Abstract
Objectives
Methotrexate is considered to be first-line therapy for rheumatoid arthritis (RA). However, a substantial proportion of treated patients do not achieve the desired goals of therapy. This analysis aimed to identify predictors of insufficient response to methotrexate in patients with early RA.
Methods
The Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab (OPTIMA) and PREMIER studies in patients with RA for <1 and <3 years, respectively, examined the efficacy of methotrexate and adalimumab in methotrexate-naive patients. This post hoc analysis included patients for whom initial methotrexate monotherapy was not successful after 6 months. Candidate predictors of insufficient response and clinically relevant radiographic progression (CRRP) included demographics, baseline disease characteristics and time-averaged disease variables over a 12-week interval. In OPTIMA, adalimumab was added to therapy after insufficient treatment response; in PREMIER, initial methotrexate therapy was continued; clinical, functional and radiologic outcomes were assessed after 1 year.
Results
Baseline 28-joint Disease Activity Score based on C-reactive protein (DAS28(CRP)) and time-averaged DAS28(CRP) over 4, 8 and 12 weeks were the strongest predictors of insufficient response to methotrexate and CRRP. Addition of adalimumab to methotrexate therapy was associated with better clinical, functional and radiographic outcomes after 1 year compared with continuing on methotrexate monotherapy.
Conclusions
In patients with early RA, baseline disease characteristics and early disease activity can predict response to methotrexate treatment and radiographic progression at 6 months. The addition of adalimumab at 6 months after methotrexate failure is associated with improved outcomes. These results support treatment-to-target strategies and timely adaptation of therapy in patients with early RA.
Trial registration number
NCT00420927, NCT00195663; Post-results.
Keywords: methotrexate, anti-TNF, rheumatoid arthritis
Introduction
Disease-modifying antirheumatic drugs (DMARDs) such as methotrexate are currently recommended as first-line therapy for the treatment of rheumatoid arthritis (RA).1 2 However, if patients do not attain the desired goal of therapy (eg, remission or at least low disease activity (LDA)) after 6 months of an initial treatment strategy, consideration must be given for adjustment of therapy.1 Furthermore, joint damage, one of the hallmarks of RA, develops in many patients during the early stages of the disease and can continue despite conventional synthetic DMARD therapy, leading to bone and cartilage loss.3–7 Adding a biologic DMARD could be considered in patients with an insufficient response to methotrexate when risk factors are present.1
The Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab (OPTIMA) and PREMIER studies examined the efficacy of methotrexate and adalimumab in methotrexate-naive patients with early RA.6 8 The studies used different protocols when patients did not achieve response to methotrexate after 6 months: in OPTIMA, adalimumab was added to therapy from week 26 onward for patients with insufficient response to methotrexate, whereas in PREMIER, initial methotrexate therapy was maintained for up to 2 years.
The objective of this post hoc analysis of data from the randomised, double-blind OPTIMA and PREMIER studies in patients with early RA was to identify predictors of insufficient response to methotrexate and clinically relevant radiographic progression (CRRP) after 6 months of methotrexate monotherapy, as well as to evaluate the subsequent benefits of treatment adjustment (OPTIMA) versus no treatment adjustment (PREMIER).
Methods
Study patients, designs and treatments
The methods and results of the trials have been described previously (see also online supplementary table 1).6 8 9 Briefly, OPTIMA was a 78-week, randomised, double-blind, double-period, phase 4 trial conducted between December 2006 and July 2010.8 9 In period 1, methotrexate-naive patients were randomised 1:1 to receive adalimumab 40 mg every other week plus methotrexate weekly (titrated to 20 mg/week by week 8) or placebo every other week plus methotrexate weekly for 26 weeks. In period 2, patients who did not achieve the stable LDA target during period 1 continued or initiated adalimumab 40 mg every other week plus methotrexate weekly. PREMIER was a 2-year, randomised, double-blind, placebo-controlled phase 3 trial conducted between December 2000 and April 2004.6 Patients were randomised 1:1:1 to receive adalimumab 40 mg every other week plus methotrexate weekly (titrated to 20 mg/week by week 9), adalimumab 40 mg every other week plus placebo weekly, or placebo every other week plus methotrexate weekly for 2 years. This post hoc analysis included only patients who received initial methotrexate monotherapy in OPTIMA or PREMIER and did not achieve stable LDA. Stable LDA was defined as achieving 28-joint Disease Activity Score based on C-reactive protein (DAS28(CRP)) <3.2 at two consecutive assessments (weeks 22 and 26 in OPTIMA and weeks 20 and 24 in PREMIER).
annrheumdis-2018-213502supp001.pdf (844KB, pdf)
Assessments
Predictors were assessed for insufficient response to methotrexate, defined as not achieving stable LDA at 6 months, or experiencing CRRP, defined as an increase in modified total Sharp score (mTSS) of >1.5 from baseline to 6 months based on a prior definition of increase >3 in 1 year.10 Stable DAS28(CRP) <3.2 was chosen as the definition of insufficient response because it was the primary endpoint at the 6-month time point in the OPTIMA trial8 and was the decisive outcome for subsequent randomisation for the phase 2 portion of that trial.9
Assessments after 6 months (week 26 in OPTIMA and week 24 in PREMIER) included clinical outcomes at week 78 for OPTIMA and week 76 for PREMIER: the proportion of patients achieving LDA (DAS28(CRP)<3.2), DAS28(CRP) <2.6, Simplified Disease Activity Index (SDAI) ≤3.3 and Clinical Disease Activity Index (CDAI) ≤2.8. Physical function was evaluated using the Health Assessment Questionnaire Disability Index (HAQ-DI) and reported as the mean score at week 78/76 and the proportion of patients achieving clinically meaningful improvement in HAQ-DI (change of ≥0.22)11 from week 26 to 78 (OPTIMA) or week 24 to 76 (PREMIER). Radiologic outcomes were assessed using the mTSS and reported as the mean score at week 78/76 and the proportion of patients achieving change in mTSS (ΔmTSS) of ≤0.5 from week 26 to 52 or 78 (OPTIMA) or week 24 to 52 or 76 (PREMIER; value at week 76 estimated from linear extrapolation between actual assessments at weeks 52 and 104).
Statistical analyses
Backward logistic regression was used to identify potential predictors of insufficient response to methotrexate therapy and CRRP during the first 6 months of therapy in a pooled analysis of patients with available data in OPTIMA and PREMIER. Two models were used. The first model included demographic and disease factors (including DAS28(CRP), SDAI or CDAI) at baseline only. The second model included baseline factors and postbaseline, time-averaged disease parameters for three time intervals (through 4, 8 and 12 weeks of methotrexate exposure; three separate models fitted). Time-averaged variables were calculated as area under the curve standardised for length of time interval. Factors were removed from the models if their significance level rose to 0.1.
The percentage of patients achieving DAS28(CRP) <3.2, DAS28(CRP) <2.6, SDAI ≤3.3 and CDAI ≤2.8 at 1 year after the 6-month assessment was analysed using non-responder imputation and Χ2 test (or Fisher’s exact test if ≥20% of the cells had expected counts ≤5). Differences in mean outcomes between patients who switched to adalimumab rescue therapy in OPTIMA and patients who continued to receive methotrexate monotherapy in PREMIER were assessed using a t-test. A multivariate logistic regression adjusted for study (OPTIMA or PREMIER) and baseline characteristics (age, sex, RA duration, rheumatoid factor status, previous DMARD use, tender joint count, swollen joint count, CRP, DAS28, HAQ-DI, mTSS and erosion score) was used to determine the odds ratios (ORs) of achieving categorical clinical, functional and radiographic outcomes between the OPTIMA and PREMIER patients.
Results
Predictors of insufficient response to methotrexate and CRRP at 6 months
Patients
The pooled analysis (PREMIER and OPTIMA) of predictors of insufficient response to methotrexate included 525 insufficient responders and 162 responders who had data for all independent variables. The pooled CRRP analysis included 171 patients with CRRP and 499 without CRRP. More patients with an insufficient response to methotrexate were women and had significantly higher baseline mean values for age, CRP, tender and swollen joint counts, DAS28(CRP), SDAI, CDAI, HAQ-DI and mTSS compared with patients achieving stable LDA with methotrexate (table 1). Similarly, patients with CRRP had significantly higher mean CRP, tender and swollen joint counts, DAS28(CRP), SDAI, CDAI and mTSS values at baseline versus patients without CRRP (table 1).
Table 1.
Baseline demographics, disease characteristics and prior therapies of the pooled predictors analysis populations
| Characteristic | Pooled for prediction of insufficient response to methotrexate (n=687) | Pooled for prediction of CRRP (n=670) | ||
| Insufficient responders (n=525) | Responders (n=162) | CRRP (n=171) | No CRRP (n=499) | |
| Demographic characteristics | ||||
| Women, n (%) | 398 (76) | 113 (70) | 126 (74) | 372 (75) |
| White, n (%) | 483 (92) | 148 (91) | 158 (92) | 456 (91) |
| Age (years) | 51.3 (13.7) | 48.8 (13.2) | 50.9 (14.9) | 50.7 (13.3) |
| RA duration (years) | 0.5 (0.6) | 0.5 (0.6) | 0.5 (0.6) | 0.5 (0.6) |
| Disease characteristics | ||||
| CRP (mg/L) | 35.7 (36.5) | 26.4 (28.2) | 50.7 (45.7) | 27.6 (28.1) |
| TJC68 | 30.1 (14.8) | 23.9 (12.7) | 30.7 (13.7) | 28.0 (14.8) |
| SJC66 | 20.4 (11.4) | 15.6 (9.1) | 22.3 (11.5) | 18.3 (10.8) |
| DAS28(CRP) | 6.2 (0.9) | 5.6 (1.0) | 6.4 (0.9) | 6.0 (1.0) |
| SDAI | 46.7 (13.8) | 38.0 (13.9) | 49.4 (14.4) | 43.1 (14.0) |
| CDAI | 43.3 (12.6) | 35.4 (12.3) | 44.4 (12.4) | 40.4 (13.0) |
| HAQ-DI (range, 0–3) | 1.6 (0.6) | 1.3 (0.7) | 1.6 (0.6) | 1.5 (0.7) |
| mTSS | 15.5 (20.6) | 12.2 (17.9) | 20.7 (22.1) | 12.4 (18.6) |
| CRRP, n (%) | 146 (28) | 25 (15) | NA | NA |
| Insufficient responders, n (%) | NA | NA | 146 (85) | 364 (73) |
| Prior therapies, n (%) | ||||
| Systemic glucocorticoids | 216 (41) | 79 (49) | 61 (36) | 225 (45) |
| ≥1 DMARD | 88 (17) | 28 (17) | 34 (20) | 78 (16) |
Values are mean (SD), unless otherwise indicated.
Insufficient response to methotrexate was defined as not achieving DAS28(CRP) <3.2 at week 24/26 for OPTIMA or week 20/24 for PREMIER.
CRRP was defined as an increase in mTSS of >1.5 from baseline to week 26 for OPTIMA or week 24 for PREMIER.
CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; CRRP, clinically relevant radiographic progression; DAS28(CRP), 28-joint Disease Activity Score based on CRP; DMARD, disease-modifying antirheumatic drug; HAQ-DI, Health Assessment Questionnaire Disability Index; mTSS, modified total Sharp score; NA, not available; OPTIMA, Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab; RA, rheumatoid arthritis; SDAI, Simplified Disease Activity Index; SJC66, swollen joint count based on 66 joints; TJC68, tender joint count based on 68 joints.
Model with baseline factors
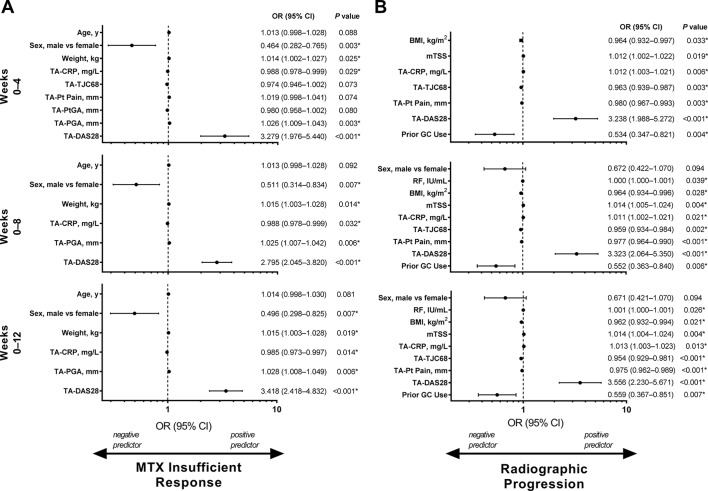
In the baseline model, DAS28(CRP) was the most statistically significant predictor that was positively associated with insufficient response to methotrexate and CRRP at 6 months (figure 1A,B). In separate analyses, SDAI and CDAI also predicted insufficient response to methotrexate at 6 months, similar to the primary analysis with DAS28(CRP), but did not predict CRRP (online supplementary figures 1 and 3). Male sex and prior use of systemic glucocorticoids were the strongest negative predictors of insufficient response to methotrexate (figure 1A; online supplementary figures 1A and 3A); prior systemic glucocorticoid use was also a negative predictor of CRRP (figure 1B; online supplementary figures 1B and 3B).
Figure 1.
Backward logistic regression analysis of baseline predictors, including DAS28(CRP), of insufficient response to methotrexate at 6 months (A) and CRRP at 6 months (B) in patients with early RA receiving methotrexate monotherapy. Insufficient response to methotrexate was defined as not achieving DAS28(CRP) <3.2 at weeks 22 and 26 (OPTIMA study) or weeks 20 and 24 (PREMIER study). CRRP was defined as increase in mTSS of >1.5 from baseline to week 26 (OPTIMA study) or week 24 (PREMIER study). Predictors analysis considered 500 methotrexate insufficient responders and 156 methotrexate responders; CRRP analysis considered 481 patients without CRRP and 163 patients with CRRP. The OR for each continuous variable (age, BMI, CRP at BL, DAS28 at BL, mTSS, PGA at BL, Pt Pain at BL, RF and weight) reflects the effect of a 1-unit change in that variable; thus, the ORs differ partly because of the different relative meaning of a 1-unit change, such as for DAS28 (scale, about 1–10) compared with SDAI (scale, 0 to about 86). The full regression models, before elimination of factors with p≥0.1, included age, race (white vs non-white), sex (male vs female), weight, RA duration, RF, BMI and mTSS; BL values for CRP, tender joint count based on 68 joints, swollen joint count based on 66 joints, Health Assessment Questionnaire Disability Index, Pt Pain, Pt Global Assessment of Disease Activity on a 100 mm visual analogue scale, PGA and DAS28; and prior use of DMARDs (yes vs no), number of prior DMARDs and prior use of GCs (yes vs no). *P<0.05; statistically significant difference. BL, baseline; BMI, body mass index; CRP, C-reactive protein; CRRP, clinically relevant radiographic progression; DAS28, 28-joint Disease Activity Score; DMARD, disease-modifying antirheumatic drug; GC, glucocorticoid; mTSS, modified total Sharp score; MTX, methotrexate; OPTIMA, Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab; OR, odds ratio; PGA, Physician Global Assessment of Disease Activity on a 100 mm visual analogue scale; Pt, patient; RA, rheumatoid arthritis; RF, rheumatoid factor; SDAI, Simplified Disease Activity Index.
Model with baseline and postbaseline factors
In the baseline and postbaseline (time-averaged) predictors model, higher time-averaged DAS28(CRP) through week 12 was the strongest positive predictor of insufficient response to methotrexate and CRRP at 6 months (p<0.001 for both; figure 2). Additionally, high values of time-averaged DAS28(CRP) through weeks 4 and 8 (p<0.001 for all) were also positive predictors of insufficient methotrexate response and CRRP. As in the model with only baseline factors, time-averaged SDAI and CDAI were also predictors of these outcomes (online supplementary figures 2 and 4). Male sex was again the strongest negative predictor of insufficient response to methotrexate (figure 2A; online supplementary figures 2A and 4A), and prior glucocorticoid was the strongest negative predictor of CRRP in the time-averaged model (figure 2B; online supplementary figures 2B and 4B).
Figure 2.
Backward logistic regression analysis of baseline and postbaseline predictors, including DAS28(CRP), of insufficient response to methotrexate at 6 months (A) and CRRP at 6 months (B) based on data from weeks 0–4, weeks 0–8 and weeks 0–12 in patients with early RA receiving methotrexate monotherapy. Week 4 analysis considered 485 methotrexate insufficient responders and 151 methotrexate responders, week 8 analysis considered 507 methotrexate insufficient responders and 160 methotrexate responders and week 12 analysis considered 500 methotrexate insufficient responders and 156 methotrexate responders. Week 4 analysis considered 471 patients without CRRP and 153 patients with CRRP, week 8 analysis considered 491 patients without CRRP and 163 patients with CRRP and week 12 analysis considered 481 patients without CRRP and 163 patients with CRRP. Insufficient response to methotrexate was defined as not achieving DAS28(CRP) <3.2 at weeks 22 and 26 (OPTIMA study) or weeks 20 and 24 (PREMIER study). CRRP was defined as an increase in modified total Sharp score of >1.5 from baseline to week 26 (OPTIMA study) or week 24 (PREMIER study). The OR for each continuous variable (age, BMI, mTSS, RF, TA-CRP, TA-DAS28, TA-PGA, TA-Pt Pain, TA-PtGA, TA-TJC68, weight) reflects the effect of a 1-unit change in that variable; thus, the ORs differ partly because of the different relative meaning of a 1-unit change, such as for DAS28 (scale, about 1–10) compared with SDAI (scale, 0 to about 86). The full regression models, before elimination of factors with p≥0.1, included age, race (white vs non-white), sex (male vs female), weight, RA duration, RF, BMI and mTSS; TA values for CRP, TJC68, swollen joint count based on 66 joints, HAQ-DI, Pt Pain, PtGA, PGA and DAS28; and prior use of DMARDs (yes vs no), number of prior DMARDs and prior use of GCs (yes vs no). *P<0.05; statistically significant difference. BMI, body mass index; CRP, C-reactive protein; CRRP, clinically relevant radiographic progression; DAS28, 28-joint Disease Activity Score; DMARD, disease-modifying antirheumatic drug; GC, glucocorticoid; HAQ-DI, Health Assessment Questionnaire Disability Index; mTSS, modified total Sharp score; MTX, methotrexate; OPTIMA, Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab; OR, odds ratio; PGA, Physician Global Assessment of Disease Activity on a 100 mm visual analogue scale; Pt, patient; PtGA, Patient Global Assessment of Disease Activity on a 100 mm visual analogue scale; RA, rheumatoid arthritis; RF, rheumatoid factor; SDAI, Simplified Disease Activity Index; TA, time averaged; TJC68, tender joint count based on 68 joints.
Efficacy at 6 months and onward among patients with an insufficient response to methotrexate
Patients
In OPTIMA, 348 patients who did not achieve the stable LDA target of DAS28(CRP) <3.2 after 26 weeks of methotrexate monotherapy (ie, had an insufficient response to methotrexate) and who advanced to adalimumab plus methotrexate rescue therapy were included in this analysis. In PREMIER, 177 patients who did not achieve the stable LDA target of DAS28(CRP) <3.2 after 24 weeks of methotrexate monotherapy and continued that regimen were included in this analysis. Most demographics and baseline disease characteristics were different between patients who had an insufficient response to methotrexate in OPTIMA and PREMIER (table 2). Mean baseline disease duration was 0.3 and 0.8 years, respectively. The mean mTSS was lower among patients who had an insufficient response to methotrexate in OPTIMA than in PREMIER (11.7 vs 23.0), and a lower percentage of patients in OPTIMA than in PREMIER (63% vs 89%) had >1 erosion at baseline.
Table 2.
Baseline demographics, disease characteristics and prior and concomitant therapies of patients who had an insufficient response to methotrexate
| Characteristic | Patients with an insufficient response to methotrexate at 6 months (n=525)* | |
| OPTIMA Adalimumab Rescue (n=348) |
PREMIER Methotrexate Monotherapy (n=177) |
|
| Demographic characteristics | ||
| Women, n (%) | 266 (76) | 132 (75) |
| White, n (%) | 313 (90) | 170 (96) |
| Age (years) | 50.7 (13.9) | 52.5 (13.3) |
| RA duration (years) | 0.3 (0.3) | 0.8 (0.9) |
| Weight (kg) | 77.5 (20.8) | 76.5 (18.6) |
| BMI (kg/m2) | 28.4 (6.8)† | 27.6 (6.6) |
| Disease characteristics | ||
| RF+, n (%) | 302 (88)‡ | 147 (83) |
| CRP (mg/L) | 32.6 (33.3) | 41.8 (41.6) |
| TJC68 | 28.6 (14.7) | 33.3 (14.7) |
| SJC66 | 19.1 (10.7) | 22.9 (12.3) |
| DAS28(CRP) | 6.1 (0.9)§ | 6.4 (0.8)¶ |
| SDAI | 45.2 (13.7)§ | 49.7 (13.5)** |
| HAQ-DI (range, 0–3) | 1.7 (0.6)† | 1.5 (0.7)¶ |
| mTSS | 11.7 (18.5)† | 23.0 (22.5)** |
| >1 erosion, n (%) | 217 (63)† | 155 (89)** |
| Prior therapies | ||
| Systemic glucocorticoids, n (%) | 133 (38) | 83 (47) |
| ≥1 DMARD, n (%) | 34 (10) | 54 (31) |
Values are mean (SD), unless otherwise indicated.
*n consists of patients treated with placebo+methotrexate who did not achieve DAS28(CRP) <3.2 at week 24/26 for OPTIMA or week 20/24 for PREMIER.
†n=347 non-missing values.
‡n=344 non-missing values.
§n=341 non-missing values.
¶n=176 non-missing values.
**n=174 non-missing values.
BMI, body mass index; CRP, C-reactive protein; DAS28(CRP), 28-joint Disease Activity Score based on CRP; DMARD, disease-modifying antirheumatic drug; HAQ-DI, Health Assessment Questionnaire Disability Index; mTSS, modified total Sharp score; NA, not available; OPTIMA, Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab; RA, rheumatoid arthritis; RF, rheumatoid factor; SDAI, Simplified Disease Activity Index; SJC66, swollen joint count based on 66 joints; TJC68, tender joint count based on 68 joints.
Disease activity
Although by definition none of the patients evaluated here had achieved stable LDA (ie, on at least two assessments) by 6 months, a small and similar percentage of patients achieved LDA (10% vs 12%) in OPTIMA and PREMIER at 6 months (week 26 or 24, respectively). The mean (SD) DAS28(CRP) scores also were similar at the same time points (4.5 (1.2) vs 4.4 (1.1); p=0.144). After 1 year of additional treatment (ie, at week 78 in OPTIMA or week 76 in PREMIER), a significantly higher percentage of patients who received adalimumab rescue therapy at week 26 in OPTIMA, versus patients who continued to receive methotrexate monotherapy in PREMIER, achieved LDA (64% vs 41%, respectively; p<0.001), and their mean DAS28(CRP) score was significantly lower (2.9 vs 3.6; p<0.001). Similar patterns of improvement were also observed in the proportion of patients achieving DAS28(CRP) <2.6 (48% vs 19%), SDAI ≤3.3 (32% vs 10%) and CDAI ≤2.8 (32% vs 10%) at week 78 (OPTIMA) or week 76 (PREMIER), respectively (all p<0.001), as well as in mean SDAI (10.3 vs 16.0; p<0.001) and mean CDAI (9.5 vs 14.9; p<0.001). In multivariate regression analyses, switching to adalimumab rescue therapy was associated with significantly greater odds of achieving LDA (ie, DAS28(CRP) <3.2) versus continuing on methotrexate (OR 3.25 (95% CI 1.83 to 5.74); p<0.001). Similar results were observed for DAS28(CRP) <2.6 (OR 3.27 (95% CI 1.74 to 6.14); p<0.001), SDAI ≤3.3 (OR 3.30 (95% CI 1.52 to 7.15); p=0.002) and CDAI ≤2.8 (OR 3.43 (95% CI 1.58 to 7.44); p=0.002).
Functional and radiographic outcomes
Functional and radiographic outcomes demonstrated similar patterns as disease activity outcomes. At week 78 (OPTIMA) or week 76 (PREMIER), significantly greater proportions of patients who switched to adalimumab rescue therapy achieved improvement in HAQ-DI of ≥0.22 (54% vs 30%; p<0.001), and these patients also had significantly greater mean changes in HAQ-DI from week 26/24 to week 78/76 (−0.3 vs −0.1; p<0.001) versus those who continued on methotrexate monotherapy. However, there was no difference in the mean HAQ-DI scores between groups at week 78/76 (0.7 vs 0.8; p=0.236), although baseline HAQ-DI scores were higher in insufficient methotrexate responders in OPTIMA versus PREMIER (1.7 vs 1.5; table 2), and therefore overall reduction from baseline was also much larger in OPTIMA. At week 52, 88% of patients who switched to adalimumab rescue therapy achieved ΔmTSS of ≤0.5 vs 57% of patients who continued on methotrexate (p<0.001); the mean changes from week 26 were 0.0 and 2.2 (p<0.001) in the two groups, respectively. Similarly, at week 78/76, patients who switched to adalimumab rescue therapy achieved ΔmTSS of ≤0.5 with significantly greater frequency (88% vs 41%; p<0.001) and had significantly less mean progression in mTSS from week 26/24 (0.1 vs 4.7; p<0.001) versus those who continued on methotrexate monotherapy. Furthermore, switching to adalimumab rescue therapy was associated with greater odds of achieving improvement in HAQ-DI of ≥0.22 at week 78/76 (OR 2.27 (95% CI 1.26 to 4.11); p=0.006), ΔmTSS of ≤0.5 at week 52 (OR 4.71 (95% CI 2.56 to 8.65); p<0.001) and ΔmTSS of ≤0.5 at week 78/76 (OR 8.96 (95% CI 4.94 to 16.27); p<0.001) versus continuing on methotrexate monotherapy.
Discussion
Although methotrexate remains the first-line treatment strategy in patients with RA,1 only 28%–44% of patients with early RA exhibit sufficient clinical response to methotrexate monotherapy within 6 months,4 12 13 and another treatment strategy must be considered in those for whom the treatment is insufficient. Because radiographic damage occurs early in the course of RA,3 timely adaptation of therapy after failure of the initial treatment strategy may be necessary to prevent irreversible damage. Current recommendations suggest adjusting therapy when patients have no improvement by 3 months or have not reached the therapeutic target by 6 months.1 Consequently, identifying patients who are at risk of methotrexate failure would facilitate a more targeted therapy strategy and allow earlier adjustment of treatment.
In our post hoc analysis of two randomised, double-blind, placebo-controlled trials in patients with early RA receiving initial methotrexate monotherapy, baseline disease activity by composite measures was the strongest positive predictor of insufficient response to the starting therapy at 6 months. Although methotrexate was the starting treatment in our analysis, prior research suggests that the predictive value of early disease activity on later outcomes is independent of the particular regimen, as correlations in a previous report were observed whether patients received methotrexate monotherapy or methotrexate plus a tumour necrosis factor (TNF) inhibitor.14 Furthermore, in our analysis, early clinical activity (ie, time-averaged DAS28(CRP), SDAI and CDAI over 4, 8 and 12 weeks) was a significant predictor of insufficient response to methotrexate, suggesting that changes in clinical disease activity as early as 4 weeks can predict response to methotrexate treatment at 6 months. Similarly, various predictors for response to methotrexate therapy in patients with RA have been identified in previous studies, including high disease activity (measured by DAS, SDAI or CDAI) and HAQ score at baseline14 15 and disease activity (measured by DAS28, SDAI or CDAI) at 3 months.14 Early disease activity has been previously found to predict later disease activity in patients with RA treated with the TNF inhibitor certolizumab pegol.16 17 An analysis of seven pivotal, randomised trials in early and established RA demonstrated that disease activity response reached at 3 months is significantly associated with reaching target at 6 months; interestingly, the type of treatment did not significantly influence the accuracy of the analysis.18 More studies are warranted to confirm these results.
Besides being predictors of insufficient response to methotrexate, baseline and time-averaged disease activity were also significant predictors of CRRP in the present analysis. Baseline DAS28 and mTSS were also identified as predictors of radiographic progression in early RA in a previous study.19 This is in line with other studies that have long found that markers of disease activity (eg, CRP, erythrocyte sedimentation rate and swollen joint count) are predictors of radiographic progression, aside from autoantibody positivity and presence of early damage.7 20–26 Our findings also showed that glucocorticoid treatment at baseline was a negative predictor of CRRP in the baseline and time-averaged models (and also of insufficient response to methotrexate in the baseline models), similar to established understanding.27 Overall, these results are in line with the treatment-to-target strategy and support timely adaptation of therapy with TNF inhibitors in patients who have insufficient response to methotrexate.1 2
When assessing response to methotrexate, more consistent results have been obtained in other studies when using sex as a predictor. Men respond better to methotrexate therapy than women, and studies in early RA have identified female sex as a predictor of poor response to methotrexate.12 28 29 Greater body mass index also has been shown previously to negatively predict radiographic progression.30 31 Our findings were consistent with these patterns. Overall, these data suggest that women with early RA who receive methotrexate may benefit from frequent early monitoring of disease activity, allowing for the opportunity to adjust treatment within the first 3–6 months for those with insufficient response. Indeed, it is currently recommended to change treatment regimen if disease activity has not improved by at least 50% within 3 months after treatment start.1 18
When assessed for long-term outcomes, patients who received adalimumab rescue therapy at week 26 (OPTIMA) had significantly greater improvements and odds of achieving LDA at week 78 compared with patients who received methotrexate monotherapy (PREMIER) for 76 weeks. Nonetheless, 41% of patients who received methotrexate monotherapy achieved LDA at week 76. A previous study demonstrated delayed response to methotrexate monotherapy versus methotrexate combined with adalimumab.32 Taken together, these results suggest that, although more patients receiving combination therapy achieve LDA earlier, some patients receiving methotrexate monotherapy will achieve treatment response later. Future analyses should explore what factors predict successful long-term methotrexate treatment in some patients, despite an insufficient response at 6 months, to identify who might not require adaptation of therapy.
In this post hoc analysis, integration of the populations randomised to receive methotrexate in the OPTIMA and PREMIER trials allowed identification of outcomes and predictors of insufficient response to methotrexate and CRRP. However, because population differences can impact treatment response,33 the possible heterogeneity between pooled study populations is a limitation of this analysis. Other limiting factors included possible patient selection bias and multiple comparisons and the fact that the analyses compared the results of two separate trials that were not conducted head to head, were done in different calendar years and had non-identical designs and baseline characteristics. Also, smoking status, citrullinated peptide positivity and erosions (except as part of mTSS) were not included in the predictor regression models. Furthermore, addition of glucocorticoids when methotrexate treatment failed was not part of the study protocols. However, because (based on recent insights) conducting a 2-year methotrexate monotherapy study in patients with an insufficient response to methotrexate is not in line with current ethical considerations, this analysis provides data not attainable in future studies.
In patients with early RA, baseline disease characteristics and patient demographics as well as early disease activity can predict response to methotrexate treatment and radiographic progression at 6 months. In patients who did not achieve LDA after 6 months of initial methotrexate monotherapy, the addition of adalimumab to the treatment regimen was associated with better clinical, functional and radiographic outcomes. These results support, and indirectly confirm, treatment-to-target strategies and timely adaptation of therapy with TNF inhibitors in patients with early RA and identify risk factors of patients who are not responding sufficiently to methotrexate.
Acknowledgments
AbbVie funded the studies, contributed to their designs and was involved in the collection, analysis, and interpretation of the data and in the writing, review and approval of the publication. AbbVie and the authors thank the patients who participated in the clinical trial and all study investigators for their contributions.
Footnotes
Handling editor: Dimitrios T Boumpas
Contributors: All authors made contributions to the planning, conduct and/or reporting of the work. Medical writing assistance was provided by Michael J Theisen, PhD, and Maria Hovenden, PhD, of Complete Publication Solutions (North Wales, PA), a CHC Group company, and was supported by AbbVie.
Funding: The OPTIMA and PREMIER studies and this post hoc analysis were funded by AbbVie (NCT00420927, NCT00195663).
Competing interests: JSS and AK provided remunerated expert advice to and received grant/research support for his institution from AbbVie. RFvV has received grants and research support from AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, Roche and UCB; and consulting fees and honoraria from AbbVie, AstraZeneca, Biotest, Bristol-Myers Squibb, Celgene, Crescendo, GlaxoSmithKline, Janssen, Lilly, Merck, Novartis, Pfizer, Roche, UCB and Vertex. SF, SC and JLS are employees of AbbVie and may hold stock or stock options.
Patient consent: Not required.
Ethics approval: The original clinical studies were approved by a central institutional review board or independent ethics committee at each participating site. This post hoc analysis of data from those studies did not require additional approval.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymised, individual and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 3. Lindqvist E, Jonsson K, Saxne T, et al. Course of radiographic damage over 10 years in a cohort with early rheumatoid arthritis. Ann Rheum Dis 2003;62:611–6. 10.1136/ard.62.7.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rezaei H, Saevarsdottir S, Forslind K, et al. In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2-year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Ann Rheum Dis 2012;71:186–91. 10.1136/annrheumdis-2011-200038 [DOI] [PubMed] [Google Scholar]
- 5. van der Heijde D, Klareskog L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum 2006;54:1063–74. 10.1002/art.21655 [DOI] [PubMed] [Google Scholar]
- 6. Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- 7. Vastesaeger N, Xu S, Aletaha D, et al. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology 2009;48:1114–21. 10.1093/rheumatology/kep155 [DOI] [PubMed] [Google Scholar]
- 8. Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis 2013;72:64–71. 10.1136/annrheumdis-2011-201247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321–32. 10.1016/S0140-6736(13)61751-1 [DOI] [PubMed] [Google Scholar]
- 10. Keystone EC, Breedveld FC, van der Heijde D, et al. Longterm effect of delaying combination therapy with tumor necrosis factor inhibitor in patients with aggressive early rheumatoid arthritis: 10-year efficacy and safety of adalimumab from the randomized controlled PREMIER trial with open-label extension. J Rheumatol 2014;41:5–14. 10.3899/jrheum.130543 [DOI] [PubMed] [Google Scholar]
- 11. Wells GA, Tugwell P, Kraag GR, et al. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol 1993;20:557–60. [PubMed] [Google Scholar]
- 12. van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YP, et al. Limited efficacy of conventional DMARDs after initial methotrexate failure in patients with recent onset rheumatoid arthritis treated according to the disease activity score. Ann Rheum Dis 2007;66:1356–62. 10.1136/ard.2006.066662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreland LW, O’Dell JR, Paulus HE, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum 2012;64:2824–35. 10.1002/art.34498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aletaha D, Funovits J, Keystone EC, et al. Disease activity early in the course of treatment predicts response to therapy after one year in rheumatoid arthritis patients. Arthritis Rheum 2007;56:3226–35. 10.1002/art.22943 [DOI] [PubMed] [Google Scholar]
- 15. Romão VC, Canhão H, Fonseca JE. Old drugs, old problems: where do we stand in prediction of rheumatoid arthritis responsiveness to methotrexate and other synthetic DMARDs? BMC Med 2013;11:17 10.1186/1741-7015-11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curtis JR, Luijtens K, Kavanaugh A. Predicting future response to certolizumab pegol in rheumatoid arthritis patients: features at 12 weeks associated with low disease activity at 1 year. Arthritis Care Res 2012;64:658–67. 10.1002/acr.21600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van der Heijde D, Keystone EC, Curtis JR, et al. Timing and magnitude of initial change in disease activity score 28 predicts the likelihood of achieving low disease activity at 1 year in rheumatoid arthritis patients treated with certolizumab pegol: a post-hoc analysis of the RAPID 1 trial. J Rheumatol 2012;39:1326–33. 10.3899/jrheum.111171 [DOI] [PubMed] [Google Scholar]
- 18. Aletaha D, Alasti F, Smolen JS. Optimisation of a treat-to-target approach in rheumatoid arthritis: strategies for the 3-month time point. Ann Rheum Dis 2016;75:1479–85. 10.1136/annrheumdis-2015-208324 [DOI] [PubMed] [Google Scholar]
- 19. Bombardier C, Chen M, Li X, et al. A risk model for the prediction of radiographic progression: results from SONORA study. J Rheumatol 2010;61:1281. [Google Scholar]
- 20. Fautrel B, Granger B, Combe B, et al. Matrix to predict rapid radiographic progression of early rheumatoid arthritis patients from the community treated with methotrexate or leflunomide: results from the ESPOIR cohort. Arthritis Res Ther 2012;14:R249 10.1186/ar4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis 2010;69:1333–7. 10.1136/ard.2009.121160 [DOI] [PubMed] [Google Scholar]
- 22. Smolen JS, Van Der Heijde DM, St Clair EW, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum 2006;54:702–10. 10.1002/art.21678 [DOI] [PubMed] [Google Scholar]
- 23. Amos RS, Constable TJ, Crockson RA, et al. Rheumatoid arthritis: relation of serum C-reactive protein and erythrocyte sedimentation rates to radiographic changes. Br Med J 1977;1:195–7. 10.1136/bmj.1.6055.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharp JT, Lidsky MD, Duffy J. Clinical responses during gold therapy for rheumatoid arthritis. Changes in synovitis, radiologically detectable erosive lesions, serum proteins, and serologic abnormalities. Arthritis Rheum 1982;25:540–9. 10.1002/art.1780250508 [DOI] [PubMed] [Google Scholar]
- 25. Möttönen TT. Prediction of erosiveness and rate of development of new erosions in early rheumatoid arthritis. Ann Rheum Dis 1988;47:648–53. 10.1136/ard.47.8.648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Leeuwen MA, van der Heijde DM, van Rijswijk MH, et al. Interrelationship of outcome measures and process variables in early rheumatoid arthritis. A comparison of radiologic damage, physical disability, joint counts, and acute phase reactants. J Rheumatol 1994;21:425–9. [PubMed] [Google Scholar]
- 27. Kavanaugh A, Wells AF. Benefits and risks of low-dose glucocorticoid treatment in the patient with rheumatoid arthritis. Rheumatology 2014;53:1742–51. 10.1093/rheumatology/keu135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saevarsdottir S, Wallin H, Seddighzadeh M, et al. Predictors of response to methotrexate in early DMARD naive rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis 2011;70:469–75. 10.1136/ard.2010.139212 [DOI] [PubMed] [Google Scholar]
- 29. Wessels JA, van der Kooij SM, le Cessie S, et al. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis Rheum 2007;56:1765–75. 10.1002/art.22640 [DOI] [PubMed] [Google Scholar]
- 30. Joo YB, Bang SY, Ryu JA, et al. Predictors of severe radiographic progression in patients with early rheumatoid arthritis: A prospective observational cohort study. Int J Rheum Dis 2017;20:1437–46. 10.1111/1756-185X.13054 [DOI] [PubMed] [Google Scholar]
- 31. Baker JF, Ostergaard M, George M, et al. Greater body mass independently predicts less radiographic progression on X-ray and MRI over 1-2 years. Ann Rheum Dis 2014;73:1923–8. 10.1136/annrheumdis-2014-205544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smolen JS, Bu X, Wang X, et al. Characteristics of patients with early rheumatoid arthritis who have a delayed response to treatment with methotrexate in monotherapy or in combination with adalimumab [abstract]. Arthritis Rheumatol 2017;69(suppl 10 abstr 1419). [Google Scholar]
- 33. Griffiths B, Situnayake RD, Clark B, et al. Racial origin and its effect on disease expression and HLA-DRB1 types in patients with rheumatoid arthritis: a matched cross-sectional study. Rheumatology 2000;39:857–64. 10.1093/rheumatology/39.8.857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2018-213502supp001.pdf (844KB, pdf)