Abstract
Intensity-modulated radiation therapy (IMRT) is a conformal irradiation technique that enables steep dose gradients. In head and neck tumours this approach spares parotid-gland function without compromise to treatment efficacy. Anatomical and molecular imaging modalities may be used to tailor treatment by enabling proper selection and delineation of target volumes and organs at risk, which in turn lead to dose prescriptions that take into account the underlying tumour biology (eg, human papillomavirus status). Therefore, adaptations can be made throughout the course of radiotherapy, as required. Planned dose increases to parts of the target volumes may also be used to match the radiosensitivity of tumours (so-called dose-painting), assessed by molecular imaging. For swift implementation of tailored and adaptive IMRT, tools and procedures, such as accurate image acquisition and reconstruction, automatic segmentation of target volumes and organs at risk, non-rigid image and dose registration, and dose summation methods, need to be developed and properly validated.
Introduction
Intensity-modulated radiation therapy (IMRT) is an irradiation technique that combines beams with non-uniform fluence-intensity-generating steep-dose gradients, even in targets with concave shape.1 Although Brahme and colleagues2 described the concept 25 years ago, and although IMRT has been progressively introduced into the clinics over the past 10 years, in particular for the treatment of malignant head and neck disease, only in the past few years has sparing of the parotid gland been validated.2–4 IMRT has enabled a substantial reduction in parotid-gland irradiation, which has led to subjective and objective improvements in parotid function without a loss of efficacy. A prospective, non-randomised study also showed that after switching from three-dimensional conformal radiation therapy to IMRT for the treatment of locally advanced oral and pharyngolaryngeal squamous-cell carcinoma, late xerostomia and acute mucositis were decreased (possibly because of reductions in dose per fraction) and an association was seen with improved quality of life.5 In silico studies have also shown that IMRT allows sparing of the anatomical structures involved in swallowing,6,7 and a randomised study is underway to assess whether the dose to the auditory apparatus can be reduced with use of IMRT.
The proper delivery of IMRT for head and neck tumours requires a thorough knowledge of the complex anatomy of the region and the intricate physiology of swallowing, speech, and auditory functions, among others. A clear understanding of the pathophysiology of local and regional tumour spread is also required. Knowledge of the tolerance of normal tissues to irradiation, whether or not combined with chemotherapy or targeted agents, is required to keep complications to a minimum. Over the past decade seminal studies and guidelines have been published that have helped to standardise the delivery of IMRT for head and neck tumours. Consensus guidelines endorsed by the major scientific radiation oncology societies and by clinical research cooperative groups have been published for target-volume selection and delineation in the node-negative neck.8 These guidelines have been extended to the selection and delineation of the target volumes in the node-positive and the postoperative neck.9 A comprehensive review of the irradiation tolerance of the major organs at risk in the head and neck area has been published by the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) initiative,10–12 and additional data on irradiation tolerance for swallowing function have also been published.13
Despite the guidelines, some changes to practice are still required. Hong and colleagues14 asked radiation oncologists at 20 centres with established head and neck oncology expertise to delineate the clinical target volumes from a precontoured gross tumour volume. Major differences were noted between the centres, which illustrates the need for more stringent application of the guidelines mentioned above. In a similar study in the Netherlands, after guidelines had been available for a longer period, more consistent target-volume delineation was seen.15 This finding illustrates a learning curve to the understanding of proper implementation of IMRT. Variability between radiation oncologists is likely to decrease further as the education of younger radiation oncologists provided by academic centres improves and the number and quality of educational sessions at international conferences increases, and maybe also by the use of anatomical atlases for the delineation of organs at risk.16,17 To limit the consequences of variations in delineation between radiation oncologists, the use of conformal avoidance IMRT has been proposed. With this approach only structures for which dose-volume constraints are needed are delineated (eg, gross tumour volume, the spinal cord, and the parotid glands).18 From an academic point of view, such practice cannot be recommended, as it leads to higher doses being delivered to non-target tissues than to target tissues, and to irradiation of the whole primary-tumour subsite. The amount of normal mucosa around the gross tumour volume that needs to be included in the clinical target volume is unclear, but even in the IMRT area most primary-tumour failures typically occur in the gross tumour volume and not in the surrounding mucosal area.19 A way around these uncertainties is the delineation of an extra clinical target volume that is intermediate to the so-called elective and therapeutic clinical target volumes for the nodes and the primary tumour that would be exposed to a medium radiation dose, such as 60 Gy. Unfortunately, no undisputable argument can be made in favour of one or other proposal.
In this Review, we explore issues that need to developed further to improve radiotherapy for head and neck tumours, such as delineation of target volumes, biological tailoring of radiotherapy, adaptive radiation delivery, and radiation dose-painting.
Image-tailored IMRT for head and neck cancer
One of the first steps of a radiotherapy treatment plan is to properly select and delineate the target volume or volumes and the organs at risk. In head and neck tumours, planning has typically been done with anatomical imaging, such as CT and MRI. An electronic map of densities created by the CT images is used for dose calculation. MRI was added because it was thought the exquisite soft-tissue contrast would complement CT. Quantitative comparison between CT and MRI for delineation of pharyngolaryngeal gross tumour volume, compared with pathological findings, showed similar results for these two modalities, although subjective assessment was in favour of MRI. Both modalities, however, substantially overestimated the gross tumour volume,20 which illustrates the low specificity of iodine or gadolinium contrast-enhanced features.
In head and neck cancers, as for tumours at other sites, molecular imaging with fluorodeoxyglucose (FDG) PET has been increasingly used over the past few years in diagnosis and staging. FDG PET does not improve accuracy for nodal staging of head and neck tumours to a clinically relevant degree when compared with CT or MRI,21 but integration of FDG PET with CT, MRI, or both, can alter the primary delineation of gross target volume.22–24 In some studies, though, the gross target volumes were larger with FDG PET than without, whereas in other studies they were smaller. These discrepancies are probably caused partly by the use of suboptimum methods to reconstruct and segment the PET images. FDG PET was no better than anatomical imaging for delineation of the cervical lymph node. So far, only one study has reported a comparison between anatomical and molecular imaging in comparison with pathology.20 That study showed that in locally advanced squamous cell carcinoma, gross target volumes with FDG PET were significantly closer to those defined by pathological assessment of macroscopic tissue sections than were volumes delineated by CT or MRI. All imaging modalities in that study typically missed the small mucosal infiltration detectable by clinical examination, which emphasises the importance of the clinical information in delineation of gross target volumes,20,25 especially for small primary tumours, for which acquisition and reconstruction uncertainties with PET images outweighed any benefits. These findings justify the need to define clinical target volumes around the gross target volumes to ensure microscopic infiltration is included and to take into account the margin of uncertainty in delineation of gross volumes.
The use of PET for delineation of gross target volumes requires adequate data for acquisition, reconstruction, and segmentation of the images.26 Owing to technological differences between commercially available PET systems, the quality and properties of the images vary substantially.27 Several studies have shown that resolution is crucial to delineation tasks; the lower the resolution is, the more blurred the images are.28,29 Beyond visual discomfort, blur also smoothes and distorts iso-uptake contours, which can lead to statistical uncertainty (because of excess noise) and bias (because of the limited resolution) if delineation is based on uptake thresholds.30 Only specific, iterative delineation methods that proceed with several successive approximations of the target contours can help to resolve some of the issues caused by low resolution.31 Of more complex delineation methods, the most accurate are those that typically entail statistical or optical modelling of blur related to resolution. For instance, the method developed by Geets and colleagues32 includes image-processing steps that lessen noise and correct the images for resolution blurring. Next, delineation proceeds with gradient-crest detection instead of an uptake threshold, which helps to deal with heterogeneous signals within and around the target. Most of the segmentation techniques focus on delineation of gross target volumes with FDG PET. For images acquired with other tracers (eg, hypoxic tracers, such as 18F-fluoromisonidazole [Miso] or 18F-fluoroazomycin arabinoside [FAZA]), the reduced ratio of signal to background noise might further hinder delineation. Similarly, resolution issues become worse for delineation of heterogeneous areas within tumours than for assessment of the whole volume (see the discussion of dose-painting below).
Validation of FDG PET for treatment planning should ideally come from randomised trials that compare molecular and anatomical imaging. The complexity of doing such studies, including ethical issues, however, could justify the use of surrogates of efficacy. FDG-PET-based gross target volumes have been shown to translate into smaller clinical and planning target volumes than those delineated with CT and clinical information only.33 Furthermore, comparative planning studies showed that conformity of dose distribution could be increased when planning target volumes were based on FDG PET.33 Finally, preliminary results of a multicentre, prospective, phase 2 study of the use of planning target volumes based on FDG PET to guide treatment of locally advanced pharyngolaryngeal squamous-cell carcinoma have confirmed the previous volumetric and dosimetric results: smaller volumes have been delineated with FDG PET than with CT. The difference in dose distributions has, however, been small owing to the complexity of the treatment plan, which includes prophylactic nodal irradiation (Grégoire V, unpublished).
PET tracers for specific cellular pathways involved in radiation response (eg, hypoxia and proliferation) have also been investigated for delineation of target volumes. The use of PET tracers for hypoxia pathways has been studied in several proof-of-concept planning studies.34–37 Although hypoxic subvolumes can be delineated and substantially boosted without exceeding the tolerance dose to the surrounding normal tissues, various features, such as spatial resolution and temporal variation, need to be more clearly understood before hypoxia-targeted IMRT can be used routinely.38,39 Tumour-cell proliferation tracers, such as 3′-deoxy-3′-18F-fluorothymidine (18FLT), have been tested, but no real clinical benefit has yet been seen, probably because these tracers do not distinguish proliferation of tumour cells from that of surrounding normal cells.40 11C-methionine has been tested as a tracer of protein synthesis in target-volume selection and delineation, but no added value was shown because uptake by mucosal and salivary glands was high.41
Diffusion-weighted MRI is emerging as an imaging technique that measures water mobility in the tumour microenvironment.42 It has shown value in pretreatment nodal staging, in prediction of response to radiation treatment, and as an early marker of recurrence during follow-up. For pretreatment delineation of target volume, however, usefulness is limited. Whether it has a role during radiotherapy to identify non-responding patients who might need a boost dose remains to be investigated.
Biological tailoring of radiotherapy
Epidemiological and clinical data indicate that survival among patients with oropharyngeal cancer associated with human papillomavirus (HPV) is notably better than that for smoking-related head and neck cancers.43–45 Patients with HPV-related oropharyngeal cancer are generally light smokers or have never smoked and consume little alcohol. They are also frequently younger—of working and reproductive age—than are patients with other head and neck cancers. Reduction of the risk of treatment-related toxic effects without compromise to cure is important for patients with HPV-related oropharyngeal cancer, but the undertaking of non-inferiority trials, where event rates are low, is challenging.
In the RTOG 0129 trial, Ang and colleagues46 reported that tumour stage T4 and nodal status N2b–N3, accumulated tobacco exposure (>10 pack-years), and a negative test for HPV were predictors of poor outcomes in patients with oropharyngeal cancer. The investigators used these parameters to stratify the cohort into three groups according to risk of death.46 The observation that the prognosis differs between subgroups of patients with HPV-related oropharyngeal cancer is exciting because it suggests the tailoring of treatment for this disease could be possible. Heavy smokers, however, might still develop smoking-related disorders independent of cancer risk or be at risk of harmful effects from continuing to smoke during radiotherapy. Such effects might be independent of resistant biology related to heavy smoking that is acquired by the tumour during pathogenesis.47–50 Participants in the RTOG 1016 trial that is underway, which is assessing HPV-related oropharyngeal cancer, have been stratified by smoking status (>10 pack years exposure) so that non-smokers and light smokers may be assessed in the same trial as heavy smokers (NCT01302834).
Strategies to replace cisplatin with cetuximab (eg, as assessed in the RTOG 1016 trial, which also uses accelerated radiotherapy in both arms) or induction cetuximab with other agents to select patients for a reduced radiotherapy dose, underpin the first trials specific for HPV-related oropharyngeal cancer (NCT01302834 and NCT01084083). Other HPV trials specifically addressing a potential role for cetuximab in the treatment of oropharyngeal cancer, such as the UK De-ESCAlate HPV trial, other anti-EGFR strategies, and ways to reduce treatment intensity, are being considered. No randomised trial has yet addressed de-escalation with radiotherapy alone for patients with oropharyngeal cancer, despite meta-analysis showing that intensified hyperfractionated radiotherapy and chemoradiotherapy lead to similar survival benefits in patients with oropharyngeal or other head and neck cancers.51,52 This finding was, however, unexpected because benefits achieved with moderately accelerated radiotherapy were independent of HPV status in the DAHANCA 6 & 7 trials.53 Other data, albeit retro-spective, support very high survival among non-smokers or light smokers with HPV-related oropharyngeal cancers, even stage IV cancers (mostly related to N2 neck involvement), when altered fractionation radiotherapy is used alone.54 Evidence has also suggested that nodal status is an unreliable prognostic indicator in HPV-related oropharyngeal cancer.55
Infection with HPV is not the only biological parameter that affects treatment response, and thus potentially dosing. Toustrup and colleagues56 reported a predictive 15-gene hypoxic signature that could predict whether patients with locally advanced supraglottic or pharyngeal squamous-cell carcinoma would benefit from combined nimorazole and radiotherapy.51 Patients positive for HPV did not benefit from nimorazole, irrespective of their hypoxia signature.
A final issue in relation to the tailoring of radiobiological approaches to head and neck cancers is management of the neck during IMRT through the use of simultaneous integrated boosts. The use of low doses per fraction (eg, 1 · 5 Gy) is convenient and potentially beneficial in elective management of subclinical neck disease, compared with the traditional 2·0 Gy per fraction dose, as long-term toxic effects might be avoided, especially in patients who receive concurrent chemotherapy. Seung and colleagues57 noted no regional failures in the clinically negative neck treated with a median dose of 1 · 65 Gy per fraction. Similarly, Bedi and colleagues58 saw no recurrences with 50 ·0 Gy administered at 1·43 Gy per fraction, and suggest that this dose is sufficient to electively treat low-risk neck lymphatics with chemotherapy. This tailored approach is applicable to all head and neck cancers where the risk of regional neck relapse warrants elective treatment.
Adaptive IMRT
Adaptive radiotherapy involves changes to the radio-therapy plan during treatment on the basis of patient-specific observations that were not taken into account during initial planning. Adaptive radiotherapy should also include corrections for set-up variations. In head and neck tumours, discrepancies can be kept to a minimum with the use of various immobilisation systems and on-board imaging.59 Nevertheless, margins of 3–5 mm between the planning and clinical target volumes have been recommended to account for uncertainties in residual positioning after corrections for systematic and random deviations.60 Frequently, though, the term adaptive radiotherapy is used to refer to different procedures used throughout the course of a treatment to account for anatomical and functional variations that can affect the dose distribution. Relevant unspecific anatomical variations might be seen in the patient’s weight, the tumour volume, position or function of a specific organ at risk, or target volume.
Studies of head and neck cancers have mainly focused on variations in the volumes and positions of the parotid glands and in target volumes throughout the treatment course. Progressive shrinkage of around 1% per treatment day and displacement of 3–4 mm by the end of treatment toward the mid-sagittal plane have been consistently reported for the ipsilateral parotids.61 Smaller variations have been noted for the contralateral parotids. On average, nodal and primary-tumour gross target volumes assessed on repeated planning CT shrink by 2–3% per treatment day, which translates into a change in the associated clinical target volumes. The centre of mass also moves a few millimetres, although the direction is not consistent.61,62 Similar findings for gross target volumes were seen on FDG PET.63
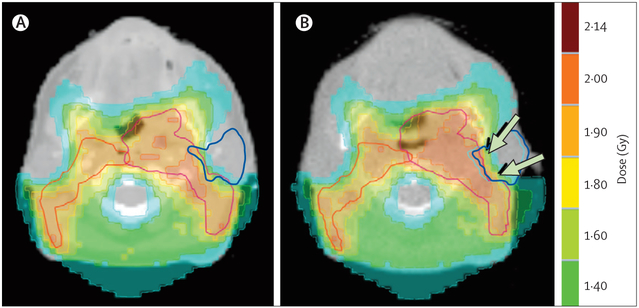
Volumetric and positional changes of organs at risk and target volumes are generally associated with progressive increase in the delivered dose compared with the planned dose,64 typically because of shrinkage of the gross target volume owing to tumour tissue loss (figure 1). The volumetric and positional changes of organs at risk and target volumes have also been shown to lead to increased mean doses to the ipsilateral and controlateral parotid glands, by 15% and 10%, respectively, and to small increases to the spinal cord and the oral cavity.64 Other studies have consistently reported increases in parotid-gland dose throughout the treatment course.64–67 For target volumes, variations in dose metrics have also been reported but are not consistent, which probably reflects variations in treatment procedures (eg, no margin between planning and clinical volumes) and methods (eg, megavoltage CT vs kilovoltage CT with contrast to assess target volumes).64–66 We have found a marginally significant decrease in the planning target volume nearminimum dose (D98), but dose metrics in the clinical target volume were not affected, which illustrates the usefulness of the planning target volume concept.
Figure 1: Comparison between the planned isodose distribution and the dose distribution actually delivered on the 25th fraction.
(A) Kilovoltage CT with contrast enhancement and (B) megavoltage CT without contrast enhancement for a patient with a T2-N3 oropharyngeal squamous-cell carcinoma. The patient was treated with chemoradiotherapy. Planned radiotherapy dose was 50·0 Gy administered as 25 fractions of 2·0 Gy, and planning target volumes are delineated in red. The left parotid gland is delineated in blue. Arrows indicate the overdose of the left parotid gland compared with the planned dose.
In view of the issues outlined above, incorporation of adaptive dose distributions in planning can be useful. Planning studies have shown that dose adaptation can recover the extra dose delivered to the irradiated volume, and in particular to the parotid glands.64,68,69 At a population level, however, the dose recovered is small and benefits only 20–30% of patients.64,68 Various factors, such as parotid shift toward the mid-sagittal plane, but not weight loss, help to select patients in whom adaptation will be useful.
For adaptation of target volumes, the so-called elective clinical target volume (eg, prophylactic nodal irradiation) must be distinguished from the so-called therapeutic clinical target volume around a gross target volume. As change in the elective clinical target volume is directly related to anatomical modifications, adaptation throughout treatment seems justified. For the clinical target volume around the gross target volume, however, although planning studies show consistently that shrinkage of the gross target volume enables isodose reduction, implementation of this procedure is not recommended. The limited resolution of the imaging modalities raises the risk of underdosing to part of the surrounding normal tissues still infiltrated by tumour cells.63–69
The concept of adaptive planning has been validated, albeit in one study of 22 patients treated with IMRT for stage III–IV oropharyngeal primary tumours.70 Median follow-up was 31 months, and local and regional control at 2 years reached 100% and 95%, respectively. Thus, with use of proper methodology, adaptive treatment without changes to target volume is safe. In that study, however, no margin was used between the planning and clinical target volumes to limit the high dose distribution to organs at risk around the clinical target volume (eg, the parotid glands), which is not recommended because the residual positioning uncertainties after correction for deviations are not taken into account.
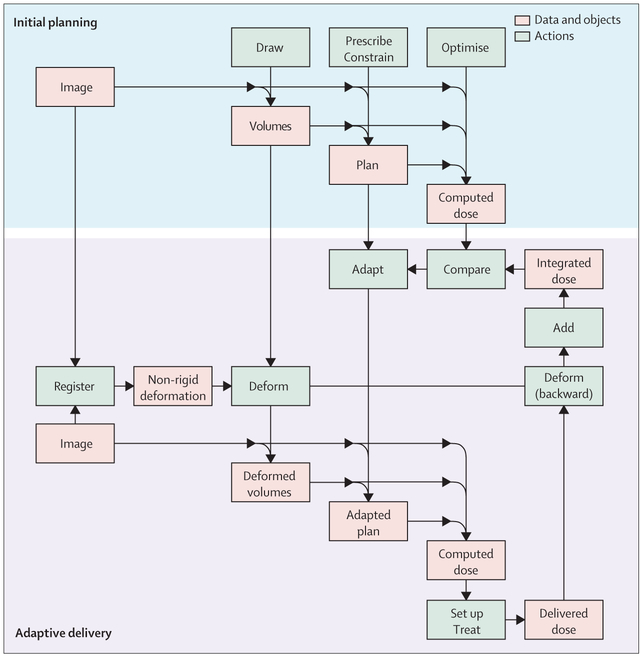
Technical implementation of IMRT adaptive planning is also challenging (figure 2). Adaptive treatment relies on several hardware and software tools, such as on-board imaging, image registration algorithms, image segmentation techniques, and dose summation.64,65 These tools, respectively, provide the raw data, connect them within a reference coordinate system, propagate the contours of the target volumes and organs at risk from the pretreatment planning CT to the per-treatment images, and integrate the dose distributions. Some processes can be done offline (between fractions) but others should be done online during a treatment session. In head and neck tumours, anatomical changes are mainly progressive over the treatment course and, therefore, offline adaptation is probably most realistic, as the immediacy of online adaptation is generally not required.67 The development of automated procedures, accompanied by efficient decision tools (eg, thresholds to trigger adaptation) can aid work flow. Automation is also expected to decrease variability within and between observers.67
Figure 2: Typical diagrammatic representation of an adaptive treatment strategy.
The main difference between adaptive and classic treatment strategies is that images acquired during treatment may be used for set-up and dose re-calculation. The diagram relies on two assumptions. First, the quality of in-room imaging is sufficient to compute a provisional dose just before set-up and delivery. Second, the dose effectively received by the patient can be measured after delivery. If not, a natural surrogate would be the provisional dose. Set-up may be guided by the images (image-guided adaptive radiotherapy) and the computed provisional dose (dose-guided adaptive radiotherapy).
Image registration requires the development of algorithms that can identify non-rigid (or elastic) deformation.65,67,71,72 The quality of on-board imaging devices, however, remains an issue for these algorithms because of various factors, such as inaccurate calibration of the Hounsfield units, low signal-to-noise ratio, strong artifacts, and low soft-tissue contrast. Although the current registration algorithms can easily catch smooth deformations, discontinuous variations— eg, tongue sliding or occlusion of air cavities—are more difficult to model.71–73 The effect on delineation of the gross target volume is especially important, where substantial changes, up to tissue disappearance, are expected to occur. Despite these limitations, non-rigid registration algorithms remain a cornerstone in the implementation of adaptive radiotherapy. They provide spatial mapping and thus enable contours and dose distributions to be deformed and propagated from one image to another,61,64,72 which yields a good approximation of the total dose delivered. Finer radiobiological models of dose summation do exist,74 but are not used in practice because correction for dose per fraction is of much less importance than the remaining inaccuracy in image registration.
Dose-painting
In theory, delivery of a uniform dose would be optimum if the tumour radiosensitivity were uniform.75,76 Studies suggest that dose escalation for radioresistant parts of tumours and dose decrease for radiosensitive parts would lead to improved tumour control and maintain the integral delivered dose.77 This approach is termed biologically conformal radiotherapy or dose-painting. Consensus indicates that the only realistic way to identify biological heterogeneity of tumours in vivo is with biological imaging techniques, which has become possible in the past decade. The biological properties considered most frequently for selective dose boosting are regions that display high metabolism, increased tumour hypoxia, or increased cell proliferation.78
Dose-painting aims to deliver a non-uniform dose throughout the target volume. The technique involves four distinct steps: determination of the correlation between the underlying tumour biology and molecular imaging; determination of dose prescription function based on molecular imaging data; planning of the treatment and dose delivery; and assessment of the clinical outcomes in comparison with standard treatments. Each step is associated with specific challenges and uncertainties that need to be resolved before dose-painting can be safely administered to patients.
A distinction is usually made between dose-painting by volume, which takes into account subvolumes within a gross target volume for dose prescription, and that by number, where each voxel receives a different dose based on the intensity of a given image parameter.78 The method is decided according to technical choices and limitations, but is rather artificial, as the use of a high number of subvolumes will mimic dose-painting by number. Treatment planning systems, however, do not easily manage a large number of intricate contours and the granularity of dose-painting by volume and number is limited. The resolution of dose delivery also has a limiting effect.
Most understanding of tumour biology that leads to increased radiation resistance is known on a spatial scale (eg, microns), which is much smaller than the resolution that can be achieved with any imaging techniques (ie, millimetres).39 This disparity leads to underestimation of tracer uptake and an overestimation of object size.79 Similarly, no molecular imaging technique can directly depict the distribution of radiosensitivity within the tumour, which leads to differential response to radiation. Most of the current dose-painting targets are, therefore, based partly on clinical evidence80 and theoretical arguments,34,81,82 without direct connection between imaging data and radiosensitivity. Consequently, the selection of molecular imaging agent is frequently driven by convenience (eg, FDG PET) and availability at a given institution, rather than by strong clinical evidence.
Treatment induces substantial spatial and temporal changes in tumour biology.63 Good understanding of these dynamics is essential to determine the optimum time of assessment and of the dose-painting targets, how these features might change through the course of therapy, and, therefore, whether target reassessment will be required. Furthermore, molecular imaging decisions related to specific tumour phenotypes that have increased radioresistance (eg, hypoxia, proliferation) typically require advanced compartmental modelling to reliably extract biological information of interest from imaging data.35 While the techniques for this extraction are known, its implementation is likely not to be straightforward because complex imaging protocols, such as dynamic PET imaging, and expertise in advanced image analysis will be required. Furthermore, molecular imaging techniques are typically only partly related to biological phenomena. For instance, 18F-FAZA is required to show hypoxia, but FDG is required for metabolism and 18F-FLT for proliferation. Multiple tracers exist, but each has its own limitations related to uptake and retention mechanisms, which need to be understood to ensure proper interpretation.83 One of the most important issues in dose-painting, however, is to determine the correlation between the molecular imaging information and dose to compensate for differential spatial radiosensitivity. Dose-painting prescription can be assessed indirectly by correlation of particular biological phenotypes and clinical outcomes, or directly by correlation of molecular imaging information and clinical outcomes. The latter approach is preferable, but no reliable clinical trial data that enable direct comparison yet exist.
Multiple studies have shown that the planning of dosepainting delivery is feasible, but most have assumed optimum delivery conditions.84–87 To target small regions within tumours, positioning and motion uncertainties must be taken into account in a way similar to that for treating the entire tumour,88 although the same margins should not be used for subvolumes. Such application lowers the theoretical advantage of dose-painting over traditional uniform delivery. In head and neck tumours, however, positioning errors can be minimised with image-guided radiotherapy techniques, and motion is not typically a major issue.
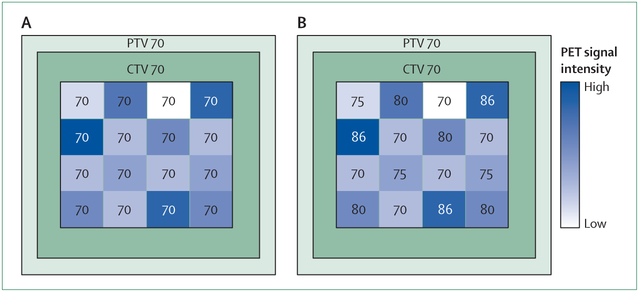
Clinical trials to compare clinical benefits of dose painting with those of standard treatment need to be carefully designed. A two-phase approach is probably best. Technical feasibility and safety would need to be established in the first phase, followed by a larger randomised trial, with local control and late toxic effects as the combined primary endpoint, to compare biologically conformal dose escalation with a simple geometrically defined boost plan to deliver the same integral dose to the target volume (figure 3).78
Figure 3: Typical schematic treatment plans for a dose-painting phase 3 trial protocol.
Numbers represent doses in Gy. (A) In the standard arm a median dose of 70 Gy is homogeneously delivered to the GTV. (B) In the experimental arm boost doses are delivered to the GTV that range from 70 to 86 Gy and are based on the PET-signal intensity in each voxel. In both arms a median dose of 70 Gy is administered to the CTV and PTV. PTV=planning target volume. CTV=clinical target volume. GTV=gross target volume.
Because of the number of challenges that need to be overcome, clinical adoption of dose-painting has been slow. Most clinical studies have focused on the feasibility and safety of dose escalation. In one trial in head and neck tumours, two dose-boosting escalation levels to the pretreatment FDG PET or CT-avid areas were tested (physical doses of 72·5 Gy or 77·5 Gy).89 Unfortunately, the study was terminated because of a treatment-related death at the high-dose boost level. Of nine recurrences, four were in the boost regions. This finding suggests that even higher doses might be warranted, if they do not exceed acceptable toxicity. In a follow-up trial by the same group, median dose boosts of 80·9 Gy or 85·9 Gy with adaption of delivery after ten fractions based on a repeat FDG PET or CT scans, and delivery of a uniform dose after 20 fractions, resulted in no grade 4 acute toxic effects.90 These findings indicate that dose-painting is technically and clinically feasible, but that a careful approach is needed.
Conclusions and future directions
Radiotherapy has become an indisputable part of the multidisciplinary management of head and neck cancer. Selection and delineation of target volumes, use of stringent dose-volume constraints to steer the calculation engines, and modern equipment permit high dose delivery with sharp dose gradients. The tailoring of treatment seems feasible in the near future through the use of molecular imaging with PET and MRI to select and delineate gross target volume, biological profiling of the tumour to select patients who might benefit from adaptive treatment intensity (eg, decreases for HPV-positive tumours or escalated for hypoxic tumours), application of dose-painting with doses decided on the basis of tumour-response parameters (eg, number of stem cells, hypoxia, and proliferation), and the monitoring of parameters and variations in the patient’s anatomy to trigger dose adaptation. Before this scenario becomes reality, however, proof of concept must be tested in clinical studies. Randomised studies of dose escalation based on molecular imaging are being designed, and those of de-escalation will start soon for patients with oropharyngeal squamous-cell carcinoma related to HPV. Whether to assess molecular profiling of hypoxia to select patients for treatment intensification in studies is also being debated. In the meantime, new software (eg, delineation atlases, image segmentation algorithms, and non-rigid dose summation algorithms) and hardware (eg, faster calculation engines) are being used to enable dose-painting and dose adaptation. Important technological and methodological issues still need to be addressed, however, before the dose-painting concept can be validated. For example, low resolution and statistical noise in PET images do not take into account intrinsic variation of biological phenomena, and uncertainties in dose calculation and delivery might mean that dose distribution does not match the underlying tumour biology. The next few years will be crucial to radiation treatment of head and neck cancers. Irrespective of the improvements already made or those expected, though, patients with head and neck tumours will certainly be best managed by radiation oncologists who specialise in these cancers.
Footnotes
Conflicts of interest
We declare that we have no conflicts of interest.
Contributor Information
Prof Vincent Grégoire, Department of Radiation Oncology, Université Catholique de Louvain, Cliniques, Universitaires Saint-Luc, Brussels, Belgium.
Robert Jeraj, Department of Medical Physics, University of Wisconsin, Madison, WI, USA.
John Aldo Lee, Centre for Molecular Imaging, Radiotherapy and Oncology, Université Catholique de Louvain, Cliniques, Universitaires Saint-Luc, Brussels, Belgium.
Prof Brian O’Sullivan, Department of Radiation Oncology, Princess Margaret Hospital, University of Toronto, Toronto, ON, Canada.
References
- 1.Brahme A Design principles and clinical possibilities with a new generation of radiation therapy equipment. A review. Acta Oncol 1987; 26: 403–12. [DOI] [PubMed] [Google Scholar]
- 2.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 2006; 66: 981–91. [DOI] [PubMed] [Google Scholar]
- 3.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol 2007; 25: 4873–79. [DOI] [PubMed] [Google Scholar]
- 4.Nutting CM, Morden JP, Harrington KJ, et al. , on behalf of the PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011; 12: 127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys 2009; 74: 1–8. [DOI] [PubMed] [Google Scholar]
- 6.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys 2007; 68: 1289–98. [DOI] [PubMed] [Google Scholar]
- 7.van der Laan HP, Christianen ME, Bijl HP, Schilstra C, Langendijk JA. The potential benefit of swallowing sparing intensity modulated radiotherapy to reduce swallowing dysfunction: an in silico planning comparative study. Radiother Oncol 2012; 103: 76–81 [DOI] [PubMed] [Google Scholar]
- 8.Grégoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol 2003; 69: 227–36. [DOI] [PubMed] [Google Scholar]
- 9.Grégoire V, Eisbruch A, Hamoir M, Levendag P. Proposal for the delineation of the nodal CTV in the node-positive and the post-operative neck. Radiother Oncol 2006; 79: 15–20. [DOI] [PubMed] [Google Scholar]
- 10.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 2010; 76 (3 suppl): S58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 2010; 76 (3 suppl): S42–49. [DOI] [PubMed] [Google Scholar]
- 12.Rancati T, Schwarz M, Allen AM, et al. Radiation dose-volume effects in the larynx and pharynx. Int J Radiat Oncol Biol Phys 2010; 76 (3 suppl): S64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christianen ME, Schilstra C, Beetz I, et al. Predictive modelling for swallowing dysfunction after primary (chemo)radiation: results of a prospective observational study. Radiother Oncol 2011; published online September 21 DOI: 10.1016/j.radonc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Hong TS, Tomé WA, Harari PM. Heterogeneity in head and neck IMRT target design and clinical practice. Radiother Oncol 2012; 103: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasch CR, Steenbakkers R, Duppen J, van Herk M. 3-Dimensional observer variation in international guideline based delination of neck lymph node levels. Int J Radiat Oncol Biol Phys 2007; 69 (suppl 1): S205. [Google Scholar]
- 16.Sims R, Isambert A, Grégoire V, et al. A pre-clinical assessment of an atlas-based automatic segmentation tool for the head and neck. Radiother Oncol 2009; 93: 474–78. [DOI] [PubMed] [Google Scholar]
- 17.van de Water TA, Bijl HP, Westerlaan HE, Langendijk JA. Delineation guidelines for organs at risk involved in radiation-induced salivary dysfunction and xerostomia. Radiother Oncol 2009; 93: 545–52. [DOI] [PubMed] [Google Scholar]
- 18.Harari PM, Song S, Tomé WA. Emphasizing conformal avoidance versus target definition for IMRT planning in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2010; 77: 950–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisbruch A, Grégoire V. Balancing risk and reward in target delineation for highly conformal radiotherapy in head and neck cancer. Semin Radiat Oncol 2009; 19: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daisne JF, Duprez T, Weynand B, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology 2004; 233: 93–100. [DOI] [PubMed] [Google Scholar]
- 21.Kyzas PA, Evangelou E, Denaxa-Kyza D, Ioannidis JP. 18F-fluorodeoxyglucose positron emission tomography to evaluate cervical node metastases in patients with head and neck squamous cell carcinoma: a meta-analysis. J Natl Cancer Inst 2008; 100: 712–20. [DOI] [PubMed] [Google Scholar]
- 22.Paulino AC, Koshy M, Howell R, Schuster D, Davis LW. Comparison of CT- and FDG-PET-defined gross tumor volume in intensity-modulated radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2005; 61: 1385–92. [DOI] [PubMed] [Google Scholar]
- 23.Riegel AC, Berson AM, Destian S, et al. Variability of gross tumor volume delineation in head-and-neck cancer using CT and PET/CT fusion. Int J Radiat Oncol Biol Phys 2006; 65: 726–32. [DOI] [PubMed] [Google Scholar]
- 24.Guido A, Fuccio L, Rombi B, et al. Combined 18F-FDG-PET/CT imaging in radiotherapy target delineation for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2009; 73: 759–63. [DOI] [PubMed] [Google Scholar]
- 25.Thiagarajan A, Caria N, Schöder H, et al. Target volume delineation in oropharyngeal cancer: impact of PET, MRI, and physical examination. Int J Radiat Oncol Biol Phys 2011; published online October 26 D0I: 10.1016/j.ijrobp.2011.05.060. [DOI] [PubMed] [Google Scholar]
- 26.Schinagl DA, Vogel WV, Hoffmann AL, van Dalen JA, Oyen WJ, Kaanders JH. Comparison of five segmentation tools for 18F-fluoro-deoxy-glucose-positron emission tomography-based target volume definition in head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 69: 1282–89. [DOI] [PubMed] [Google Scholar]
- 27.Bailey DL, Townsend DW, Valk PE, Maisey MN, eds. Positron emission tomography: basic science and clinical practice. London: Springer, 2004. [Google Scholar]
- 28.Kessler RM, Ellis JR Jr, Eden M. Analysis of emission tomographic scan data: limitations imposed by resolution and background. J Comput Assist Tomogr 1984; 8: 514–22. [DOI] [PubMed] [Google Scholar]
- 29.King MA, Long DT, Brill AB. SPECT volume quantitation: influence of spatial resolution, source size and shape, and voxel size. Med Phys 1991; 18: 1016–24. [DOI] [PubMed] [Google Scholar]
- 30.Lee JA. Segmentation of positron emission tomography images: some recommendations for target delineation in radiation oncology. Radiother Oncol 2010; 96: 302–07. [DOI] [PubMed] [Google Scholar]
- 31.van Dalen JA, Hoffmann AL, Dicken V, et al. A novel iterative method for lesion delineation and volumetric quantification with FDG PET. Nucl Med Commun 2007; 28: 485–93. [DOI] [PubMed] [Google Scholar]
- 32.Geets X, Lee JA, Bol A, Lonneux M, Grégoire V. A gradient-based method for segmenting FDG-PET images: methodology and validation. Eur J Nucl Med 2007; 34: 1427–38. [DOI] [PubMed] [Google Scholar]
- 33.Geets X, Daisne JF, Tomsej M, Duprez T, Lonneux M, Grégoire V. Impact of the type of imaging modality on target volumes delineation and dose distribution in pharyngo-laryngeal squamous cell carcinoma: comparison between pre- and per-treatment studies. Radiother Oncol 2006; 78: 291–97 [DOI] [PubMed] [Google Scholar]
- 34.Chao KS, Bosch WR, Mutic S, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2001; 49: 1171–82. [DOI] [PubMed] [Google Scholar]
- 35.Thorwarth D, Eschmann SM, Paulsen F, Alber M. Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol Biol Phys 2007; 68: 291–300. [DOI] [PubMed] [Google Scholar]
- 36.Lee NY, Mechalakos JG, Nehmeh S, et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys 2008; 70: 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendrickson K, Phillips M, Smith W, Peterson L, Krohn K, Rajendran J. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother Oncol 2011; 101: 369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin Z, Mechalakos J, Nehmeh S, et al. The influence of changes in tumor hypoxia on dose-painting treatment plans based on 18F-FMISO positron emission tomography. Int J Radiat Oncol Biol Phys 2008; 70: 1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christian N, Lee JA, Bol A, De Bast M, Jordan B, Grégoire V. The limitation of PET imaging for biological adaptive-IMRT assessed in animal models. Radiother Oncol 2009; 91: 101–06. [DOI] [PubMed] [Google Scholar]
- 40.Troost EG, Schinagl DA, Bussink J, Oyen WJ, Kaanders JH. Clinical evidence on PET-CT for radiation therapy planning in head and neck tumours. Radiother Oncol 2010; 96: 328–34. [DOI] [PubMed] [Google Scholar]
- 41.Geets X, Daisne JF, Gregoire V, Hamoir M, Lonneux M. Role of 11-C-methionine positron emission tomography for the delineation of the tumor volume in pharyngo-laryngeal squamous cell carcinoma: comparison with FdG-PET and CT. Radiother Oncol 2004; 71: 267–73. [DOI] [PubMed] [Google Scholar]
- 42.Vandecaveye V, De Keyzer F, Dirix P, Lambrecht M, Nuyts S, Hermans R. Applications of diffusion-weighted magnetic resonance imaging in head and neck squamous cell carcinoma. Neuroradiology 2010; 52: 773–84. [DOI] [PubMed] [Google Scholar]
- 43.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000; 92: 709–20. [DOI] [PubMed] [Google Scholar]
- 44.Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol 2010; 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 2007; 121: 1813–20. [DOI] [PubMed] [Google Scholar]
- 46.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farshadpour F, Kranenborg H, Calkoen EV, et al. Survival analysis of head and neck squamous cell carcinoma: influence of smoking and drinking. Head Neck 2011; 33: 817–23. [DOI] [PubMed] [Google Scholar]
- 48.Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys 2011; 79: 414–19. [DOI] [PubMed] [Google Scholar]
- 49.Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer 2008; 122: 2656–64. [DOI] [PubMed] [Google Scholar]
- 50.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus-associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006; 24: 736–47. [DOI] [PubMed] [Google Scholar]
- 51.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006; 368: 843–54. [DOI] [PubMed] [Google Scholar]
- 52.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92: 4–14. [DOI] [PubMed] [Google Scholar]
- 53.Lassen P, Eriksen JG, Krogdahl A, et al. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother Oncol 2011; 100: 49–55. [DOI] [PubMed] [Google Scholar]
- 54.O’Sullivan B, Huang SH, Perez-Ordonez B, et al. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother Oncol 2012; 103: 49–56. [DOI] [PubMed] [Google Scholar]
- 55.Straetmans JM, Olthof N, Mooren JJ, de Jong J, Speel EJ, Kremer B. Human papillomavirus reduces the prognostic value of nodal involvement in tonsillar squamous cell carcinomas. Laryngoscope 2009; 119: 1951–57. [DOI] [PubMed] [Google Scholar]
- 56.Toustrup K, Sørensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J, Danish Head and Neck Cancer Group (DAHANCA). Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol 2012; 102: 122–29. [DOI] [PubMed] [Google Scholar]
- 57.Seung S, Bae J, Solhjem M, et al. Intensity-modulated radiotherapy for head-and-neck cancer in the community setting. Int J Radiat Oncol Biol Phys 2008; 72: 1075–81. [DOI] [PubMed] [Google Scholar]
- 58.Bedi M, Firat S, Semenenko VA, et al. Elective lymph node irradiation with intensity-modulated radiotherapy: is conventional dose fractionation necessary? Int J Radiat Oncol Biol Phys (in press). [DOI] [PubMed] [Google Scholar]
- 59.Coffey M, Vaandering A. Patient setup for PET/CT acquisition in radiotherapy planning. Radiother Oncol 2010; 96: 298–301. [DOI] [PubMed] [Google Scholar]
- 60.van Herk M Margins and margin recipes In: Palta JR, Mackie TR, eds. Uncertainties in external beam radiation therapy (AAPM 2011 summer school). College Park, MD: Medical Physics Publishing Corp, 2011: 169–90. [Google Scholar]
- 61.Castadot P, Geets X, Lee JA, Christian N, Grégoire V. Assessment by a deformable registration method of the volumetric and positional changes of target volumes and organs at risk in pharyngo-laryngeal tumors treated with concomitant chemo-radiation. Radiother Oncol 2010; 95: 209–17 [DOI] [PubMed] [Google Scholar]
- 62.Barker JL Jr, Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys 2004; 59: 960–70. [DOI] [PubMed] [Google Scholar]
- 63.Geets X, Tomsej M, Lee JA, et al. Adaptive biological image-guided IMRT with anatomic and functional imaging in pharyngo-laryngeal tumors: impact on target volume delineation and dose distribution using helical tomotherapy. Radiother Oncol 2007; 85: 105–15. [DOI] [PubMed] [Google Scholar]
- 64.Castadot P, Geets X, Lee JA, Grégoire V. Adaptive functional image-guided IMRT in pharyngo-laryngeal squamous cell carcinoma: is the gain in dose distribution worth the effort? Radiother Oncol 2011; 101: 343–50. [DOI] [PubMed] [Google Scholar]
- 65.Wu Q, Chi Y, Chen PY, Krauss DJ, Yan D, Martinez A. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Phys 2009; 75: 924–32. [DOI] [PubMed] [Google Scholar]
- 66.Ahn PH, Chen CC, Ahn AI, et al. Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol Biol Phys 2011; 80: 677–85. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz DL, Dong L. Adaptive radiation therapy for head and neck cancer—can an old goal evolve into a new standard? J Oncol 2011; 2011: 690595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee C, Langen KM, Lu W, et al. Assessment of parotid gland dose changes during head and neck cancer radiotherapy using daily megavoltage computed tomography and deformable image registration. Int J Radiat Oncol Biol Phys 2008; 71: 1563–71. [DOI] [PubMed] [Google Scholar]
- 69.Simone CB 2nd, Ly D, Dan TD, et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol 2011; 101: 376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys 2011; published online December 2 D0I: 10.1016/j.ijrobp.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brock KK, Kaus MR. Deformable image registration for radiation therapy planning: algorithms and applications In Leondes CT, ed. Biomechanical systems technology—volume 1: computational methods. Singapore: World Scientific, 2007: 1–28. [Google Scholar]
- 72.Castadot P, Lee JA, Parraga A, Geets X, Macq B, Grégoire V. Comparison of 12 deformable registration strategies in adaptive radiation therapy for the treatment of head and neck tumors. Radiother Oncol 2008; 89: 1–12. [DOI] [PubMed] [Google Scholar]
- 73.Brock KK, Deformable Registration Accuracy Consortium. Results of a multi-institution deformable registration accuracy study (MIDRAS). Int J Radiat Oncol Biol Phys 2010; 76: 583–96. [DOI] [PubMed] [Google Scholar]
- 74.Orban de Xivry J, Castadot P, et al. Evaluation of the radiobiological impact of anatomic modifications during radiation therapy for head and neck cancer: can we simply summate the dose? Radiother Oncol 2010; 96: 131–38. [DOI] [PubMed] [Google Scholar]
- 75.Brahme A, Ågren AK. Optimal dose distribution for eradication of heterogeneous tumours. Acta Oncol 1987; 26: 377–85. [DOI] [PubMed] [Google Scholar]
- 76.Webb S, Nahum AE. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell density. Phys Med Biol 1993; 38: 653–66. [DOI] [PubMed] [Google Scholar]
- 77.Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys 2000; 47: 551–60. [DOI] [PubMed] [Google Scholar]
- 78.Bentzen S, Gregoire V. Molecular imaging-based dose painting: a novel paradigm for radiation therapy prescription. Semin Radiat Oncol 2011; 21: 101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barbee DL, Flynn RT, Holden JE, Nickles RJ, Jeraj R. A method for partial volume correction of PET-imaged tumor heterogeneity using expectation maximization with a spatially varying point spread function. Phys Med Biol 2010; 55: 221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dirix P, Vandecaveye V, De Keyzer F, Stroobants S, Hermans R, Nuyts S. Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with 18F-FDG PET, 18F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med 2009; 50:1020–27 [DOI] [PubMed] [Google Scholar]
- 81.Vanderstraeten B, Duthoy W, De Gersem W, De Neve W, Thierens H. [18F]fluoro-deoxy-glucose positron emission tomography ([18F]FDG-PET) voxel intensity-based intensity-modulated radiation therapy (IMRT) for head and neck cancer. Radiother Oncol 2006. 79: 249–58. [DOI] [PubMed] [Google Scholar]
- 82.Troost EG, Bussing J, Hoffmann AL, et al. , 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med 2010; 51: 866–74. [DOI] [PubMed] [Google Scholar]
- 83.Bowen SR, van der Kogel AJ, Nordsmark M, Bentzen SM, Jeraj R. Characterization of positron emission tomography hypoxia tracer uptake and tissue oxygenation via electrochemical modeling. Nucl Med Biol 2011; 38: 771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brahme A Biologically optimized 3-dimensional in vivo predictive assay-based radiation therapy using positron emission tomography-computerized tomography imaging. Acta Oncol 2003; 42: 123–36. [DOI] [PubMed] [Google Scholar]
- 85.Alber M, Paulsen F, Eschmann SM, Machulla HJ. On biologically conformal boost dose optimization. Phys Med Biol 2003; 48: N31–35. [DOI] [PubMed] [Google Scholar]
- 86.Deveau MA, Bowen SR, Westerly DC, Jeraj R. Feasibility and sensitivity study of helical tomotherapy for dose painting plans. Acta Oncol 2010; 49: 991–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korreman SS, Ulrich S, Bowen S, Deveau M, Bentzen SM, Jeraj R. Feasibility of dose painting using volumetric modulated arc optimization and delivery, Acta Oncol 2010; 49: 964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCall KC, Barbee DL, Kissick MW, Jeraj R. PET imaging for the quantification of biologically heterogeneous tumours: measuring the effect of relative position on image-based quantification of dose-painting targets. Phys Med Biol 2010; 55: 2789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Madani I, Duthoy W, Derie C, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2007; 68: 126–35. [DOI] [PubMed] [Google Scholar]
- 90.Duprez F, De Neve W, De Gersem W, Coghe M, Madani I. Adaptive dose painting by numbers for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2011; 80: 1045–55. [DOI] [PubMed] [Google Scholar]