Abstract
Standard-of-care imaging for initial staging of prostate cancer (PCa) underestimates disease burden. Prostate-specific membrane antigen (PSMA) PET/CT detects PCa metastasis with superior accuracy, having a potential impact on the planning of definitive radiation therapy (RT) for nonmetastatic PCa. Our objectives were to determine how often definitive RT planning based on standard target volumes covers 68Ga-PSMA-11 PET/CT–defined disease and to assess the potential impact of 68Ga-PSMA-11 PET/CT on definitive RT planning. Methods: This was a post hoc analysis of an intention-to-treat population of 73 patients with localized PCa without prior local therapy who underwent 68Ga-PSMA PET/CT for initial staging as part of an investigational new drug trial. Eleven of the 73 were intermediate-risk (15%), 33 were high-risk (45%), 22 were very-high-risk (30%), and 7 were N1 (9.5%). Clinical target volumes (CTVs), which included the prostate, seminal vesicles, and (in accord with the Radiation Therapy Oncology Group consensus guidelines) pelvic lymph nodes (LNs), were contoured on the CT portion of the PET/CT images by a radiation oncologist masked to the PET findings. 68Ga-PSMA-11 PET/CT images were analyzed by a nuclear medicine physician. 68Ga-PSMA-11–positive lesions not covered by planning volumes based on the CTVs were considered to have a major potential impact on treatment planning. Results: All patients had one or more 68Ga-PSMA-11–positive primary prostate lesions. Twenty-five (34%) and 7 (9.5%) of the 73 patients had 68Ga-PSMA-11–positive pelvic LN and distant metastases, respectively. The sites of LN metastases in decreasing order of frequency were external iliac (20.5%), common iliac (13.5%), internal iliac (12.5%) obturator (12.5%), perirectal (4%), abdominal (4%), upper diaphragm (4%), and presacral (1.5%). The median size of the LN lesions was 6 mm (range, 4–24 mm). RT planning based on the CTVs covered 69 (94.5%) of the 73 primary lesions and 20 (80%) of the 25 pelvic LN lesions, on a per-patient analysis. Conclusion: 68Ga-PSMA-11 PET/CT had a major impact on intended definitive RT planning for PCa in 12 (16.5%) of the 73 patients whose RT fields covered the prostate, seminal vesicles, and pelvic LNs and in 25 (37%) of the 66 patients whose RT fields covered the prostate and seminal vesicles but not the pelvic LNs.
Keywords: prostate cancer, PSMA, PET/CT, initial staging, definitive radiotherapy
The definitive treatment for patients with localized prostate cancer (PCa) is either radical prostatectomy or radiation therapy (RT) with or without androgen deprivation therapy (ADT). Ten-year progression-free survival after definitive RT for localized PCa (N0M0) ranges from 60% to 80% or higher depending on clinicopathologic features (1,2). The effectiveness of definitive RT depends on accurate staging to determine disease extent, including direct extraprostatic extension and pelvic lymph node (LN) metastasis.
Durable disease control depends on adequate dose delivery to target volumes covering both visible and occult disease. 99mTc bone scans and CT or MRI of the abdomen and pelvis are currently the standard of care for initial staging of PCa (3,4). The combined staging accuracy of these scans for metastasis detection is low, resulting in underestimation of disease burden (5–7). 68Ga-labeled prostate-specific membrane antigen (68Ga-PSMA-11) PET/CT is more sensitive for detecting pelvic and extrapelvic metastasis (8–10). Therefore, 68Ga-PSMA-11 PET/CT might improve the success rate of prostate RT by identifying patients with occult metastases and by modifying target volume delineations and dose to adequately cover local disease.
The clinical target volumes (CTVs) for definitive RT of PCa in patients without pathologic or radiographic evidence of pelvic LN metastases encompass the prostate and the seminal vesicles. Currently, there is no consensus on the value of pelvic LN RT in patients with intermediate-to high-risk PCa when the results of standard-of-care imaging are negative. Retrospective analyses suggest that outcomes improve when pelvic LNs are irradiated (11,12). Radiation Therapy Oncology Group (RTOG) 9413 was a 4-arm trial randomizing high-risk patients to prostate RT with versus without elective pelvic LN RT (up to L5/S1), and to neoadjuvant and concurrent versus adjuvant hormone therapy (13). There was an improvement in progression-free survival in patients who underwent pelvic LN RT (13) with neoadjuvant and concurrent hormone therapy, but this observation disappeared at further follow-up (14).
GETUG-01 randomized patients to prostate RT with versus without partial pelvic LN inclusion (superior field border at S1/S2) and showed no differences in outcomes (15). However, inadequate dose delivery to at-risk LN volumes, inadequate treatment of the primary tumor, and inclusion of patients at low risk of LN involvement may have reduced the power of these studies. RTOG 0924, currently accruing, may offer additional insights. Although not assessed by prospective randomized phase III trials, current guidelines support the inclusion of pelvic LNs for patients with radiographic N1 disease (16).
Small retrospective studies have suggested a major impact of 68Ga-PSMA-11 PET/CT on definitive RT planning for PCa in more than a third of patients (17–21). Here, we present a post hoc analysis of an intention-to-treat population of 73 patients with localized PCa by standard-of-care imaging (CT and bone scans) who are in an ongoing prospective study of 68Ga-PSMA-11 PET/CT (NCT03368547). This cohort of patients is representative of those who are routinely offered definitive RT in the absence of known extrapelvic disease. We mapped the location of 68Ga-PSMA-11 PET/CT–defined disease, determined how often planning of definitive RT based on standard-of-care imaging would fail to cover PSMA-positive disease, and simulated the impact of 68Ga-PSMA-11 PET/CT on definitive RT.
MATERIALS AND METHODS
Patients and Data Management
From December 2016 to December 2017, 73 patients with intermediate- or high-risk PCa (prostate-specific antigen [PSA] > 10 ng/mL, ≥T-stage 2b, or Gleason score > 6) without extrapelvic metastasis were enrolled into NCT03368547 at our institution. All underwent a 68Ga-PSMA-11 PET/CT scan for initial staging before intended radical prostatectomy with pelvic LN dissection, and all gave written consent to undergo the procedure (UCLA institutional review board approval 16-001684). The clinical data and DICOM files of the patients were anonymized and imported into a dedicated RT contouring workstation (MIM, version 6.7.5; MIM Software Inc.). The requirement to obtain informed consent was waived for this analysis (UCLA institutional review board approval 17-001824).
68Ga-PSMA-11 PET/CT Image Acquisition
68Ga-PSMA-11 (Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)]) was used as the PSMA ligand (22). The median injected activity was 196 MBq (range, 151–222 MBq). To reduce bladder activity, 37 patients without contraindications (50.5%) received 20 mg of furosemide at the time of tracer injection. The median uptake period was 60 min (range, 45–85 min). Images were acquired using a 64-detector PET/CT scanner (2007 Biograph 64 Truepoint or 2010 Biograph mCT 64; Siemens). A diagnostic CT scan (200–240 mAs, 120 kV) was obtained after intravenous contrast injection (n = 68/73 [93%], 115 mL of iohexol [Omnipaque; GE Healthcare], 350 mg of iodine/mL, injection speed of 2 mL/s, portal venous phase + 80 s after injection) and oral contrast ingestion (n = 72/73 [99%], 600 mL of 2.1% barium sulfate [Readi-Cat 2; Bracco]). The PET image acquisition included a whole-body scan, (pelvis to vertex, 2–4 min/bed position depending on patient weight), 1 dedicated pelvic scan after voiding (same bed time as used for the whole body), and 1 dedicated scan of the lower extremities (pelvis to toes, 1 min/bed position). All PET images were reconstructed with corrections for attenuation, dead-time, random events, and scatter, using iterative ordered-subsets expectation maximization in an axial 168 × 168 matrix for the Biograph 64 Truepoint (2 dimensions, 2 iterations, 8 subsets, 5.0-mm gaussian filter) or a 200 × 200 matrix for the Biograph mCT 64 (3 dimensions, 2 iterations, 24 subsets, 5.0-mm gaussian filter).
Simulation of RT Planning
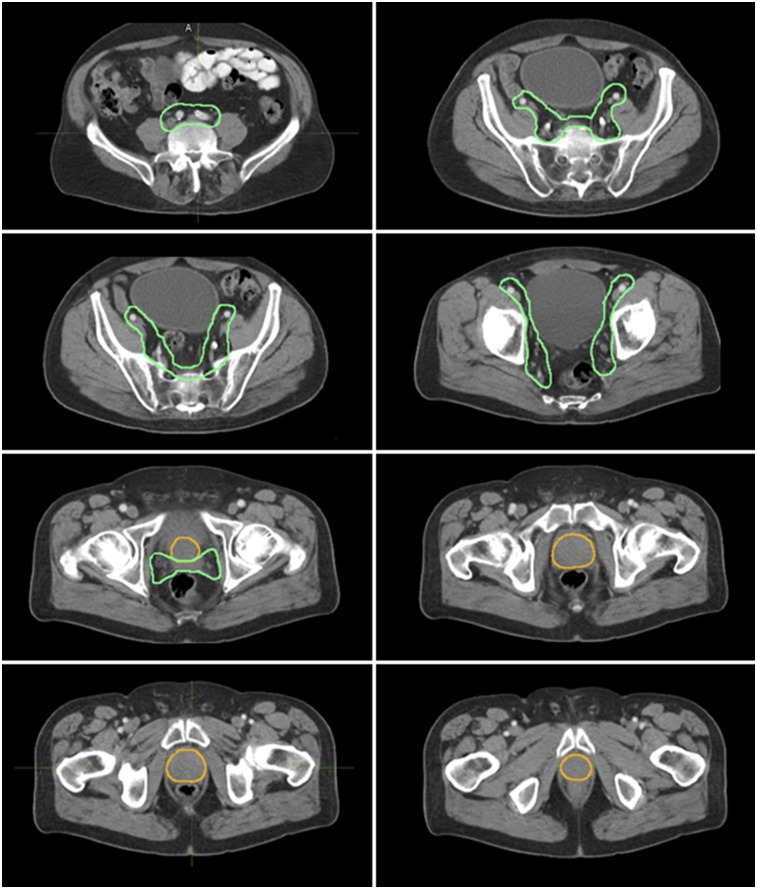
CTVs including the prostate, seminal vesicles, and pelvic LNs were contoured on the CT dataset of the PET/CT scan for all 73 patients by an experienced radiation oncologist masked to the 68Ga-PSMA-11 PET findings (Fig. 1). The prostate and seminal vesicle CTVs included the anatomic structures themselves and any tumor visible on the CT scan. Consensus RTOG contouring guidelines were used for the pelvic LN CTVs (23) except for the upper limit of the field: L4/L5 (rather than L5/S1). Briefly, the pelvic LN CTV included the presacral, distal common iliac, internal iliac, external iliac, and obturator LNs.
FIGURE 1.
Axial CT views of prostate CTV (yellow) and of pelvic LN and seminal vesicle CTV (green). CTVs were contoured on CT dataset of PET/CT for all 73 patients by experienced radiation oncologist who was masked to 68Ga-PSMA-11 PET findings. Pelvic LN CTV included presacral, distal common iliac, internal iliac, external iliac, and obturator LNs (upper limit, L4/L5).
68Ga-PSMA-11 PET/CT Image Analysis
All 68Ga-PSMA-11 PET/CT images were analyzed by an experienced nuclear medicine physician according to recent recommendations (24,25): any focal uptake of 68Ga-PSMA-11 above the surrounding background level and not associated with physiologic uptake or known pitfalls (26–29) was suspected of indicating malignancy. Distinction between malignant and inflammatory LNs (reactive, granuloma) was based on the degree of 68Ga-PSMA-11 uptake (typically intermediate or low for inflammation) and location (typically perihilar, axillary, or inguinal for inflammation). Based on TNM staging, the following regions were systematically analyzed (30): prostate/seminal vesicles (T), pelvic LNs (N) (internal iliac, obturator, external iliac, perirectal, presacral, common iliac), extrapelvic LNs (M1a) (abdominal, inguinal, upper diaphragm), bone (M1b), and other visceral organs (M1c).
68Ga-PSMA-11 PET Lesion Contouring
68Ga-PSMA-11–positive lesions were contoured to delineate the PET-based target volume of the primary tumor, in 2 steps. First, a volume of interest containing the whole prostate and excluding bladder activity was placed on the PET images to automatically generate the contours using a threshold-based segmentation method with a fixed cutoff of 40% of SUVmax (31,32). Manual adjustments were used for lesions with low tumor-to-background ratios, for obvious misregistration, or for correction of the CT contours of the positive lesions (fat-tissue borders, rectum, bladder). Positive LN contours were delineated by following the anatomic contours of the LN on CT.
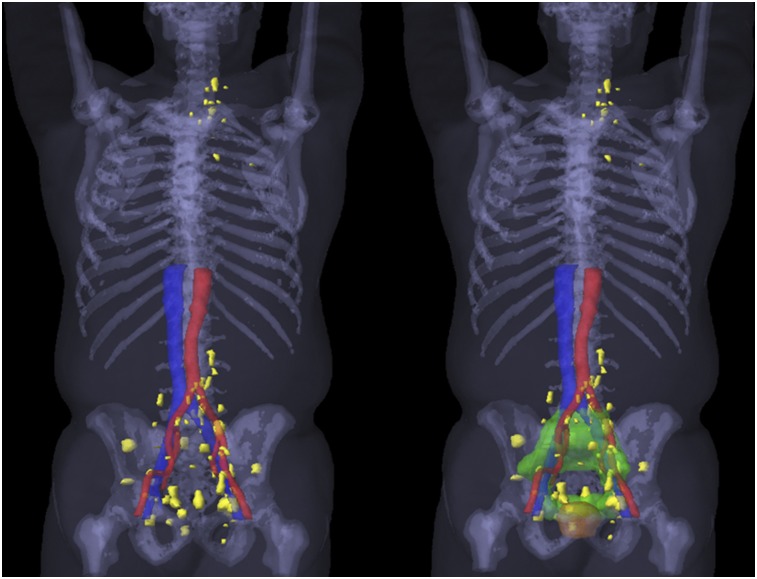
Second, a 3-dimensional rendering of all positive lesions across the entire study population was generated (Fig. 2). This step was achieved by rigid registration of each patient CT scan to a template patient CT scan, followed by transfer of each positive lesion contour onto the template patient CT using MIM.
FIGURE 2.
(Left) Three-dimensional rendering of all 68Ga-PSMA-11–positive lesions (yellow) in patients with extraprostatic metastasis: 20 N1M0 lesions (5 with out-of-field positive lesions), 3 N1M1a lesions, 2 N0M1b lesions, 1 N1M1aM1b lesion, and 1 N1M1bM1c lesion. (Right) Three-dimensional rendering of targeted volumes for prostate (yellow) and for pelvic LN plus seminal vesicles (green).
Assessment of 68Ga-PSMA-11 PET Lesion Coverage by CTVs
68Ga-PSMA-11–positive lesion contours were compared with the target volumes for each patient to assess whether positive lesions were within the irradiated volumes (Fig. 3) or outside them (Fig. 4).
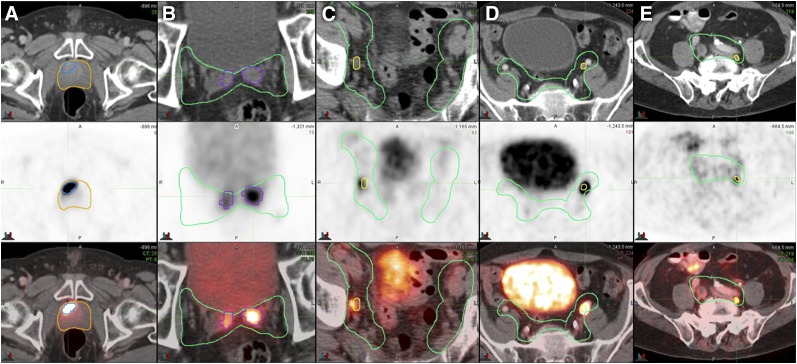
FIGURE 3.
Examples of 68Ga-PSMA-11–positive disease within radiation fields on axial CT (top), PET (middle), and PET/CT (bottom). Once positive lesions were identified on PET, contours of prostate CTV (yellow) and pelvic LN CTV (green) were drawn on the basis of CT. (A) Primary prostate tumor (MTV, 4 cm3; SUVmax, 34.6). (B) Invaded seminal vesicles (SUVmax, 18.0). (C) Right obturator LN (short axis, 6 mm; SUVmax, 4.6). (D) Left external iliac LN (short axis, 7 mm; SUVmax, 22.3). (E) Left common iliac LN (short axis, 5 mm; SUVmax, 4.1).
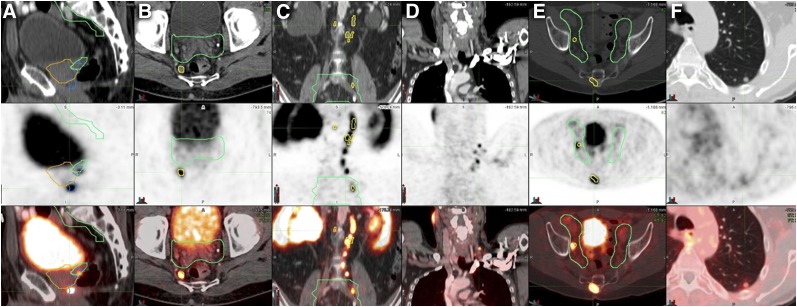
FIGURE 4.
Examples of 68Ga-PSMA-11–positive disease outside radiation fields on CT (top), PET (middle), and PET/CT (bottom). Once positive lesions were identified on PET, contours of prostate CTV (yellow) and pelvic LN CTV (green) were drawn on the basis of CT. (A) Primary prostate tumor (MTV, 3 cm3; SUVmax, 12) without CT correlate, located more than 1 cm below CTV. (B) Right perirectal LN (short axis, 8 mm; SUVmax, 6.1). (C) Multiple abdominal LNs (short axis, 4–7 mm; SUVmax, 4.7–17.2). (D) Multiple left subclavicular LNs (short axis, 3–4 mm; SUVmax, 3.0–9.1). (E) Sacral bone metastasis without CT correlate (SUVmax, 8.4). (F) Left lung nodule (short axis, 7 mm; SUVmax, 1.5).
Regarding the final planning target volumes, only positive lesion contours at least 10 mm away from the CTV were considered inadequately covered. Because many centers use CTV to plan target-volume expansions of much less than 10 mm, this analysis yields a maximally generous estimate of how often planning based on these CTVs offers coverage.
Potential Impact of 68Ga-PSMA-11 PET/CT on RT Planning
There is no official consensus on the indication for elective inclusion of radiographically negative pelvic LNs during definitive RT for PCa. Therefore, for patients without evidence of N1 disease on standard-of-care imaging, we analyzed the data for, first, intended treatment of the prostate and seminal vesicles along with the pelvic LNs and, second, intended treatment of the prostate and seminal vesicles but not the pelvic LNs.
Positive lesions not covered by planning volumes based on the CTVs were considered to have a major potential impact on treatment planning. This major impact was further subclassified as adding or extending the CTV to cover positive lesions within the pelvis; as adding metastasis-directed stereotactic body RT for extrapelvic oligometastatic disease (1–5 extrapelvic sites that were M1a or M1b); or as indicating that RT would be futile because of the presence of visible polymetastatic disease (>5 M1a or M1b lesions) or any visceral metastatic disease (M1c).
If positive lesions were covered by planning volumes based on the CTVs, the potential impact of 68Ga-PSMA-11 PET/CT on treatment planning was defined as minor (potential for dose escalation to gross disease [visibly positive]).
Negative studies were considered to have no impact on RT planning.
Statistical Analysis
We performed a post hoc analysis of this intention-to-treat population and simulated the impact of 68Ga-PSMA-11 PET/CT on intended definitive RT planning. Descriptive statistics were used (median, mean, and range). The Kruskal–Wallis rank sum test was used to compare the detection rate of extraprostatic 68Ga-PSMA-11–positive lesions (N1 or M1) by PSA level (<10, 10–20, >20 ng/mL).
RESULTS
Patient Characteristics
Table 1 summarizes the clinical and pathologic characteristics of the 73 patients. In brief, median age was 66 y (range, 45–91 y) and median serum PSA level was 13.9 ng/mL (mean, 42.69 ng/mL; range, 0.22–909 ng/mL). Seven of 73 patients (9.5%) had started ADT within a median of 5 wk before the 68Ga-PSMA-11 PET/CT study (mean, 8 wk; range, 1–28 wk). Eleven of 73 patients (15%) were National Comprehensive Cancer Network–defined intermediate-risk, 33 (45%) were high-risk, 22 (30%) were very-high-risk, and 7 (9.5%) were N1 by standard-of-care imaging (National Comprehensive Cancer Network risk groups are defined in Table 1).
TABLE 1.
Clinical and Pathologic Characteristics of the 73 Patients
| Characteristic | Data |
| Age at PET/CT (y) | |
| Median | 66 |
| Range | 45–91 |
| PSA before surgery | |
| Median (ng/mL) | 13.9 |
| Range (ng/mL) | 0.22–909 |
| 10 ng/mL (n) | 28 (38.5%) |
| ≥10 to <20 ng/mL (n) | 18 (24.5%) |
| ≥20 ng/mL (n) | 27 (37%) |
| Gleason score (n) | |
| ≤6 | 2 (2.5%) |
| 7 | 27 (37%) |
| ≥8 | 44 (60%) |
| Initial tumor stage* (n) | |
| T1–T2a | 14 (19%) |
| T2b–T2c | 8 (11%) |
| T3a | 20 (27.5%) |
| T3b–T4 | 8 (11%) |
| N1 | 6 (8%) |
| Unknown | 17 (23%) |
| NCCN risk group (n) | |
| Intermediate | 11 (15%) |
| High | 33 (45%) |
| Very high | 22 (30%) |
| N1 | 7 (9.5%) |
| Prior ADT (n) | 7 (9.5%) |
Clinical examination and CT/MRI.
NCCN (National Comprehensive Cancer Network) risk groups: intermediate (T2b–T2c, or Gleason score 3 + 4 = 7 [grade group 2], or Gleason score 4 + 3 = 7 [grade group 3], or PSA = 10–20 ng/mL); high (T3a, or Gleason score 8 [grade group 4], or Gleason score 9–10 [grade group 5], or PSA > 20 ng/mL); very high (T3b–T4, or primary Gleason pattern 5 [grade group 5], or >4 cores with Gleason score 8–10 [grade group 4 or 5]).
Upstaging by 68Ga-PSMA-11 PET/CT
Table 2 shows the 68Ga-PSMA-11 PET/CT–based staging of the 73 patients. Seven patients (9.5%) were M1: 4 with oligometastatic disease, 1 with a 68Ga-PSMA-11–positive lung lesion (M1c), and 2 with polymetastatic disease. Twenty-five patients (34%) were N1. The 3 patients with extrapelvic positive LNs also harbored positive pelvic LNs (N1M1a). Two patients with positive bone lesions had no positive LNs (N0M1b). Figure 2 displays a 3-dimensional rendering of all positive lesions in the 27 patients (37%) with extraprostatic metastasis (N1M0, N1M1, N0M1) coregistered on a template patient CT image. We found that extraprostatic positive disease was associated with PSA level. For a PSA level of less than 10, 10–20, and more than 20 ng/mL, extraprostatic (N1 or M1) positive lesions were seen in 6 of 28 patients (21.5%), 7 of 18 patients (39%), and 14 of 27 patients (52%), respectively (Kruskal–Wallis rank sum test, P = 0.020).
TABLE 2.
68Ga-PSMA-11 PET/CT Findings and Patterns
| Parameter | Total population (n = 73) | Patients without radiographic N1 disease (n = 66) |
| PSMA-positive findings* | ||
| N1 | 25 (34%) | 19 (29%) |
| M1 | 7 (9.5%) | 7 (10.5%) |
| M1a | 4 (5.5%) | 4 (6%) |
| M1b | 4 (5.5%) | 4 (6%) |
| M1c | 1 (1.5%) | 1 (1.5%) |
| PSMA patterns | ||
| N0M0 | 46 (63%) | 45 (68%) |
| N1M0 | 20 (27.5%) | 14 (21%) |
| N1M1a | 3 (4%) | 3 (4.5%) |
| N0M1b | 2 (2.5%) | 2 (3%) |
| N1M1aM1b | 1 (1.5%) | 1 (1.5%) |
| N1M1bM1c | 1 (1.5%) | 1 (1.5%) |
Percentages do not add up to 100 because multiple disease locations per patient were possible.
Data are number of patients.
68Ga-PSMA-11 PET/CT Findings and CTV Coverage
Table 3 details the 68Ga-PSMA-11–positive lesion locations and their coverage by the radiation fields. Twelve of 73 patients (16.5%) had at least 1 positive lesion not covered by either the prostate consensus CTV or the pelvic LN consensus CTV. Four of 73 (5.5%) had at least 1 cm of the primary prostate tumor not covered by the prostate CTV (Fig. 4A), 5 of 73 (7%) had at least 1 pelvic LN not covered by the pelvic LN CTV (Fig. 4B), and 7 of 73 (9.5%) had extrapelvic metastasis (Figs. 4C–4F). The anatomic location of the positive pelvic LNs was, in decreasing order, external iliac (20.5%), common iliac (13.5%), internal iliac (12.5%), obturator (12.5%), perirectal (4%), and presacral (1.5%). The perirectal and upper common iliac LNs were the 2 most common pelvic LN sites not covered by the CTVs.
TABLE 3.
Anatomic Repartition and Radiation Field Coverage of 68Ga-PSMA-11 PET/CT–Positive Findings, per Patient and per Lesion
| Patients (n) |
Lesions (n) |
Volume (cm3) or size (mm) |
SUVmax |
|||||
| Lesion site | PSMA-positive | Outside CTV | PSMA-positive | Outside CTV | Median | Range | Median | Range |
| Prostate gland (T+) | 73 (100%) | 4 (5.5%) | 107 | 4 | 7.46 cm3 | 1–65 cm3 | 11.2 | 3–53 |
| Pelvic LNs (N+) | 25 (34%) | 5 (7%) | 73 | 11 | 6.0 mm | 3.0–24.0 mm | 4.6 | 1.7–58.2 |
| External iliac | 15 (20.5%) | 1 (1.5%) | 27 | 1 | 8.0 mm | 4.0–24.0 mm | 5.8 | 1.7–31.5 |
| Common iliac | 10 (13.5%) | 3 (4%) | 15 | 5 | 5.0 mm | 3.5–12.0 mm | 3.9 | 2.0–25.6 |
| Internal iliac | 9 (12.5%) | 0 (0%) | 10 | 0 | 5.0 mm | 4.0–12.0 mm | 4.9 | 1.7–11.4 |
| Obturator | 9 (12.5%) | 0 (0%) | 14 | 0 | 6.0 mm | 4.0–11.0 mm | 4.8 | 2.9–16.5 |
| Perirectal | 3 (4%) | 3 (4%) | 4 | 4 | 9 mm | 5–17.0 mm | 11.4 | 2.1–58.2 |
| Presacral | 1 (1.5%) | 1 (1.5%) | 3 | 1 | 7.0 mm | 3.0–7.0 mm | 8.9 | 3.5–16.2 |
| Extrapelvic LNs (M1a) | 4 (5.5%) | 4 (5.5%) | 27 | 27 | 4.0 mm | 3.0–7.0 mm | 4.3 | 1.7–17.2 |
| Abdominal | 3 (4%) | 3 (4%) | 13 | 13 | 4.0 mm | 3.0–7.0 mm | 4.3 | 1.7–17.2 |
| Upper diaphragm | 3 (4%) | 3 (4%) | 14 | 14 | 4.5 mm | 3.5–7.0 mm | 3.4 | 2.7–9.1 |
| Bone (M1b) | 4 (5.5%) | 4 (5.5%) | 6 | 6 | NA | 4.0 | 3.0–8.0 | |
| Lung (M1c) | 1 (1.5%) | 1 (1.5%) | 1 | 1 | 7.0 mm | 1.50 | 1.50 | |
+ = positive; NA = not applicable.
Percentages do not add up to 100 because multiple disease locations per patient were possible.
Potential Impact of 68Ga-PSMA-11 PET/CT on Definitive RT Planning
Table 4 summarizes the potential impact of 68Ga-PSMA-11 PET/CT on prostate RT planning based on CTVs covering the prostate, seminal vesicles, and pelvic LNs (Table 4A) or the prostate and seminal vesicles alone (Table 4B).
TABLE 4.
Potential Impact of 68Ga-PSMA-11 PET/CT on RT Planning Based on CTVs Treating Prostate and Seminal Vesicles With or Without Pelvic LNs
| Parameter | n | Out-of-field PSMA-positive findings | PSMA pattern |
| RT to prostate and seminal vesicles with pelvic LNs | 73 | ||
| Major impact on RT planning outside CTV | 12 (16.5%) | ||
| Extension of prostate CTV | 1 (1.5%) | 1 T out | N0M0 |
| Extension of consensus pelvic LN CTV | 2 (2.5%) | 2 N out | N1M0 |
| Extension of both prostate CTV and consensus pelvic LN CTV | 2 (2.5%) | 2 T out + N out | N1M0 |
| Oligometastasis-directed SBRT (≤5 M1a or M1b) | 3 (4%) | 2 M1b | N0M1b |
| 1 M1a + M1b | N1M1aM1b | ||
| Oligometastasis-directed SBRT + extension of prostate CTV | 1 (1.5%) | 1 T out + M1b | N1M1a |
| RT futile because of polymetastatic or visceral disease | 3 (4%) | 1 M1a | N1M1a |
| 1 N out + M1a | N1M1a | ||
| 1 M1b + M1c | N1M1bM1c | ||
| RT to prostate and seminal vesicles without pelvic LNs | 66 | ||
| Major impact on RT planning outside CTV | 21 (32%) | ||
| Addition of whole pelvic LN CTV | 13 (19.5%) | 13 N out | N1M0 |
| Extension of prostate CTV | 1 (1.5%) | 1 T out | N0M0 |
| Extension of both prostate and consensus pelvic LN CTV | 1 (1.5%) | 1 T out + N out | N1M0 |
| Oligometastasis-directed SBRT (≤5 M1a or M1b) | 3 (4.5%) | 2 M1b | N0M1b |
| 1 M1a + M1b | N1M1aM1b | ||
| Oligometastasis-directed SBRT + extension of prostate CTV | 1 (1.5%) | 1 T out + M1a | N1M1a |
| RT futile because of polymetastatic or visceral disease | 3 (4.5%) | 1 M1a | N1M1a |
| 1 N out + M1a | N1M1a | ||
| 1 M1b + M1c | N1M1bM1c |
SBRT = stereotactic body RT.
Potential Major Impact on the 73 M0 Patients with Intention to Treat Prostate, Seminal Vesicles, and Pelvic LNs
Twelve of the 73 patients (16.5%) with M0 disease on standard-of-care imaging had at least 1 68Ga-PSMA-11–positive lesion not covered by the CTVs. One patient (1.5%) had a primary lesion not covered and could have benefited from an extended prostate CTV. Two patients (2.5%) had positive pelvic LNs that could have benefited from an extended pelvic LN CTV. Two patients (2.5%) had positive disease not covered both for the primary lesion and in the pelvis and would have benefited from extension of both the prostate CTV and the pelvic LN CTV. Four patients (5.5%) had oligometastatic M1a or M1b (≤5 metastatic sites) and could possibly have benefited from metastasis-directed therapy. Three patients (4.5%) had diffuse or visceral metastases.
Potential Major Impact on the 66 N0M0 Patients with Intention to Treat Prostate and Seminal Vesicles Alone
Twenty-one of the 66 patients (32%) with N1M0 disease on standard-of-care imaging had at least 1 68Ga-PSMA-11–positive lesion not covered by the CTVs. RT based on CTVs covering the prostate and seminal vesicles would not be curative for these patients. Thirteen of the 66 (19.5%) could have benefited from the addition of pelvic LN RT.
Potential Minor Impact
All patients had at least 1 68Ga-PSMA-11–positive lesion covered by the CTVs and might have benefited from focal dose escalation.
DISCUSSION
Most reports on the impact of 68Ga-PSMA-11 PET/CT on prostate RT have focused on patients with biochemical recurrence (21). Our prior investigation on a homogeneous cohort of 270 patients with biochemical recurrence and a PSA level of less than 1 ng/mL suggested a potential major impact on salvage RT planning in 19% of patients, even when generous coverage of the prostate bed and pelvic LNs was considered (33). Less is known about the impact of 68Ga-PSMA-11 PET/CT on definitive RT planning for the intact prostate. Prior published studies suggest that 68Ga-PSMA-11 PET/CT changed the definitive RT plan in 26%–33% of cases, but these studies included few patients (17–21). In the current post hoc analysis of 73 prospective patients with M0 localized PCa, we found that 68Ga-PSMA-11 PET/CT would have had a major impact in 16.5%–37%, depending on whether elective pelvic LN RT was initially intended.
Seven (9.5%) of our patients were M1 by 68Ga-PSMA-11 PET/CT, 25 (34%) were N1, 20 (27.5%) were N1M0, and 5 (7%) were N1M1. These percentages are consistent with prior reports of 108 patients who underwent 68Ga-PSMA-11 PET/CT for initial staging (34), with positive pelvic LNs and metastatic lesions being identified in 25% and 6% of patients, respectively.
The CTVs for definitive RT in intermediate- to high-risk patients always include the entire prostate drawn on the CT simulation scan. Contouring aided by prostate MRI, which more accurately identifies the extent of intraprostatic disease, is increasingly used. However, contouring based on MRI typically reduces rather than extends prostate CTVs. The extent of seminal vesicle inclusion varies by practitioner, but often the seminal vesicles are covered at least proximally if not entirely. We found that most 68Ga-PSMA-11–defined primary disease was covered by contouring on CT alone (69/73 patients [94.5%]). In 3 of the 4 patients in whom primary disease was insufficiently covered, distant positive disease was also present.
Many radiation oncologists follow guidelines when contouring pelvic LNs (23). RT planning based on standard RTOG pelvic LN CTVs would have covered all positive disease in 20 of the 25 68Ga-PSMA-11 N1 patients (80%). In total, 73 positive pelvic LNs were identified, 11 (15%) of which would not have been covered. Most of the positive LNs not covered were perirectal (n = 4) or common iliac (n = 4). The median size of positive LNs was 6 mm, underscoring the known fact that most PCa LN metastases will be missed on the basis of size or morphologic criteria on CT or MRI.
When the initial intent was to cover the prostate, seminal vesicles, and pelvic LNs, the presence of 68Ga-PSMA-11 M1 lesions (7 of 73 patients [9.5%]) accounted for the main impact of 68Ga-PSMA-11 PET/CT on definitive RT planning, whereas 5 of 73 patients (7%) had local positive pelvic disease that could be covered by extension of standard CTVs. Notably, 4 of the 7 patients with M1 disease had oligometastatic disease, suggesting a possible role for metastasis-directed therapy in most M1 patients in this cohort.
When the initial intent was to cover the prostate and seminal vesicles alone, the addition of a standard pelvic LN CTV (13/66 patients [19.5%]) accounted for the main impact of 68Ga-PSMA-11 PET/CT on definitive RT planning. Given this finding, it is somewhat surprising that results from prior phase III trials of elective pelvic LN RT are controversial (13–15). The commonly prescribed dose of 45 Gy may be insufficient to control gross disease in the pelvic LNs, which was present in a third of patients in our study. An alternative to dose escalation is radiosensitization with concurrent ADT (35,36). Notably, in RTOG 9413, the progression-free-survival benefit with the addition of elective pelvic LN RT was observed only in the arm that received neoadjuvant and concurrent ADT and was absent from the arm that received purely adjuvant ADT. Enhanced radiosensitization with newer agents (e.g., abiraterone acetate or enzalutamide) in combination with gonadotropin-releasing hormone analogs is another strategy to increase dose efficacy without compromise to adjacent organs at risk.
This study had 2 major limitations. It was a post hoc retrospective analysis. The design precluded analysis of the actual impact of 68Ga-PSMA-11 PET/CT on RT planning and outcomes. To minimize bias, drawing of consensus CTVs was masked to the 68Ga-PSMA-11 PET images.
The other main limitation was the absence of lesion verification. Therefore, we cannot formally exclude potential 68Ga-PSMA-11 false-positive findings such as follicular hyperplasia or granulomatosis in LNs and benign fracture, fibrous dysplasia, or Paget disease in bones (26–29). However, the interpreting nuclear medicine physician was careful to avoid known pitfalls such as ganglia (29) and to examine both the 68Ga-PSMA-11 PET scan and the CT scan. Moreover, we focused on the potential impact of 68Ga-PSMA-11 PET/CT on RT planning based on conventional imaging that rarely involves metastatic biopsies. It is unlikely that a treating radiation oncologist would ignore positive disease during planning, even allowing for some frequency of false-positive lesions.
CONCLUSION
This post hoc analysis of an intention-to-treat cohort of 73 patients with localized PCa representative of those who are routinely offered curative-intent prostate RT suggests a potential major impact of 68Ga-PSMA-11 PET/CT on definitive RT planning in 12 of the 73 patients (16.5%) for whom RT was planned to cover the prostate, seminal vesicles, and pelvic LNs and in 25 of the 66 patients (37%) for whom RT was planned to cover the prostate and seminal vesicles alone.
DISCLOSURE
Jeremie Calais is the recipient of a grant from the Philippe Foundation Inc. Wolfgang Fendler received a scholarship (grant 807122) from the Deutsche Forschungsgemeinschaft (DFG). Matthias Eiber was supported by the DFG (sonderforschungsbereich 824, project B11). Nicholas Nickols received a Young Investigator Award from the Prostate Cancer Foundation, a level 2 Career Development Award (5IK2BX002520) from the U.S. Department of Veterans Affairs, and a Research Career Development Award from STOP CANCER. Johannes Czernin received a grant (DE SC0012353) from the U.S. Department of Energy, a 2017 Challenge Award (17CHAL02) from the Prostate Cancer Foundation, and NIH-NCI Cancer Center Support Grant P30 CA016042 to the Jonsson Comprehensive Cancer Center. He is also a founder and board member, and holds equity in, Sofie Biosciences and Trethera Therapeutics. Intellectual property was patented by the University of California and licensed to Sofie Biosciences and Trethera Therapeutics. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank Remy Niman, Bill Carruthers, and Joe Meyers (MIM Software Inc.) and Zhouhuizi Shen for assistance with generating the 3-dimensional rendering map.
REFERENCES
- 1.Kishan AU, Shaikh T, Wang P-C, et al. Clinical outcomes for patients with Gleason score 9-10 prostate adenocarcinoma treated with radiotherapy or radical prostatectomy: a multi-institutional comparative analysis. Eur Urol. 2017;71:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alicikus ZA, Yamada Y, Zhang Z, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117:1429–1437. [DOI] [PubMed] [Google Scholar]
- 3.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. December 15, 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199:990–997. [DOI] [PubMed] [Google Scholar]
- 5.Lecouvet FE, Geukens D, Stainier A, et al. Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol. 2007;25:3281–3287. [DOI] [PubMed] [Google Scholar]
- 6.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 7.Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. [DOI] [PubMed] [Google Scholar]
- 8.Gupta M, Choudhury PS, Hazarika D, Rawal S. A comparative study of 68gallium-prostate specific membrane antigen positron emission tomography-computed tomography and magnetic resonance imaging for lymph node staging in high risk prostate cancer patients: an initial experience. World J Nucl Med. 2017;16:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinsensia M, Chyoke PL, Hadaschik B, et al. 68Ga-PSMA PET/CT and volumetric morphology of PET-positive lymph nodes stratified by tumor differentiation of prostate cancer. J Nucl Med. 2017;58:1949–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Öbek C, Doğanca T, Demirci E, et al. The accuracy of 68Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1806–1812. [DOI] [PubMed] [Google Scholar]
- 11.Seaward SA, Weinberg V, Lewis P, Leigh B, Phillips TL, Roach M. Identification of a high-risk clinically localized prostate cancer subgroup receiving maximum benefit from whole-pelvic irradiation. Cancer J Sci Am. 1998;4:370–377. [PubMed] [Google Scholar]
- 12.Pan CC, Kim KY, Taylor JMG, McLaughlin PW, Sandler HM. Influence of 3D-CRT pelvic irradiation on outcome in prostate cancer treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2002;53:1139–1145. [DOI] [PubMed] [Google Scholar]
- 13.Roach M, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21:1904–1911. [DOI] [PubMed] [Google Scholar]
- 14.Lawton CA, DeSilvio M, Roach M, et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int J Radiat Oncol Biol Phys. 2007;69:646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pommier P, Chabaud S, Lagrange JL, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol. 2007;25:5366–5373. [DOI] [PubMed] [Google Scholar]
- 16.James ND, Spears MR, Clarke NW, et al. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: data from patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2016;2:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterzing F, Kratochwil C, Fiedler H, et al. 68Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewes S, Schiller K, Sauter K, et al. Integration of 68Ga-PSMA-PET imaging in planning of primary definitive radiotherapy in prostate cancer: a retrospective study. Radiat Oncol. 2016;11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shakespeare TP. Effect of prostate-specific membrane antigen positron emission tomography on the decision-making of radiation oncologists. Radiat Oncol. 2015;10:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt-Hegemann N-S, Fendler WP, Buchner A, et al. Detection level and pattern of positive lesions using PSMA PET/CT for staging prior to radiation therapy. Radiat Oncol. 2017;12:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calais J, Cao M, Nickols NG. The utility of PET/CT in the planning of external radiation therapy for prostate cancer. J Nucl Med. 2018;59:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eder M, Schäfer M, Bauder-Wüst U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–697. [DOI] [PubMed] [Google Scholar]
- 23.Lawton CAF, Michalski J, El-Naqa I, et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. 68Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging—version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 26.Schwarzenboeck SM, Rauscher I, Bluemel C, et al. PSMA ligands for PET imaging of prostate cancer. J Nucl Med. 2017;58:1545–1552. [DOI] [PubMed] [Google Scholar]
- 27.Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–217. [DOI] [PubMed] [Google Scholar]
- 28.Fendler WP, Calais J, Allen-Auerbach M, et al. 68Ga-PSMA-11 PET/CT interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–1623. [DOI] [PubMed] [Google Scholar]
- 29.Rischpler C, Beck TI, Okamoto S, et al. 68Ga-PSMA-HBED-CC uptake in cervical, coeliac and sacral ganglia as an important pitfall in prostate cancer PET imaging. J Nucl Med. January 25, 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Eiber M, Herrmann K, Calais J, et al. PROstate cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 31.Zamboglou C, Schiller F, Fechter T, et al. 68Ga-HBED-CC-PSMA PET/CT versus histopathology in primary localized prostate cancer: a voxel-wise comparison. Theranostics. 2016;6:1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boellaard R, Delgado-Bolton R, Oyen WJG, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging—version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calais J, Czernin J, Cao M, et al. 68Ga-PSMA PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roach PJ, Francis R, Emmett L, et al. The impact of 68 Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med. 2018;59:82–88. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin JF, Schiewer MJ, Dean JL, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3:1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]