Abstract
Background
The goal of this study was to provide up-to-date and comprehensive statistics on incidence, survival, and prevalence rates for selected malignant brain and other CNS tumors in adults.
Methods
The current study used data from the Central Brain Tumor Registry of the United States, provided by the Centers for Disease Control and Prevention, to examine incidence and data from the Surveillance, Epidemiology, and End Results program to examine survival and prevalence in 16 distinct malignant brain and other CNS histologies in adults (aged 20 y and older at diagnosis) from 2000–2014 overall and by sex, age group, race, and ethnicity.
Results
Glioblastoma had the highest incidence (4.40 per 100000) and prevalence (9.23 per 100000). Ependymal tumors had the highest 5- and 10-year relative survivals (87.8% and 84.5%, respectively), while glioblastoma had the lowest 5- and 10-year relative survivals (5.4% and 2.7%, respectively). Females generally had better survival and lower prevalence than males. Younger adults tended to have better survival than older adults, and prevalence varied greatly by age and histology. While survival did not vary significantly by race, white adults had higher prevalence than the other race groups. Hispanics generally had better survival rates and lower prevalence than non-Hispanics.
Conclusions
Survival varied greatly by age and ethnicity. Prevalence differed by sex, age, race, and ethnicity.
Keywords: adults, brain and CNS tumors, incidence, prevalence, survival
Importance of the study
This study presents overall incidence rates, survival, and prevalence rates, as well as survival and prevalence rates by sex, 10-year age group, race, and Hispanic ethnicity in adults ages 20 years and older by malignant brain and other CNS tumor histology. These population-based measures are important for characterizing disease burden. Although some past literature has described similar statistics, few studies have focused explicitly on adult populations and on specific brain and other CNS tumor histologies. We found that survival varied greatly by age and ethnicity, while survival did not vary as much by race or sex. Prevalence rates differed greatly by sex, race, and ethnicity. Prevalence rates also differed by age group, but these differences were primarily histology driven. Although this study cannot determine the reasons for these differences, it does provide the most comprehensive and up-to-date information for these tumors and may provoke future investigations into their causes.
Primary central nervous system (CNS) tumors are currently classified by the World Health Organization into a broad range of distinct categories based upon histologic morphology, molecular biomarkers, and clinical phenotype.1 Such tumors can be further classified as malignant or nonmalignant based upon histologic analysis of invasive potential. Furthermore, certain tumors are known to preferentially affect adult populations over pediatric populations.
Malignant brain and other CNS tumors account for approximately 31.5% of all brain tumors, of which gliomas account for approximately 80%.2 The average age-adjusted mortality rate overall has been reported as 4.33 per 100000 population, and 5- and 10-year relative survival rates for all malignant brain and other CNS tumors have been reported as 34.9% and 29.3%, respectively.2 These values vary considerably based upon histologic classification of the tumor. For example, 5-year relative survival of pilocytic astrocytoma has been reported as 94.1%, while the 5-year relative survival of glioblastoma has been reported as 5.5%.2 Individual-level demographics, particularly age and sex, and genetic factors, such as isocitrate dehydrogenase (IDH) mutation and 1p/19q codeletion status, have been implicated as relevant for risk and for prognosis after diagnosis; however, these factors vary between tumors of different histologic classifications and within tumors of the same histologic classification.3–10
In this study, we used data provided by the Centers for Disease Control and Prevention’s National Program of Cancer Registries to the Central Brain Tumor Registry of the United States (CBTRUS) to characterize the incidence rates, as well as data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program to calculate survival and prevalence rates for selected malignant brain tumors in adults.
Materials and Methods
The CBTRUS is the largest collection of population-based data for primary brain and other CNS tumors in the United States and represents approximately 99.9% of the US population.2 The CBTRUS obtains incidence data for cases of malignant and nonmalignant (benign and uncertain) primary brain and other CNS tumors from 52 central cancer registries (CCR) of which 47 CCR are from the National Program of Central Registries (NPCR) and 5 CCR are from SEER. Using these data, 16 malignant brain and other CNS tumor histologies were selected for this more detailed analysis based on frequency, with a cutoff of at least 1000 adult (ages 20 y and older) cases or more for the 15-year period of 2000–2014. Our analyses included newly diagnosed malignant brain and other CNS tumors with International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histologies as shown in Supplementary Table S1.11 Note that the World Health Organization (WHO) classification listed pilocytic astrocytoma as having uncertain behavior (ICD-O-3, behavior code /1) but this histology is included in population-based cancer registry reporting as CNS tumor with malignant behavior (ICD-O-3, behavior code /3). Age-adjusted incidence rates (IR) per 100000 population with corresponding 95% CIs were calculated for these 16 malignant histologies using SEER*Stat 8.3.2 statistical software (http://seer.cancer.gov/seerstat/).12 The standard population used to calculate these age-adjusted incidence rates is an estimate of the 2000 US population.
Survival data for the selected malignant histologies were obtained from 18 SEER registries for the same time period of 2000–2014. This dataset provides population-based information for approximately 26% of the US population and is a subset of the CBTRUS data used for incidence calculations. Survival information derived from active patient follow-up is not available in the data that CBTRUS receives from NPCR registries, so the SEER data were used for the survival calculations.12 Survival was estimated for ICD-O-3 topography sites: brain (C71.0–C71.9), meninges (C70.0–C70.9), spinal cord, cranial nerves, and other parts of the CNS (C72.0–72.9), pituitary and pineal glands (C75.1–C75.3), and olfactory tumors of the nasal cavity (C30.0 [ICD-O-3 codes 9522–9523]). SEER*Stat 8.3.2 statistical software was used to estimate 1-, 2-, 3-, 4-, 5-, and 10-year relative survival rates for the selected malignant histologies overall, as well as 1-, 2-, 5-, and 10-year relative survival rates for the same histologies by sex (male and female), 10-year age groups (20–29 y, 30–39 y, 40–49 y, 50–59 y, 60–69 y, 70–79 y, and 80 y and older), race (white, black, and Other, including Asian or Pacific Islander [API] and American Indian/Alaska Native [AIAN], and Hispanic ethnicity (Hispanic and non-Hispanic).
Limited duration prevalence estimates for 2014 were calculated using methodology previously described by Zhang et al, using incidence data from the CBTRUS analytic file from 2000–2014 and survival data from SEER 18 from 2000–2014.13 Annual counts for each histologic group were generated for single year age groups overall and by sex, race, and ethnicity. For any group that did not have any incidence cases for a given year, 0.5 cases were used as case count. Observed survival probabilities were generated for each histologic group for the entire period of 2000–2014 using 5-year age groups overall and by sex, race, and ethnicity. For any group that did not have the appropriate number of cases to calculate group-specific survival, the overall age-specific survival for that histology was used. Crude prevalence rates were calculated using all prevalent cases in those patients age 20 years and older in 2014 using the estimated US population for 2014 obtained from SEER (estimated using the 2010 decennial census).14 Age-adjusted prevalence rates (PR) adjusted to the 2000 US standard population were calculated by histology group using the R package ‘asht’ overall, by sex, age groups, race, and ethnicity.15
All figures were created using R v3.4.3 statistical software.16 Statistics were suppressed when counts were fewer than 16 within a cell but included in totals except when data were suppressed from only one cell within a category to prevent identification of the number in the suppressed cell. Please note that prevalence counts within the API and AIAN race groups may not necessarily add up to the Other race category, as these numbers were rounded to the nearest ones place.
Results
Overall Incidence, Survival, and Prevalence
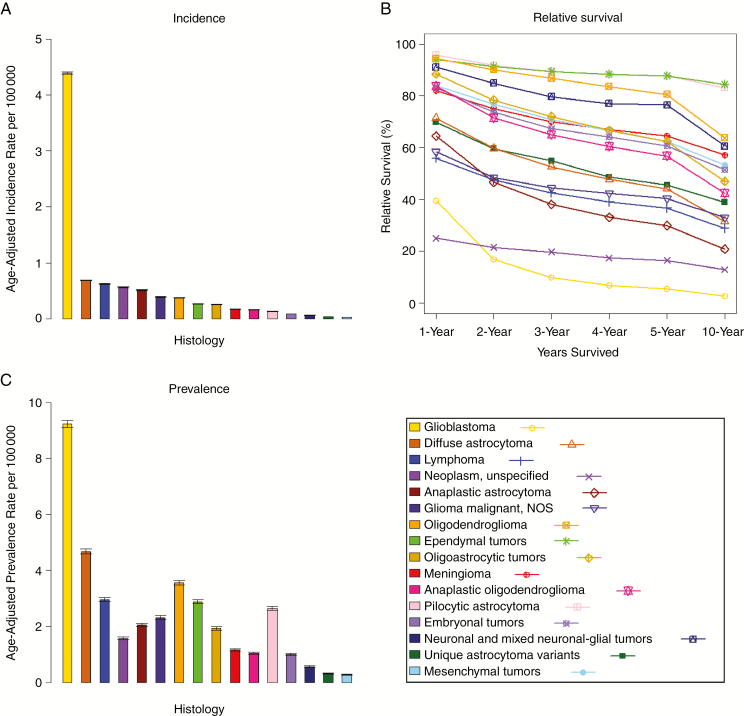
The malignant histology with the highest age-adjusted incidence in those age 20 years and older was glioblastoma (IR = 4.40 per 100000 [95% CI: 4.38–4.42]) (Table 1, Fig. 1A). The histologies with the next greatest age-adjusted incidence in adults were diffuse astrocytoma (IR = 0.68 per 100000 [95% CI: 0.68–0.69]), lymphoma (IR = 0.62 per 100000 [95% CI: 0.61–0.63]), neoplasm unspecified (IR = 0.56 per 100000 [95% CI: 0.55–0.57]), and anaplastic astrocytoma (IR = 0.51 per 100000 [95% CI: 0.50–0.52]).
Table 1.
Fifteen-year total, annual average total, and annual average age-adjusted incidence rates for adults (age 20+ y), brain and other CNS tumors by selected malignant histology, CBTRUS, 2000–2014
| Histology | Fifteen-Year Total | Annual Average | Rate per 100000 | 95% CI |
|---|---|---|---|---|
| Glioblastoma | 150 399 | 10 027 | 4.40 | 4.38–4.42 |
| Diffuse astrocytoma | 22 445 | 1496 | 0.68 | 0.68–0.69 |
| Lymphoma | 20 687 | 1379 | 0.62 | 0.61–0.63 |
| Neoplasm, unspecified | 18 866 | 1258 | 0.56 | 0.55–0.57 |
| Anaplastic astrocytoma | 16 948 | 1130 | 0.51 | 0.50–0.52 |
| Glioma malignant, NOS | 12 918 | 861 | 0.39 | 0.38–0.40 |
| Oligodendroglioma | 11 976 | 798 | 0.37 | 0.37–0.38 |
| Ependymal tumors | 8778 | 585 | 0.27 | 0.26–0.27 |
| Oligoastrocytic tumors | 8273 | 552 | 0.26 | 0.25–0.26 |
| Meningioma | 6027 | 402 | 0.18 | 0.17–0.18 |
| Anaplastic oligodendroglioma | 5544 | 370 | 0.17 | 0.16–0.17 |
| Pilocytic astrocytoma | 4281 | 285 | 0.13 | 0.13–0.14 |
| Embryonal tumors | 2922 | 195 | 0.09 | 0.09-0.09 |
| Neuronal and mixed neuronal-glial tumors | 2147 | 143 | 0.06 | 0.06–0.07 |
| Unique astrocytoma variants | 1287 | 86 | 0.04 | 0.04-0.04 |
| Mesenchymal tumors | 1007 | 67 | 0.03 | 0.03-0.03 |
Fig. 1.
(A) annual average age-adjusted incidence rates with 95% CIs for adults (age 20+ y), brain and other CNS tumors by selected malignant histology, CBTRUS, 2000–2014; (B) 1-, 2-, 3-, 4-, 5-, and 10-year relative survival rates for adults (age 20+ y), brain and other CNS tumors by selected malignant histology, SEER 18 registries, 2000–2014; (C) age-adjusted prevalence rates with 95% CIs for adults (age 20+ y), brain and other central nervous system tumors by histology, SEER 18 registries, 2014.
Relative survival varied greatly by histology. The malignant histologies with the highest survival rates were ependymal tumors, with a 5-year relative survival of 87.8% (95% CI: 86.0–89.5) and a 10-year relative survival of 84.5% (95% CI: 81.9–86.8); pilocytic astrocytoma, with a 5-year relative survival of 87.6% (95% CI: 85.0–89.7) and a 10-year relative survival of 83.1% (95% CI: 79.7–85.9); and oligodendroglioma, with a 5-year relative survival of 80.6% (95% CI: 78.9–82.2) and a 10-year relative survival of 63.8% (95% CI: 61.3–66.1) (Supplementary Table S2, Fig. 1B). The malignant histologies with the lowest survival rates were glioblastoma, with a 5-year relative survival of 5.4% (95% CI: 5.1–5.7) and a 10-year relative survival of 2.7% (95% CI: 2.5–3.0); neoplasm unspecified, with a 5-year relative survival of 16.5% (95% CI: 14.8–18.2) and a 10-year relative survival of 12.9% (95% CI: 11.1–14.9); and anaplastic astrocytoma, with a 5-year relative survival of 29.9% (95% CI: 28.3–31.6) and a 10-year relative survival of 20.8% (95% CI: 19.1–22.6).
Glioblastoma also had the highest age-adjusted prevalence rate of all histologies examined (PR = 9.23 per 100000 [95% CI: 9.11–9.36]) (Supplementary Table S3, Fig. 1C). The histologies with the next greatest age-adjusted prevalence rate in adults were diffuse astrocytoma (PR = 4.68 per 100000 [95% CI: 4.59–4.77]), oligodendroglioma (PR = 3.57 per 100000 [95% CI: 3.49–3.65]), lymphoma (PR = 2.96 per 100000 [95% CI: 2.89–3.03]), and ependymal tumors (PR = 2.88 per 100000 [95% CI: 2.81–2.95]).
Survival and Prevalence by Sex
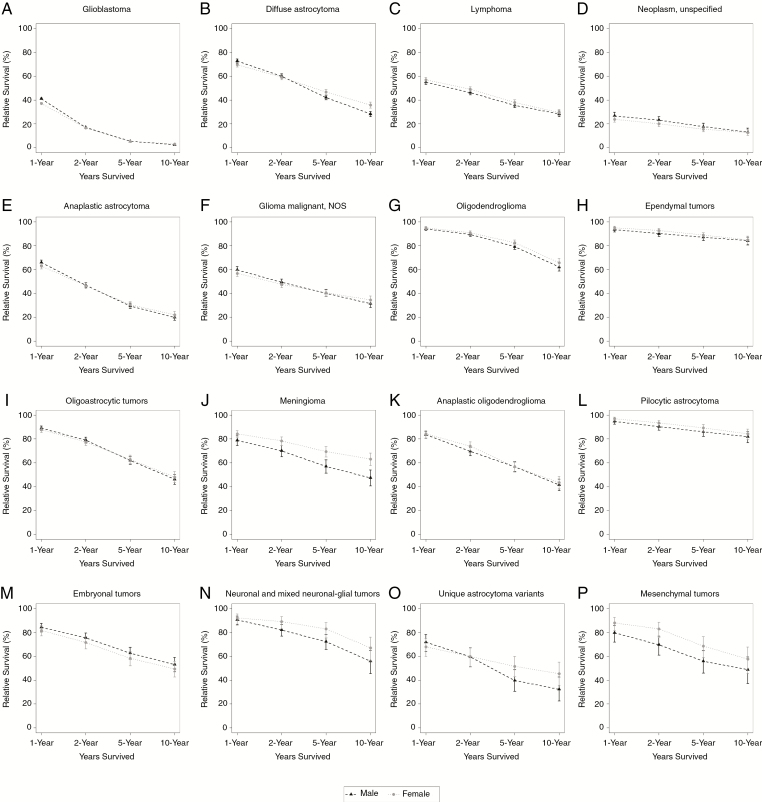
Females generally had better survival outcomes than males, although this varied by histology. For malignant meningioma, females had significantly higher 5-year relative survival (69.4% [95% CI: 64.9–73.5]) than males (57.0% [95% CI: 51.3–62.3]), as well as significantly higher 10-year relative survival (63.0% [95% CI: 57.6–68.0]) compared with males (47.4% [95% CI: 40.5–54.0]) (Supplementary Table S4, Fig. 2). For diffuse astrocytoma, females had significantly higher 5-year relative survival (46.6% [95% CI: 44.3–48.8]) than males (42.2% [95% CI: 40.2–44.2]), as well as significantly higher 10-year relative survival (35.7% [95% CI: 33.1–38.2]) compared with males (28.3% [95% CI: 26.1–30.4]). No other statistically significant differences were found between males and females in terms of relative 5- and 10-year survival.
Fig. 2.
One-, 2-, 5-, and 10-year relative survival rates for adults (age 20+ y), brain and other CNS tumors by sex and selected malignant histology, SEER 18 registries, 2000–2014.
Male adults generally had higher prevalence rates than female adults, although this varied by histology. In the following malignant histologies, males had significantly higher prevalence rates than females: embryonal tumors, glioblastoma, oligoastrocytic tumors, lymphoma, diffuse astrocytoma, anaplastic astrocytoma, oligodendroglioma, neuronal and mixed neuronal-glial tumors, anaplastic oligodendroglioma, glioma malignant NOS, and neoplasm unspecified (Supplementary Table S5). Malignant meningioma was the only histology where PR was significantly higher in females (female PR: 1.31 per 100000 [95% CI: 1.25–1.38]; male PR: 0.95 per 100000 [95% CI: 0.90–1.02]). There were no statistically significant differences in PR between males and females with mesenchymal tumors, unique astrocytoma variants, pilocytic astrocytoma, and ependymal tumors.
Survival and Prevalence by 10-Year Age Group
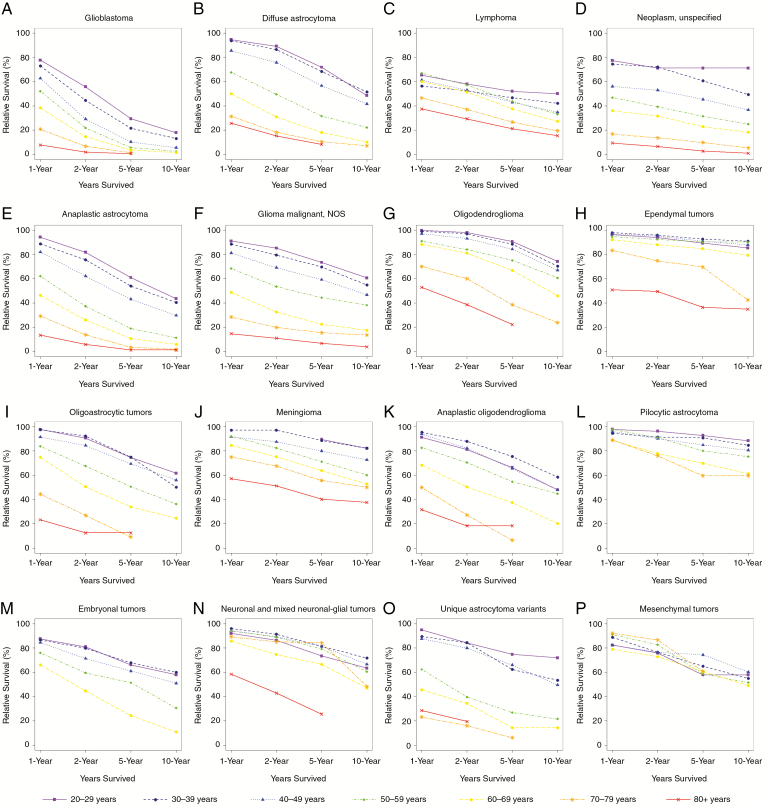
Survival varied greatly by age group, with younger adults typically having better relative survival than older adults. The malignant histologies with the largest variation in relative survival by age group were anaplastic astrocytoma, glioblastoma, glioma malignant NOS, oligoastrocytic tumors, and diffuse astrocytoma (Supplementary Table S6, Fig. 3). For adults age 20–29 years, the histology with the highest relative survival rate was pilocytic astrocytoma. For adults age 30–39 years, 40–49 years, 50–59 years, and 60–69 years, the histology with the highest relative survival rate was ependymal tumors. For adults age 70–79 years, the histology with the highest relative survival rates was neuronal and mixed neuronal-glial tumors. For adults age 80+ years, the histology with the highest relative survival rates was malignant meningioma. Glioblastoma had the lowest survival rate for every 10-year age group.
Fig. 3.
One-, 2-, 5-, and 10-year relative survival rates for adults (age 20+ y), brain and other CNS tumors by age group and selected malignant histology, SEER 18 registries, 2000–2014.
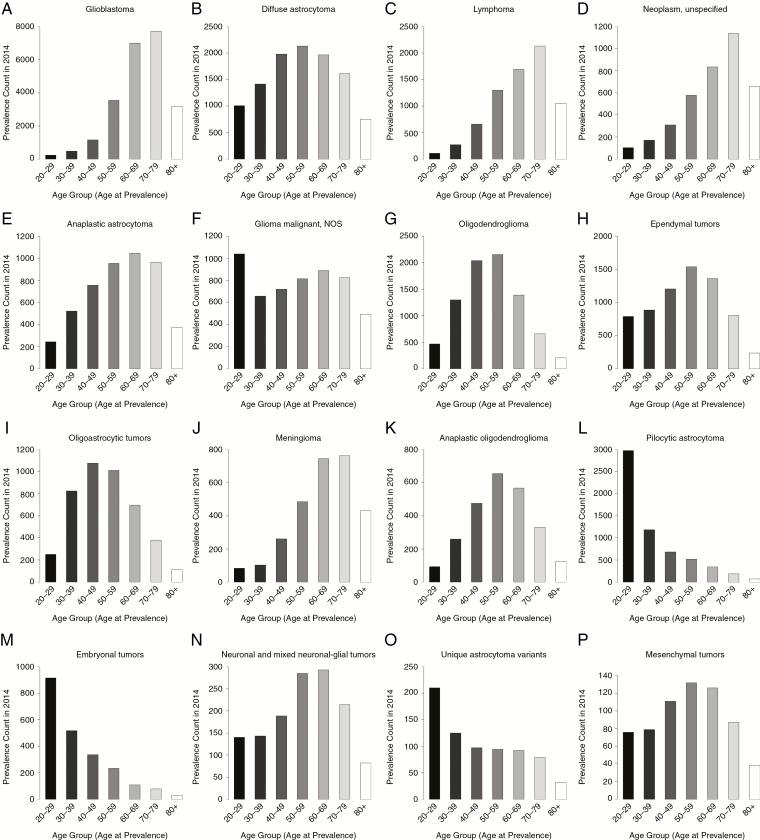
The total estimated prevalent cases in 2014 varied greatly by histology. The highest number of prevalent cases in older adults were in neoplasm unspecified (70–79 y: N = 1138; 80+ y: N = 656), glioblastoma (70–79 y: N = 7719; 80+ y: N = 3191), and lymphoma (70–79 y: N = 2135; 80+ y: N = 1052), while pilocytic astrocytoma (20–29 y: N = 2979; 30–39 y: N = 1187), embryonal tumors (20–29 y: N = 915; 30–39 y: N = 517), and unique astrocytoma variants (20–29 y: N = 210; 30–39 y: N = 125) were more prevalent in younger adults (Supplementary Table S7, Fig. 4). Prevalence rates varied significantly by age group for glioblastoma, lymphoma, neoplasm unspecified, and anaplastic astrocytoma (Supplementary Table S7). For diffuse astrocytoma, there were significant differences in PR between all of the age groups except for adults 40–49 and 50–59 years.
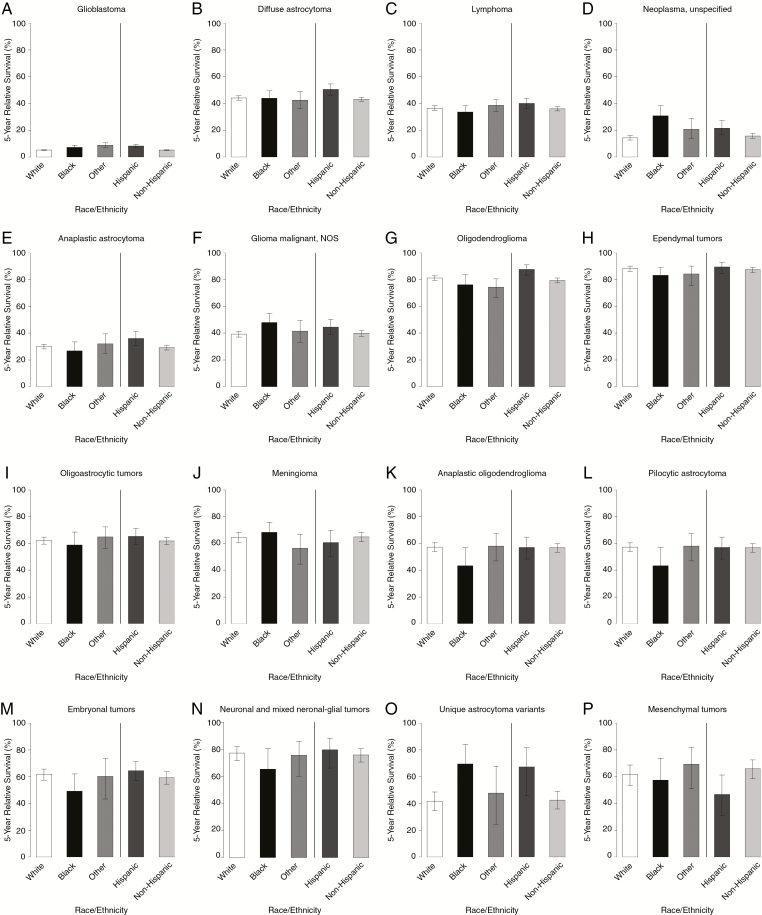
Fig. 4.
Five-year relative survival rates for adults (age 20+ y), brain and other CNS tumors by race, ethnicity, and selected malignant histology, SEER 18 registries, 2000–2014.
Survival and Prevalence by Race
Survival did not vary substantially by race (Supplementary Table S8, Fig. 5). For glioblastoma, Other races (8.7% [95% CI: 7.0–10.6]) and blacks (7.2% [95% CI: 5.8–8.7]) had significantly higher 5-year relative survival than whites (5.1% [95% CI: 4.8–5.4]), although their 10-year relative survival rates were not significantly different from each other.
Fig. 5.
Prevalence counts for adults (age 20+ y), brain and other CNS tumors by age group and selected malignant histology, SEER 18 registries, 2014.
Prevalence rates varied significantly by race, with whites having the highest prevalence for most histologies (Supplementary Table 9). Similarly for glioblastoma, whites had the greatest PR of 10.04 per 100000 (95% CI: 9.90–10.18), followed by blacks with a PR of 5.10 per 100000 (95% CI: 4.82–5.39), followed by Others with a PR of 3.87 per 100000 (95% CI: 3.54–4.22). For oligodendroglioma, whites had the greatest PR of 4.02 per 100000 (95% CI: 3.92–4.11), followed by Others with a PR of 2.00 per 100000 (95% CI: 1.79–2.24), followed by blacks with a PR of 1.48 per 100000 (95% CI: 1.34–1.63). Similarly for oligoastrocytic tumors, whites had the greatest PR of 2.16 per 100000 (95% CI: 2.09–2.23), followed by Others with a PR of 1.12 per 100000 (95% CI: 0.96–1.30), followed by blacks with a PR of 0.82 per 100000 (95% CI: 0.71–0.93). For anaplastic oligodendroglioma, whites had the greatest PR of 1.14 per 100000 (95% CI: 1.09–1.19), followed by Others with a PR of 0.70 per 100000 (95% CI: 0.58–0.84), followed by blacks with a PR of 0.47 per 100000 (95% CI: 0.39–0.56). Whites also had significantly higher PR than the other 2 race groups with anaplastic astrocytoma, diffuse astrocytoma, pilocytic astrocytoma, ependymal tumors, and embryonal tumors. Blacks had significantly higher PR than the other 2 race groups with meningioma. Finally, the race category Others had significantly lower PR than the other two race groups with neoplasm unspecified and with lymphoma.
Survival and Prevalence by Hispanic Ethnicity
Survival varied by ethnicity, with Hispanics generally surviving longer than non-Hispanics. For glioblastoma, Hispanics had significantly higher 5-year relative survival (8.1% [95% CI: 7.0–9.4]) than non-Hispanics (5.1% [95% CI: 4.8–5.4]), as well as significantly higher 10-year relative survival (4.8% [95% CI: 3.7–6.0]) compared with non-Hispanics (2.5% [95% CI: 2.2–2.8]) (Supplementary Table S10, Fig. 5). For diffuse astrocytoma, Hispanics had significantly higher 5-year relative survival (50.5% [95% CI: 46.1–54.7]) than non-Hispanics (43.2% [95% CI: 41.6–44.8]). Similarly for oligodendroglioma, Hispanics had significantly higher 5-year relative survival (87.8% [95% CI: 83.4–91.1]) than non-Hispanics (79.5% [95% CI: 77.7–81.3]). For unique astrocytoma variants, Hispanics had significantly higher 10-year relative survival (67.4% [95% CI: 46.0–81.9]) compared with non-Hispanics (35.1% [95% CI: 27.7–42.6]). Similar for anaplastic astrocytoma, Hispanics had significantly higher 10-year relative survival (27.8% [95% CI: 22.3–33.6]) compared with non-Hispanics (20.0% [95% CI: 18.1–21.9]). Non-Hispanics did not have significantly greater relative survival than Hispanics for any of the malignant histologies.
Non-Hispanic adults generally had higher prevalence rates than Hispanic adults, although this varied by histology. In the following malignant histologies, non-Hispanics had significantly higher PR than Hispanics: pilocytic astrocytoma, oligoastrocytic tumors, anaplastic astrocytoma, oligodendroglioma, diffuse astrocytoma, anaplastic oligodendroglioma, glioblastoma, glioma malignant NOS, and ependymal tumors (Supplementary Table S11). For embryonal tumors, however, Hispanics (PR = 1.13 per 100000 [95% CI: 1.02–1.24]) had a significantly higher PR than non-Hispanics (PR = 0.96 per 100000 [95% CI: 0.91–1.01]).
Discussion
This study aimed to comprehensively characterize the overall incidence, relative survival, and prevalence of selected malignant brain cancer histologies in adults over 20 years old for the 15-year time period between 2000 and 2014. The histologies included in this report were selected based on incidence (1000 cases or more per histology group). This report presents a more comprehensive view of specific histologies and can be used as a companion to the annual CBTRUS Statistical Report.2 Relative survival and prevalence with respect to sex, 10-year age group, race, and ethnicity were also assessed.
Glioblastoma was by far the most common malignant brain tumor in individuals over 20 years old, with an age-adjusted incidence rate of 4.40 per 100000, over 6 times higher than that of diffuse astrocytoma, the second most common histology, with an incidence rate of 0.68 per 100000. Lymphoma was only slightly lower than diffuse astrocytoma, with an incidence of 0.62 per 100000. Although few studies could be found that focused exclusively on adults over 20 years old or that considered the large number of tumor histologies we considered, all studies of incidence rates in primary malignant brain tumors showed a similar pattern of glioblastoma incidence overpowering the other histologies considered. For instance, a Dutch study focusing on gliomas in patients over 18 years old between 1989 and 2010 reported an incidence rate for glioblastoma of 2.5 per 100000 inhabitants compared with that of anaplastic astrocytoma, the next highest incidence, of 0.6 per 100000, a 4.2-fold difference.17 A small population-based study in Switzerland which sought to characterize incidence and survival rates of primary astrocytic and oligodendrogliomas in 987 cases over 15 years (1980–1994) reported the incidence rate of glioblastoma as 3.55 per 100000 (European standard population) compared with pilocytic astrocytoma, the next most common, with an incidence rate of 0.37 per 100000, a 9.6-fold difference.3 Reasons for discrepancies among incidence rates among databases from different countries are likely attributed to differences in collection procedures between countries and provide some insight into differences in global incidence trends in these tumors.18
The relative survival rates in our study also followed a similar pattern, as demonstrated in prior literature, with pilocytic astrocytoma reported as having the highest relative survival rate at 10 years and glioblastoma reported as having the lowest relative survival rate at 10 years. In the Swiss study, the 10-year overall relative survival rate of pilocytic astrocytoma was reported as 96%, while that of glioblastoma was reportedly 0%.3 In the Dutch study, 5-year overall relative survival rates were reported for oligodendroglioma and ependymal tumors as 72% and 84%, respectively.17 Discrepancies between these values and our own may be attributed to geographic differences in survival of brain cancer; however, it is more likely due to differences in how the data were collected, including clinical outcomes data, by country. For example, our study reports a 10-year overall survival rate of 83% for pilocytic astrocytoma compared with the Swiss study, which reported a rate of 96%.3 Since our study included only individuals over the age of 20 and because survival of pilocytic astrocytoma decreases with increasing age, it is not surprising that our result is lower than that of the Swiss study, whose sample size for pilocytic astrocytoma, which tends to affect younger populations that experience higher survival rates, was relatively small (n = 56).3
Although such discrepancies exist, general patterns in survival and prevalence that emerged from this study were similar to those demonstrated in prior literature. In the EUROCARE-5 study, which analyzed survival of European adults greater than 15 years old with primary malignant brain cancer, results showed similar trends in 5-year survival by age group, that is, most malignant cancers demonstrated decreased 5-year survival rates with increasing age.19 This is similarly demonstrated in the Swiss and Dutch studies.3,17 Moreover, our data support prior literature demonstrating no difference between overall survival rates by race,20 although other studies have indicated increased survival rates in black populations.21 Previous studies have also shown increased prevalence of malignant brain cancers, particularly lymphoma and glioblastoma, in white populations.21
Other notable features of a disease may be ascertained by considering incidence, prevalence, and survival data together. For instance, although the age-adjusted incidence rates of diseases like pilocytic astrocytoma and ependymal tumors are low (<0.4 per 100000), their high 10-year relative survival rates (>80% at 10 years) and increased prevalence suggest an increased morbidity burden of these diseases. That is, although survival rates are high, patients are left living and coping with these diseases for longer periods of time than those diagnosed with more aggressive brain cancer histologies. Moreover, the high prevalence in glioblastoma found in this study could be attributed to the high incidence and low survival rates of this aggressive histology.
This study, drawing from CBTRUS data during the 15-year time period 2000–2014, represents the most up-to-date and comprehensive analysis of these selected histology groups of malignant brain tumors in adult populations over the age of 20 years in the United States. This is supported by the fact that CBTRUS data contain brain and other CNS tumors from approximately 99.9% of the US population. Furthermore, the diagnostic capabilities in the time period studied allowed for optimum diagnostic imaging as well as molecular information to supplement pathological diagnosis and vastly improve the diagnostic precision of these data compared with those of previous timeframes. While comprehensive for baseline demographics and incidence for brain and other CNS tumors, CBTRUS does not include data on presenting symptoms, treatment modalities, or clinical course, including survival, thereby preventing analysis of these variables. Survival estimates stem from analysis of SEER data, which is a subset of the CBTRUS dataset, representing approximately 26% of the US population and may not be a complete representation of the actual survival rates in the US. However, future analyses using SEER will include data from a larger population coverage. Data received from these surveillance partners are not subject to central pathology reviews. Furthermore, as a member of the surveillance community in the US, CBTRUS is bound by the rules and regulations guiding cancer collection at the national level, such as including pilocytic astrocytoma in the reporting of malignant brain and other CNS tumors to preserve the historical reporting of these tumors.22
Conclusions
The overall incidence, relative survival, and prevalence of selected malignant histologies in adults ages 20 years and older from the 15-year period of 2000 to 2014 have been described, along with relative survival and prevalence by sex, 10-year age group, race, and ethnicity. These measures vary significantly by histology. Survival rates varied greatly by age group and ethnicity, with younger adults and Hispanics generally surviving longer than older adults and non-Hispanics. Survival did not vary as much by race or sex. Prevalence rates differed greatly by sex, race, and ethnicity, with males, whites, and non-Hispanics tending to have higher overall prevalence than females, blacks, Others, and Hispanics. Prevalence rates also differed by age group, but these differences were primarily histology driven. Reasons for these differences cannot be determined by this study. Future analyses will be needed for further comparison.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 2016-M-9030, The Sontag Foundation, Novocure, AbbVie, along with the Musella Foundation, the National Brain Tumor Society, the Pediatric Brain Tumor Foundation, and the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in kind donations. Contents are solely the responsibility of the authors and do not necessarily reflect the official views of the CDC. Q.T.O. is supported by a Research Training Grant from the Cancer Prevention and Research Institute of Texas (CPRIT; RP160097T).
Conflict of interest statement. The authors declare that they have no conflict of interest.
Authorship statement. Experimental design: Haley Gittleman, Jill Barnholtz-Sloan. Experimental implementation: Haley Gittleman, Yi Fritz, Carol Kruchko, Jill Barnholtz-Sloan. Statistical analyses/interpretation: Haley Gittleman, Quinn Ostrom. Manuscript writing: Haley Gittleman, Alexander Boscia, Quinn Ostrom, Gabrielle Truitt, Yi Fritz, Carol Kruchko, Jill Barnholtz-Sloan. Reading and approving final version: Haley Gittleman, Alexander Boscia, Quinn Ostrom, Gabrielle Truitt, Yi Fritz, Carol Kruchko, Jill Barnholtz-Sloan.
Supplement sponsorship. This supplement was funded through an independent medical educational grant from AbbVie.
Supplementary Material
References
- 1. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. [DOI] [PubMed] [Google Scholar]
- 4. Olar A, Wani KM, Alfaro-Munoz KD, et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II-III diffuse gliomas. Acta Neuropathol. 2015;129(4):585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reuss DE, Mamatjan Y, Schrimpf D, et al. IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gittleman H, Cote DJ, Ostrom QT, et al. Do race and age vary in non-malignant central nervous system tumor incidences in the United States?J Neurooncol. 2017;134(2):269–277. [DOI] [PubMed] [Google Scholar]
- 8. Ostrom QT, Rubin JB, Lathia JD, Berens ME, Barnholtz-Sloan JS. Females have the survival advantage in glioblastoma. Neuro Oncol. 2018;20(4):576–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ceccarelli M, Barthel FP, Malta TM, et al. ; TCGA Research Network Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Melin BS, Barnholtz-Sloan JS, Wrensch MR, et al. ; GliomaScan Consortium Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49(5): 789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S, eds. International Classification of Diseases for Oncology. Third edition. Maryland Heights, MO: Matthews Book Company; 2000. [Google Scholar]
- 12. Surveillance Epidemiology and End Results (SEER) Program. SEER*Stat software version 8.3.2 2016; www.seer.cancer.gov/seerstat. .Accessed MAY 16,2018).
- 13. Zhang AS, Ostrom QT, Kruchko C, Rogers L, Peereboom DM, Barnholtz-Sloan JS. Complete prevalence of malignant primary brain tumors registry data in the United States compared with other common cancers, 2010. Neuro Oncol. 2017;19(5):726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Surveillance, Epidemiology, and End Results (SEER) Program www.seer.cancer.gov. SEER*Stat Database: Populations – Total U.S. (1990–2016), National Cancer Institute, DCCPS, Surveillance Research Program, released November 2017. Accessed May 16, 2018.
- 15. Michael P.Fay. asht: Applied Statistical Hypothesis Tests. R package version 0.9.3 https://CRAN.R-project.org/package=asht. Accessed May 16, 2018 2017.
- 16. R Core Team. R: A Language and Environment for Statistical Computing. Vienne, Austria: R Foundation for Statistical Computing; https://www.R-project.org/. Accessed May 16, 2018 2017. [Google Scholar]
- 17. Ho VK, Reijneveld JC, Enting RH, et al. ; Dutch Society for Neuro-Oncology (LWNO) Changing incidence and improved survival of gliomas. Eur J Cancer. 2014;50(13):2309–2318. [DOI] [PubMed] [Google Scholar]
- 18. Leece R, Xu J, Ostrom QT, Chen Y, Kruchko C, Barnholtz-Sloan JS. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro Oncol. 2017;19(11):1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Visser O, Ardanaz E, Botta L, Sant M, Tavilla A, Minicozzi P; EUROCARE-5 Working Group Survival of adults with primary malignant brain tumours in Europe; Results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2231–2241. [DOI] [PubMed] [Google Scholar]
- 20. Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer. 2003;98(3):603–609. [DOI] [PubMed] [Google Scholar]
- 21. Gabriel A, Batey J, Capogreco J, et al. Adult brain cancer in the U.S. black population: a Surveillance, Epidemiology, and End Results (SEER) analysis of incidence, survival, and trends. Med Sci Monit. 2014;20:1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chapter 111: Standards for Tumor Inclusion and Reportability, Table 2. NAACCR Layout Version 18: Comparison of Reportable Cancers: CoC, SEER, NPCR and CCCR http://datadictionary.naaccr.org/?c=3. Accessed May 16, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.