Abstract
Background
The objective of this study was to assess, whether right atrial (RA) and ventricular (RV) size is related to RV pump function at rest and during exercise in patients with pulmonary arterial hypertension (PAH).
Methods
We included 54 patients with invasively diagnosed PAH that had been stable on targeted medication. All patients underwent clinical assessments including right heart catheterization and echocardiography at rest and during exercise. RV output reserve was defined as increase of cardiac index (CI) from rest to peak exercise (∆CIexercise). Patients were classified according to the median of RA and RV-area. RV pump function and further clinical parameters were compared between groups by student’s t-test. Uni- and multivariate Pearson correlation analyses were performed.
Results
Patients with larger RA and/or RV-areas (above a median of 16 and 20cm2, respectively) showed significantly lower ∆CIexercise, higher mean pulmonary arterial pressure, pulmonary vascular resistance at rest and NT-proBNP levels. Furthermore, patients with higher RV-areas presented with a significantly lower RV stroke volume and pulmonary arterial compliance at peak exercise than patients with smaller RV-size. RV area was identified as the only independent predictor of RV output reserve.
Conclusion
RV and RA areas represent valuable and easily accessible indicators of RV pump function at rest and during exercise. Cardiac output reserve should be considered as an important clinical parameter. Prospective studies are needed for further evaluation.
Keywords: Pulmonary hypertension, Right ventricular output reserve, Pump function, Right ventricular size, Right atrial size
Background
Pulmonary arterial hypertension (PAH) is a complex cardiopulmonary disorder, characterized by progressive changes affecting both the pulmonary vasculature and the right heart [1, 2]. Although the initial pathological changes occur on pulmonary arterioles causing increased pulmonary vascular resistance (PVR), adaptation of right ventricular (RV) pump function is a key determinant of survival [2, 3]. Rising attention is drawn to the concept of RV-arterial coupling, a composite measure of RV pump function and ventricular load [4–6].
Right atrial (RA) [7–9] and RV size have repeatedly been proven of prognostic significance in pulmonary hypertension [2, 10], whereas their impact on RV contractility remains to be determined. Recent studies using magnetic resonance imaging (MRI) have shown, that increased RV-endsystolic or diastolic volumes were significantly related to a worse outcome and reduced RV stroke volume (SV) [11]. In a further study enlargement of RV volumes during follow-up was associated with further clinical signs of disease progression [12].
RV output reserve (∆CIexercise) defined as increase of cardiac output/cardiac index (CI) during exercise with normal or elevated PVR measured by right heart catheterization (RHC) is an emerging parameter which has shown to be prognostically important in patients with PAH [13, 14]. It solely displays the capacity of the right ventricle to adjust its systolic function to a given level of pulmonary loading4. Pulmonary arterial compliance (PAC) reflects the elasticity of the pulmonary arteries. For estimation of pulmonary arterial compliance (or capacitance) the measurement of SV/pulse pressure (cardiac output/heart rate)/(systolicPAP-diastolicPAP) by RHC has been shown to be the most simple and practical method [15, 16].
The objective of the study was to investigate the correlation between right heart size (measured as right atrial and ventricular area by echocardiography) and RV pump function at rest and during exercise (assessed by RHC) and further hemodynamic and clinical parameters. Furthermore, this study aimed to detect correlations and determining factors of RV pump function.
Methods
Patient selection
We retrospectively reviewed all incident (i.e. newly diagnosed) patients aged ≥18 to 80 years with idiopathic, heritable or drug- and toxin-induced or connective tissue disease associated PAH who were diagnosed at the PH-center in Heidelberg between January 1st, 2016 and November 31st, 2016. Inclusion required RHC at rest (confirming PAH, defined as a mean pulmonary arterial pressure ⩾25 mmHg, pulmonary arterial wedge pressure ⩽15 mmHg and PVR > 3 Wood units [17], and during exercise. Diagnosis of PAH was performed according to the ESC/ERS guidelines [17].
Patients were excluded if they lacked a complete evaluation including medical history, WHO/NYHA functional class assessment, physical examination, electrocardiogram, transthoracic 2D-echocardiography at rest, lung function test, arterial blood gases, 6-min walking distance (6MWD) under standardized conditions [18], laboratory testing including NT-proBNP levels. All examinations were performed at the Thoraxklinik at Heidelberg University Hospital by experienced physicians within 48 h from the right heart catheterization.
Right heart catheterization
The hemodynamic values have been obtained by the charts. The right heart catheterization has been performed in a standardized way in a supine position using the transjugular access with a triple-lumen 7F-Swan-Ganz thermodilution catheter at rest and during exercise as previously described [19]. Patients had been examined on a variable load supine bicycle ergometer by experienced investigators (CN, BE, SH). Pressures were continuously recorded and averaged over several respiratory cycles during spontaneous breathing, both at rest and during exercise. Cardiac output (CO) was measured by thermodilution at least in triplicate with a variation of less than 10% between the measured values. The zero reference point for pressure recordings was set at ½ of the thoracic diameter below the anterior thorax surface [20]. After the hemodynamic measurement at rest, the supine position was changed to a 45° position. Calibration for exercise measurements were performed as previously described [21]. The exercise test was started with a workload of 25 W. Workload was incrementally increased by 25 W every 2 min to an exercise capacity or symptom limited maximum.
Echocardiography
Resting two dimensional transthoracic echocardiography (TTE) Doppler examinations were performed by experienced cardiac sonographers (EG, CN, BE, SH) with commercially available equipment (Vivid 7, GE Healthcare, Milwaukee, Wisconsin) according to standardized protocol as described previously [9, 22]. TTE measurements were obtained off line from stored DICOM data according to the European Association of Cardiovascular Imaging (EACVI) Guidelines [23]. Specific indices included RA-/RV-area, TAPSE and PASP at rest. For all calculations the mean value of at least 3 measurements was used. PASP was estimated from peak tricuspid regurgitation jet velocities (TRV) according to the equation: PASP = 4 (V) [2] + right atrial pressure, where V is the peak velocity (in m/s) of tricuspid regurgitation jet (TRV) [24]. Right atrial pressure was estimated from characteristics of the inferior vena cava [18]. If it was < 20 mm in diameter and decreased during inspiration we added 5 mmHg, ≥20 mm we added 10 mmHg and 15 mmHg if no decrease of diameter during inspiration occurred.
Cardiopulmonary exercise testing
Patients were examined on a variable load supine bicycle ergometer (model 8420; KHL Corp., Kirkland, Washington) in Heidelberg as described previously [25]. Workload was increased by 25 W every 2 min to an exercise capacity or symptom limited maximum. Peak VO2 was defined as the highest 30-s average value of oxygen uptake during the last minute of the exercise test.
Ethics statement
The Ethics Committee of the Medical Faculty, University of Heidelberg had no obligation against the conduct of the study (internal number S425/2016). All data were anonymized and the study was conducted in accordance with the amended Declaration of Helsinki.
Statistical methods
Statistical analyses were conducted by two biometricians (CF, NE). Data are described as means ± standard deviations or number and respective percentage. Patients were divided into two groups according to their RV size (larger or smaller RA and/or RV area with value above or below the median of the complete sample). A receiver operating characteristic (ROC) curve analysis for RA and RV area with CI increase below the median of the sample as outcome parameter for further validation of the cut-off values was performed. Quantitative characteristics between the two groups including demographics, hemodynamics and parameters of echocardiography and cardiopulmonary exercise testing were compared by two-sided student’s t-tests and nonparametric tests if needed. Frequency distributions were compared by chi-square test or Fisher’s exact test. A sensitivity analysis with a threshold of 18 cm2 for RV area according to the cut-off proposed by the guidelines17 was performed.
Right heart size (RA and RV area) was compared between patients with higher vs. lower ∆CIexercise (according to the median of the complete sample).
Differences of the course of CI and SV increase during exercise between patients with smaller vs. larger RA and RV area were analysed with mixed ANOVA. To investigate the associations between clinical parameters, right heart size and output reserve, Pearson’s correlation analysis was performed. To identify independent predictors of RV output reserve, multivariate analysis was performed by stepwise forward selection method of logistic regression with the dichotomous variable of the two groups (high or low ∆CIexercise) as outcome variable. Parameters for correlation analysis included demographics, hemodynamics, echocardiographic parameters and measures of cardiopulmonary exercise testing according to clinical significance.
Pulmonary arterial compliance (PAC) was calculated according the formula PAC = SV/ pulse pressure with SV = CO/Heart rate and pulse pressure = sPAP-dPAP. Stroke volume index was calculated with SVI = CI / heart rate.
All tests were two-sided and a pointwise p-value of 0.05 was considered statistically significant. All analyses have been performed using IBM SPSS 23 (SPSS Statistics V23, IBM Corporation, Somers, New York).
Results
Study population (Table 1)
Table 1.
Characteristics of the study population
| mean ± SD or n (%) | ||||||
|---|---|---|---|---|---|---|
| Demographics | Age | (years) | 53 | ± | 14.65 | |
| BMI | (kg/m 2 ) | 27.9 | ± | 5.69 | ||
| Gender | male | n (%) | 21 | (38.9) | ||
| female | n (%) | 33 | (61.1) | |||
| Diagnosis | IPAH | n (%) | 31 | (57.4) | ||
| HPAH | n (%) | 8 | (14.8) | |||
| APAH | n (%) | 12 | (22.2) | |||
| CTEPH | n (%) | 3 | (5.6) | |||
| WHO functional class | I | n (%) | 1 | (1.9) | ||
| II | n (%) | 36 | (66.7) | |||
| III | n (%) | 17 | (31.5) | |||
| PAH-targeted medication | Endothelin receptor antagonist | 40 | (74.1) | |||
| Phosphodiesterase-5-inhibitors | 38 | (70.4) | ||||
| Soluble guanylate cyclase-stimulator | 8 | (13.0) | ||||
| Prostanoids | 6 | (14.8) | ||||
| Calcium channel blockers | 2 | (03.7) | ||||
| Combination therapy | ||||||
| Mono | n (%) | 18 | (33.3) | |||
| Double | n (%) | 31 | (57.4) | |||
| Triple | n (%) | 5 | (9.3) | |||
| RHC | Rest | mPAP | (mmHg) | 35.5 | ± | 11.69 |
| sPAP | (mmHg) | 57.6 | ± | 20.87 | ||
| dPAP | (mmHg) | 23 | ± | 7.87 | ||
| PCWP | (mmHg) | 10 | ± | 3.54 | ||
| PVR | (dyn*sec*cm −5 ) | 393.4 | ± | 235.03 | ||
| CO | (l/min) | 5.8 | ± | 1.61 | ||
| CI | (l/min/m 2 ) | 3 | ± | 0.73 | ||
| SVI | (ml/m 2 ) | 41.1 | ± | 10.2 | ||
| 25 W | ∆ CI | (l/min/m 2 ) | 1.2 | ± | 0.67 | |
| 50 W | ∆ CI | (l/min/m 2 ) | 2 | ± | 0.93 | |
| 75 W | ∆ CI | (l/min/m 2 ) | 2.6 | ± | 1.15 | |
| Peak | mPAP | (mmHg) | 56.5 | ± | 15.91 | |
| sPAP | (mmHg) | 90.2 | ± | 28.36 | ||
| dPAP | (mmHg) | 36.1 | ± | 11.56 | ||
| CO | (l/min) | 10.2 | ± | 3.49 | ||
| CI | (l/min/m 2 ) | 5.3 | ± | 1.59 | ||
| SVI | (ml/m 2 ) | 47.2 | ± | 13.9 | ||
| Echocardiography | RV area | (cm 2 ) | 20.1 | ± | 5.59 | |
| RA area | (cm 2 ) | 16.8 | ± | 6.62 | ||
| TAPSE | (cm) | 2.3 | ± | 0.38 | ||
| Cardiopulmonary exercise testing (CPET) | peak V’O2 | (ml/min) | 1126 | ± | 428.88 | |
| peak V’O2/kg | (ml/min/kg) | 14.1 | ± | 3.92 | ||
| sPAP Max | (mmHg) | 81.8 | ± | 27.87 | ||
| 6-MWD | (m) | 423 | ± | 113.09 | ||
| Laboratory analysis | NT-proBNP | (pg/ml) | 470.3 | ± | 856.74 | |
| Pulmonary function test (PFT) | DLCOc SB | (% Soll) | 58.6 | ± | 17.25 | |
| DLCOc VA | (% Soll) | 70.42 | ± | 20.00 | ||
IPAH = idiopathic pulmonary arterial hypertension, HPAH = heritable PAH, APAH = associated PAH, CTEPH = chronic thromboembolic PH, RHC = right heart catheter, BMI = Body Mass Index, RV = right ventricular, RA = right atrial, TAPSE = tricuspid annular plane systolic excursion, VO’2 = oxygen consumption, NT-proBNP = N-terminal pro brain natriuretic peptide, DLCOc SB = diffusing capacity transfer factor, DLCOc / VA = diffusing capacity transfer coefficient, mPAP = mean pulmonary arterial pressure, sPAP = systolic PAP, dPAP = diastolic PAP, PCWP = pulmonary capillary wedge pressure, PVR = pulmonary vascular resistance, CI = Cardiac Index, SVI = stroke volume index, HR = heart rate, SV = stroke volume, ∆ = difference
We included 54 patients diagnosed with moderate to severe PAH who fulfilled the inclusion criteria (21 males and 33 females, mean age 53 ± 15 years, 66.7% WHO functional class II, 57.4% double combination therapy; Table 1).
The study cohort presented with a median RA of 16cm2 and RV of 20 cm2. ROC curve analysis for RA and RV area with CI increase < 2.1 l/min/m2 (median of the sample for CI increase) further supported these proposed cutoff-values of 16cm2 for RA and 20cm2 for RV area (Fig. 1). For RV area, 20cm2 showed a sensitivity of 75% and specificity of 73.1%; an RA area of 16cm2 presented with a sensitivity of 75% and specificity of 57.7%.
Fig. 1.
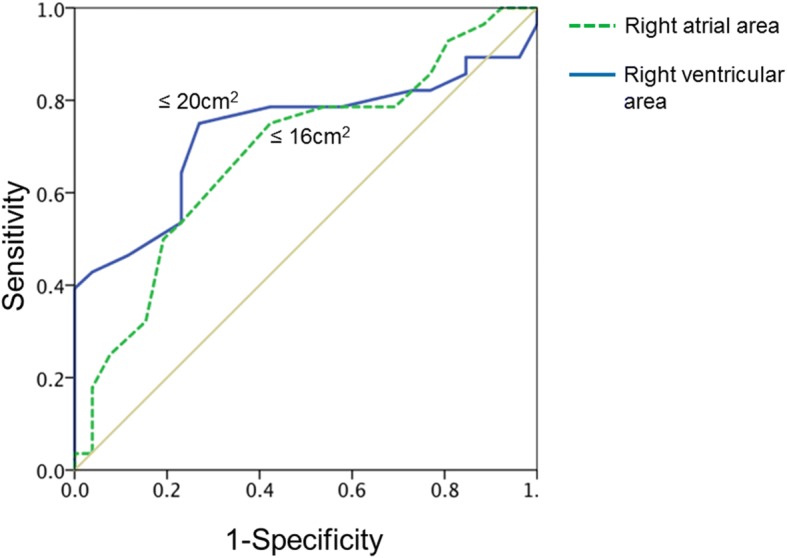
ROC curve analysis. For RV area, 20cm2 showed a sensitivity of 75% and specificity of 73.1%; an RA area of 16cm2 presented with a sensitivity of 75% and specificity of 57.7%
Characteristics of groups with small and large right heart size: According to the median RA and RV area, two subgroups were defined for both RA and RV area: 1) “enlarged right heart size” (RA >16cm2, RV >20cm2) and 2) “normal/smaller right heart size” (RA ≤16cm2, RV ≤20cm2; Table 2).
Table 2.
Comparison of patients with small and large right heart size
| n | RA area ≤ 16 cm2 | n | RA area > 16 cm2 | p-value | n | RV area ≤ 20 cm2 | n | RV area > 20 cm2 | p-value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | Age | (years) | 33 | 53.6 | ± | 14.4 | 21 | 52.1 | ± | 15.3 | 0.71 | 30 | 51.4 | ± | 13.7 | 24 | 55.1 | ± | 15.8 | 0.354 | |||
| BMI | (kg/m 2 ) | 33 | 28.3 | ± | 6.5 | 21 | 27.3 | ± | 4.3 | 0.537 | 30 | 27.5 | ± | 5.5 | 24 | 28.4 | ± | 6.1 | 0.591 | ||||
| 6-MWD | (m) | 32 | 429 | ± | 124 | 21 | 414 | ± | 97 | 0.655 | 30 | 436 | ± | 127 | 23 | 406 | ± | 92 | 0.350 | ||||
| PAH-targeted medication | ERA | 24 | (72.7%) | 16 | (76.2%) | 1.0 | 22 | (73.3%) | 18 | (75%) | 1.0 | ||||||||||||
| PDE5-I | 22 | (66.7%) | 16 | (76.2%) | 0.549 | 21 | (70%) | 17 | (70.8%) | 1.0 | |||||||||||||
| sGC stimulator | 3 | (9.1%) | 5 | (23.8%) | 0.238 | 3 | (10%) | 5 | (20.8%) | 0.443 | |||||||||||||
| Prostanoids | 3 | (9.1%) | 3 | (14.3%) | 0.667 | 2 | (6.7%) | 4 | (16.7%) | 0.389 | |||||||||||||
| Calcium channel blockers | 2 | (6.1%) | 0 | 0.516 | 2 | (6.7%) | 0 | 0.497 | |||||||||||||||
| Combination therapy | Mono | n (%) | 13 | (39.4%) | 5 | (23.8%) | 0.102 | 11 | (36.7%) | 7 | (29.2%) | 0.414 | |||||||||||
| Double | n (%) | 17 | (51.5%) | 14 | (66.7%) | 17 | (56.7%) | 14 | (58.3%) | ||||||||||||||
| Triple | n (%) | 3 | (9.1%) | 2 | (9.5%) | 2 | (6.6%) | 3 | (12.5%) | ||||||||||||||
| Echocardiography | RV area | (cm 2 ) | 33 | 17.3 | ± | 3.9 | 21 | 24.6 | ± | 4.9 | < 0.0001 | * | 30 | 16.3 | ± | 3.1 | 24 | 25.0 | ± | 4.0 | < 0.0001 | * | |
| RA area | (cm 2 ) | 33 | 13.2 | ± | 2.7 | 21 | 22.4 | ± | 7.1 | < 0.0001 | * | 30 | 13.3 | ± | 3.2 | 24 | 21.1 | ± | 7.2 | < 0.0001 | * | ||
| sPAP | (mmHg) | 33 | 42 | ± | 16 | 21 | 57 | ± | 17 | 0.003 | * | 30 | 42 | ± | 12 | 24 | 56 | 20 | 0.006 | * | |||
| TAPSE | (cm) | 33 | 2.4 | ± | 0.4 | 21 | 2.3 | ± | 0.4 | 0.362 | 30 | 2.4 | ± | 0.4 | 24 | 2.3 | ± | 0.4 | 0.204 | ||||
| Cardiopulmonary Exercise Testing | peak V’O2 | (ml/min) | 33 | 1155 | ± | 495 | 21 | 1080 | ± | 302 | 0.536 | 30 | 1144 | ± | 435 | 24 | 1103 | ± | 429 | 0.730 | |||
| peak V’O2/kg | (ml/min/kg) | 33 | 14.5 | ± | 4.1 | 21 | 13.4 | ± | 3.7 | 0.321 | 30 | 14.7 | ± | 3.6 | 24 | 13.2 | ± | 4.2 | 0.166 | ||||
| Laboratory analysis | NT-proBNP | (pg/ml) | 33 | 191 | ± | 231 | 20 | 931 | ± | 1266 | 0.018 | * | 30 | 145 | ± | 125 | 23 | 895 | ± | 1191 | 0.006 | * | |
| Pulmonary function test | DLCOc SB | (% Soll) | 30 | 58.6 | ± | 18.6 | 18 | 58.6 | ± | 15.2 | 0.988 | 27 | 60.5 | ± | 15.3 | 21 | 56.2 | ± | 19.6 | 0.398 | |||
| DLCOc VA | (% Soll) | 30 | 69.0 | ± | 21.2 | 18 | 72.8 | ± | 18.1 | 0.522 | 27 | 70.1 | ± | 19.3 | 21 | 70.8 | ± | 21.3 | 0.906 | ||||
| RHC | Rest | mPAP | (mmHg) | 33 | 32 | ± | 10 | 21 | 41 | ± | 13 | 0.012 | * | 30 | 32 | ± | 9 | 24 | 40 | ± | 13 | 0.014 | * |
| sPAP | (mmHg) | 33 | 51 | ± | 17 | 21 | 68 | ± | 22 | 0.002 | * | 30 | 51 | ± | 16 | 24 | 66 | ± | 24 | 0.013 | * | ||
| dPAP | (mmHg) | 33 | 21 | ± | 6 | 21 | 26 | ± | 9 | 0.032 | * | 30 | 21 | ± | 6 | 24 | 26 | ± | 9 | 0.018 | * | ||
| PAWP | (mmHg) | 33 | 10 | ± | 3 | 21 | 10 | ± | 4 | 0.514 | 30 | 10 | ± | 3 | 24 | 10 | ± | 4 | 0.852 | ||||
| PVR | (dyn*sec*cm − 5 ) | 33 | 335 | ± | 201 | 21 | 486 | ± | 259 | 0.03 | * | 30 | 311 | ± | 149 | 24 | 496 | ± | 282 | 0.006 | * | ||
| CI | (l/min/m 2 ) | 33 | 3.08 | ± | 0.63 | 21 | 2.93 | ± | 0.88 | 0.470 | 30 | 3.19 | ± | 0.67 | 24 | 2.82 | ± | 0.77 | 0.062 | ||||
| HR | (1/min) | 33 | 74 | ± | 12 | 21 | 76 | ± | 12 | 0.480 | 30 | 75 | ± | 10 | 24 | 75 | ± | 13 | 0.927 | ||||
| SV | (ml) | 33 | 79.4 | ± | 21.2 | 21 | 77.1 | ± | 25.4 | 0.716 | 30 | 80.1 | ± | 19.0 | 24 | 76.4 | ± | 27.0 | 0.556 | ||||
| SVI | (ml/m 2 ) | 33 | 42.2 | ± | 8.4 | 21 | 39.3 | ± | 12.6 | 0.317 | 30 | 42.9 | ± | 8.4 | 24 | 38.8 | ± | 11.9 | 0.142 | ||||
| 25 W | ∆ CI | (l/min/m 2 ) | 31 | 1.4 | ± | 0.7 | 20 | 0.9 | ± | 0.5 | 0.008 | * | 28 | 1.3 | ± | 0.7 | 23 | 1.0 | ± | 0.6 | 0.114 | ||
| HR | (1/min) | 31 | 91 | ± | 18 | 20 | 93 | ± | 12 | 0.572 | 28 | 91 | ± | 18 | 23 | 93 | ± | 13 | 0.623 | ||||
| ∆ SV | (ml) | 31 | 14.6 | ± | 20.4 | 20 | 6.2 | ± | 12.3 | 0.105 | 28 | 15.0 | ± | 21.5 | 23 | 6.9 | ± | 11.5 | 0.112 | ||||
| 50 W | ∆ CI | (l/min/m 2 ) | 31 | 2.2 | ± | 1.0 | 18 | 1.6 | ± | 0.7 | 0.027 | * | 28 | 2.28 | ± | 0.91 | 21 | 1.51 | ± | 0.76 | 0.003 | * | |
| HR | (1/min) | 31 | 103 | ± | 17 | 18 | 105 | ± | 17 | 0.639 | 28 | 102 | ± | 18 | 21 | 106 | ± | 15 | 0.345 | ||||
| ∆ SV | (ml) | 31 | 18.0 | ± | 22.7 | 18 | 7.2 | ± | 13.1 | 0.041 | * | 28 | 21.0 | ± | 22.5 | 21 | 4.7 | ± | 12.0 | 0.002 | * | ||
| 75 W | ∆ CI | (l/min/m 2 ) | 18 | 2.9 | ± | 1.2 | 9 | 2.0 | ± | 0.6 | 0.043 | * | 16 | 2.93 | ± | 1.17 | 11 | 2.09 | ± | 0.96 | 0.060 | ||
| HR | (1/min) | 18 | 113 | ± | 16 | 9 | 112 | ± | 12 | 0.813 | 16 | 111 | ± | 17 | 11 | 115 | ± | 10 | 0.541 | ||||
| ∆ SV | (ml) | 18 | 22.2 | ± | 18.1 | 9 | 7.4 | ± | 18.1 | 0.045 | * | 16 | 25.0 | ± | 14.2 | 11 | 6.0 | ± | 18.2 | 0.005 | * | ||
| Peak | mPAP | (mmHg) | 33 | 54 | ± | 15 | 21 | 61 | ± | 17 | 0.115 | 30 | 54 | ± | 15 | 24 | 60 | ± | 17 | 0.124 | |||
| sPAP | (mmHg) | 33 | 84 | ± | 26 | 21 | 100 | ± | 30 | 0.049 | * | 30 | 85 | ± | 25 | 24 | 97 | ± | 31 | 0.144 | |||
| dPAP | (mmHg) | 33 | 35 | ± | 12 | 21 | 38 | ± | 11 | 0.254 | 30 | 34 | ± | 12 | 24 | 39 | ± | 10 | 0.111 | ||||
| CI | (l/min/m 2 ) | 33 | 5.62 | ± | 1.57 | 21 | 4.80 | ± | 1.52 | 0.049 | * | 30 | 5.92 | ± | 1.43 | 24 | 4.52 | ± | 1.45 | 0.001 | * | ||
| ∆ CI | (l/min/m 2 ) | 33 | 2.54 | ± | 1.42 | 21 | 1.86 | ± | 0.83 | 0.033 | * | 30 | 2.73 | ± | 1.34 | 24 | 1.7 | ± | 0.88 | 0.002 | * | ||
| SV | (ml) | 33 | 93.3 | ± | 26.7 | 21 | 86.0 | ± | 33.8 | 0.383 | 30 | 0.1 | ± | 0.027 | 24 | 0.08 | ± | 0.029 | 0.007 | * | |||
| PAC | (ml/mmHg) | 33 | 39.0 | ± | 13.7 | 21 | 33.1 | ± | 12.4 | 0.108 | 30 | 39.5 | ± | 11.2 | 24 | 33.2 | ± | 15.3 | 0.027 | * | |||
| SVI | (l/m2) | 33 | 49.4 | ± | 11.5 | 21 | 43.7 | ± | 16.6 | 0.141 | 30 | 53.1 | ± | 11.1 | 24 | 39.8 | ± | 13.7 | < 0.001 | * | |||
ERA = Endothelin receptor antagonist, PDE5-I = Phosphodiesterase-5-inhibitors, sGC stimulator = Soluble guanylate cyclase-stimulator, RHC = right heart catheter, BMI = Body Mass Index, RV = right ventricular, RA = right atrial, TAPSE = tricuspid annular plane systolic excursion, VO’2 = oxygen consumption, NT-proBNP = N-terminal pro brain natriuretic peptide, DLCOc SB = diffusion capacity transfer factor, DLCOc / VA = diffusion capacity transfer coefficient, mPAP = mean pulmonary arterial pressure, sPAP = systolic PAP, dPAP = diastolic PAP, PAWP = pulmonary arterial wedge pressure, PVR = pulmonary vascular resistance, CI = Cardiac Index, SVI = stroke volume index, HR = heart rate, SV = stroke volume, ∆ = difference
* = significant at level 0.05.; values are given as mean ± standard deviation or n (%)
Both groups did not significantly differ in their demographics (age and BMI), 6MWD, diffusion capacity and peak VO2 for both RA and RV area. PH-targeted treatment and distribution of combination treatment did also not significantly differ between groups (Table 2).
Patients with enlarged RA- (n = 21) and/or RV-area (n = 24) had significantly higher mean, systolic and diastolic pulmonary arterial pressures, mean pulmonary vascular resistance, and NT-proBNP levels than patients with normal or smaller right heart size.
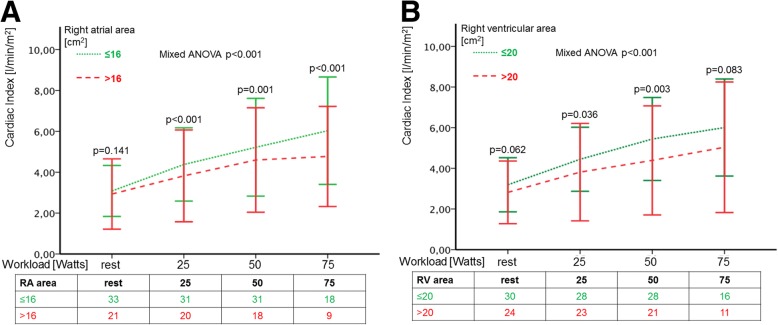
Both groups of RA and RV size had well preserved RV function at rest, represented by regular CI and SV, even though PVR and mean pulmonary arterial pressures were elevated in patients with enlarged right heart size. Increase of CI during exercise was significantly smaller in patients with enlarged RA- or RV-areas (Fig. 2a and b). Furthermore, patients with higher RV-area, but not RA-area, presented with a significantly lower SV, SVI and pulmonary arterial compliance at peak exercise than patients with smaller RV-size (Table 2).
Fig. 2.
Course of CI during exercise according to RA (a) and RV area (b). Patients with smaller (RA ≤ median 16 cm2, RV ≤ median 20 cm2, dotted line) right heart size showed significantly higher CI during exercise, than patients with larger right heart size (RA > median 16 cm2, RV > median 20 cm2, dashed line; mixed ANOVA p < 0.001). Bars indicate 2 standard deviations of the mean
SV failed to increase in accordance with the exposed exercise level in patients with large RA- (p = 0.031) and/or RV area (p < 0.001; Fig. 3). Likewise, SVI was significantly higher in patients with small right heart size, compared to patients with enlarged RA and/or RV area (ANOVA RV p < 0.001, RA p = 0.001). Furthermore, patients with smaller RV, but not RA, presented with significantly higher peak PAC than patients with RV area above the median (39.5 ± 11.2 ml/mmHg vs. 33.2 ± 15.3 ml/mmHg, p = 0.027).
Fig. 3.
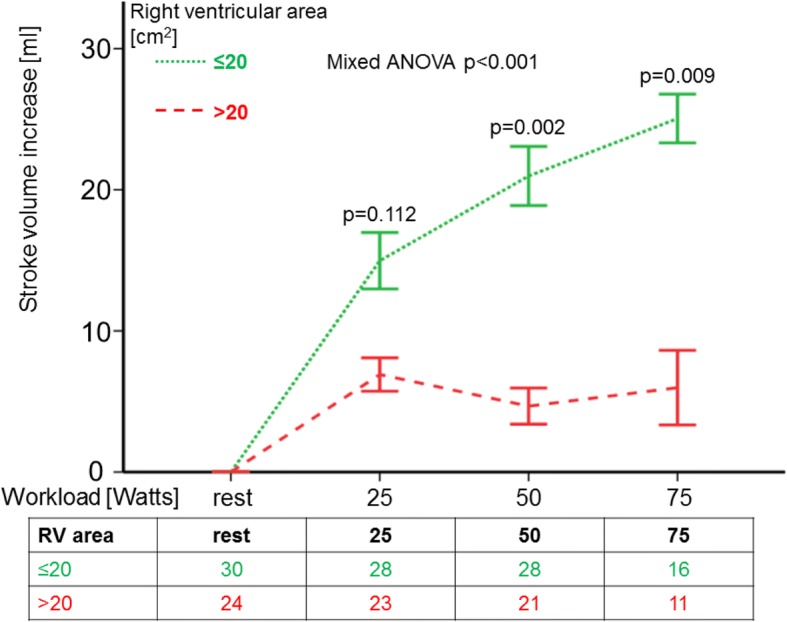
Course of stroke volume increase during exercise according to RV area. Patients with smaller (≤ median 20 cm2, dotted line) RV area showed significantly higher SV increase during exercise, than patients with larger RV area (> median 20 cm2; dashed line; mixed ANOVA p < 0.001). Bars indicate the standard errors of the mean difference
Sensitivity analysis with a threshold of 18 cm2 for RV area led to the same differences between groups with small and large right heart size. Furthermore, CI increase showed a statistically significant difference for each workload level.
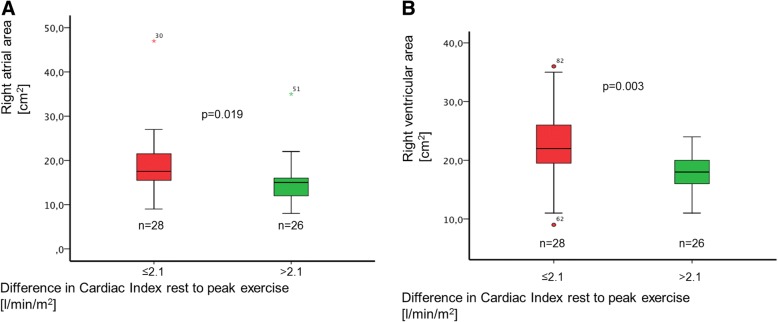
When dichotomising the patient cohort according to RV output reserve (high and low ∆CIexercise) echocardiography showed considerable differences in RV and RA area (p = 0.003 and p = 0.019 respectively; Fig. 4a and b).
Fig. 4.
Difference of right heart size in patients with high and low ∆CI. Right heart size significantly differed between patients with high and low ∆CIexercise according to the median of the complete sample of 2.1 L/min/m2 (a) RA area p = 0.019, (b) RV area p = 0.003; identical p-values for nonparametric and parametric testing)
Factors associated with right heart size and RV output reserve (Tables 3 and 4)
Table 3.
Correlation analysis of right heart size and clinical parameters
| Right atrial area | Right ventricular area | |||||||
|---|---|---|---|---|---|---|---|---|
| n | pearson’s R | p-value | n | pearson’s R | p-value | |||
| Univariate analysis | ||||||||
| Age | 54 | 0.193 | 0.162 | 54 | - 0.048 | 0.209 | ||
| Body mass index | 54 | 0.181 | 0.190 | 54 | 0.733 | 0.129 | ||
| 6-min walking distance | 53 | - 0.108 | 0.441 | 53 | - 0.121 | 0.387 | ||
| NT-proBNP | 53 | 0.539 | < 0.001 | * | 53 | 0.538 | < 0.001 | * |
| Echocardiography | ||||||||
| Systolic pulmonary arterial pressure | 54 | 0.307 | 0.024 | * | 54 | 0.567 | < 0.001 | * |
| Right atrial area | – | 54 | 0.703 | < 0.001 | * | |||
| Right ventricular area | 54 | 0.703 | < 0.001 | * | – | |||
| Tricuspid annular plane systolic excursion | 54 | - 0.128 | 0.356 | 54 | - 0.082 | 0.554 | ||
| Cardiopulmonary exercise testing | ||||||||
| Peak oxygen consumption (V’O2) | 54 | 0.042 | 0.736 | 54 | 0.051 | 0.713 | ||
| Peak oxygen consumption/kg (V’O2/kg) | 54 | - 0.199 | 0.149 | 54 | - 0.227 | 0.099 | ||
| Right heart catheter | ||||||||
| rest | ||||||||
| Mean pulmonary arterial pressure | 54 | 0.176 | 0.202 | 54 | 0.544 | < 0.001 | * | |
| Cardiac Output | 54 | - 0.028 | 0.839 | 54 | - 0.052 | 0.709 | ||
| Cardiac Index | 54 | - 0.209 | 0.129 | 54 | - 0.281 | 0.040 | * | |
| Pulmonary arterial wedge pressure | 54 | 0.025 | 0.857 | 54 | - 0.101 | 0.467 | ||
| Pulmonary vascular resistance | 54 | 0.175 | 0.206 | 54 | 0.508 | < 0.001 | * | |
| Stroke volume index | 54 | −0.244 | 0.076 | 54 | −0.301 | 0.027 | * | |
| exercise | ||||||||
| Mean pulmonary arterial pressure | 54 | 0.097 | 0.486 | 54 | 0.419 | 0.002 | * | |
| Cardiac Output | 54 | - 0.177 | 0.200 | 54 | - 0.223 | 0.104 | ||
| Cardiac Index | 54 | - 0.344 | 0.011 | * | 54 | - 0.427 | 0.001 | * |
| ∆ CI peak | 54 | - 0.313 | 0.021 | * | 54 | - 0.376 | 0.005 | * |
| Stroke volume index | 54 | −0.264 | 0.054 | 54 | −0.407 | 0.002 | * | |
| Lung function / Diffusing capacity | ||||||||
| DLCOc SB | 48 | - 0.052 | 0.723 | 48 | - 0.003 | 0.982 | ||
| DLCOc /VA | 48 | 0.176 | 0.231 | 48 | 0.137 | 0.352 | ||
CI = Cardiac Index, NT-proBNP = N-terminal pro brain natriuretic peptide, DLCOc SB = diffusion capacity transfer factor, DLCOc /VA = diffusion capacity transfer coefficient
* = significant at level 0.05
Table 4.
Uni- and multivariate regression analysis of RV output reserve
| Univariate analysis (∆ CI Peak) | n | pearson’s R | p-value | |
|---|---|---|---|---|
| Age | 54 | 0.424 | 0.001 | * |
| Body mass index | 54 | 0.092 | 0.506 | |
| 6 min walking distance | 54 | 0.278 | 0.044 | * |
| NT-proBNP | 54 | - 0.360 | 0.008 | * |
| Echocardiography | ||||
| Systolic pulmonary arterial pressure | 54 | - 0.462 | < 0.001 | * |
| Right atrial area | 54 | - 0.313 | 0.021 | * |
| Right ventricular area | 54 | - 0.376 | 0.005 | * |
| Tricuspid annular plane systolic excursion | 54 | 0.065 | 0.64 | |
| Cardiopulmonary exercise testing | ||||
| peak oxygen consumption (V’O2) | 54 | 0.466 | < 0.001 | * |
| peak oxygen consumption/kg (V’O2/kg) | 54 | 0.380 | 0.005 | * |
| Right heart catheter | ||||
| rest | ||||
| mean pulmonary arterial pressure | 54 | - 0.288 | 0.035 | * |
| Cardiac Output | 54 | 0.282 | 0.039 | * |
| Cardiac Index | 54 | 0.223 | 0.106 | |
| Pulmonary arterial wedge pressure | 54 | - 0.016 | 0.906 | |
| pulmonary vascular resistance | 54 | - 0.366 | 0.006 | * |
| exercise | ||||
| mean pulmonary arterial pressure | 54 | - 0.073 | 0.598 | |
| Cardiac Output | 54 | 0.839 | < 0.001 | * |
| Cardiac Index | 54 | 0.894 | < 0.001 | * |
| Lung function / Diffusing capacity | ||||
| DLCOc SB | 48 | 0.361 | 0.012 | * |
| DLCOc / VA | 48 | 0.342 | 0.017 | * |
| Multivariate analysis | ||||
| Logistic Regression (stepwise forward selection) | ||||
| ∆ CI exrcise (dichotomous) | Exp (B) | |||
| Right ventricular area | 47 | 0.863 | 0.027 | * |
| Linear Regression (stepwise forward selection) | ||||
| ∆ CI Peak (continuous) | pearson’s R | |||
| Right ventricular area | 47 | - 0.360 | 0.003 | * |
| Age | 47 | - 0.412 | 0.001 | * |
CI = Cardiac Index, NT-proBNP = N-terminal pro brain natriuretic peptide, DLCOc SB = diffusing capacity transfer factor, DLCOc / VA = diffusing capacity transfer coefficient
* = significant at level 0.05, Exp (B) = Regression coefficient
Univariate analysis of right heart size and output reserve
In univariate regression analysis RV and RA area were significantly correlated with NT-proBNP, sPAP, CI during exercise, ∆CIPeak (Fig. 5) and with right heart size (Table 3). RV area additionally significantly correlated with mPAP at rest, CI at rest, PVR at rest and peak mPAP.
Figure 5.
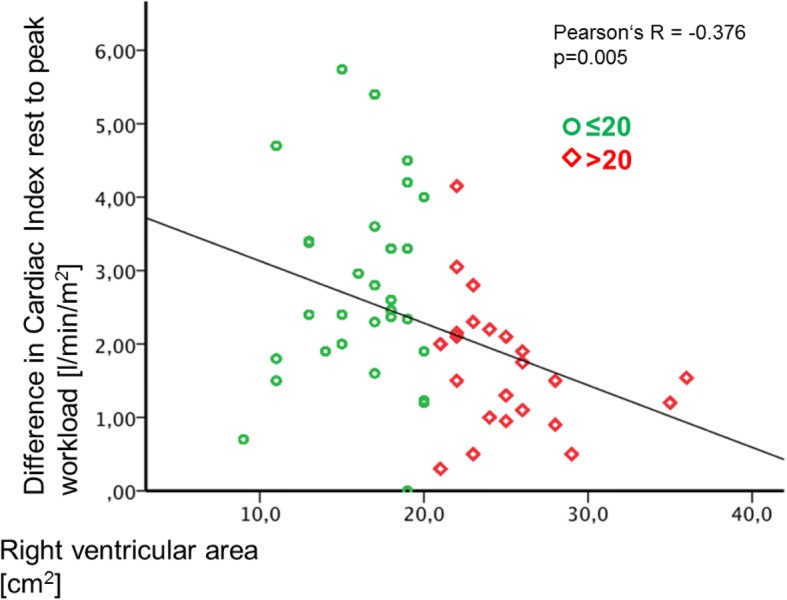
Correlation of RV-Area and ∆CIexercise. RV area showed a significant negative correlation with ∆CIexercise (p = 0.005)
∆CIexercise significantly positively correlated with age, exercise capacity (6-MWD, peak oxygen consumption, peak oxygen consumption/kg/min), hemodynamics (CO at rest; peak CO and CI during exercise) and lung diffusing capacity (transfer factor DLCOc SB and transfer coefficient DLCOc / VA) (Table 4). A negative correlation was detected between ∆CIexercise and NT-proBNP, echocardiographic parameters (sPAP, RA area, RV area) and hemodynamics at rest (mPAP, PVR).
Multivariate analysis of output reserve
Stepwise forward selection of multivariate logistic regression analysis showed RV area to be the single independent predictor for high or low ∆CIPeak (regression coefficient 0.863, p = 0.027).
Discussion
To the best of our knowledge this is the first study showing that in patents with PAH enlarged RV- or RA areas (measured by echocardiography) were associated with a significantly reduced RV pump function during exercise (lower ∆CIexercise) measured by right heart catheterization. Furthermore, the study revealed that PAH-patients with larger size of the right heart had higher pulmonary arterial pressures, pulmonary vascular resistance and NT-proBNP levels. Patients with higher RV-areas presented with a significantly lower stroke volume index and pulmonary arterial compliance at peak exercise than patients with smaller RV-size. RV area was identified as the only independent predictor of RV output reserve (lower ∆CIexercise). Thus, this study gives further evidence that assessing the right heart size by imaging techniques as echocardiography gives further important clues to RV pump function and cardiopulmonary hemodynamics.
Right heart size, pump function
This study confirms the results of previous studies using MRI which showed that enlarged RV end systolic and end-diastolic volumes were obtained in patients with lower RV stroke volumes [12, 26]. However, in the first previous MRI-study RV volumes were not directly compared with pump function but with survival [26]. Large RV end-diastolic volume and SV at baseline were associated with poorer prognosis. Further dilation of RV with further decrease of SV during follow-up predicted a poor long-term outcome [26]. Most recently these findings have been confirmed by an analysis of the French PAH registry demonstrating that SVI and right atrial pressure were independently associated with death or lung transplantation at first follow-up after initial PAH treatment [27].
Our study demonstrates for the first time a negative relationship between right heart size and RV pump function using 2-D-echocardiography for assessing the RA- and RV-areas in the four chamber view and hemodynamic values from right heart catheterization at rest and during exercise. Patients with enlarged RV area had significantly lower CI and SVI at rest and during exercise. These patients had also higher mean pulmonary arterial pressure, pulmonary vascular resistance at rest and NT-proBNP levels which reflects a more severe disease. The negative impact of RV-enlargement was also demonstrated by a MRI-study which showed in patients with increasing RV volumes during follow-up a disease progression leading to death or transplantation whereas patients with stable RV volumes remained clinically stable [12]. Changes in RV volumes were even more sensitive parameters for deterioration than the repeated measurement of hemodynamics which remained unchanged [12]. In this study, patients with enlargement of RV volumes had a decline of RV ejection fraction [12]. Two further studies demonstrated a reduction in RV volumes by targeted PAH-therapy, which suggests an improvement of RV pump function [11, 28].
RV output reserve and right heart size
RV output reserve, defined in this study as increase of CI during exercise measured by right heart catheterization, is an emerging parameter which has shown to be of prognostic importance in patients with PH [13, 14]. In this study RV area was identified as the only independent predictor of RV output reserve. This again shows that RV size may reflect the impairment of RV pump function. We hypothesize that increased PAC and reduced increase of RV output during exercise in patients with larger RV and/or RA areas, respectively is due to more severe pulmonary vascular disease. A both reproducible and clinically practical way to evaluate RV output reserve can be performed by invasive measurements15. Further prospective studies have to be conducted to evaluate the magnitude of the relation to right heart size and if non/invasive assessment of RA- and RV area or volume are useful for an estimation of RV output reserve.
Advanced PAH with increased pulmonary load leads to RV dilatation (heterometric adaptation) in order to maintain SV6. In this study RV output reserve was significantly linked to RV size.
Study limitations
The retrospective, single-center design of this study with a rather small number of subjects limits the study results. A higher sample size may have led to identification of more independent factors in the multivariate analysis.
Echocardiographic assessments of the right heart are complicated by its complex shape and morphology. Especially in obese patients, patients with chest wall deformities or COPD, the correct assessment of RV size and function becomes a challenging task. In this respect, cardiac MRI becomes particularly appealing, as it does provide a thorough assessment of right heart size and function even in complicated conditions. In our cohort, high quality recordings were used and no comorbidities were interfering the test results. As the determination of RA and RV area is a readily available assessment which is practicable in a good quality, its application in clinical practice is more common compared to cardiac MRI. Unfortunately, no MRI data is available for this patient cohort to confirm the hemodynamic data.
Invasive measurements, cardiopulmonary exercise testing and echocardiographic parameters could not be assessed in one single examination. In order to reduce the influence of inter-exam variations, we only included patients that underwent all measures within a time frame of 48 h. The assessment of CI may be complicated by tricuspid insufficiency in patients with PH. Due to the bidirectional blood flow through the tricuspid valve CI may be overestimated in some patients, which may have influenced the results.
The correlation of right heart size and function to TAPSE and other parameters and their prognostic value should be investigated in a larger-scale study.
Conclusion
The study shows that assessment of right heart size is important for RV functional characterization and may be helpful since it reflects RV pump function and RV output reserve. RV and RA area by 2-D echocardiography represented a valuable and easily accessible indicator of RV pump function at rest and during exercise. Therefore, these results may be relevant for clinical practice. RV output reserve should be considered as an important clinical parameter. However, prospective studies are needed for further evaluation.
Acknowledgements
Not applicable.
Funding
No funding was received for this study.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ∆
Difference
- 6MWD
6-min walking distance
- ANOVA
Analysis of variance
- APAH
Associated PAH
- BMI
Body Mass Index
- CI
Cardiac Index
- CO
Cardiac Output
- CTEPH
Chronic thromboembolic pulmonary hypertension
- DLCOc / VA
Diffusing capacity transfer coefficient
- DLCOc SB
Diffusing capacity transfer factor
- dPAP
Diastolic PAP
- Exp (B)
Regression coefficient
- HPAH
Heritable PAH
- HR
Heart rate
- IPAH
Idiopathic pulmonary arterial hypertension
- mPAP
Mean pulmonary arterial pressure
- MRI
Magnetic resonance imaging
- NT-proBNP
N-terminal pro brain natriuretic peptide
- PAC
Pulmonary arterial compliance
- PAH
Pulmonary arterial hypertension
- PAP
Pulmonary arterial pressure
- PASP
Pulmonary arterial systolic pressure
- PCWP
Pulmonary capillary wedge pressure
- PH
Pulmonary hypertension
- PVR
Pulmonary vascular resistance
- RA
Right atrial
- RHC
Right heart catheter
- RV
Right ventricular
- sPAP
Systolic PAP
- SV
Stroke volume
- SVI
Stroke volume index
- TAPSE
Tricuspid annular plane systolic excursion
- TRV
Tricuspid regurgitation velocity
- TTE
Transthoracic echocardiography
- VO’2
Oxygen consumption
Authors’ contributions
This work was the doctoral thesis of LF. EG, LF, CN, AMM, EB, NB, CF contributed substantially to the study conception and design. EG, LF, CN, BE, SH performed the assessments and patients examinations. LF, MK performed the data collection. NB, CF, LF performed the data analysis. All authors contributed to data interpretation and to the writing of the manuscript. All authors have read and approved the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The Ethics Committee of the Medical Faculty, University of Heidelberg had no obligation against the conduct of the study (internal number S425/2016). All data were anonymized and the study was conducted in accordance with the amended Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
LF nothing to disclose. NB received speaker honoraria and travel support from Actelion and Bayer outside the submitted work. BE nothing to disclose. SH received travel support from Actelion and OMT outside the submitted work. MK nothing to disclose. HML received consultancy / speaker fees from: AbbVie, Bristol-Myers Squibb, Roche-Chugai, UCB, MSD, GSK, Sobi, Medac, Novartis, Janssen-Cilag, AstraZeneca, Pfizer, Actelion; speakers bureau: AbbVie, Bristol-Myers Squibb, Roche-Chugai, UCB, MSD, GSK, Sobi, Medac, Novartis, Janssen-Cilag, AstraZeneca, Pfizer, Actelion outside the submitted work. NB received speaker honoraria from Actelion pharmaceuticals. AMM received grants from Italian Helthcare Ministry, grant for young researchers “Ricerca finalizzata 2016 per giovani ricercatori” n. GR-2016-02364727, personal lecture fee from Bayer Healthcare outside the submitted work;. CN reports honoraria for lectures and participation in clinical trials from Actelion, Bayer/MSD, Novartis, speaker honoraria from Boehringer, Astra Zeneca, Berlin Chemie and participation in clinical trials from GSK, United Therapeutics outside the submitted work. CF nothing to disclose. EB nothing to disclose. EG received advisory board member and speaker honoraria from Actelion, Bayer/MSD, GSK, United Therapeutics, Novartis, Pfizer, OrphaSwiss GmbH outside the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Ann Intern Med. 1991;115(5):343–355. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25):D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Amsallem M, Boulate D, Aymami M, et al. Load adaptability in patients with pulmonary arterial hypertension. Am J Cardiol. 2017;120(5):874–882. doi: 10.1016/j.amjcard.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 4.Naeije R, Manes A. The right ventricle in pulmonary arterial hypertension. Eur Respir Rev. 2014;23(134):476–487. doi: 10.1183/09059180.00007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101(1):37–43. doi: 10.1136/heartjnl-2014-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69(2):236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Bustamante-Labarta M, Perrone S, De La Fuente RL, et al. Right atrial size and tricuspid regurgitation severity predict mortality or transplantation in primary pulmonary hypertension. J Am Soc Echocardiogr. 2002;15(10 2):1160–1164. doi: 10.1067/mje.2002.123962. [DOI] [PubMed] [Google Scholar]
- 8.Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39(7):1214–1219. doi: 10.1016/S0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 9.Grunig E, Henn P, D'Andrea A, et al. Reference values for and determinants of right atrial area in healthy adults by 2-dimensional echocardiography. Circ Cardiovasc imaging. 2013;6(1):117–124. doi: 10.1161/CIRCIMAGING.112.978031. [DOI] [PubMed] [Google Scholar]
- 10.Austin C, Alassas K, Burger C, et al. Echocardiographic assessment of estimated right atrial pressure and size predicts mortality in pulmonary arterial hypertension. Chest. 2015;147(1):198–208. doi: 10.1378/chest.13-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Veerdonk MC, Huis In TVAE, Marcus JT, et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J. 2017;49(6):1700007. doi: 10.1183/13993003.00007-2017. [DOI] [PubMed] [Google Scholar]
- 12.van de Veerdonk MC, Marcus JT, Westerhof N, et al. Signs of right ventricular deterioration in clinically stable patients with pulmonary arterial hypertension. Chest. 2015;147(4):1063–1071. doi: 10.1378/chest.14-0701. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg FC, Arzt M, Lange T, Schroll S, Pfeifer M, Wensel R. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur J Heart Fail. 2013;15(7):771–775. doi: 10.1093/eurjhf/hft044. [DOI] [PubMed] [Google Scholar]
- 14.Chaouat A, Sitbon O, Mercy M, et al. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J. 2014;44(3):704–713. doi: 10.1183/09031936.00153613. [DOI] [PubMed] [Google Scholar]
- 15.Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The critical role of pulmonary arterial compliance in pulmonary hypertension. Ann Am Thorac Soc. 2016;13(2):276–284. doi: 10.1513/AnnalsATS.201509-599FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain P, Rao S, Macdonald P, et al. Diagnostic Performance of Pulmonary Capacitance at Rest and During Exercise in Idiopathic Pulmonary Arterial Hypertension. Heart Lung Circ. 2017. 10.1016/j.hlc.2017.10.019. [DOI] [PubMed]
- 17.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt GH, Pugsley SO, Sullivan MJ, et al. Effect of encouragement on walking test performance. Thorax. 1984;39(11):818–822. doi: 10.1136/thx.39.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehlken N, Lichtblau M, Klose H, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J. 2016;37(1):35–44. doi: 10.1093/eurheartj/ehv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs G, Avian A, Olschewski A, Olschewski H. Zero reference level for right heart catheterisation. Eur Respir J. 2013;42(6):1586–1594. doi: 10.1183/09031936.00050713. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs G, Herve P, Barbera JA, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50(5):1700578. doi: 10.1183/13993003.00578-2017. [DOI] [PubMed] [Google Scholar]
- 22.Grunig E, Weissmann S, Ehlken N, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation. 2009;119(13):1747–1757. doi: 10.1161/CIRCULATIONAHA.108.800938. [DOI] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657–662. doi: 10.1161/01.CIR.70.4.657. [DOI] [PubMed] [Google Scholar]
- 25.Grunig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS guidelines with updated commentary of the Cologne consensus conference 2011. Int J Cardiol. 2011;154(1):S3–12. doi: 10.1016/S0167-5273(11)70488-0. [DOI] [PubMed] [Google Scholar]
- 26.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28(10):1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 27.Weatherald J, Boucly A, Chemla D, et al. Prognostic value of follow-up hemodynamic variables after initial Management in Pulmonary Arterial Hypertension. Circulation. 2018;137(7):693–704. doi: 10.1161/CIRCULATIONAHA.117.029254. [DOI] [PubMed] [Google Scholar]
- 28.Vanderpool RR, Desai AA, Knapp SM, et al. How prostacyclin therapy improves right ventricular function in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700764. doi: 10.1183/13993003.00764-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.