ABSTRACT
Eastern Europe (EE) has been severely affected by mosquito-borne viruses (moboviruses). In this review, we summarize the epidemiology of moboviruses, with particular attention to West Nile virus (WNV). The study of WNV human cases in EE between 2010 and 2016, revealed that the epidemiology of WNV in EE is complex with the combination of introduction of different WNV strains from lineages 1 and 2, and the establishment of endemic cycles. We found a positive correlation between the risk of WNV re-emergence in an area and the number of human cases reported in the previous year. We also report the main ecological and biological characteristics of the key mosquito species vectors of moboviruses. Recent expansion of invasive mosquito species in EE, mainly Aedes albopictus but also Aedes aegypti and Culex quinquefasciatus, may result in new scenarios with an increased risk of transmission of moboviruses. Main gaps of knowledge in relation to moboviruses and their vectors in EE are identified. Understanding the epidemiology of moboviruses in EE is essential for the improvement of their surveillance and the control of the diseases they cause.
KEYWORDS: Mosquito-borne viruses, Eastern Europe, West Nile virus, Culex and Aedes mosquito
Introduction
Arthropod-borne viruses (arboviruses) are a group of viruses that are transmitted by arthropod vectors and cause disease in humans and animals. Therefore, disease occurrence relies on the presence of the virus, susceptible hosts and competent vectors. Diseases caused by arboviruses have been reported for centuries, but in the recent years, the notification of outbreaks of arboviral diseases has dramatically increased [1]. Human population growth, deforestation, urbanization, movement of people, animals and vectors have contributed to dissemination of arboviruses. The impact of climate change on vector-borne diseases is controversial since it can influence arthropod vectors abundance and virus transmission in different ways. In fact, the effects of the climate are considered species-specific and location-specific [2]. Some arboviruses that had been neglected for a long time, have emerged in the last decades as important health threats, for example Zika virus (ZIKV) in South and Central America [3], Yellow fever virus (YFV) in Brazil [4] and West Nile virus (WNV) lineage 2 in Southern and Eastern Europe [5]. Nowadays arboviruses have a worldwide distribution, being present in all continents except Antarctica. However, each arbovirus will generally have a focal distribution because of its specific requirements in relation to vertebrate hosts, vectors and ecological factors needed to maintain its transmission cycle [1].
Arboviruses that are transmitted to vertebrates via competent hematophagous mosquitoes (Diptera: Culicidae) are called mosquito-borne viruses (moboviruses). Competent mosquitoes are those capable of first acquiring the virus through feeding on a viraemic host, then replicate the virus and finally deliver it to a new host through the saliva in the next blood feeding. Vertical transmission, i.e. the transmission of a pathogen from a parent to its progeny, represents another mechanism for arbovirus transmission and amplification in nature. Additionally, the mechanisms by which most arboviruses are trans-seasonally maintained (i.e. overwintering) remain unclear, and vertical transmission offers a likely explanation for the persistence of arboviruses during period of adverse environmental conditions.
Moboviruses affecting humans are concentrated in four families: Flaviviridae (genus Flavivirus), Togaviridae (genus Alphavirus), Peribunyaviridae (genus Orthobunyavirus) and Phenuiviridae (genus Phlebovirus). The virus species names included in the present review have been updated following the International Committee on Taxonomy of Viruses (ICTV). The present review briefly summarizes the group of moboviruses present in Eastern Europe (EE) (Figure 1). For more details on virus species see for example [1,6–8]. In the case of WNV, being the main mobovirus present in EE, we have focused on its distribution and spread in recent years using data on the human cases provided by the European Centre for Disease Prevention and Control (ECDC). Finally, the review also gives an overview of the main biological and ecological characteristics of the mosquito species involved in moboviruses transmission in EE. As there is not a clear classification of the countries included within EE, we based our choice on commonly accepted geographical criteria. Accordingly, we included Albania, Belarus, Bulgaria, Greece, Kosovo, the Former Yugoslav Republic of Macedonia (from here onwards, Macedonia), Moldova, Montenegro, Romania, Russia, Serbia, Turkey and Ukraine as belonging to EE.
Figure 1.
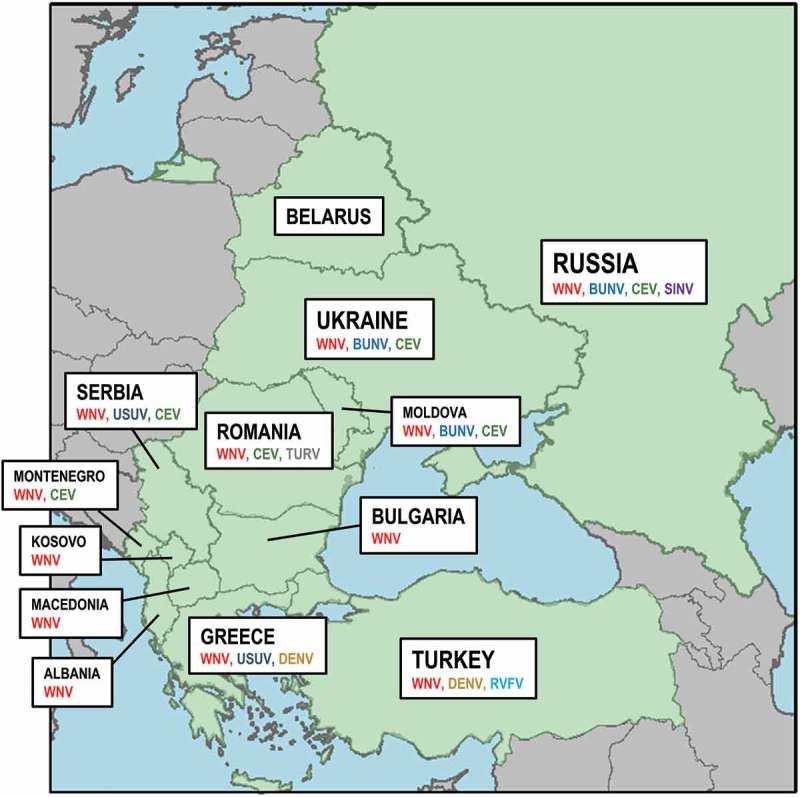
Distribution of the different moboviruses in the EE countries included in the study: West Nile virus (WNV), Usutu virus (USUV), Dengue virus (DENV), Bunyamwera orthobunyavirus (BUNV), California encephalitis virus (CEV), Turlock orthobunyavirus (TURV), Rift Valley fever phlebovirus (RVFV) and Sindbis virus (SINV).
Mosquito-borne viruses circulating in EE
The distribution of the different moboviruses in the EE countries included in the study is shown in Figure 1.
Flaviviruses (Genus Flavivirus, Family Flaviviridae)
West nile virus (WNV)
Before 2004. WNV has been responsible for repeated disease outbreaks in both horses and humans in Europe for more than 50 years [5]. Most of those outbreaks were caused by lineage 1 strains of the European Mediterranean/Kenyan cluster characterized by a moderate pathogenicity for horses and humans, and limited or no pathogenicity for birds [9]. However, in 1996 the first major West Nile fever (WNF) epidemic in humans in Europe occurred in Romania, and was caused by WNV lineage 1 strain of the Israeli/American cluster, considered as highly pathogenic for horses, humans and birds [10]. It occurred in south-eastern Romania, in the Danube valley and Bucharest, causing 352 neurological cases with 17 deaths in humans [9,11]. Culex pipiens was probably the main mosquito species involved in the epidemic [11]. Twelve further cases of WNV infection occurred in Bucharest and the lower Danube delta in 1997, and one more in Bucharest in 1998, which were indicative of persistent WNV transmission in south-eastern Romania [12]. Lineage 1 strain was reported to have continued circulating in Romania after 1996, up to 2009 [13].
In 1999, 84 cases of meningoencephalitis, 40 of which were fatal, occurred in Volgograd Region (Russia). The strain had a high degree of similarity to the strain isolated in Romania (Bucharest region) in 1996, and to that isolated in New York in 1999 [14]. Between 2000 and 2003 WNV lineage 1 circulated in Volgograd and Astrakhan regions [15].
Between 2004 and 2010. Before 2004, all WNV outbreaks in Europe had been caused by lineage 1, until a case of encephalitis in a wild goshawk (Accipiter gentilis) in Hungary resulted in the isolation of WNV lineage 2 [16]. Lineage 2 strains were thought to be restricted to sub Saharan Africa and were considered as low pathogenic [9]. Between 2004 and 2007 only sporadic cases of WNV lineage 2 in birds of prey and mammals were detected in Hungary [16]. However, in 2008 the virus expanded westwards and southwards causing mortality in birds of prey, neurological disease in horses and humans in Hungary, and reaching the eastern part of Austria, where it was detected in dead wild birds [17]. In 2009 further outbreaks were detected in Hungary and Austria [17,18].
In 2007, RNA of WNV lineage 2 was detected in brain and blood samples from humans in the Volgograd region, after which WNF epidemics with large number of human cases were observed in Russia [13]. However, retrospectively, WNV lineage 2 was identified in the sera of two patients from Rostov region affected by WNF in 2004 [15]. Russian outbreaks were caused by a WNV lineage 2 strain, but it belonged to a clade different to the cluster where the Hungarian strain was grouped [19]. Phylogeographic studies suggest at least two different introductions of WNV lineage 2 from Africa into Europe, one into Central Europe (Hungary) and the other to Russia probably linked to different bird migration routes [20].
Furthermore, in 2005, WNV lineage 1 reappeared in Astrakhan region [15].
After 2010. For the period 2010–2016, data on human cases of WNF occurred in the EE countries included in our study were obtained from the European Centre for Disease Prevention and Control (ECDC) through a request to The European Surveillance System (TESSy). ECDC WNF cases include both probable and confirmed cases of WNV infection, as defined in the Commission Decision 2008/426/EC. According to that legislation, a probable case refers to any person meeting the clinical criteria and with either an epidemiological link or a laboratory test for a probable case (i.e. presence of WNV specific antibodies). In contrast, a confirmed case refers to any person meeting the laboratory criteria for case confirmation (e.g. isolation of WNV or detection of WNV nucleic acid from blood or cerebrospinal fluid).
According to the ECDC data, 2873 WNV human cases were reported in those countries within that period. Russia was the country with the most cases reported, but Greece, Serbia, and to a lesser extent Romania were also severely affected by WNV epidemics in humans (Table 1). Of the countries included in our study, only Belarus, Moldova and Montenegro did not report any case of WNV in humans between 2010 and 2016.
Table 1.
Number of human cases of WNV reported between 2010 and 2016 in the EE countries under study
| Country | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | Total |
|---|---|---|---|---|---|---|---|---|
| Albania | 2 | 2 | ||||||
| Belarus | ||||||||
| Bulgaria | 2 | 3 | 1 | 6 | ||||
| Greece | 189 | 99 | 157 | 85 | 15 | 545 | ||
| Kosovo | 4 | 4 | ||||||
| Macedonia | 4 | 6 | 1 | 11 | ||||
| Moldova | ||||||||
| Montenegro | ||||||||
| Romania | 57 | 11 | 14 | 24 | 23 | 32 | 93 | 254 |
| Russia | 519 | 153 | 412 | 177 | 29 | 39 | 135 | 1464 |
| Serbia | 68 | 302 | 76 | 28 | 41 | 515 | ||
| Turkey | 47 | 3 | 50 | |||||
| Ukraine | 8 | 12 | 1 | 1 | 22 | |||
| Total | 812 | 280 | 675 | 590 | 143 | 102 | 271 | 2873 |
There was also quite a lot of variation among years, with 2010 and 2012 as the years with the most cases reported (812 and 675, respectively), mainly due to the circulation of WNV in Russia, where 519 and 412 cases were reported on those years respectively (Table 1). In 2013 there were also a significant number of cases reported (590), of which 302 occurred in Serbia.
Central/Southern-European lineage 2 cluster. In 2010, a major WNF epidemic occurred in the city of Thessaloniki (Greece) causing 197 neurological cases in humans with 33 deaths [5]. The analysis of serum samples collected in 2007 from residents of rural areas of northern Greece evidenced that WNV (or a closely antigenically related flavivirus) was already circulating in that area by that time [21]. WNV reappeared in the years after the 2010 epidemic, and spread both south and east of the Thessaloniki area, causing 99 human cases in 2011, 157 in 2012 and 85 in 2013 (Table 1 & Figure 2). After only 15 cases in 2014, no further cases were detected in 2015 and 2016. The complete genome analysis of the WNV circulating in 2010 showed a close genetic relationship to the lineage 2 strain that had emerged in Hungary in 2004 [22]. Phylogenetic studies carried out between 2011 and 2014 in Greece showed the similarity to the strains previously isolated in Greece, and therefore the establishment of an endemic cycle [23–25].
Figure 2.
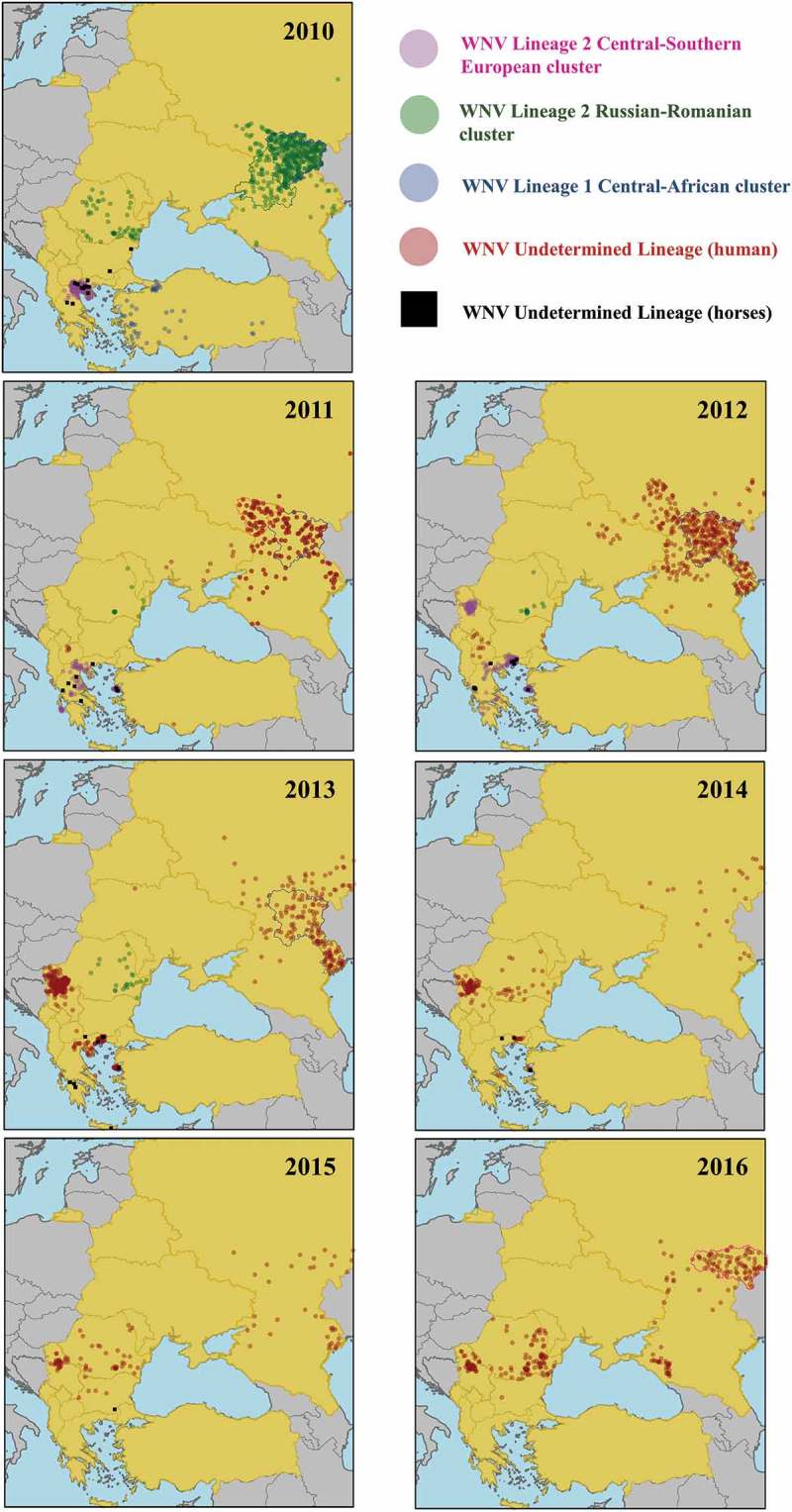
Cases of human WNV-infections registered by the ECDC in the countries under study for each year between 2010 and 2016. Distribution of cases is only an approximation as the location is randomly generated within the minimum geographical unit available in the ECDC data (NUTS3 level or equivalent unit for non-EU countries). Official outbreaks in horses reported through the EU Animal Disease Notification System (ADNS) and collected to the RASVE system of the Spanish Ministry of Agriculture, Fisheries, Food and Environment are also represented.
In 2011, WNV spread to Albania and Macedonia where it caused 2 and 4 human cases, respectively, and in 2012 it also reached Kosovo causing 4 further human cases (Table 1 and Figure 2). Although there is no information on the strain involved, given the proximity, the spread of the strain that was affecting Greece seems the most likely explanation.
In 2012, 68 human cases were reported in Serbia, 50 of which were detected in City of Belgrade district (Figure 2). In 2013, WNV re-emerged causing 302 human cases, the majority of which occurred in City of Belgrade (171 cases) but also surrounding districts (Figure 2). In the following years, the number of cases decreased (76 in 2014, 28 in 2015, and 41 in 2016) but the areas affected remained the same. Phylogenetic analyses of Serbian isolates from 2012 evidenced their close relation to Hungarian and Greek isolates [26].
In 2012 two human cases of WNF were detected in Bulgaria, where there had been two cases reported in horses in 2010 (Figure 2). There was no further detection until 2015 when 3 human cases were reported. Phylogenetic analysis showed that the virus was closely related to the WNV lineage 2 strain isolated in Greece [27].
In 2014, also WNV lineage 2, similar to the Greek isolate, was detected in the brain of a horse with severe clinical signs in Bursa region in eastern Turkey [28], but so far no human cases have been reported.
Russian/Romanian lineage 2 cluster. In 2010, 519 human cases were reported in Russia, 413 of which were in the Volgograd region (blue area in Figure 2) and 59 in the Rostov area (green area in Figure 2). The strain was the same responsible for the cases between 2004 and 2007 [15]. In 2011, only 153 cases were reported, mainly in the same areas affected in 2010 (Figure 2). In 2012 cases increased to 412, 210 of which were in the Volgograd region (blue area in Figure 2) and 60 to its south in the Astrakhan region (grey area in Figure 2). In 2013, 177 cases were registered the majority of which occurred in the Volgograd and Astrakhan regions. In 2014 and 2015, only 29 and 39 cases were reported respectively. However, in 2016, cases increased to 135, with Saratov region, to the north of Volgograd, as the most severely affected region with 87 cases (red area in Figure 2). Considering the pattern of Russian WNV outbreaks since 2010 (Figure 2), in which WNV tended to reappear in the previously-affected regions, with occasional expansion to neighbouring regions, outbreaks from 2011 onwards are likely be the result of the establishment of an endemic cycle.
WNV lineage 2 was also detected in Romania in 2010 resulting in 57 human cases, with the peculiarity that cases were distributed throughout most of the territory (Figure 2), contrary to what occurs in most countries where disease is usually clustered in some areas. Between 2011 and 2015, there were few cases (between 11 and 32) every year (Table 1), distributed in different regions (Figure 2). In 2016, the number of cases increased to 93 with some clustering of disease in the eastern part of the country. Molecular investigations revealed that the viruses involved in cases of 2010 and later years (2011–2013) were related to the Volgograd 2007 strain [29–31]. Similar to what seems to have happened in Russia; cases between 2014 and 2016 are likely to have been caused by the overwintering of the strain that was detected in Romania in previous years.
In Ukraine WNV emerged in 2011 with 8 human cases distributed in different areas south and east in the country, and further cases were detected in 2012 (12) and 2013 (1), although in areas different to those affected in 2011 (Figure 2). The location of Ukraine between Russia and Romania, lead us to think that the virus involved might belong to WNV Russian/Romanian lineage 2 cluster.
Lineage 1 Central African cluster. Between July and November 2010, 47 human cases of WNV infection were identified in Turkey, mainly in the western part of the country (Figure 2). In 2011, 3 further cases were identified in the same areas, which may be indicative of endemicity [32] (Figure 2). Sequencing of WNV from Turkey indicated close relationships to WNV lineage 1 strain ArB310/67 from the Central African Republic, and distinct from other WNV circulating in the Mediterranean Basin, EE, and the Middle East [33]. These findings suggest independent introductions of WNV strains from Africa.
Overwintering and seasonality of cases of WNV in EE. Countries affected by WNV in a given year normally had repeated cases in the following year, and generally cases reappeared in those regions that had been affected in the previous year. We evaluated the probability of WNV reoccurrence in a given area as a function of the cases occurred in the previous year. The results evidence that the higher the number of cases in a region in a year, the more likely WNV reappeared in that region in the following year (Table 2). In fact, 100% of the occasions in which a region had more than 15 human cases in a year, it was affected in the following year. If it had between 10 and 15 cases in a year, the probability of recurrence in the following year was 73%, which decreased to 40% if it had between 4 and 9 human cases. In contrast, only 16% of the times a region had less than 4 human cases in a year it was affected in the following year. Therefore, the number of human cases in an area in a given year seems to be positively correlated with the risk of WNV re-emergence in that area in the following year.
Table 2.
Probability of WNV re-emergence in a given geographical unit (NUTS3 level or equivalent unit for non-EU countries) in a year, according to the number of human cases of WNV reported in that geographical unit in the previous year.
| Number of WNV human cases in a given region and year | Number of times that number of WNV human cases in a given region and year have been detected | Probability of WNV re-emergence in that geographical unit in the next year |
|---|---|---|
| >15 | 24 | 100% |
| 10–15 | 11 | 73% |
| 4–9 | 42 | 40% |
| <4 | 115 | 16% |
| Total | 192 | 35% |
The evaluation of the seasonality of WNV transmission in EE indicates that usually cases started to be reported between beginnings to mid-July, although in some occasions first cases were reported as early as the end of May or as late as the end of August (Figure 3). Transmission usually continued through August, September, and in some cases until October and even later. However, the period of WNV transmission in Figure 3 is probably an underestimation as only the first case in each area is available in the ECDC data, so the actual date of the last case may be later than reported in the Figure 3.
Figure 3.
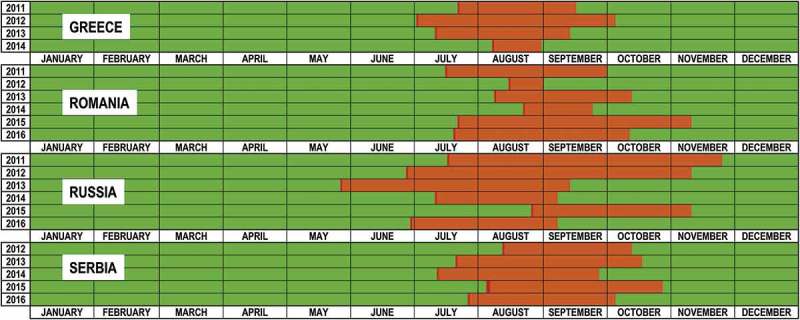
In dark red dates when reporting of new cases start for different countries and years. In orange, period between first and last report for that country and year (proxy of the period of WNV transmission). Only the first case in each area is available in the ECDC tables, so the actual date of the last case may be later than reported in the figure.
The relatively short duration of the WNV transmission season evidences that overwintering, whatever the mechanism, needs to allow the virus to survive many months in the absence of transmission by vectors. WNV infection in some bird species seems to result in the persistence of the virus in some tissues (even several months) after the initial infection [34]. Experimental infections in different bird species demonstrated that birds may become infected by ingestion of dead animals (mammals and birds infected with WNV), and that subsequent high level viremia was observed [35]. Therefore, combination of persistent infection and predation may offer a possible mechanism for WNV overwintering. Also, in case of persistent infection and immune impairment of the host, a recrudescence of the viremia may occur, offering an alternative explanation for overwintering [36]. On the other hand, WNV persistence in overwintering female mosquitoes in frost-free places has also been demonstrated in Europe [37]; this is the most likely way how the virus ‘survives’ the European winter.
In Russia, higher temperatures in May-June, and (to a lesser extent) in August-September were associated with an increase of human WND incidence [38]. Studies carried out in different countries affected by WNV outbreaks (France, Italy, Serbia and Greece) evidenced a strong positive correlation between the mean temperatures and the abundance of Cx. pipiens, and therefore also the risk of WNV transmission [39].
Areas of WNV transmission in EE. Results from official reporting in horses, or seroprevalence studies carried out in birds and horses, evidence that in some cases WNV transmission occurred in areas where human cases were not reported. In fact, while ECDC data includes both probable and confirmed cases, it should be taken into account that the majority of people infected with WNV are asymptomatic or show mild-nonspecific symptoms, which are unlikely to be detected, and that may result in the underestimation of the level of infection in the human population.
The data indicates that in EE large WNV human epidemics occurred in highly populated urban areas located such as Bucharest, Volgograd, Thessaloniki and Belgrade. That resulted in hundreds of WNV human cases in the last few years. In fact, the vast majority of WNV human cases in Europe have occurred in EE. The drivers of human epidemics, in particular large epidemics, are not clear.
Usutu virus (USUV)
USUV was isolated for the first time from Culex neavei mosquitoes in South Africa in 1959 [40], and it has dispersed through the African continent [41]. USUV has been repeatedly introduced into Europe by migratory birds over the last decades [42]. In Europe, USUV was identified for the first time in 2001 after a considerable mortality of Eurasian blackbirds (Turdus merula) in Vienna, Austria [43]. However its introduction could be traced back to at least 1996, when USUV was identified retrospectively in a bird die-off event in the Tuscany region (Italy) [44]. In subsequent years, USUV was detected in neighbouring countries, including Italy, Germany, Spain, Hungary, Switzerland, Poland, England, Czech Republic, Belgium, Serbia and Greece [45,46]. Clinical disease in humans associated to USUV infections have been reported both in Africa and Europe, with symptoms ranging from fever and rash to meningoencephalitis [47]. Recently, USUV was also detected in blood donations in Austria [48]. Of the EE countries included in the study, one serum of domestic pigeon was positive for neutralizing antibodies directed against USUV in Veria city (Central Greece) in November 2010, suggesting the virus circulation in that region, although the geographical spread of USUV within Greece is still unknown [49]. In 2015, USUV specific IgG antibodies were detected in 7 (out of 93) healthy persons subjected to routine serological tests in the South Bačka district of northern Serbia, confirming the circulation of the virus in the area [50]. USUV circulation both in Greece and in Serbia occurred in areas and periods in which WNV was also circulating. The effect that coinfections of different arboviruses, in both the hosts and in the vectors, may have, deserves further attention [19]. The recurrence of USUV in several European countries suggest the establishment of an endemic cycle in some of the affected areas [46,51,52], possibly through overwintering mosquitoes [53].
Dengue virus (DENV)
Dengue is the most widespread arboviral disease affecting humans, and it is caused by four dengue virus serotypes (DENV 1–4) [54]. The historical origin of DENV remains unclear. It is present mostly in the tropics and subtropics, being endemic in more than 100 countries in south-eastern Asia, the Americas, the western Pacific, Africa and the eastern Mediterranean regions [54]. Additionally, DENV is considered one of the most important arboviruses because of the high morbidity it causes: estimated in more than 390 million cases per year [55]. The primary vectors of DENV are Ae. aegypti and Ae. albopictus. The infection with any of the DENV serotypes can be either asymptomatic (in 75% of the cases), or result in one of the three clinical forms with increasing severity: dengue fever, dengue hemorrhagic fever, and dengue shock syndrome.
A disease clinically compatible with dengue was reported in Athens in 1928. This would have been the last major dengue epidemic in Europe, with roughly 1 million cases and 1000 deaths [56]. Since then, no transmission of Dengue was reported in Europe until 2010, when one autochthonous Dengue case were recorded in Croatia [57] and two other cases in France [58]. In 2012, after the identification of two autochthonous dengue fever cases, up to 1,891 cases were reported in the Portuguese island of Madeira [59]. Since DENV usually produces asymptomatic infection, it is possible that it may have been circulating in other European countries with no clinical manifestations. In fact, data from blood donors in Turkey indicate exposure to DENV despite the lack of reporting of clinical cases [60]. Considering the increasing frequency of dengue epidemics worldwide and the growing number of people travelling abroad, the number of DENV-infected viraemic travellers arriving to EE is likely to increase in the near future. That, and the presence of vectors competent for DENV transmission (both Ae. aegypti and Ae. albopictus) in large areas of south-eastern Europe, which are likely to expand, indicate that autochthonous cases are likely to occur in the area.
Orthobunyaviruses (genus Orthobunyavirus, family Peribunyaviridae)
Bunyamwera orthobunyavirus (BUNV)
BUNV has also been known as Batai or Calovo virus in EE. BUNV was first isolated in 1943 from Aedes mosquitoes in Uganda [61]. In the 1970s, BUNV was detected in An. maculipennis s.l. in Ukraine and Moldova [7,8]. In humans, BUNV has been associated with influenza-like illness accompanied by malaise, myalgia, and anorexia [62,63]. Vertebrate hosts of BUNV include pigs, horses and ruminants, but it has also been isolated from different species of birds. BUNV is thought to be pathogenic for sheep and goats, likely to cause stillbirths and congenital abnormalities, and therefore may be relevant to monitor it when those problems are detected in domestic ruminants in Europe [8]. In the European area of Russia, antibodies in humans and in bovine sera have been found in the Saratov province [64,65] and in the estuary of Kuban River [66]. Molecular analysis of BUNV have demonstrated that the strains isolated in Russia grouped into a European cluster together with isolates from Ukraine and the Czech Republic, and they were distinct to Asian and African strains [67,68]. While serological data indicate that BUNV is endemic in different regions of EE [8], a broader geographical distribution of BUNV remains without confirmation by virus isolation. Further research on pathogenesis and transmission of BUNV to animals and humans is necessary to better understanding its epidemiology.
California encephalitis orthobunyavirus (CEV)
CEV has also been historically known as Tahyna virus, Snowshoe Hare virus and Inkoo virus in EE. CEV was originally isolated from Aedes vexans and Aedes caspius in Slovakia in 1958 [69]. Later it was also isolated from mosquitoes in Moldova [7]. This was the first mobovirus pathogenic for humans isolated in Europe [8]. Human disease caused by CEV, called ‘Valtice fever’ is an influenza-like illness affecting mainly children. CEV has been isolated from mosquitoes, humans and rodents in the Central and southern territories of the Russian plain situated in the southern taiga, mixed forest, broad-leaved forest, forest-steppe, steppe and semiarid zones [70]. Cases of CEV were documented in the 1980s and 1990s in the European area of Russia, Ukraine [71] and Serbia and Montenegro [72]. The disease has a well apparent seasonal pattern (July-August) [73]. The highest infection rate of mosquitoes was observed at the end of the epidemic season in all regions [74]. Human antibodies to CEV have been found in Rumania [75] and in Saratov province (Russia) [64]. The vertebrate hosts of CEV are lagomorphs (hares and rabbits), hedgehogs, and rodents [8]. However, virus-neutralizing antibodies to CEV have been found in gulls, terns and in bald-coots in Russia [66] as well as in wild ungulates in Romania, Hungary and Austria [76]. Animal disease caused by CEV is unknown in EE.
Turlock orthobunyavirus (TURV)
TURV has also been known as Lednice virus in EE. Its pathogenicity for humans and animals is still unknown. Presence of antibodies to TURV was evaluated in migratory birds of the Danube Delta (Romania), and from humans and domestic birds of three Romanian counties [77]. Antibodies were detected only in migratory birds, especially in wild geese and ducks, but because these migrate, a geographic source of transmission remains unknown [77]. Regarding vectors, Culex modestus was implicated in TURV transmission in Slovakia [78]. More information on TURV biology would be needed to determine its potential impact in animals and humans.
Phleboviruses (genus Phlebovirus, family Phenuiviridae)
Rift valley fever phlebovirus (RVFV)
RVFV was described for the first time in 1931 in Kenya [79]. Since then, RVFV caused large animal and human epidemics in several African countries [80]. RVFV infection of animals may occur by the bite of an infected mosquito (mainly of the Culex or Aedes genera), or through direct contact with infected animal tissues or fluids [81]. The pathogenesis of the disease varies depending on breed, species and age. Newborn lambs, goats and calfs frequently develop an acute/hyperacute form of the disease with high mortalities (up to 100%) [82]. Older sheep, goats and cattle are more resistant to clinical disease, but can exhibit high fever, anorexia, depression, lymphadenitis, vomiting, weakness, nasal and ocular discharges, diarrhoea, abortions at any stage of pregnancy, and necrotic hepatitis (mortality rates 10–30%). Camels and wild ruminants usually develop subclinical infections; however, the disease can lead to sudden mortality and abortions [82,83]. In humans, the disease causes a flu-like syndrome in most infected individuals, but can also cause severe encephalitic or haemorrhagic forms and even death [84]. In 2000, RVFV was first reported outside Africa, in Saudi Arabia and Yemen [85]. Serological evidence for RVFV infection was also detected in Turkey: one out of 71 camel samples and 35 out of 410 buffalo samples from 2000 and 2001 were positive for RVFV-specific antibodies [86]. It was suggested that RVF infection was introduced into Turkey at the time of outbreaks in Saudi Arabia and Yemen through uncontrolled movement of viraemic domestic or wild animals or vectors, through the southern border with Syria and Iraq. No clinical or suspected RVF cases were reported in humans or animals in Turkey, but RVFV can circulate with mild or even no clinical signs, remaining undetected in an area, and emerge when eco-climatic conditions are favourable for transmission [87]. EE is at risk of RVF because of both the presence of competent vectors and the climatic conditions in the area, which are favourable for RVFV transmission [88].
Alphaviruses (genus Alphavirus, family Togaviridae)
Sindbis virus (SINV)
SINV was first isolated in 1952 from Cx. pipiens and Cx. univittatus mosquitoes captured in the Sindbis Health District, north of Cairo [89]. SINV has a worldwide distribution including Eurasia, Africa, and Oceania. It is transmitted among its natural bird hosts, mainly Passeriformes, via mosquitoes. In birds, the virus can cause sporadic illness (pigeon-encephalitis) and irregular deaths in old chickens [8]. SINV is thought to have entered Europe from Africa through Israel, reflecting migratory bird pathways [6]. First European SINV isolation was reported from a reed warbler caught in Slovakia in 1971 [90]. Human disease caused by SINV infection has been reported mainly in South Africa and in Northern Europe. The disease has different names depending on the region affected. In north-western Russia, it was known as ‘Karelian’ fever [91]. Symptoms include fever, malaise, rash and musculoskeletal pain. In a significant portion of patients, the debilitating musculoskeletal symptoms persist for years [92]. Silent circulation in more recent years should not be discarded since most symptoms of SINV infection are compatible with other human infectious diseases. Antibodies to SINV were detected in Saratov province (Russia) in the end-1990s [60]. The most recent report of SINV circulation in EE was from the Kuvan River, in the north-west Caucasus region of Russia in 2006–2007. In this wetland, virus-neutralizing antibodies to SINV were found in European hares and in herons [66].
Ecological/biological aspects of the mosquito vectors of arboviruses in EE
The capacity of a pathogen to transmit within a population is frequently measured by the basic reproduction number (R0), defined as the number of secondary cases which one case would produce in a completely susceptible population. R0 varies considerably for different infectious diseases but also for the same disease in different populations [93]. For vector-borne diseases, R0 is typically estimated according to the Ross-MacDonald formula [94], which takes into account: the ratio of mosquitoes to hosts, the rate at which mosquitoes bite the hosts, the daily probability of survival for mosquitoes, the vector competence, the extrinsic incubation period, the host infectiousness, and the host’ recovery rate. Therefore, in order to estimate the risk of transmission posed by a mobovirus, it is essential to understand all the biological and ecological factors that drive viral transmission, and which include the following factors related to the vectors: (i) abundance and dynamics during the season; (ii) biting behaviour; (iii) dispersal capacity; (iv) vector competence; (v) availability and type of larval breeding sites. Knowledge of those parameters is also crucial for efficient vector control. In the present manuscript, we highlight the main biological and ecological aspects of the mosquito species present in EE, to better estimate the risk of mosquito-borne transmission in the region.
Nine mosquito species: Culex (Culex) pipiens, Culex (Barraudius) modestus, Culex (Culex) perexiguus, Culex (Culex) quinquefasciatus, Aedes (Aedimorphus) vexans, Aedes (Ochlerotatus) caspius, Aedes (Stegomyia) albopictus, Aedes (Stegomyia) cretinus, Aedes (Stegomyia) aegypti were considered as the main mosquito species (on the basis of their proven or potential role as vectors of moboviruses in EE), and their distribution is shown in Table 3.
Table 3.
Distribution of the nine main mosquito species vectors of moboviruses in EE.
| Albania | Belarus | Bulgaria | Greece | Kosovo | Macedonia | Moldova | Montenegro | Romania | Russia | Serbia | Ukraine | Turkey | TOTAL | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | Sub-genus | Species | ||||||||||||||
| Culex | Culex | pipiens | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
| Culex | Barraudius | modestus | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 12 |
| Culex | Culex | perexiguus | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 5 |
| Culex | Culex | quinquefasciatus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Aedes | Aedimorphus | vexans | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
| Aedes | Ochlerotatus | caspius | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
| Aedes | Stegomyia | albopictus | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 9 |
| Aedes | Stegomyia | cretinus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 |
| Aedes | Stegomyia | aegypti | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| Total out of 9 vector species present in the country: | 6 | 4 | 6 | 7 | 3 | 5 | 4 | 5 | 5 | 7 | 6 | 4 | 9 | |||
Culex (culex) pipiens linnaeus 1758
Culex pipiens is widespread in the Holarctic region, and it is found in all 13 countries of EE included in the study (Table 3). This species comprises two morphologically identical biotypes, pipiens and molestus, as well as their hybrids. No comprehensive data of the occurrence of biotypes pipiens, molestus and their hybrids throughout EE region is available. The two biotypes and the hybrid form of Cx. pipiens are all morphologically similar to Cx. torrentium with whom are sympatric in much of Central, Eastern and Northern Europe [95]. Cx. pipiens larvae can inhabit nearly every kind of water collection, artificial and natural, even tolerating a small amount of salinity. This species can develop up to several generations per year depending on climatic conditions.
From the data obtained in European countries, Cx. pipiens is the mosquito species most commonly associated with both WNV [96] and USUV [97–99].
Culex pipiens biotype pipiens linnaeus 1758
Culex pipiens biotype pipiens is strongly avian-seeking (ornithophilic) and therefore plays a major role in both WNV and USUV transmission [100].
Females overwinter in diapause. In the spring, when average daily temperature rises above 10°C, females search for blood and lay their eggs on the water surface glued in an egg raft of 150–240 eggs. The larvae hatch within one or two days and complete their development to adults in between one week and one month depending on the temperature.
Culex pipiens biotype molestus forskal 1775
The biotype molestus occurs more frequently in human settlements. The most common larval habitats are road drains, flooded basements, underground sewage constructions, and other human-made water containers. The biotype molestus is mammalophilic and bites humans both indoors and outdoors, after sunset and during the night. As it is autogenous, it may lay eggs without a blood meal, although much fewer (10–50 per egg raft) when it feeds on blood. It can reproduce throughout the winter in warm urban habitats containing water, or may overwinter in human-made shelters. There are records of biotype molestus even from very northern cities in the European parts of Russia.
Hybrids between the two biotypes have an intermediate host preference and are therefore ideal bridge vectors of WNV and USUV from birds to mammals [100]. In terms of vector competence, European Cx. pipiens hybrid forms have been shown to be able to transmit WNV [101] and RVFV [102].
Culex (barraudius) modestus ficalbi 1889
Culex modestus has been reported in 12 out of 13 EE countries (Table 3). However, its distribution throughout the EE region is patchy and usually limited to fresh or slightly saline waters of marshes, irrigation canals and inundation areas of rivers and rice fields. Dispersal ability of Cx. modestus is very low, so the aggressive females remain localized around larval breeding sites [103], which diminishes its importance in the transmission of moboviruses to humans in semi-urban and urban surroundings. However, it could serve as important enzootic and a bridge vector in natural/rural wetland environments across EE.
The seasonal maximum of the adult population might occur from the beginning of July to late September depending on weather conditions. The females readily bite humans outdoors, close to the breeding sites, often during the day, even at sun and in wind-exposed places [104]. Balenghien and collaborators [105] regarded Cx. modestus as the primary vector of WNV in wetland areas of southern France after demonstrating high dissemination and transmission rates of the virus in the laboratory, and considering its biting behaviour (ornithophilic and mammalophilic). Apart from WNV, this species has been implicated as a vector of CEV and TURV [78].
Culex (culex) perexiguus theobald 1903
Culex perixiguus species has been found in 5 out of 13 EE countries (Table 3). The larvae can be found in many kinds of stagnant water collections, from clean to moderately polluted swamps, ponds, streams, pools, wells, usually with emergent vegetation, and occasionally in human-made containers. The species is common during summer and autumn in natural/rural habitats.
Cx. perexiguus has been implicated in WNV circulation since they feed mainly on birds, although it may also be implicated in WNV transmission to horses, since horse blood has also been identified in this species [106]. On the other hand, Gad and collaborators [107] indicated that Cx. perexiguus may have served as a bridge vector of RVFV between bovines and humans in Egypt, since mixed blood meals from humans and RVFV susceptible animals were identified in Cx. perexiguus.
Culex (culex) quinquefasciatus say 1823
Culex quinquefasciatus, the southern house mosquito, is one of the most troublesome mosquitoes in the world, and it is widespread throughout the tropics and subtropics [104]. Of the countries included in the study, it has only been recorded in Turkey (Table 3). In 2015, Gunay and collaborators [108] recorded the wide geographical distribution of Cx. quinquefasciatus across most of southern Turkey, while hybrids between this species and Cx. pipiens had been detected in the Greek island of Kos, close to the Turkish coast in 2012 [109].
The larvae breed in any artificial and natural water collection, ranging from fresh and clear to saline and polluted. The females are ferocious and bite humans at night, both indoors and outdoors. They also frequently attack birds and, to a lesser extent, domestic animals, such as dogs, cats, and pigs. The range of hosts varies depending on the characteristics of the local population. The females develop throughout the whole year [110].
Cx. quinquefasciatus is incriminated as primary vector of WNV [111], and therefore the expansion of Cx. quinquefasciatus and its hybrids north and west of current distribution represents a new threat to EE countries.
Aedes (aedimorphus) vexans (meigen 1830)
Aedes vexans is a native species widely distributed through EE (present in all 13 countries, see Table 3). They may have several generations per year depending on the number of water fluctuations, and when the temperature of the water in the breeding sites exceeds 9°C [104]. The preferred breeding sites are floodplains of rivers and lakes holding temporary water bodies. The females lay the eggs 5–8 days after the blood meal into the ground that is subjected to flooding. In temperate climates, egg diapause lasts from September to March. Frost toleration of eggs is extraordinary; they can survive several days with temperatures below −10°C. The hatching rate is particularly elevated at high water temperatures. If there are no water fluctuations above the level where the eggs were laid during following season, the eggs can survive for a long time (more than five years). Ae. vexans frequently emerges en masse during the summer months depending on the water fluctuations, and is often the most critical nuisance mosquito around rivers and lakes. The females usually disperse long distances from their breeding sites (10–30 km).
Ae. vexans are mainly mammalophilic and have been involved in the transmission of CEV [112]. Even though it has been found naturally infected with WNV in Serbia [113], its role in WNV transmission is not clear. In the north-eastern United States Ae. vexans was described as a vector of minor importance for WNV transmission [111]. However, in the laboratory assays, WNV vector competence of Ae. vexans was similar to that of Cx. pipiens. In addition, CEV was isolated from Ae. vexans in South Moravia (Czech Republic) [114], and Ae. vexans was implicated in RVFV epidemiology in Senegal and Saudi Arabia [115]. Recently, O’Donnell and collaborators [116] found that Ae. vexans was able to transmit ZIKV, which together with its abundance and capacity for multiple feeding on humans, may result in a high vectorial capacity for ZIKV.
Aedes (ochlerotatus) caspius (pallas 1771)
Aedes caspius is a native species and widely distributed through EE (present in all 13 countries, see Table 3). Ae. caspius may develop several generations per year depending on the characteristics of the breeding site but sometimes only one generation per year is produced. Similar to Ae. vexans, the species overwinters in the egg stage. Eggs hatch after submersion with water due to the fluctuation of the water level in a breeding site. The hatching time in spring varies with the latitude, and occurs in March in most of the countries of EE. The larvae develop in freshwater bodies, but also in saline water common in Mediterranean coastal marshes [104]. The females feed on humans and animals both in rural and urban areas. They are crepuscular feeders, most actively searching for a blood meal at dusk, but may bite during the day and night. They normally feed outdoors, but may feed in houses and animal shelters when abundant. Females hunt for blood at temperatures ranging from 11.5 to 36°C and relative humidity from 47 to 92%, and may disperse up to 10 km [117,118].
Both WNV and CEV were detected in natural populations of Ae. caspius in Russia [119]. Ae. caspius was found to be an inefficient vector of WNV in the laboratory and, despite its high densities, was assigned a minor role in WNV transmission in southern France [105]. Gad and collaborators [107] indicated that Ae. caspius may have served as a bridge vector of RVFV between bovines and humans in Egypt. However, it was found to be much less efficient in the dissemination of RVFV in laboratory conditions than Cx. pipiens [120].
Aedes (stegomyia) albopictus (skuse1895)
Aedes albopictus is native to south-east Asia and Oceania, therefore its popular name, the ‘Asian tiger mosquito’. Its worldwide spread is considered to have started in Albania where it was introduced in 1979, or even before [121]. In 1985, it was discovered in Houston, Texas (the first in America), which was the beginning of its rapid spread to other parts of the world [122]. Since 1999 Ae. albopictus has been introduced and established in 30 European countries according to ECDC, including nine of the 13 countries included in our study (Table 3). Local climatic conditions combined with passive dispersal mechanisms are likely to result in further expansion of Ae. albopictus to naive EE countries [113,123]. Even though they are weak flyers, Ae. albopictus can spread rapidly within countries through passive mechanisms [124]. In Europe, this species is confined to human settlements, and the immature stages breed in a variety of small natural and artificial containers. In temperate climatic zones of EE, populations of Ae. albopictus overwinter in the cold-resistant egg stage. The eggs are also resistant to desiccation, which facilitates their transport around the world. Adult females predominantly feed on humans, but may also bite other mammals and occasionally birds [104]. Such feeding behaviour makes Ae. albopictus an excellent vector of a variety of arboviruses that use mammals and birds as their reservoir hosts [122]. The females bite man during the daytime, outdoors in urban and semi-urban environments, but may also feed inside houses during the day and night.
Ae. albopictus is a competent vector to 26 arboviruses in laboratory conditions [125] including WNV [101] and RVFV [102]. It has also been found infected with USUV in the field [126]. Ae. albopictus was in 2007, the first invasive mosquito species involved in transmitting an exotic virus in Europe (CHIKV in Italy [127]. Later on, Ae. albopictus was also implicated in the transmission of CHIKV in France in 2010 [128] and 2014 [129], and also DENV in Croatia in 2010 [57] and in France in 2010 [58], 2013 [130] and 2015 [131].
Aedes (stegomyia) cretinus edwards 1921
Aedes cretinus has been recorded in 3 out of 13 EE countries, although the record of Russia dates from 1931 (Table 3). Abundant populations have been reported in Athens (Greece) [132] and Antalya (Turkey) [133]. Elsewhere it seems to be an uncommon species, although its distribution range could also be underestimated by the preference of Ae. cretinus for rural habitats and to the lack of knowledge on its bio-ecology [134]. No vector competence studies have been carried out on Ae. cretinus, so its vectorial capacity for the different moboviruses is still unknown [135].
Aedes (stegomyia) aegypti (linnaeus 1762)
In Europe, before 1945, all Mediterranean countries and most major port cities had reported at least occasional introductions of Ae. aegypti [122]. It seems that it was eliminated from Europe thanks to the sanitation of urban water collections and the anti-malaria campaigns with DDT during the 1950s. Since then, different countries in EE (e.g. Albania, Greece and Turkey) have sporadically reported Ae. aegypti, although without evidence of established populations [135]. It was not considered established in EE until 2007 when it was found in the Black Sea coast of Russia and in Georgia [124,136]. In 2015, Ae. aegypti was detected in north-eastern Turkey [137], and since then has spread westwards along the Black Sea coast of Turkey (Akiner, personal communication). Currently it is recorded in two out of 13 EE countries studied, Turkey and Russia (Table 3). Its range of distribution is apparently limited by the 10°C January isotherm in the northern hemisphere, although several records from Europe (Brest, Odessa) corresponded to much lower temperatures [138]. It is intriguing that the areas of its reestablishment in Russia, Georgia and north-eastern Turkey are zones with lower January temperatures. If the population introduced in Russia is adapted to colder temperature conditions, then much broader areas of Europe would be at risk of invasion by Ae. aegypti.
In Europe, this species is confined to human settlements. The female lays eggs resistant to desiccation in artificial human-made containers and water recipients of all kinds, both in- and outdoors. Eggs, larvae and adults are not able to overwinter under conditions of temperate climate, but might use warm, protected sites in urban environments (cellars of human houses) that can provide shelters, blood sources and oviposition sites. They might travel west across the Black Sea in artificial water collection sites on ships. The larvae spend a long time underwater feeding on the bottom of their breeding sites, which may make their detection difficult for untrained personnel. At a temperature of 27 to 30°C the adults emerge from the pupae 9–10 days after the eggs have been laid. The females feed predominantly during the day, outdoors in shaded places but can also feed and rest indoors. Human blood seems to be preferred to that of domestic animals, and the females prefer to take multiple blood meals (feeding on more than one person for obtaining full blood meal); and the blood feeding interval is only about 2–4 days [94,124]. Its feeding behaviour makes Ae. aegypti an excellent vector of human arboviruses. Females do not fly over long distances, often not more than several hundred meters away from breeding sites.
Aedes aegypti is the principal vector of yellow fever virus (‘yellow fever mosquito’), but it is also an important vector of DENV, CHIKV and ZIKV, which threaten the EE [6].
Conclusions
The epidemiology of WNV in EE is rather complex with the combination of the repeated introduction of WNV strains from both lineages 1 and 2, but also the establishment of endemic cycles. In many cases those WNV strains overwinter in affected areas and re-emerge in the following season in the same areas after a period that lasts many months, and in some occasions, they expand to neighboring regions. The risk of WNV re-emergence in a given area increases proportionally to the number of human cases in the previous year, which was probably related to the level of viral circulation. This complex scenario resulted in 2873 human cases in the EE countries included in the study between 2010 and 2016.
Other moboviruses reviewed such as BUNV, CEV, TURV, SINV, and even USUV, are considered to be established in EE. However, many aspects of their epidemiology including their current distribution, their vectors and hosts, or their pathogenicity for animals and humans are not clear. The overlapping of different arboviruses in EE is likely to result in coinfections, in both the hosts and in the vectors, the effect of which are unknown.
The case of DENV is a bit different, as it is considered exotic to EE, although some autochthonous cases have been reported in other areas of Europe. However, the risk of epidemics in EE is likely to increase in near future as a result of: a) the proliferation of DENV epidemics worldwide, with an increase in the number of DENV-infected viraemic travelers arriving to EE, and b) to presence of vectors competent for DENV transmission (Ae. aegypti and Ae. albopictus) in large areas of south-eastern Europe. Whether that may result in the establishment of an endemic cycle remains to be seen.
RVFV also has a known zoonotic potential and it has traditionally been considered exotic to EE. However, some serological evidence for RVFV infection in Turkey in 2017, and both the presence of competent vectors and the favourable climatic conditions for RVFV transmission in EE, evidence the need to remain vigilant.
Reported distribution of moboviruses in EE is likely to be biased by the differences in the intensity of surveillance among countries, which is highly influenced by the resources available. For example, in Belarus no circulation of moboviruses has been reported, and in countries such as Kosovo, Macedonia, Albania and Bulgaria, only WNV has been detected.
While the competence of some mosquito species for the transmission of some moboviruses has been clearly demonstrated (for example Culex (Culex) pipiens for WNV transmission), there are many knowledge gaps in relation to the role of several mosquito species present in EE for the transmission of the moboviruses present in the area.
Current expansion of invasive mosquito species, mainly Ae. albopictus but also Ae. aegypti, in EE, very efficient vectors of human moboviruses such as DENV, CHIKV and ZIKV may result in new scenarios for the emergence of those diseases in EE. Additionally, recent detection in Turkey and Greece of Cx. quinquefasciatus or its hybrids, represents a cause for concern, as this species is one of the most troublesome mosquitoes in tropical and subtropical areas of the world, and it is a primary vector of WNV.
As a result of the current increasing trend in many anthropogenic factors such as human population, deforestation, urbanization and movements of people, animals and vectors, as well as, to some extent, the effect of climate change, further spread of moboviruses in EE is to be expected in the future.
Funding Statement
This work was supported by the INFRAVEC2 Project [731060];
Acknowledgments
IRTA is supported by CERCA Programme/Generalitat de Catalunya. This publication was supported by the project, Research Infrastructures for the Control of Vector-borne Diseases (Infravec2), which received funding from the European Union Horizon 2020 Research and Innovation programme.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Vasilakis N, Gubler DJ.. Arboviruses : molecular Biology, Evolution and Control. UK (Poole).: Caister Academic Press; 2016. [Google Scholar]
- [2].Roiz D, Ruiz S, Soriguer R, et al. Climatic effects on mosquito abundance in Mediterranean wetlands. Parasit Vectors. 2014;7:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Petersen LR, Jamieson DJ, Powers AM, et al. Zika virus. N Engl J Med. 2016;374:1552–1563. [DOI] [PubMed] [Google Scholar]
- [4].WHO [Internet]. Yellow Fever - Brazil 2017. [cited 2017 Nov 11]. Available from: http://www.who.int/csr/don/13-january-2017-yellow-fever-brazil/en/
- [5].Hernández-Triana LM, Jeffries CL, Mansfield KL, et al. Emergence of west nile virus lineage 2 in europe: a review on the introduction and spread of a mosquito-borne disease. Front Public Health. 2014;2:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Braack L, Gouveia De Almeida AP, Cornel AJ, et al. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. . Parasit Vectors. 2018;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Failloux AB, Bouattour A, Faraj C, et al. Surveillance of Arthropod-Borne Viruses and Their Vectors in the Mediterranean and Black Sea Regions Within the MediLabSecure Network. Curr Trop Med Rep. 2017;4(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hubálek Z. Mosquito-borne viruses in Europe. Parasitol Res. 2008;103(Suppl 1):S29–43. [DOI] [PubMed] [Google Scholar]
- [9].Calistri P, Giovannini A, Hubalek Z, et al. Epidemiology of West Nile in Europe and in the Mediterranean basin. Open Virol J. 2010;4:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Komar N, Panella NA, Boyce E. Exposure of domestic mammals to West Nile virus during an outbreak of human encephalitis, New York City, 1999. Emerg Infect Dis. 2001;7(4):736–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tsai TF, Popovici F, Cernescu C, et al. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352(9130):767–771. [DOI] [PubMed] [Google Scholar]
- [12].Cernescu C, Nedelcu NI, Tardei G, et al. Continued transmission of West Nile virus to humans in southeastern Romania, 1997–1998. J Infect Dis. 2000;181(2):710–712. [DOI] [PubMed] [Google Scholar]
- [13].Di Sabatino D, Bruno R, Sauro F, et al. Epidemiology of West Nile disease in Europe and in the Mediterranean Basin from 2009 to 2013. Biomed Res Int. 2014;2014:907852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Platonov AE, Shipulin GA, Shipulina OY, et al. Outbreak of West Nile virus infection, Volgograd Region, Russia, 1999. Emerg Infect Dis. 2001;7(1):128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Platonov AE, Karan LS, Shopenskaia TA, et al. [Genotyping of West Nile fever virus strains circulating in southern Russia as an epidemiological investigation method: principles and results]. Zh Mikrobiol Epidemiol Immunobiol.2011;(2):29–37. Russian. [PubMed] [Google Scholar]
- [16].Bakonyi T, Ivanics E, Erdélyi K, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12(4):618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bakonyi T, Ferenczi E, Erdélyi K, et al. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet Microbiol. 2013;165(1–2):61–70. [DOI] [PubMed] [Google Scholar]
- [18].Wodak E, Richter S, Bagó Z, et al. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet Microbiol. 2011;149(3–4):358–366. [DOI] [PubMed] [Google Scholar]
- [19].Rizzoli A, Jimenez-Clavero MA, Barzon L, et al. The challenge of West Nile virus in Europe: knowledge gaps and research priorities. Euro Surveill. 2015;20(20):pii: 21135. [DOI] [PubMed] [Google Scholar]
- [20].Ciccozzi M, Peletto S, Cella E, et al. Epidemiological history and phylogeography of West Nile virus lineage 2. Infect Genet Evol. 2013;17:46–50. [DOI] [PubMed] [Google Scholar]
- [21].Papa A, Perperidou P, Tzouli A, et al. West Nile virus–neutralizing antibodies in humans in Greece. Vector Borne Zoonotic Dis. 2010;10(7):655–658. [DOI] [PubMed] [Google Scholar]
- [22].Papa A, Bakonyi T, Xanthopoulou K, et al. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg Infect Dis. 2011;17(5):920–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chaskopoulou A, Dovas C, Chaintoutis S, et al. Evidence of enzootic circulation of West Nile virus (Nea Santa-Greece-2010, lineage 2), Greece, May to July 2011. Euro Surveill. 2011;16(31):pii: 19933. [PubMed] [Google Scholar]
- [24].Chaintoutis SC, Chaskopoulou A, Chassalevris T, et al. West Nile virus lineage 2 strain in Greece, 2012. Emerg Infect Dis. 2013;19(5):827–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barzon L, Papa A, Lavezzo E, et al. Phylogenetic characterization of Central/Southern European lineage 2 West Nile virus: analysis of human outbreaks in Italy and Greece, 2013–2014. Clin Microbiol Infect. 2015;21(12):1122.e1–10. [DOI] [PubMed] [Google Scholar]
- [26].Petrović T, Blazquez AB, Lupulović D, et al. Monitoring West Nile virus (WNV) infection in wild birds in Serbia during 2012: first isolation and characterisation of WNV strains from Serbia. Euro Surveill. 2013;18(44):pii: 20622. [DOI] [PubMed] [Google Scholar]
- [27].Baymakova M, Trifonova I, Panayotova E, et al. Fatal Case of West Nile Neuroinvasive Disease in Bulgaria. Emerg Infect Dis. 2016;22(12):2203–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Monaco F, Çizmeci Ş, Polci A, et al First evidence of West Nile virus lineage 2 circulation in Turkey. Vet Ital. 2016;52(1):77–81. [DOI] [PubMed] [Google Scholar]
- [29].Sirbu A, Ceianu CS, Panculescu-Gatej RI, et al. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro Surveill. 2011;16(2):pii:19762. [PubMed] [Google Scholar]
- [30].Dinu S, Cotar AI, Pănculescu-Gătej IR, et al. West Nile virus circulation in South-Eastern Romania, 2011 to 2013. Euro Surveill. 2015;20(20):pii: 21130. [DOI] [PubMed] [Google Scholar]
- [31].Kolodziejek J, Marinov M, Kiss BJ, et al. The complete sequence of a West Nile virus lineage 2 strain detected in a Hyalomma marginatum marginatum tick collected from a song thrush (Turdus philomelos) in eastern Romania in 2013 revealed closest genetic relationship to strain Volgograd 2007. PLoS One. 2014;9(10):e109905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kalaycioglu H, Korukluoglu G, Ozkul A, et al. Emergence of West Nile virus infections in humans in Turkey, 2010 to 2011. Euro Surveill. 2012;17(21):pii: 20182. [PubMed] [Google Scholar]
- [33].Ergunay K, Bakonyi T, Nowotny N, et al. Close relationship between West Nile virus from Turkey and lineage 1 strain from Central African Republic. Emerg Infect Dis. 2015;21(2):352–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bakonyi T, Gajdon GK, Schwing R, et al. Chronic West Nile virus infection in kea (Nestor notabilis). Vet Microbiol. 2016;183:135–139. [DOI] [PubMed] [Google Scholar]
- [35].Komar N, Langevin S, Hinten S, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9(3):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pérez-Ramírez E, Llorente F, Jiménez-Clavero MA. Experimental infections of wild birds with West Nile virus. Viruses. 2014;6(2):752–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rudolf I, Betášová L, Blažejová H, et al. West Nile virus in overwintering mosquitoes, central Europe. Parasit Vectors. 2017October2;10(1):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Platonov AE, Tolpin VA, Gridneva KA, et al. The incidence of West Nile disease in Russia in relation to climatic and environmental factors. Int J Environ Res Public Health. 2014;11(2):1211–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Groen TA, L’Ambert G, Bellini R, et al. Ecology of West Nile virus across four European countries: empirical modelling of the Culex pipiens abundance dynamics as a function of weather. Parasit Vectors. 2017;10(1):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McIntosh BM. Usutu (SA Ar 1776), nouvel arbovirus du groupe B. International Catalogue of Arboviruses. 1985;3:1059–1060. [Google Scholar]
- [41].Nikolay B, Diallo M, Boye C, et al. Usutu virus in Africa. Vector Borne Zoonotic Dis. 2011;11(11):1417–1423. [DOI] [PubMed] [Google Scholar]
- [42].Engel D, Jöst H, Wink M, et al. Reconstruction of the Evolutionary History and Dispersal of Usutu Virus, a Neglected Emerging Arbovirus in Europe and Africa. mBio. 2016Jan-Feb;7(1):e01938–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weissenböck H, Kolodziejek J, Url A, et al. Emergence of Usutu virus, an African Mosquito-Borne Flavivirus of the Japanese Encephalitis Virus Group, Central Europe. Emerg Infect Dis. 2002;8(7):652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Weissenböck H, Bakonyi T, Rossi G, et al. Usutu virus, Italy, 1996. Emerg Infect Dis. 2013;19(2):274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bakonyi T, Busquets N, Nowotny N. Comparison of complete genome sequences of Usutu virus strains detected in Spain, Central Europe, and Africa. Vector Borne Zoonotic Dis. 2014;14(5):324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ashraf U, Ye J, Ruan X, et al. Usutu virus: an emerging flavivirus in Europe. Viruses. 2015;7(1):219–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pecorari M, Longo G, Gennari W, et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Euro Surveill. 2009December17;14(50):19446. [PubMed] [Google Scholar]
- [48].Bakonyi T, Jungbauer C, Aberle SW, et al. Usutu virus infections among blood donors, Austria, July and August 2017 - Raising awareness for diagnostic challenges. Euro Surveill. 2017;22:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chaintoutis SC, Dovas CI, Papanastassopoulou M, et al. Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Comp Immunol Microbiol Infect Dis. 2014;37(2):131–141. [DOI] [PubMed] [Google Scholar]
- [50].Cvjetković IH, Petrović T, Petrić D, et al. Seroprevalence of mosquito-born and tick-born microorganisms in human population of South Backa District. Arhiv Veterinarske Medicine. 2016;9(1):23–30. [Google Scholar]
- [51].Cadar D, Lühken R, Van Der Jeugd H, et al. Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Euro Surveill. 2017;22(4):pii: 30452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bakonyi T, Erdélyi K, Brunthaler R, et al. Usutu virus, Austria and Hungary, 2010–2016. Emerg Microbes Infect. 2017;6(10):e85 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vazquez A, Jimenez-Clavero M, Franco L, et al. Usutu virus: potential risk of human disease in Europe. Euro Surveill. 2011;16(31):pii:19935. [PubMed] [Google Scholar]
- [54].Guzman MG, Harris E. Dengue. Lancet. 2015;385(9966):453–465. [DOI] [PubMed] [Google Scholar]
- [55].Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rosen L. Dengue in Greece in 1927 and 1928 and the pathogenesis of dengue hemorrhagic fever: new data and a different conclusion. Am J Trop Med Hyg. 1986;35(3):642–653. [DOI] [PubMed] [Google Scholar]
- [57].Schmidt-Chanasit J, Haditsch M, Schoneberg I, et al. Dengue virus infection in a traveller returning from Croatia to Germany. Euro Surveill. 2010;15(40):pii:19677. [DOI] [PubMed] [Google Scholar]
- [58].La Ruche G, Souarès Y, Armengaud A, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15(39):19676. [PubMed] [Google Scholar]
- [59].Sousa CA, Clairouin M, Seixas G, et al. Ongoing outbreak of dengue type 1 in the Autonomous Region of Madeira, Portugal: preliminary report. Euro Surveill. 2012;17(49):pii:20333. [DOI] [PubMed] [Google Scholar]
- [60].Ergünay K, Saygan MB, Aydoğan S, et al. West Nile virus seroprevalence in blood donors from Central Anatolia, Turkey. Vector Borne Zoonotic Dis. 2010;10(8):771–775. [DOI] [PubMed] [Google Scholar]
- [61].Smithburn KC, Haddow AJ, Mahaffy AF. A neurotropic virus isolated from Aedes mosquitoes caught in the Semliki forest. Am J Trop Med Hyg. 1946;26:189–208. [DOI] [PubMed] [Google Scholar]
- [62].Bárdos V, Sluka F, Cupkova E, et al. Serological study on the medical importance of Calovo virus In: Bardos V, ed. Arboviruses of the California complex and the Bunyamwera group. Bratislava: Slovak Academy of Science; 1969. p. 333–336. [Google Scholar]
- [63].Lundström JO.Mosquito-borne viruses in western Europe: a review. J Vector Ecol. 1999;24(1):1–39. [PubMed] [Google Scholar]
- [64].Shcherbakova SA, Vyshemirskiĭ OI, Liapin MN. [A circulation study of California serogroup and Batai viruses (fam. Bunyaviridae, genus Bunyavirus) on the territory of Saratov Province]. Med Parazitol (Mosk).. 1997;(1):51–52. Russian. [PubMed] [Google Scholar]
- [65].Shcherbakova SA, Golovinskaia ON, Kliueva EV, et al. [Anti-arbovirus antibodies positive serum screening in population of Saratov]. Vopr Virusol. 2002;47(3):32–34. Russian. [PubMed] [Google Scholar]
- [66].L’vov DK, Shchelkanov M, Kolobukhina LV, et al. [Serological monitoring of arbovirus infections in the estuary of the Kuban River (the 2006–2007 data)]. Vopr Virusol. 2008;53(4):30–35. [PubMed] [Google Scholar]
- [67].Terekhin SA, Grebennikova TV, Khutoretskaia NV, et al. [Molecular-genetic analysis of the Batai virus strains isolated from mosquitoes in Volgograd Region of the Russian Federation, West Ukraine, and Czech Republic]. Mol Gen Mikrobiol Virusol. 2010;1:27–29. Russian [PubMed] [Google Scholar]
- [68].Dufkova L, Pachler K, Kilian P, et al. Full-length genome analysis of Čalovo strains of Batai orthobunyavirus (Bunyamwera serogroup): implications to taxonomy. Infect Genet Evol. 2014;27:96–104. [DOI] [PubMed] [Google Scholar]
- [69].Bardos V, Danielova V. The Tahyna virus-a virus isolated from mosquitoes in Czechoslovakia. J Hyg Epidemiol Microbiol Immunol. 1959;3:264–276. [PubMed] [Google Scholar]
- [70].L’vov DK, Gromashevskiĭ VL, Skvortsova TM, et al. [Circulation of viruses of the California serocomplex (Bunyaviridae, Bunyavirus) in the central and southern parts of the Russian plain]. Vopr Virusol. 1998;43(1):10–14. Russian. [PubMed] [Google Scholar]
- [71].Lozyns’kyĭ IM, Vynohrad IA. [Arboviruses and arbovirus infections in the forest steppe zone of Ukraine]. Mikrobiol Z. 1998;60(2):49–60. Ukranian. [PubMed] [Google Scholar]
- [72].Vesenjak-Hirjan J, Punda-Polić V, Dobe M. Geographical distribution of arboviruses in Yugoslavia. J Hyg Epidemiol Microbiol Immunol. 1991;35(2):129–140. [PubMed] [Google Scholar]
- [73].Kolobukhina LV, L’vov SD. [Arborviruses of the California encephalitis serogroup 1N Russia and their contribution to infectious pathology]. Vestn Ross Akad Med Nauk. 2011;(5):41–45.Russian. [PubMed] [Google Scholar]
- [74].L’vov SD, Gromashevskiĭ VL, Morozova TN, et al. [Distribution of viruses from the Californian encephalitis serogroup (Bunyaviridae, Bunyavirus) in the northern expanses of Russia]. Vopr Virusol. 1997;42(5):229–235. Russian. [PubMed] [Google Scholar]
- [75].Drăgănescu N, Gîrjabu E. Investigations on the presence of antibodies to Tahyna virus in Romania. Virologie. 1979;30(2):91–93. [PubMed] [Google Scholar]
- [76].Camp JV, Haider R, Porea D, et al. Serological surveillance for Tahyna virus (California encephalitis orthobunyavirus, Peribunyaviridae) neutralizing antibodies in wild ungulates in Austria, Hungary and Romania. Zoonoses Public Health. 2018;65(4):459–463. [DOI] [PubMed] [Google Scholar]
- [77].Drăgănescu N, Málková D, Girjabu E, et al. Serological investigations into the presence of antibodies to Yaba 1–lednice 110 virus in Romania. Virologie. 1981;32(1):19–21. [PubMed] [Google Scholar]
- [78].Lundström JO. Vector competence of Western European mosquitoes for arboviruses: A review of field and experimental studies. Bull Soc Vector Ecol. 1994;19:23–36. [Google Scholar]
- [79].Daubney R, Hudson JR, Garnham PC. Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from East Africa. J PatholBacteriol. 1931;34:545–579. [Google Scholar]
- [80].Boshra H, Lorenzo G, Busquets N, et al. Rift valley fever: recent insights into pathogenesis and prevention. J Virol. 2011;85(13):6098–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Pepin M, Bouloy M, Bird BH, et al. Rift Valley fever virus (Bunyaviridae: phlebovirus): anupdate on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].FAO [Internet]. Recognizing Rift Valley Fever 2003. [cited 2017 Nov 11]. Available from: http://www.oie.int/fileadmin/Home/esp/Health_standards/tahm/2.01.18_Fiebre_Valle_Rif.pdf
- [83].OIE [internet]. Rift Valley fever 2016. [cited 2017 Nov 11]. Available from: http://www.oie.int/fileadmin/Home/esp/Health_standards/tahm/2.01.18_Fiebre_Valle_Rif.pdf
- [84].CDC [internet] Rift Valley Fever. 2013. [cited 2017 Nov 11]. Available from: https://www.cdc.gov/vhf/rvf/resources/index.html
- [85].Ahmad K. More deaths from Rift Valley fever in Saudi Arabia and Yemen. Lancet. 2000;356:1422. [DOI] [PubMed] [Google Scholar]
- [86].Gür S, Kale M, Erol N, et al. The first serological evidence for Rift Valley fever infection in the camel, goitered gazelle and Anatolian water buffaloes in Turkey. Trop Anim Health Prod. 2017;49(7):1531–1535. [DOI] [PubMed] [Google Scholar]
- [87].Chevalier V. Relevance of Rift Valley fever to public health in the European Union. Clin Microbiol Infect. 2013;19(8):705–708. [DOI] [PubMed] [Google Scholar]
- [88].Chevalier V, Pepin M, Plee L, et al. Rift Valley fever- a threat for Europe?. Euro Surveill. 2010;15:19506. [PubMed] [Google Scholar]
- [89].Taylor RM, Hurlbut HS, Work TH, et al. Sindbis virus: a newly recognized arthropod transmitted virus. Am J Trop Med Hyg. 1955;4(5):844–862. [DOI] [PubMed] [Google Scholar]
- [90].Ernek E, Kozuch O, Gresíková M, et al. Isolation of Sindbis virus from the reed warbler (Acrocephalus scirpaceus) in Slovakia. Acta Virol. 1973;17(4):359–361. [PubMed] [Google Scholar]
- [91].L’vov DK, Skvortsova TM, Kondrashina NG, et al. [Etiology of Karelia fever-a new arbovirus infection]. Vopr Virusol. 1982;27:690–692. Russian [PubMed] [Google Scholar]
- [92].Adouchief S, Smura T, Sane J, et al. Sindbis virus as a human pathogen-epidemiology, clinical picture and pathogenesis. Rev Med Virol. 2016;26(4):221–241. [DOI] [PubMed] [Google Scholar]
- [93].Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res. 1993;2(1):23–41. [DOI] [PubMed] [Google Scholar]
- [94].Smith DL, Battle KE, Hay SI, et al. Ross, Macdonald, and a Theory for the Dynamics and Control of Mosquito-Transmitted Pathogens. PLoS Pathog. 2012;8(4):e1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Weitzel T, Braun K, Collado A, et al. Distribution and frequency of Culex pipiens and Culex torrentium (Culicidae) in Europe and diagnostic allozyme markers. European Mosquito Bulletin. 2011;29:22–37. [Google Scholar]
- [96].Engler O, Savini G, Papa A, et al. European surveillance for West Nile virus in mosquito populations. Int J Environ Res Public Health. 2013;10(10):4869–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Busquets N, Alba A, Allepuz A, et al. Usutu virus sequences in Culex pipiens (Diptera: culicidae), Spain. Emerg Infect Dis. 2008;14(5):861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Calzolari M, Bonilauri P, Bellini R, et al. Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PLoS One. 2010;5(12):e14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jöst H, Bialonski A, Maus D, et al. Isolation of usutu virus in Germany. Am J Trop Med Hyg. 2011;85(3):551–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Fritz ML, Walker ED, Miller JR, et al. Divergent host preferences of above- and below-ground Culex pipiens mosquitoes and their hybrid offspring. Med Vet Entomol. 2015;29(2):115–123. [DOI] [PubMed] [Google Scholar]
- [101].Brustolin M, Talavera S, Santamaría C, et al. Culex pipiens and Stegomyia albopicta (= Aedes albopictus) populations as vectors for lineage 1 and 2 West Nile virus in Europe. Med Vet Entomol. 2016;30(2):166–173. [DOI] [PubMed] [Google Scholar]
- [102].Brustolin M, Talavera S, Nuñez A, et al. Rift Valley fever virus and European mosquitoes: vector competence of Culex pipiens and Stegomyia albopicta (= Aedes albopictus). Med Vet Entomol. 2017;31(4):365–372. [DOI] [PubMed] [Google Scholar]
- [103].Mouchet J, Rageau J, Laumond C, et al. Epidémiologie du virus West Nile: étude d’un foyer en Camargue V. Le vecteur: culex modestus Ficalbi Diptera; Culicidae. Ann Inst Pasteur Paris. 1970;118:839–855. [PubMed] [Google Scholar]
- [104].Becker N, Petrić D, Zgomba M, et al. Mosquitoes and their control. Heidelberg, Dordrecht (NY): Springer; 2010. [Google Scholar]
- [105].Balenghien T, Vazeille M, Grandadam M, et al. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis. 2008;8:589–595. [DOI] [PubMed] [Google Scholar]
- [106].Muñoz J, Ruiz S, Soriguer R, et al. Feeding Patterns of Potential West Nile Virus Vectors in South-West Spain. PLoS One. 2012;7(6):e39549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gad AM, Farid HA, Ramzy RR, et al. Host feeding of mosquitoes (Diptera: culicidae) associated with the recurrence of Rift Valley fever in Egypt. Med Vet Entomol. 1999;36:709–714. [DOI] [PubMed] [Google Scholar]
- [108].Gunay F, Alten B, Simsek F, et al. Barcoding Turkish Culex mosquitoes to facilitate arbovirus vector incrimination studies reveals hidden diversity and new potential vectors. Acta Trop. 2015;143:112–120. [DOI] [PubMed] [Google Scholar]
- [109].Shaikevich EV, Vinogradova EB. The discovery of a hybrid population of mosquitoes of the Culex pipiens L. complex (Diptera, Culicidae) on the Kos Island (Greece) by means of molecular markers. Entomol Rev. 2014;94:35–39. [Google Scholar]
- [110].Gutsevich AV, Monchadskii AS, Shtakelberg AA. Fauna of the USSR Diptera volume 3 (4). Family Culicidae: Mosquitoes; 1974. [Google Scholar]
- [111].Kilpatrick AM, Kramer LD, Campbell SR, et al. West Nile Virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 2005;11:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gligić A, Adamović ŽR. Isolation of Tahyna virus from Aedes vexans mosquitoes in Serbia. Mikrobiologija, Beograd. 1976;13:119–129. [Google Scholar]
- [113].Petrić D, Petrović T, Hrnjaković Cvjetković I, et al. West Nile virus ‘circulation’ in Vojvodina, Serbia: mosquito, bird, horse and human surveillance. Mol Cell Probes. 2017;31:28–36. [DOI] [PubMed] [Google Scholar]
- [114].Hubálek Z, Rudolf I, Bakonyi T, et al. Mosquito (Diptera: culicidae) surveillance for arboviruses in an area endemic for West Nile (Lineage Rabensburg) and Tahyna viruses in Central Europe. J Med Entomol. 2010;47(3):466–472. [DOI] [PubMed] [Google Scholar]
- [115].Linthicum KJ, Britch SC, Anyamba A. Rift Valley Fever: an Emerging Mosquito-Borne Disease. Annu Rev Entomol. 2016;61:395–415. [DOI] [PubMed] [Google Scholar]
- [116].O'Donnell KL, Bixby MA, Morin KJet al. Potential of a Northern Population of Aedes vexans (Diptera: culicidae) to Transmit Zika Virus. J Med Entomol. 2017;54 (5):1354–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Petric D.Seasonal and diel biting activity of mosquitoes in the Vojvodina Province [PhD thesis], University of Novi Sad, Faculty of Agriculture; 1989. Serbian [Google Scholar]
- [118].Veronesi R, Gentile G, Carrieri M, et al. Seasonal pattern of daily activity of Aedes caspius, Aedes detritus, Culex modestus, and Culex pipiens in the Po Delta of northern Italy and significance for vector-borne disease risk assessment. J Vector Ecol. 2012;37(1):49–61. [DOI] [PubMed] [Google Scholar]
- [119].Detinova TS, Smelova VA. [Medical importance of mosquitoes (Culicidae, Diptera) from the fauna of the Soviet Union]. Med Parazitol (Mosk).. 1973;42(4):455–471. Russian. [PubMed] [Google Scholar]
- [120].Moutailler S, Krida G, Schaffner F, et al. Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector Borne Zoonotic Dis. 2008;8:749–754. [DOI] [PubMed] [Google Scholar]
- [121].Adhami J. Albania’s mosquitoes (Diptera: culicidae), the Culicini tribe. Medical. 1987;1–2:82–95. Albanian. [Google Scholar]
- [122].Mitchell CJ. Geographic spread of Aedes albopictus and potential for involvement in arbovirus cycles in the Mediterranean basin. J Vector Ecol. 1995;20:44–58. [Google Scholar]
- [123].Roiz D, Neteler M, Castellani C, et al. Climatic factors driving invasion of the tiger mosquito (Aedes albopictus) into new areas of Trentino, Northern Italy. PLoS One. 2011;6:e14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Medlock JM, Hansford KM, Schaffner F, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonot Dis. 2012;12:435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Paupy C, Delatte H, Bagny L, et al. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect Inst Pasteur. 2009;11(14–15):1177–1185. [DOI] [PubMed] [Google Scholar]
- [126].Calzolari M, Bonilauri P, Bellini R, et al. Usutu Virus Persistence and West Nile Virus Inactivity in the Emilia-Romagna Region (Italy) in 2011. PLoS ONE. 2013;8(5):e63978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Rezza G, Nicoletti L, Angelini R, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370(9602):1840–1846. [DOI] [PubMed] [Google Scholar]
- [128].Grandadam M, Caro V, Plumet S, et al. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17(5):910–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Delisle E, Rousseau C, Broche B, et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill. 2015;20(17):pii: 21108. [DOI] [PubMed] [Google Scholar]
- [130].Marchand E, Prat C, Jeannin C, et al. Autochthonous case of dengue in France, October 2013. Eur Surveill. 2013;18(50):20661. [DOI] [PubMed] [Google Scholar]
- [131].Succo T, Leparc-Goffart I, Ferré J, et al. Autochthonous dengue outbreak in Nîmes, South of France, July to September 2015. Euro Surveill. 2016;21(21):30240. [DOI] [PubMed] [Google Scholar]
- [132].Samanidou A. Aedes cretinus: is it a threat to the Mediterranean Countries?. European Mosquito Bulletin. 1998;1(8):8. [Google Scholar]
- [133].Alten B, Bellini R, Caglar SS, et al. Species composition and seasonal dynamics of mosquitoes in the Belek region of Turkey. J Vector Ecol. 2000;25:146–154. [PubMed] [Google Scholar]
- [134].Ramsdale CD, Alten B, Caglar SS, et al. A revised, annotated checklist of the mosquitoes (Diptera, Culicidae) of Turkey. Eur Mosq Bull. 2001;9:18–27. [Google Scholar]
- [135].Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European Region: past, present, and scenarios for the future. Lancet Infect Dis. 2015;14(12):1271–1280. [DOI] [PubMed] [Google Scholar]
- [136].Yunicheva YU, Ryabova TE, Markovich NY, et al. [First data on the presence of breeding populations of the Aedes aegypti L. mosquito in Greater Sochi and various cities of Abkhazia]. Med Parazitol Parazit Bol. 2008;3:40–43. Russian [PubMed] [Google Scholar]
- [137].Akiner MM, Demirci B, Babuadze G, et al. Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea region increases risk of Chikungunya, Dengue, and Zika outbreaks in Europe. PLoS Negl Trop Dis. 2016;10(4):e0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Christophers SR. Aedes aegypti. The yellow fever mosquito. Its life history, bionomics and structure. London: Cambridge University Press; 1960. p. 738. [Google Scholar]


