Abstract
Background
Interleukin-22 (IL-22) is one of the cytokines secreted by T-helper 17 (Th17) cells. It belongs to the IL-10 cytokine family and influences a variety of immune reactions. Studies have indicated that IL-22 can promote cancer progression and metastases. However, the function of IL-22 in osteosarcoma (OS) remains unclear.
Material/Methods
In this study, the expression of IL-22 in the OS cell line was detected by qRT-PCR. The role of IL-22 in proliferation and invasion in OS cells was tested by MTT and Transwell assays. The protein expression of STAT3, phospho-STAT3, AKT, and phospho-AKT was detected by Western blot analysis.
Results
The results showed that IL-22 was upregulated in OS cells. IL-22 dose-independently promoted OS cells proliferation and invasion, which could be reversed by IL-22 antibody or STAT3 siRNA. Furthermore, IL-22 exposure of OS cells resulted in dose-independently increased levels of phosphorylated STAT3 protein kinases. Interestingly, IL-22 did not influence the expression of phosphorylated AKT.
Conclusions
These results suggest that IL-22 promotes OS cells proliferation and invasion and its effect is mediated by activation of the STAT3 pathway. These findings demonstrate that IL-22 may serve as a promising molecular biomarker for diagnosis and therapy for OS patients.
MeSH Keywords: Cell Proliferation, Neoplasm Invasiveness, Osteosarcoma, STAT3 Transcription Factor
Background
Osteosarcoma (OS) is the most prevalent primary malignancy of the bones and joints worldwide, accounting for ~2.4% of all malignancies in children and adolescents [1,2]. The annual incidence in children under 15 years old is 5.6 cases per million [3]. OS cells are characteristically aggressive, with rapid growth and early metastasis. The current therapy is a combination of surgical excision, multi-agent neoadjuvant chemotherapy, and adjuvant chemotherapy [4,5]. Although treatment has progressed, the prognosis is still poor. One of the most important causes of ineffective chemotherapy treatment of OSA is multidrug resistance (MDR) [6]. In addition, the presence of synchronous metastases is a factor that significantly reduces the probability of survival [7]. Up to 20% of osteosarcoma patients present with imagological evidence of metastases at the time of diagnosis, with the lungs as the most prevalent metastatic location [8]. The 5-year overall survival rate for non-metastatic patients is 60–70% [9,10]. Many molecular alterations are involved in the critical signal transduction pathways that contribute to OS pathogenesis via promoting key molecular targets [11]. Therefore, it is important to understand the potential molecular mechanisms of rapid growth and early metastasis of OS and to identify new treatment options to prevent early-stage metastasis.
Interleukin-22 (IL-22) is a member of IL-10 cytokine family and is preferentially produced by activated immune cells Th17 and Th22 cells [12]. Increasing evidence shows that IL-22 is involved in host defense, tissue regeneration, and protection against injury [13–15]. Recent studies have reported that IL-22 acts as a tumor promoting cytokine and is closely related to different cancers, including breast, lung, and gastric cancers, and promotes progression and metastasis in many cancer [16,17]. IL-22 induces phosphorylation of kinase Jak1 and Tyk2 and STAT3 transcription factors through the Jak-STAT signaling pathway [18,19], which plays a key role in tumor progression [20]. A previous study has reported that IL-22 overexpression protects lung cancer cell lines from apoptosis by activating STAT3 and its downstream anti-apoptotic proteins Bcl-2 and Bcl-xL and inhibiting MAP kinases [21]. However, the role of IL-22 in osteosarcoma is not clear.
Therefore, in the present study, we investigated the role of IL-22 in OS cell proliferation and invasion. In addition, we further clarified potential mechanisms by which IL-22 stimulates OS cell migration and invasion.
Material and Methods
Antibodies and reagents
Recombinant human IL-22 and anti-human IL-22 neutralizing antibody was obtained from R&D Systems (Minneapolis, MN, USA). The primary antibodies of total STAT3 and phospho-STAT3, total AKT and phospho-AKT, and GAPDH were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Cell culture
Human osteosarcoma cell lines SOSP-9607, MG-63, U2OS, and SAOS-2 and the normal osteoblast cell line hFOB1.09 were obtained from the American Type Culture Collection (Manassas, VA). These cells were cultured in RPMI 1640 medium (Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Invitrogen), 100 U/mL penicillin, and 0.1g/mL streptomycin (Hyclone) in a humidified 37°C incubator with 5% CO2.
Cell proliferation assay
Cell proliferation of MG63 and U2OS cells was measured by MTT assay. We cultured 5x103 cells per well into 96-well plates with 5% CO2 at 37°C and 100 μl of MTT solution was added into each well and cultured for 4 h at 37°C. Then, 150 μl DMSO was added and we detected the absorbance at 570 nm using a microplate reader (Thermo Scientific).
Cell invasion assay
For the invasion assay, the invasion ability of MG-63 and U2OS cells was measured using Transwell assay as the reference [22]. Briefly, 3×105 MG-63 and U2OS cells were seeded onto the upper chamber of a 24-well Matrigel Transwell invasion insert (BD bioscience, America) containing 0.1% FBS, and the lower chamber was filled with DMEM containing 20% FBS. After 24 h of incubation, a cotton swab was used to remove the non-invading cells and the invaded cells were stained with crystal violet. The number of invaded cells was counted using a light microscope, and the average value was determined through examination of 5 different randomly selected fields.
Real-time PCR
Total RNA was extracted from MG-63 and U2OS cell lines using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. We reverse transcribed 2 μg of total RNA using cDNA Reverse Transcription Kits (Applied Biosystems). Quantitative RT-PCR reaction was performed using SYBR Green Master Mix (Roche). The primer sequences were as follows: Human IL-22 forward: 5′-GCTAAGGAGGCTAGCTTG-3′ and reverse: 5′-CAGCAAATCCAGTTCTCC-3′; GAPDH forward: 5′-GGCTGCTTTTAACTCTGGTA-3′ and reverse: 5′-ATGCCAGTGAGCTTCCCGT-3′. GAPDH was used as internal control and the expression of IL-22 was quantified using the 2−ΔΔCt method.
Western blotting
MG-63 and U2OS cells were lysed using lysis buffer containing protease inhibitor cocktail (Thermo Fisher Scientific, Inc.). The BCA Protein Assay kit (Pierce, USA) was used to detect the total concentration of protein. Protein extract (50 mg) was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and subsequently transferred onto polyvinylidene difluoride membranes (PVDF, EMD Millipore). The membranes were incubated with the appropriate primary antibody against STAT3 (1: 1000), phospho-STAT3 (1: 1000), AKT (1: 1000), phospho-AKT (1: 1000), or GAPDH (1: 1000) overnight at 4°C. Further, the membranes were incubated with the anti-mouse or rabbit peroxidase-conjugated secondary antibodies (HRP; 1: 5000; Santa Cruz Biotechnology) for 1 h at room temperature. Proteins were visualized using an enhanced chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK) and quantified using Image Lab Software version 4.1 (BIO RAD).
Statistical analysis
Statistical analysis was conducted using SPSS 17.0 (IBM, Armonk, NY, USA). The difference between 2 groups was assessed using one-way ANOVA. Data are presented as the means ± standard error of mean (SEM). P<0.05 was considered significant.
Results
IL-22 was upregulated in osteosarcoma cell lines
To confirm the potential role of IL-22 in osteosarcoma progression, we first detected the expression of IL-22 in the 4 osteosarcoma cells lines MG63, SOSP-9607, U2OS, SAOS2 and normal osteoblast cell line hFOB1.09 using qRT-PCR. The results revealed that IL-22 mRNA expression in the osteosarcoma cell lines was higher than in the normal osteoblast cell line hFOB1.09 (Figure 1).
Figure 1.
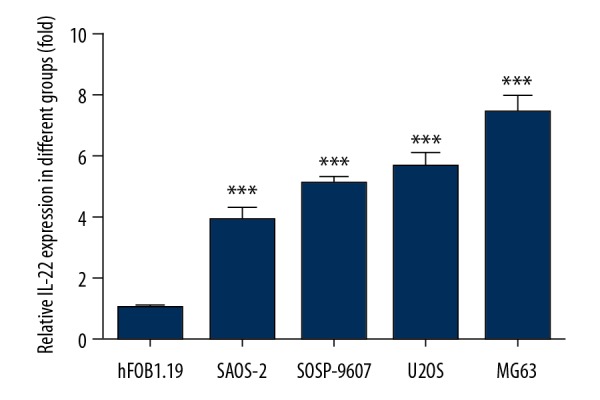
IL-22 was upregulated in osteosarcoma cell lines. The levels of IL-22 in the 4 osteosarcoma cells lines MG63, SOSP-9607, U2OS, and SAOS2 and normal osteoblast cell line hFOB1.09 were detected by qRT-PCR. Data are expressed as mean ± standard error. *** P<0.01 vs. hFOB1.09 cells.
IL-22 promotes the proliferation and invasion of osteosarcoma cells
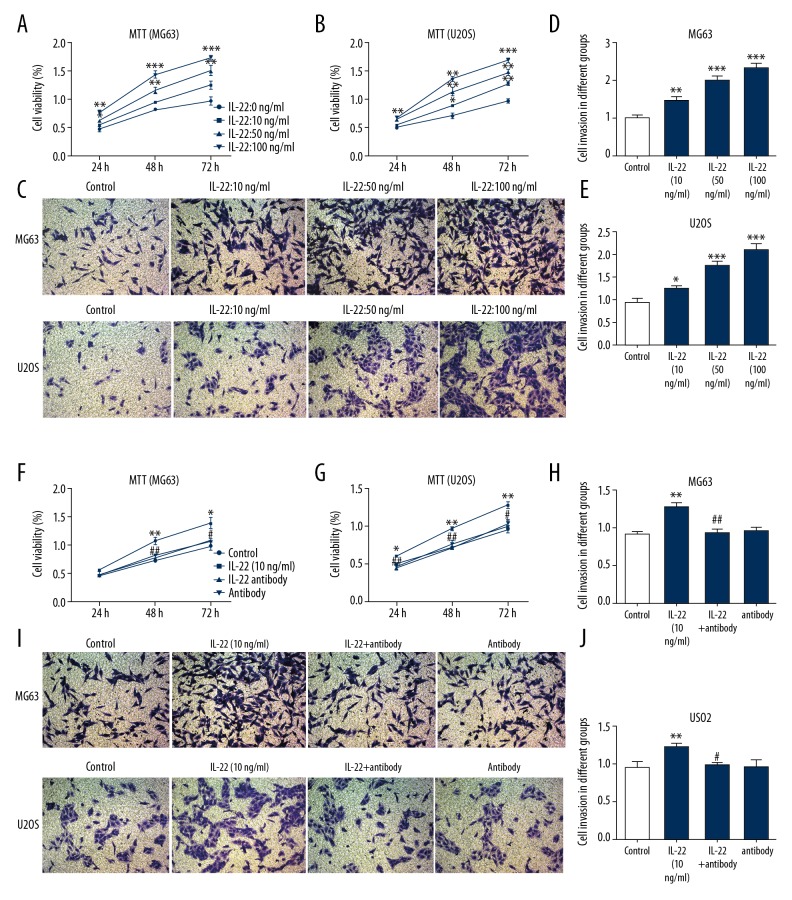
To detect the effects of IL-22 on osteosarcoma cell proliferation and invasion, MG63 and U2OS cells were pretreated with different doses of IL-22 (0, 10, 50, and 100 ng/ml). MTT results showed that IL-22 significantly increased the proliferation abilities of MG63 (Figure 2A) and U2OS cells (Figure 2B) in a concentration-dependent manner. In addition, when osteosarcoma cells were pretreated with IL-22, the invasive ability of MG63 and U2OS cells increased significantly in a concentration-dependent manner (Figure 2C–2E). Then, MG63 and U2OS cells were pretreated simultaneously with anti-IL-22 antibody (10 ng/ml) to inhibit the effect of IL-22. IL-22 antibody treatment reduced IL-22-induced proliferation and invasion of MG63 and U2OS cells (Figure 2F–2H).
Figure 2.
IL-22 promotes the proliferation and invasion of osteosarcoma cells. MG63 and U2OS cells were pretreated with different dosages of IL-22 (0, 10, 50, 100 ng/ml). Then, in order to inhibit the effect of IL-22, MG63 and U2OS cells were treated concomitantly with anti-IL-22 antibody (10ng/ml). MTT assay was used to examine cell proliferation of MG63 (A, F) and U2OS cells (B, G). Transwell assay was used to examine cell invasion of MG63 and U2OS cells (C–E, H–J). Data are expressed as mean ± standard error. * P<0.05, ** P<0.01, *** P<0.001 vs. control. # P<0.05, ## P<0.01 vs. IL-22 (10 ng/ml) group.
IL-22 stimulation activates the phosphorylation of STAT3 in osteosarcoma cells
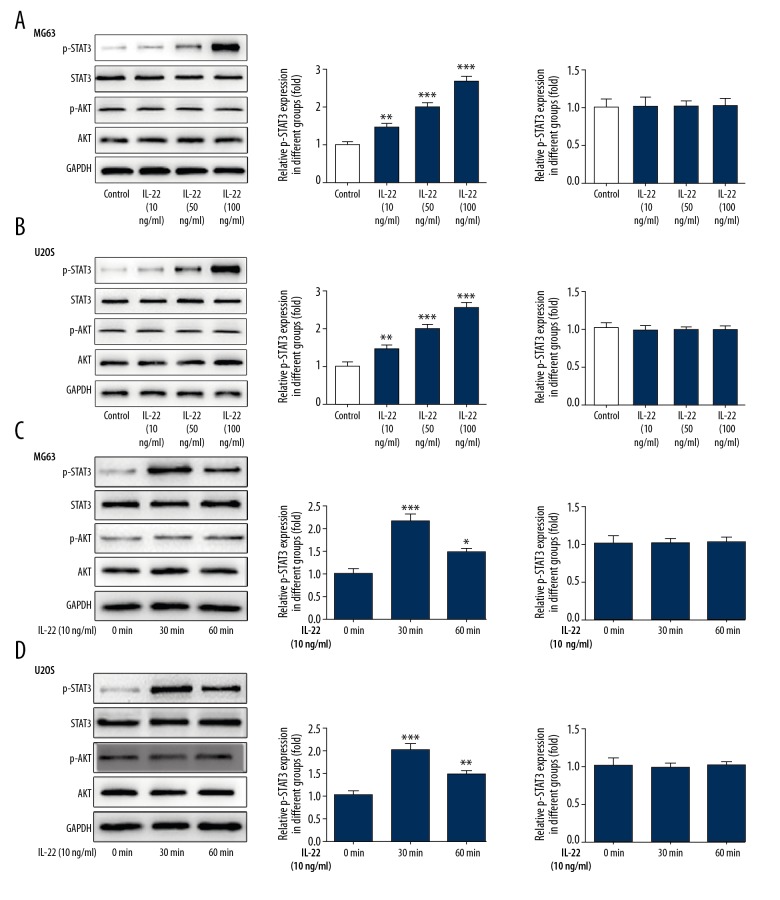
We further studied the molecular mechanism pathways potentially involved in the effect of IL-22 on the proliferation and invasion of osteosarcoma. The expression of p-STAT3 was markedly and dose-dependent increased in MG63 and U2OS cells stimulated with IL-22. Interestingly, IL-22 did not influence the expression of phosphorylated AKT (Figure 3A, 3B). Furthermore, IL-22 (10 ng/ml) stimulation for 30 min markedly induced the phosphorylation of STAT3 without affecting phosphorylation of AKT (Figure 3C, 3D).
Figure 3.
IL-22 stimulation activates the phosphorylation of STAT3 in osteosarcoma cells. Western blot analysis was used to examine the protein expression levels of P-STAT3, STAT3, P-AKT, and AKT in MG63 (A) and U2OS cells (B) pretreated with different dosages of IL-22 (0, 10, 50, 100 ng/ml). Western blot analysis was used to examine the protein expression levels of P-STAT3, STAT3, P-AKT, and AKT in MG63 (C) and U2OS cells (D) pretreated with IL-22 (10 ng/ml) for 0 min, 30 min, and 60 min. Data are expressed as mean ± standard error. * P<0.05, ** P<0.01, *** P<0.001 vs. control.
IL-22 stimulation promotes osteosarcoma cell proliferation and invasion via STAT3 signaling
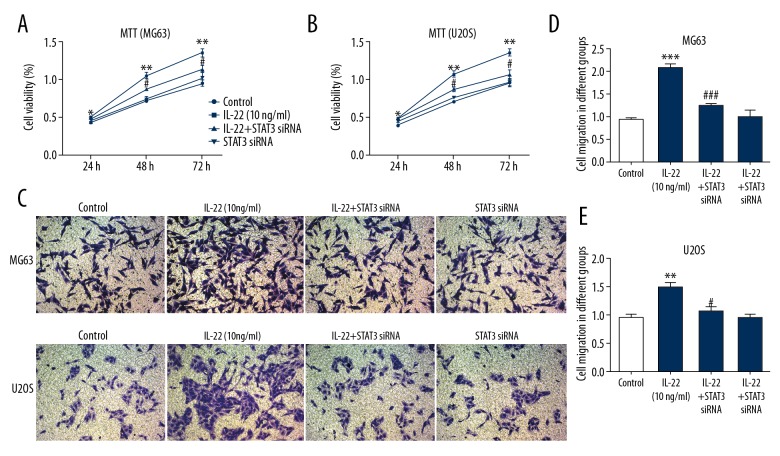
To determine whether IL-22 regulates osteosarcoma cell proliferation and invasion via STAT3 signaling, MG63 and U2OS cells were treated with IL-22 or STAT3 siRNA. The results showed that inhibition of STAT3 signaling by STAT3 siRNA significantly inhibited the proliferation and invasion of MG63 and U2OS cells promoted by IL-22 treatment (Figure 4A–4E). These findings suggest that IL-22 promotes MG63 and U2OS cell proliferation and invasion via STAT3 signaling.
Figure 4.
IL-22 stimulation promotes osteosarcoma cell proliferation and invasion via STAT3 signaling. MG63 and U2OS cells were treated with IL-22 or STAT3 siRNA. MTT assay was used to examine cell proliferation of MG63 (A) and U2OS cells (B). Transwell assay was used to examine cell invasion of MG63 and U2OS cells (C–E). Data are expressed as mean ± standard error. ** P<0.01, *** P<0.001 vs. control. # P<0.05, ### P<0.001 vs. IL-22 (10 ng/ml) group.
Discussion
Osteosarcoma is a universally prevalent primary malignant bone cancer with heterogeneous pathogenesis [1]. Inflammatory mediators and cellular effectors are important components of the local environment of tumors and are important factors in inducing tumorigenesis [23]. IL-22 plays an important role in various diseases, including autoimmune diseases, malignant tumors, and infectious diseases [24]. Levels of IL-22 are upregulated in a variety of human tumors, including prostate, ovarian, and hepatocellular cancers [25]. In addition, experimental data revealed that increased levels of IL-22 are related to tumor growth and metastasis of gastric cancer [26]. In the present study, our data first confirmed that IL-22 was upregulated in the osteosarcoma cells. Furthermore, cell proliferation and invasion tests were performed to assess the effects of IL-22 on the proliferation and invasiveness of OS cell lines MG63 and U2OS. The results showed that IL-22 treatment significantly increased OS cell migration and invasion in a dose-independent manner and its effect could be reversed by IL-22 antibody.
Previous studies indicated that the activity of most inflammatory cytokines is focused on STAT3 [27]. STAT molecules are inherently latent transcription factors that in stay the cytoplasm (inactivated) until they are activated by JAK (Janus kinase) [28]. STATs play a crucial role in normal cell signaling. However, recent evidence has suggested another possible role of STATs in the development of cancer. According to these studies, the constitutively activated STATs, especially STAT3, are the main culprits in the development of cancer [29,30]. It has been reported that multiple signal pathways, such as STAT3, MAPK, and NF-κB, can be activated by IL-22 in different types of cells [31–33]. There is increasing evidence that IL-22 treatment increases cell proliferation and suppresses apoptosis through activation of STAT3 and/or ERK signaling [34,35]. A study reported that IL-22 produced by cancer-associated fibroblasts promotes invasion of gastric cancer cells by activating STAT3 and ERK signaling [34]. IL-22 promotes epithelial cell transformation and breast tumorigenesis by activating MAP3K8 [35]. In the present study we ought to determine whether IL-22 promotes proliferation and invasion of osteosarcoma cells as mediated by STAT3, MG63, and U2OS cells pretreated with si-STAT3. Our results showed that si-STAT3 abolished the effects of IL-22 on proliferation and invasion ability of osteosarcoma cells. This result suggests that STAT3 activation plays important role in IL-22-induced tumor growth.
Conclusions
In conclusion, we found that IL-22 increases osteosarcoma cell proliferation and invasion via STAT3 activation. IL-22 may serve as a molecular biomarker for diagnosis and treatment of OS patients.
Acknowledgment
Thanks to Dr. Sun Xiangjie, Department of Pathology, Shanghai Cancer Center, Fudan University, for valuable discussions and helping with the experiments.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–34. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–43. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abate ME, Longhi A, Galletti S, et al. Non-metastatic osteosarcoma of the extremities in children aged 5 years or younger. Pediatr Blood Cancer. 2010;55:652–54. doi: 10.1002/pbc.22567. [DOI] [PubMed] [Google Scholar]
- 4.Qu W, Wang Y, Wu Q, Hao D, Li D. Emodin impairs radioresistance of human osteosarcoma cells by suppressing sonic hedgehog signaling. Med Sci Monit. 2017;23:5767–73. doi: 10.12659/MSM.907453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ESMO/European Sarcoma Network Working Group. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii113–23. doi: 10.1093/annonc/mdu256. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari S, Serra M. An update on chemotherapy for osteosarcoma. Expert Opin Pharmacother. 2015;16(18):2727–36. doi: 10.1517/14656566.2015.1102226. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Liu J, Zhang Y. Role of epithelial cell transforming sequence 2 (ECT2) in predicting prognosis of osteosarcoma. Med Sci Monit. 2017;23:3861–68. doi: 10.12659/MSM.905951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang L, Zhao YD, Chen WX. The function of the novel mechanical activated ion channel Piezo1 in the human osteosarcoma cells. Med Sci Monit. 2017;23:5070–82. doi: 10.12659/MSM.906959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther. 2016;3(2):221–43. doi: 10.1007/s40744-016-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PosthumaDeBoer J, Witlox MA, Kaspers GJ, van Royen BJ. Molecular alterations as target for therapy in metastatic osteosarcoma: A review of literature. Clin Exp Metastasis. 2011;28:493–503. doi: 10.1007/s10585-011-9384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diao CY, Guo HB, Ouyang YR, et al. Screening for metastatic osteosarcoma biomarkers with a DNA microarray. Asian Pac J Cancer Prev. 2014;15:1817–22. doi: 10.7314/apjcp.2014.15.4.1817. [DOI] [PubMed] [Google Scholar]
- 12.Trifari S, Kaplan CD, Tran EH, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–71. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 13.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 14.Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–15. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 16.Jiang R, Wang H, Deng L, et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer. 2013;13:59. doi: 10.1186/1471-2407-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim K, Kim G, Kim JY, et al. Interleukin-22 promotes epithelial cell transformation and breast tumorigenesis via MAP3K8 activation. Carcinogenesis. 2014;35:1352–61. doi: 10.1093/carcin/bgu044. [DOI] [PubMed] [Google Scholar]
- 18.Pestka S, Krause CD, Sarkar D, et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 19.Xie MH, Aggarwal S, Ho WH, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–39. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 20.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi Y, Cao J, Jin S, et al. Interleukin-22 promotes lung cancer cell proliferation and migration via the IL-22R1/STAT3 and IL-22R1/AKT signaling pathways. Mol Cell Biochem. 2016;415:1–11. doi: 10.1007/s11010-016-2663-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu P, Feng Y, Dong D, et al. Enhanced renoprotective effect of IGF-1 modified human umbilical cord-derived mesenchymal stem cells on gentamicin-induced acute kidney injury. Sci Rep. 2016;6:20287. doi: 10.1038/srep20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 24.Martin JC, Wolk K, Beriou G, et al. Limited presence of IL-22 binding protein, a natural IL-22 inhibitor, strengthens psoriatic skin inflammation. J Immunol. 2017;198:3671–78. doi: 10.4049/jimmunol.1700021. [DOI] [PubMed] [Google Scholar]
- 25.Eyerich K, Dimartino V, Cavani A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur J Immunol. 2017;47:607–14. doi: 10.1002/eji.201646723. [DOI] [PubMed] [Google Scholar]
- 26.Ji Y, Yang X, Li J, et al. IL-22 promotes the migration and invasion of gastric cancer cells via IL-22R1/AKT/MMP-9 signaling. Int J Clin Exp Pathol. 2014;7:3694–703. [PMC free article] [PubMed] [Google Scholar]
- 27.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 28.O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shan D, Shang Y, Hu T. MicroRNA-411 inhibits cervical cancer progression by directly targeting STAT3. Oncol Res. 2018 doi: 10.3727/096504018X15247361080118. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Zhao J, Du P, Cui P, et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018 doi: 10.1038/s41388-018-0250-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Chen Y, Wei H, et al. Antiapoptotic activity of autocrine interleukin-22 and therapeutic effects of interleukin-22-small interfering RNA on human lung cancer xenografts. Clin Cancer Res. 2008;14:6432–39. doi: 10.1158/1078-0432.CCR-07-4401. [DOI] [PubMed] [Google Scholar]
- 32.Lejeune D, Dumoutier L, Constantinescu S, et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem. 2002;277:33676–82. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 33.Bi Y, Cao J, Jin S, et al. Interleukin-22 promotes lung cancer cell proliferation and migration via the IL-22R1/STAT3 and IL-22R1/AKT signaling pathways. Mol Cell Biochem. 2016;415:1–11. doi: 10.1007/s11010-016-2663-8. [DOI] [PubMed] [Google Scholar]
- 34.Fukui H, Zhang X, Sun C, et al. IL-22 produced by cancer-associated fibroblasts promotes gastric cancer cell invasion via STAT3 and ERK signaling. Br J Cancer. 2014;111:763–71. doi: 10.1038/bjc.2014.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K, Kim G, Kim JY, et al. Interleukin-22 promotes epithelial cell transformation and breast tumorigenesis via MAP3K8 activation. Carcinogenesis. 2014;35:1352–61. doi: 10.1093/carcin/bgu044. [DOI] [PubMed] [Google Scholar]