive effects of cancer systemic therapy: Implications for the care of older patients and survivors. J Clin Oncol 32:2617-2626, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loh KP, Janelsins MC, Mohile SG, et al. : Chemotherapy-related cognitive impairment in older patients with cancer. J Geriatr Oncol 7:270-280, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brandt J, Benedict RHB: Hopkins Verbal Learning Test-Revised professional manual. Lutz, FL, Psychological Assessment Resources, 2001 [Google Scholar]
- 61.Reynolds CR: Comprehensive Trail-Making Test professional manual. Austin, TX, Pro-ed, 2002 [Google Scholar]
- 62.Benton AL, Hamsher KD, Sivan AB: Controlled Oral Word Association Multilingual Aphasia Examination professional manual. Lutz, FL, Psychological Assessment Resources, 1978 [Google Scholar]