Abstract
Background
Developmental dysplasia of the hip (DDH) remains common. If detected early, DDH can usually be corrected with conservative management. Late presentations often require surgery and have worse outcomes.
Objective
We estimated the risk of undergoing surgery for DDH by age 3 years before and after the introduction of enhanced DDH detection services.
Design
Retrospective cohort study.
Setting
Scotland, 1997/98–2010/11.
Patients
All children.
Methods
Using routinely collected national hospital discharge records, we examined rates of first surgery for DDH by age 3 by March 2014. Using a difference in difference analysis, we compared rates in two areas of Scotland before (to April 2002) and after (from April 2005) implementation of enhanced DDH detection services to those seen in the rest of Scotland.
Results
For children born in the study period, the risk of first surgery for DDH by age 3 was 1.18 (95% CI 1.11 to 1.26) per 1000 live births (918/777 375).
Prior to April 2002, the risk of surgery was 1.13 (95% CI 0.88 to 1.42) and 1.31 (95% CI 1.16 to 1.46) per 1000 live births in the intervention and non-intervention areas, respectively. In the intervention areas, from April 2005, this risk halved (RR 0.47; 95% CI 0.32 to 0.68). The risk remained unchanged in other areas (RR 1.01; 95% CI 0.86 to 1.18). The ratio for the difference in change of risk was 0.46 (95% CI 0.31 to 0.70).
Conclusions
The implementation of enhanced DDH detection services can produce substantial reductions in the number of children having surgical correction for DDH.
Keywords: paediatric surgery, screening
What is already known on this topic?
Developmental dysplasia of the hip is the most common congenital lower limb problem affecting children.
If identified early, conservative management with an abduction harness usually results in a good outcome, requiring no further intervention. Nonetheless, the role of screening is contested.
What this study adds?
The introduction of enhanced newborn screening for developmental dysplasia of the hip was associated with a halving of rates of subsequent corrective surgery.
On the basis of this evidence, wider implementation of enhanced developmental dysplasia of the hip screening appears to be justifiable.
Introduction
Developmental dysplasia of the hip (DDH), ranges from a physiologically immature hip joint that spontaneously resolves to an irreducible dislocation of the hip joint requiring surgical intervention. It is the most common congenital lower limb problem affecting children.1
If diagnosed within the first 10 weeks of life, most cases requiring treatment can be managed conservatively with abduction harnessing resulting in a good outcome, requiring no further intervention.2 After this age, there is a progressive increase in the need for surgery and the risk of poorer outcomes,3 4 which identifies DDH as a potential target for screening.
There is a lack of adequately powered clinical trials concerning how children should be screened for DDH,5 however. Consequently, international practice varies,6–9 and there is also disagreement between professional bodies. The US Preventive Services Task Force statement from 2006,8 which has not been updated, states that ‘evidence is insufficient to recommend routine screening for DDH in infants as a means to prevent adverse outcomes’. In contradiction, the American Academy of Paediatrics recommends that all newborns should be screened by physical examination.7
Evidence from case series and historical comparisons does suggest that enhanced models of DDH detection involving expert examiners or increased use of ultrasound imaging may improve early diagnosis and reduce the likelihood of children requiring surgical treatment.9–12 In the light of this, two of the 14 geographical National Health Service (NHS) entities within Scotland have independently introduced enhanced DDH detection services. In 2002, NHS Lothian appointed an extended scope physiotherapist to lead a DDH detection service and in 2004 NHS Fife employed a physician with an interest in DDH.
In Scotland, national data are not collected on newborn physical examinations, nor on radiological investigations or conservative treatment for DDH provided in outpatient settings. National hospital discharge records are available, however, providing information on day case and inpatient surgical procedures. A surgical procedure for DDH in childhood indicates that a child has either failed conservative treatment or, more likely, presented too late for such treatment to be attempted. Rates of surgical intervention can therefore be considered as a surrogate marker for late presentation.13
Therefore, we used nationally available hospital discharge records (1) to estimate the risk of surgery for DDH before, during and after the introduction of enhanced DDH detection services in the two geographic areas, compared with the rest of Scotland and (2) to describe the risk of surgery according to sex, deprivation and year of birth.
Methods
Population
Children were included in the study population if they were born in Scotland between April 1997 and March 2013. The number of live births in each financial year 1997/1998 to 2012/13 was obtained from statutory birth registration records held by National Records for Scotland.14
Outcomes
All surgical operations in Scotland are recorded in a national database (Scottish Morbidity Record 01) alongside a unique identifier for each individual as well as their age, sex and area of residence as well as up to six diagnostic codes (WHO Tenth International Statistical Classification of Diseases and Related Health Problems (ICD 10)) and four procedure codes (Office of Population Censuses and Surveys Fourth Classification of Interventions and Procedures).15 Using this database, we defined surgical intervention for DDH as any record with a relevant diagnostic code (ICD-10 Q65.0–9) and procedure code (T20.2, T20.5, W13.4, W14.4, W16.4, W16.9, W28.1, X22.1–9, W65-6). Code labels are provided in etable 1 in the online Supplementary data. Records identified using these codes were tested against an operative database in NHS Lothian covering 2008–2013 and found to have a 98% concordance. First surgery for DDH was defined as the first DDH procedure for any child in the study population any time up to March 2014. Birth data were matched to first operation data by sex, year of birth, area of residence and deprivation quintile.
archdischild-2017-314354supp001.docx (194KB, docx)
Intervention
In line with national guidance,16 standard care in Scotland is that children are examined using the Ortolani and Barlow tests shortly after birth in maternity units (by junior doctors, physiotherapists and/or midwives) and again during the 6–8 week universal child health review (usually by primary care physicians). Children with abnormal findings at a newborn or 6–8 week examination or with recognised risk factors for DDH (eg, breech presentation, family history or moulding abnormality) are referred to specialist services for further investigation, although the detail of service pathways varies between areas.
In two areas of Scotland, enhanced models of DDH detection involving expert examiners or increased use of ultrasound imaging were introduced. NHS Fife serves a population of 350 000 with approximately 3900 births per year, with all maternity services provided from a single large secondary centre. NHS Lothian serves a population of 765 000 with approximately 9000 births per year, with maternity services provided from a large tertiary centre and a smaller district general hospital. NHS Lothian is somewhat more affluent than the Scottish average, but otherwise these areas are fairly typical of the Scottish population (see Appendix in the In 2002, in NHS Lothian Supplementary data).
In 2002, in NHS Lothian, after a 2-year training period, a physiotherapist was appointed to an enhanced role where 75% of their time was assigned to the prevention and treatment of DDH. This included a presence on postnatal wards examining newborns, training and supporting paediatric medical staff performing hip examination, promoting awareness of DDH in the postnatal setting and delivering a weekly clinic for secondary examinations with ultrasound support and harnessing treatment. Less than 25% of this time was spent on direct patient contact, with the majority being spent providing training and promoting awareness. The service was enhanced in 2008 with the addition of a second extended scope physiotherapist.
In 2004, in NHS Fife, a physician with broad responsibilities for delivering paediatric orthopaedic care began a weekly hip examination and ultrasound clinic for detection and treatment of DDH and provided routine training and education for the paediatric and maternity teams responsible for newborn physical examinations. Expert examination and ultrasound were performed in 31% of all live births in Fife between 2004 and 2013.
The time period for the introduction of the intervention to both areas (hereafter, introduction period) was defined as the financial years from April 2002 to April 2005. The incidence of surgery for DDH was compared before, during and after this period. For the purposes of the analysis, any child born after the introduction period and who was resident in either of the two NHS areas within Scotland where the quality improvement programmes were initiated was assumed to have received the intervention. To test the robustness of our findings, we also varied the definition of both the start and end of the introduction period by ±1 year and ±2 years.
Deprivation
Deprivation quintile was defined using the Scottish Index of Multiple Deprivation, a small area-based measure of multiple material deprivation, with children assigned to a population-based deprivation quintile based on their postcode of residence at birth or surgery.17
Statistical analysis
The risk of surgery per 1000 live births and 95% CI by patient characteristics was estimated by sampling from a beta distribution with a Jeffrey’s prior, as this has excellent frequentist properties.18 The change in the 3 year odds of surgery for children born in the intervention areas before and after the intervention period was compared with the change in odds for children born in other areas using a logistic regression model, with a period/area interaction term to obtain a difference in difference comparison. Logistic regression was also used to model the risk of a surgical event for girls versus boys, each deprivation quintile (vs most deprived quintile) and each year of birth (vs 1997/1998). Analyses were undertaken both unadjusted and adjusted for each of the other covariates: adjustment made minimal difference to results and hence only unadjusted results are reported. As the risk of the outcome was low, these OR may be interpreted as relative risks (RR) (ie, risk ratios) and are presented as such to aid interpretability.
All analyses were performed within the Information Services Division of NHS National Services Scotland, with only aggregate results subsequently released, and hence ethical approval was not required. Missing covariate data were extremely uncommon and so we used complete case analysis. Analyses were undertaken using SPSS V.21.0 and R V.3.2.0 (R Foundation, Vienna, Austria).
Results
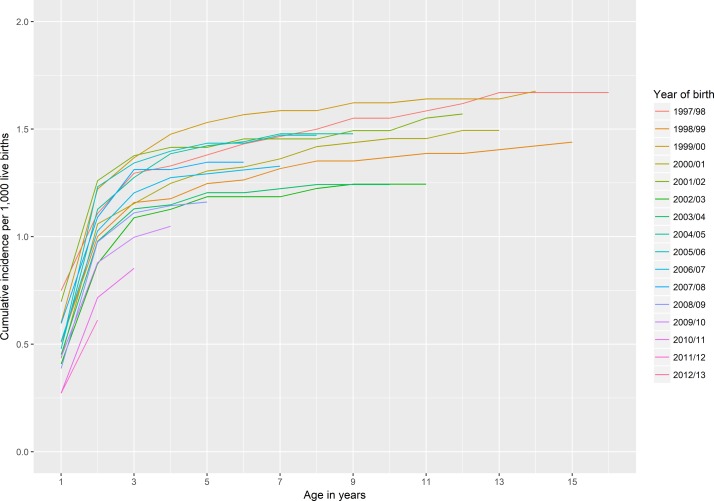
There were 896 594 live births and 1103 first surgical interventions for DDH in Scotland up to end March 2014 among children born in the financial years 1997/1998 to 2012/2013 inclusive. For children born in 1997/1998, the risk of surgery by age 16 years was 1.67 per 1000 live births, or around one in 600 births.
Around 80% of operations occurred in children aged 3 years and younger (see etable 2 in the online Supplementary data, figure 1). Among the 780 475 live births in the period 1997/1998 to 2010/2011 (ie, the period where data were available for all children up to the age of 3), there were 927 cases of first surgery by age 3 years. For 3100 (0.4%) live births and for nine children who had surgery, there was missing data on sex, deprivation or area of residence. Among the remaining 777 375 births, the risk of surgery by age 3 years was 1.18 (95 %CI 1.11 to 1.26) per 1000 births. Risk was more than six times higher in girls than boys (RR 6.20; 5.16 to 7.46) and was lowest in the most deprived quintile compared with the remaining deprivation quintiles (RR for most deprived quintile vs middle quintile 0.71; 95% CI 0.58 to 0.87, table 1).
Figure 1.
Cumulative incidence of first surgical intervention for developmental dysplasia of the hip by age up to 16 years, children born 1997/1998 to 2012/2013, Scotland.
Table 1.
Risk of first surgical intervention for developmental dysplasia of the hip by age 3 years, Scotland, children born 1997/1998–2010/2011, by sex, deprivation quintile and year of birth
| Live births | Number of children | Risk per 1000 live births (95% CI) | Relative risk (95% CI) | |
| Male | 398 036 | 133 | 0.34 (0.28 to 0.40) | 1.00 (ref) |
| Female | 379 339 | 785 | 2.07 (1.93 to 2.22) | 6.20 (5.16 to 7.46) |
| Deprivation | ||||
| 1 (most deprived) | 193 500 | 183 | 0.95 (0.82 to 1.09) | 1.00 (ref) |
| 2 | 157 246 | 193 | 1.23 (1.07 to 1.41) | 1.30 (1.06 to 1.59) |
| 3 | 144 944 | 193 | 1.33 (1.16 to 1.53) | 1.41 (1.15 to 1.72) |
| 4 | 144 096 | 188 | 1.31 (1.13 to 1.51) | 1.38 (1.13 to 1.69) |
| 5 (least deprived) | 137 589 | 161 | 1.17 (1.00 to 1.36) | 1.24 (1.00 to 1.53) |
| Year | ||||
| 1997/1998 | 58 395 | 76 | 1.31 (1.04 to 1.61) | 1.00 (ref) |
| 1998/1999 | 56 728 | 66 | 1.17 (0.91 to 1.47) | 0.89 (0.64 to 1.24) |
| 1999/2000 | 54 636 | 74 | 1.36 (1.07 to 1.69) | 1.04 (0.76 to 1.43) |
| 2000/2001 | 52 659 | 60 | 1.15 (0.88 to 1.47) | 0.88 (0.62 to 1.23) |
| 2001/2002 | 51 341 | 70 | 1.37 (1.07 to 1.70) | 1.05 (0.76 to 1.45) |
| 2002/2003 | 51 236 | 53 | 1.05 (0.79 to 1.34) | 0.80 (0.56 to 1.13) |
| 2003/2004 | 52 939 | 59 | 1.13 (0.86 to 1.42) | 0.86 (0.61 to 1.20) |
| 2004/2005 | 53 945 | 69 | 1.29 (1.00 to 1.60) | 0.98 (0.71 to 1.36) |
| 2005/2006 | 54 178 | 72 | 1.34 (1.04 to 1.66) | 1.02 (0.74 to 1.41) |
| 2006/2007 | 56 291 | 67 | 1.20 (0.94 to 1.50) | 0.91 (0.66 to 1.27) |
| 2007/2008 | 58 476 | 77 | 1.32 (1.05 to 1.63) | 1.01 (0.74 to 1.39) |
| 2008/2009 | 59 211 | 66 | 1.12 (0.87 to 1.41) | 0.86 (0.62 to 1.19) |
| 2009/2010 | 58 957 | 59 | 1.01 (0.77 to 1.28) | 0.77 (0.55 to 1.08) |
| 2010/2011 | 58 383 | 50 | 0.87 (0.65 to 1.12) | 0.66 (0.46 to 0.94) |
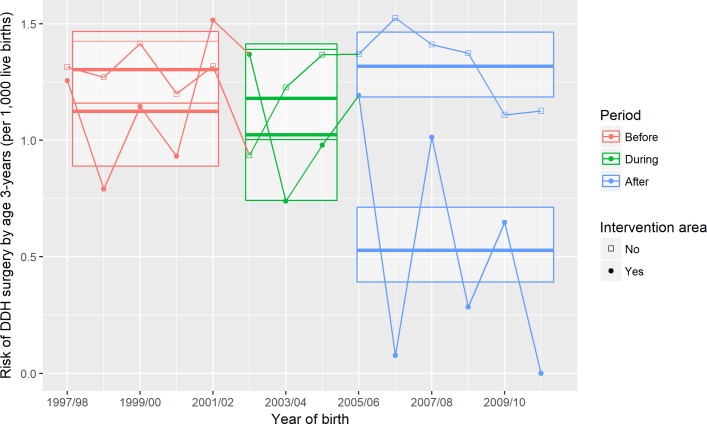
The risk of surgery at age 3 or younger before the introduction period was 1.30 and 1.13 per 1000 live births and 1.32 and 0.54 per 1000 live births after the introduction period for children born in non-intervention areas and intervention areas, respectively. For the children born in the intervention areas, this risk halved (RR after vs before introduction period 0.47; 95% CI 0.32 to 0.68, table 2, figure 2). Over this same time period, there was no evidence of a fall in risk for children born in non-intervention areas (RR 1.01; 95% CI 0.86 to 1.18). Therefore, the RR for the difference in after-introduction versus before-introduction risk in the intervention areas compared with the difference in after-introduction versus before-introduction risk in other geographic areas was 0.46 (95% CI 0.31 to 0.70).
Table 2.
Risk of first surgical intervention by age 3 years, before, during and after introduction of enhanced developmental dysplasia of the hip detection comparing intervention and non-intervention areas
| Before | During | After | ||
| Non-intervention area | Births | 212 445 | 121 991 | 264 109 |
| Surgery | 277 | 144 | 348 | |
| Risk of surgery per 1000 live births | 1.31 (1.16 to 1.46) | 1.19 (1.00 to 1.39) | 1.32 (1.18 to 1.46) | |
| Relative risk | 1 | 0.91 (0.74 to 1.11) | 1.01 (0.86 to 1.18) | |
| Intervention area | Births | 61 314 | 36 129 | 81 387 |
| Surgery | 69 | 37 | 43 | |
| Risk of surgery per 1000 live births | 1.13 (0.88 to 1.42) | 1.04 (0.73 to 1.40) | 0.54 (0.39 to 0.70) | |
| Relative risk | 1 | 0.91 (0.60 to 1.35) | 0.47 (0.32 to 0.68) | |
| Difference | Relative risk interaction | – | 1.01 (0.64 to 1.56) | 0.46 (0.31 to 0.70) |
Data shown are counts for births and surgery, risks per 1000 live births and relative risks and interaction relative risks derived from logistic regression models (assuming that, since the event rate is very low, the ORs are estimates of the relative risks, ie, risk ratios) with the main effects and an area/period interaction. Numbers in parentheses are 95% CIs.
Figure 2.
Risk of first surgical intervention by age 3 years, before, during and after introduction of enhanced developmental dysplasia of the hip (DDH) detection comparing intervention and non-intervention areas. Points represent risk of surgery per 1000 live births for each financial year by intervention versus non-intervention areas. For each combination of time period and area, the bold horizontal lines and upper and lower edges of the crossbars represent, respectively, point estimates and upper and lower 95% CIs, which were obtained from the difference-in-difference logistic regression model of risk of surgery on area and time period.
In subsequent analyses, which had not been prespecified, we estimated the difference in after-introduction versus before-introduction risk in intervention compared with non-intervention areas separately for Fife and Lothian; the RRs were 0.42 (95% CI 0.22 to 0.81) and 0.49 (95% CI 0.29 to 0.81), respectively.
In sensitivity analyses, varying the definition of both the start and end of the introduction period by ±1 year and ±2 years either side of the dates defined in the main analysis, similar associations were found. Across the sensitivity analyses, the interaction (ie, difference in difference) RRs varied from 0.33 (95% CI 0.21 to 0.52) to 0.58 (95% CI 0.39 to 0.87; see eFigure 2 in the online Supplementary data).
Discussion
In a study of all children born in Scotland over a 14-year period, we found that, following the introduction of enhanced DDH detection services, the risk of surgery for DDH in two geographic areas halved. No change in risk was seen for children born in other areas.
Our analysis exploited a natural experiment—a dramatic change in practice in selected areas of Scotland. Two of the 14 health boards in Scotland independently introduced enhanced DDH detection services; the remaining 12 areas continued with practice-as-usual. Using high-quality routine healthcare data for the whole of Scotland, we were therefore able to compare the risk of surgery for DDH across intervention and non-intervention areas before and after the introduction of these services. Therefore, this finding adds considerably to the evidence base on the effectiveness of DDH detection services.
While randomised clinical trials generally provide the strongest evidence for the effectiveness of diagnostic approaches, trials examining enhanced approaches to DDH detection have been limited by the rarity of the outcome. For example, in a 2011 Cochrane systematic review of randomised and pseudorandomised trials examining screening, only one study was identified which compared clinical examination alone to clinical examination plus targeted and/or universal ultrasound screening.5 The incidence of late surgery was lower in groups allocated (in a pseudorandomsied design) to ultrasound (RR 0.45; 95% CI 0.04 to 4.93 and 0.22; 95% CI 0.01 to 4.52 for targeted and universal ultrasound, respectively). However, the CIs were extremely wide ranging from a 20-fold protective effect to a fivefold increase in DDH surgery.19
In an update of this search, also including observational studies examining the impact of DDH screening approaches (see eResults in the online Supplementary data), we did not identify any new clinical trials and found that all but two studies addressing this question had no control group whatsoever. One previous study in a region of Australia had a historical control, comparing incidence counts between two 4-year periods before (1978–1982) and after (1993–1997) the adoption of a country-wide universal ultrasound screening programme.20 In children aged less than 1.5 years, open and closed reduction fell from 126 to 35 and 14 to 7, respectively, while children aged between 1.5 and 15 years, the count of acetabular osteotomies/varus derotation osteotomy fell from 89 to 13. However, the lack of a geographical control means that secular trends in either surgery or the recording and coding of procedures cannot be excluded as a possible explanation for the improvement.
A high-quality population-based case–control study was conducted in Germany from 1997 to 2002. The odds of having had a screening ultrasound for DDH (based on parental recall) in children who had undergone surgery for DDH aged >9 weeks and <5 years (identified via a population register) was compared with that for population-based controls born during the same period. The ORs (which in this case can be interpreted as a RR) was 0.41 (95% CI 0.31 to 0.55) suggesting that ultrasound is likely protective.21 However, universal ultrasound was offered at that time in Germany. As such, children who did and did not undergo ultrasound scanning might differ in their risk of developing DDH, or in being diagnosed and undergoing surgery between the ages of 9 weeks and 5 years. For example, parents unwilling for their child to participate in screening might also be less likely to fully engage with abduction harnessing, which depends on parental concordance.22 Alternatively, participation in the screening programme may be higher among those with one or more established risk factors for DDH (such as female sex, breech presentation and family history23), thereby increasing risk.
In contrast, to act as a confounder in our study, any increase in the prevalence of risk factors for DDH, or changes in the diagnosis and treatment of DDH would need to have differed across intervention areas and non-intervention areas and done so at the same time that the enhanced detection services were introduced. It is more probable that the introduction of enhanced DDH detection services did in fact reduce late diagnosis of DDH and hence unnecessary surgery.
In absolute terms, the estimated risk of DDH surgery aged 3 in the postintervention period was 0.54 per 1000 live births. Studies vary in definitions and follow-up. Nonetheless, this finding is consistent with rates achieved internationally: Clegg et al (Coventry, UK) 0.6/1000,11 Clarke et al (Southampton, UK) 0.74/1000,12 Duppe et al (Malmo, Sweden) 0.16/1000,10 Holen et al (Trondheim, Norway) 0.65/1000,24 Myers et al (New Zealand) 0.29/100025 and Laborie et al (Bergen, Norway) 0.38/1000.26 In each of these studies, some form of enhanced detection method was used. In the case of Clegg et al, universal ultrasound scanning was performed while Duppe, Holen and Myers describe the hip examination being carried out by experienced paediatricians or orthopaedic staff. It is notable in Clarke’s series that almost 20% of newborns were referred to specialist hip clinics where expert assessment was available.
In the context of such international comparisons, however, the estimated 3-year risk of 1.3 per 1000 live births in non-intervention areas for the same period appears to be excessively high. Indeed, if the relationship is causal, around 25 operations per year may be preventable in other regions of Scotland via the introduction of enhanced neonatal detection services similar to those introduced in NHS Lothian and Fife.
Unfortunately, as this study was not designed as an intervention study, we are unable to make strong recommendations as to the form which enhanced DDH detection should take. Nevertheless, the success of these diverse approaches support the view that the benefit of an enhanced service depend less on particular characteristics such as whether the enhanced service is weighted towards greater use of ultrasound or expert examiners than on whether it concentrates experience.19 24 Moreover, a feature common to both approaches is that direct physical examination (and/or ultrasound) by staff members with a specialist DDH role only took place in a minority of children. As such, we speculate that improved training in examination and education as to the importance of DDH detection among the wider staff may be an important component of a successful service-level intervention.
One unexpected finding in our analysis was that children in the most deprived quintile are least likely to undergo surgery. The reasons for this are unknown, but we speculate that greater involvement of health professionals (such as health visitors) in children born to socioeconomically deprived parents, and hence greater opportunities for early detection, may be one possible explanation.
One limitation of our study is that some of the children may have moved residence between DDH detection and surgery. Any such imbalance between numerator and denominator is likely to be minor, however, and appears unlikely to be differential across both time period and intervention area. Nor is there any evidence that international inward migration to the intervention areas compared with the non-intervention areas differed over the study period (see Appendix in the online Supplementary data).
We also note that we were only able to explore surgical management of DDH. Due to the nature of the administrative healthcare data used we were unable to include radiological aspects, non-surgical management or patient reported outcomes. A health-economic evaluation including the costs and quality of life impacts of both the possibility of increased ultrasound scanning and the potential increased use of abduction harnessing would be of considerable interest.
An additional limitation is the lack of a publicly deposited pre-specified analysis plan. Nonetheless, the results were extremely robust to sensitivity analyses varying the time periods, demonstrating that the choice of different cut-points would not have importantly affected the results.
Conclusion
The implementation of enhanced DDH detection services can produce substantial reductions in late diagnosis of DDH and associated requirement for surgical correction.
Acknowledgments
We would like to acknowledge the contribution of Dr James Chalmers and the Information Services Division clinical coding and quality improvement teams. We would also like to acknowledge the contribution of Dr Robert Humphries who provided data from NHS Fife. We would also like to thank Professor RW Paton of the Department of Orthopaedics, Royal Blackburn Hospital, East Lancashire Hospitals NHS Trust, UK for making useful suggestions to improve the manuscript.
Footnotes
Contributors: DM and JM contributed to study design, analysed and interpreted the data, drafted and revised the manuscript. MR revised the manuscript. CF, AM and RW designed the study, interpreted the data and revised the manuscript.
Funding: DM is funded via an Intermediate Clinical Fellowship (and Beit Fellowship) from the Wellcome Trust (201492/Z/16/Z). JM is funded via a Clinician Scientist Fellowship from the Medical Research Council. Neither sponsor had any role in the design, analysis or reporting of this work.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data can be obtained from the Information Services Division, NHS National Services Scotland via established data access policies. Contact ISD’s Electronic Data Research and Innovation Service (http://www.isdscotland.org/Products-and-Services/eDRIS/).
References
- 1. Bialik V, Bialik GM, Blazer S, et al. Developmental dysplasia of the hip: a new approach to incidence. Pediatrics 1999;103:93–9. 10.1542/peds.103.1.93 [DOI] [PubMed] [Google Scholar]
- 2. Cashman JP, Round J, Taylor G, et al. The natural history of developmental dysplasia of the hip after early supervised treatment in the Pavlik harness. A prospective, longitudinal follow-up. J Bone Joint Surg Br 2002;84:418–25. 10.1302/0301-620X.84B3.12230 [DOI] [PubMed] [Google Scholar]
- 3. Ömeroğlu H, Köse N, Akceylan A. Success of Pavlik harness treatment decreases in patients ≥ 4 months and in ultrasonographically dislocated hips in developmental dysplasia of the hip. Clin Orthop Relat Res 2016;474:1146–52. 10.1007/s11999-015-4388-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pollet V, Pruijs H, Sakkers R, et al. Results of Pavlik harness treatment in children with dislocated hips between the age of six and twenty-four months. J Pediatr Orthop 2010;30:437–42. 10.1097/BPO.0b013e3181df85ab [DOI] [PubMed] [Google Scholar]
- 5. Shorter D, Hong T, Osborn DA. Cochrane Neonatal Group. Screening programmes for developmental dysplasia of the hip in newborn infants Cochrane database of systematic reviews. 360: John Wiley & Sons, Ltd, 2011. 10.1002/14651858.CD004595.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newborn and infant physical examination screening: programme overview – GOV.UK. https://www.gov.uk/guidance/newborn-and-infant-physical-examination-screening-programme-overview (accessed 11 May 2017).
- 7. Shaw BA, Segal LS. Section on orthopaedics. Evaluation and referral for developmental dysplasia of the hip in infants. Pediatrics 2016;138:e20163107 10.1542/peds.2016-3107 [DOI] [PubMed] [Google Scholar]
- 8. Final Recommendation Statement. Developmental hip dysplasia: screening – US preventive services task force. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/developmental-hip-dysplasia-screening (accessed 11 May 2017).
- 9. von Kries R, Ihme N, Oberle D, et al. Effect of ultrasound screening on the rate of first operative procedures for developmental hip dysplasia in Germany. Lancet 2003;362:1883–7. 10.1016/S0140-6736(03)14957-4 [DOI] [PubMed] [Google Scholar]
- 10. Düppe H, Danielsson LG. Screening of neonatal instability and of developmental dislocation of the hip. A survey of 132,601 living newborn infants between 1956 and 1999. J Bone Joint Surg Br 2002;84:878–85. [DOI] [PubMed] [Google Scholar]
- 11. Clegg J, Bache CE, Raut VV. Financial justification for routine ultrasound screening of the neonatal hip. J Bone Joint Surg Br 1999;81:852–7. 10.1302/0301-620X.81B5.9746 [DOI] [PubMed] [Google Scholar]
- 12. Clarke NM, Reading IC, Corbin C, et al. Twenty years experience of selective secondary ultrasound screening for congenital dislocation of the hip. Arch Dis Child 2012;97:423–9. 10.1136/archdischild-2011-301085 [DOI] [PubMed] [Google Scholar]
- 13. Godward S, Dezateux C. Surgery for congenital dislocation of the hip in the UK as a measure of outcome of screening. MRC Working Party on Congenital Dislocation of the Hip. Medical Research Council. Lancet 1998;351:1149–52. [DOI] [PubMed] [Google Scholar]
- 14. National Records of Scotland. https://www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events Published May 31, 2013 (accessed 1 May 2017).
- 15. SMR Datasets | ISD Scotland | Data Dictionary. http://www.ndc.scot.nhs.uk/Data-Dictionary/SMR-Datasets/ (accessed 1 May 2017).
- 16. Newborn and infant physical examination screening: programme overview - GOV.UK.https://www.gov.uk/guidance/newborn-and-infant-physical-examination-screening-programme-overview (accessed 1 May 2017).
- 17. Scottish Government SAH. Scottish index of multiple deprivation. http://www.gov.scot/Topics/Statistics/SIMD (accessed 9 May 2017).
- 18. Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Stat Sci 2001;16:101–33. [Google Scholar]
- 19. Rosendahl K, Markestad T, Lie RT. Ultrasound screening for developmental dysplasia of the hip in the neonate: the effect on treatment rate and prevalence of late cases. Pediatrics 1994;94:47–52. [PubMed] [Google Scholar]
- 20. Thaler M, Biedermann R, Lair J, et al. Cost-effectiveness of universal ultrasound screening compared with clinical examination alone in the diagnosis and treatment of neonatal hip dysplasia in Austria. J Bone Joint Surg Br 2011;93:1126–30. 10.1302/0301-620X.93B8.25935 [DOI] [PubMed] [Google Scholar]
- 21. von Kries R, Ihme N, Altenhofen L, et al. General ultrasound screening reduces the rate of first operative procedures for developmental dysplasia of the hip: a case-control study. J Pediatr 2012;160:271–5. 10.1016/j.jpeds.2011.08.037 [DOI] [PubMed] [Google Scholar]
- 22. Mubarak S, Garfin S, Vance R, et al. Pitfalls in the use of the Pavlik harness for treatment of congenital dysplasia, subluxation, and dislocation of the hip. J Bone Joint Surg Am 1981;63:1239–48. 10.2106/00004623-198163080-00005 [DOI] [PubMed] [Google Scholar]
- 23. de Hundt M, Vlemmix F, Bais JM, et al. Risk factors for developmental dysplasia of the hip: a meta-analysis. Eur J Obstet Gynecol Reprod Biol 2012;165:8–17. 10.1016/j.ejogrb.2012.06.030 [DOI] [PubMed] [Google Scholar]
- 24. Holen KJ, Tegnander A, Bredland T, et al. Universal or selective screening of the neonatal hip using ultrasound? A prospective, randomised trial of 15,529 newborn infants. J Bone Joint Surg Br 2002;84:886–90. [DOI] [PubMed] [Google Scholar]
- 25. Myers J, Hadlow S, Lynskey T. The effectiveness of a programme for neonatal hip screening over a period of 40 years: a follow-up of the New Plymouth experience. J Bone Joint Surg Br 2009;91:245–8. 10.1302/0301-620X.91B2.21300 [DOI] [PubMed] [Google Scholar]
- 26. Laborie LB, Markestad TJ, Davidsen H, et al. Selective ultrasound screening for developmental hip dysplasia: effect on management and late detected cases. A prospective survey during 1991–2006. Pediatr Radiol 2014;44:410–24. 10.1007/s00247-013-2838-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
archdischild-2017-314354supp001.docx (194KB, docx)