Abstract
We report a case of Budd-Chiari syndrome occurring in a patient with coeliac disease, who presented with symptoms of increased abdominal girth, right upper quadrant pain and shortness of breath for three weeks prior to admission. Initial assessment revealed the presence of moderate ascites, hepatosplenomegaly and right-sided pleural effusion. Further diagnostic work-up established a diagnosis of chronic Budd-Chiari syndrome. Interestingly, complete screening for pro-thrombotic factors was negative. A review of the literature on this association disclosed only 28 similar cases, with the majority of them describing individuals of North African origin. Interestingly, in the majority of cases no specific thrombotic factor could be identified, suggesting that coeliac disease may play a role in this thrombotic disorder.
Keywords: coeliac disease, Budd-Chiari syndrome, hepatic vein thrombosis
Introduction
Coeliac disease (CD)—an immune-mediated enteropathy triggered by the ingestion of gluten proteins in genetically susceptible individuals—is known to be frequently associated with a variety of extra-intestinal manifestations [1]. North American and European epidemiological studies have shown that CD is a common disorder, with a prevalence ranging between 1/100 and 1/150 [2]. Similarly, studies from north Africa and the Middle East have shown that the prevalence of CD is comparable to that in western countries [3].
Over the past four decades, several CD-associated hepato-biliary disorders have been documented, including isolated hypertransaminasemia, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, and non-alcoholic fatty liver disease [4]. Budd-Chiari syndrome (BCS) comprises a heterogeneous group of disorders characterized by hepatic venous outflow obstruction, in the absence of right heart failure, constrictive pericarditis, or sinusoidal obstruction syndrome. BCS is a relatively rare condition, with an estimated prevalence at one case per 100 000 individuals [5]. Several case reports published in the past two decades suggested an association between CD and BCS [6–21]. The aim of this case report and pertinent review is to improve our understanding of the clinical, pathogenic, and epidemiological characteristics of this disease association.
Case Presentation
A 27-year old single female, a known case of CD and on a gluten-free diet (GFD) for the previous 7 years, presented with a 3-week history of increasing abdominal girth, right upper quadrant pain, and shortness of breath. Pertinent physical examination showed a patient in moderate respiratory distress, with dullness to percussion of the right hemithorax and abdomen.
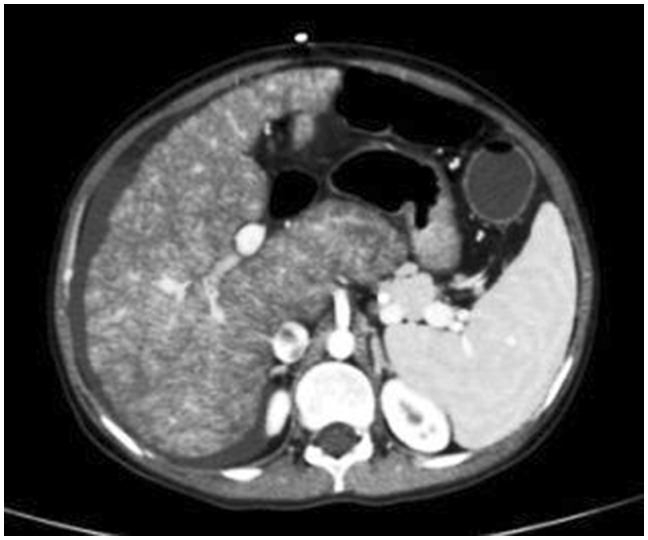
Abdominal ultrasound revealed moderate ascites, enlarged heterogenous liver with nodularity and irregular margins, and moderate right-sided pleural effusion, in addition to marked splenomegaly (long axis 16 cm). A Doppler ultrasound of the liver showed collapsed suprahepatic veins. The diagnosis of BCS was confirmed by a contrast-enhanced computed tomography (CT) of the abdomen (Figure 1), which showed an enlarged nodular liver, hypertrophied caudate lobe, non-visualization of suprahepatic veins with narrowing of the hepatic segment of the inferior vena cava. At this point a liver biopsy was performed and histopathological examination demonstrated a nodular architecture, with sinusoidal dilatation seen mainly in Zones 1 and 2, consistent with vascular insult.
Figure 1.
Abdominal CT with double contrast showing hepatosplenomegaly and free fluid in the perihepatic region. The suprahepatic veins are not opacified, while the inferior vena cava appears patent. The liver appears nodular, with hypertrophied caudate lobe.
Oesophago-gastroduodenoscopy revealed no oesophageal varices or signs of portal hypertensive gastropathy. The duodenal mucosa and folds appeared normal, while histopathological examination of biopsies taken from the bulb and the second portion of the duodenum revealed an increase in intraepithelial lymphocytes in the absence of crypt hyperplasia or villous atrophy (modified Marsh type 1). Examination of peritoneal fluid was consistent with transudative ascites and tests for spontaneous bacterial peritonitis, neoplasia, and acid-fast bacilli were all negative.
A complete blood count disclosed mild normocytic anaemia and a platelet count of 145 000/mm3 (normal: 150 000–400 000). Liver function tests revealed hypoalbuminaemia (32 g/L; normal: 35–50), normal bilirubin and transaminases, alkaline phosphatase of 274 U/L (normal: 0–240), and a gamma-glutamyl transpeptidase of 86 U/L (normal: 6–42). Pro-thrombin time and international normalized ratio (INR) were elevated at 16.9 seconds and 1.4 seconds, respectively, while partial thromboplastin time was normal. Serum iron levels were low at 3.3 μmol/L (normal: 6.6–26), while ferritin was normal. Vitamin B12 level was low at 113.4 pg/mL (normal: 208–964), and folate level was normal. Vitamin D (25-OH) was decreased at 12.89 ng/mL (normal: >29), while serum calcium levels were within normal limits. Hepatitis C antibodies and HBsAg were both non-reactive. A complete serological screen for autoimmune hepatitis (anti-nuclear antibodies and anti-smooth muscle antibodies) produced normal results. Fasting lipid profile, kidney and thyroid function tests were within normal limits.
A full screen for all known thrombophilic factors was unrevealing. Specifically homocystein level 7.8 μmol/L (normal: 4.9–11.6); wild type (normal) of pro-thrombin mutation by PCR; lupus anticoagulant 39 seconds (normal:32–42); anticardiolipin (IgG and IgA) negative; wild type of factor V Leiden by PCR (normal); antithrombin III activity 88% (normal: 80–120); protein C 95% (normal 75–165); protein S free 91% (normal: 50–120). A bone marrow biopsy—performed to rule out myeloproliferative disorder—was normal. Additionally, a test for V617F JAK2 mutation by real-time polymerase chain reaction (JAK2 MutaQuant kit, Ipsogen, Hilden, Germany) was negative.
The patient was therefore managed conservatively with anticoagulants, diuretics, and a gluten-free diet in addition to oral vitamin D supplements and vitamin B12 injections. She initially showed some improvement, but unfortunately later developed massive, refractory ascites, requiring multiple therapeutic paracentesis. At that point, she was referred to interventional radiology for porto-caval shunt, which was successfully carried out, with subsequent resolution of ascites. Six years after her shunt procedure, the patient remains well with no recurrence of ascites.
Discussion
This case describes the occurrence of BCS in a female patient with CD. She developed features of BCS despite strict adherence to a GFD and in the absence of any known pro-thrombotic factor. We searched PubMed for full articles, case reports and case series published in English, Spanish, or French-language journals in the period from January 1980 to April 2016 using the following keywords and free text terms alone or in combination: "Budd-Chiari syndrome", "celiac", "coeliac", "gluten-sensitive enteropathy", "liver abnormalities", "venous thrombosis", "coagulation", and "thrombophilia". Our literature search revealed the following points: (i) few reports of CD-associated BCS have been published, with most of the cases reported from north African countries in French-language Journals; (ii) many pro-thrombotic mechanisms have been proposed, without definite conclusions on the exact cause–effect relationship between CD and BCS; (iii) a paucity of studies on the effects of a gluten-free diet on the course of BCS and other thrombotic disorders associated with CD.
BCS in CD was first described in 1990 [6]. Since then, scattered case reports of CD-associated BCS have appeared in the literature. Our literature review revealed only 12 case reports and three case series (a total of 27 cases) on the occurrence of BCS in patients with CD [6–20], and one case of BCS detected in a case-finding study using CD serology in patients with various liver diseases (Table 1) [21]. Interestingly, 22 cases were reported from north African Arab countries [7, 8, 11, 13, 15, 17–20], while only six cases were described in other regions of the world [9, 10, 12, 14, 16, 21]. In a prospective study in Algeria, the authors assessed the aetiology of BCS in 116 consecutive patients [22]. In that study, CD was found in 10 (11.4%) of the 88 patients tested, and only 40% of those patients had an underlying thrombophilia associated with CD. Based on their results, the authors proposed a systematic search for CD in the aetiological work-up for BCS.
Table 1.
Clinical and demographic characteristics of reported BCS in CD patients
| First author; year of publication | Country | Number of cases | Gender | Presentation of BCS | Pro-thrombotic cause |
|---|---|---|---|---|---|
| Boudhina [6]; 1990 | Tunisia | 3 | 2F, 1M | Fulminant | Idiopathic |
| Hamdi [7]; 1990 | Tunisia | 1 | M | Fulminant | ATIII deficiency |
| Martheau [8]; 1994 | Algeria | 3 | 2F, 1M | 1 acute; | 1 MPD; |
| 2 chronic | 2 Idiopathic | ||||
| Aguirrebarrena [9] ; 2001 | Argentina | 1 | F | Chronic | ATIII and protein C deficiency |
| Manzano [10]; 2002 | Spain | 1 | M | Acute/sub-acute | Idiopathic |
| El Younsi [11]; 2003 | Mauritania | 1 | M | Acute/sub-acute | Idiopathic |
| Danalioğlu [12]; 2003 | Turkey | 1 | F | Chronic | Idiopathic |
| Gelsi [13]; 2004 | Algeria | 1 | F | Acute/sub-acute | Idiopathic |
| Martinez [14]; 2004 | Spain | 1 | M | Chronic | Folate deficiency |
| Kchaou [15]; 2008 | Tunisia | 1 | F | Chronic | Idiopathic |
| Kochhar [16]; 2009 | India | 1 | F | Chronic | Functional deficiency of protein C and protein S |
| Afredj [17]; 2010 | Algeria | 9 | 6F, 3M | 1 acute; | 5 Idiopathic; |
| 2 sub-acute; | 1 MPD; | ||||
| 6 chronic | 1 OCP use; | ||||
| 1 Protein C deficiency; | |||||
| 1 Factor V Leiden mutation | |||||
| Ben Hriz [18]; 2010 | Tunisia | 1 | M | Acute | Idiopathic |
| Hmami [19]; 2011 | Tunisia | 1 | F | Sub-acute | Idiopathic |
| Karim [20]; 2012 | Algeria | 1 | F | Sub-acute | Idiopathic |
| Drastich [21]; 2012 | Czech Republic | 1 | F | Chronic | Idiopathic |
BCS = Budd-Chiari syndrome; CD = Celiac disease; F = Female; M = Male; ATIII = Antithrombin III; MPD = Myeloproliferative disorder; OCP = Oral contraceptive pills.
Our case was the first to be reported from Middle-Eastern Arab countries. It thus seems that the association between CD and BCS is relatively common in north African countries, but rather infrequent in other parts of the world. It has been proposed, by some authors, that genetic and environmental factors (geophagia) explain this predilection for north African individuals, but nothing of this sort has been established [6, 7]. Others have attempted to implicate the passage of hepatotoxic substances, present in north African food, through an excessively permeable intestinal mucosa into the portal circulation [23]. Conversely, the high prevalence of CD in north Africa could also account for the elevated frequency of this pathological association, suggesting that the association could be fortuitous [24].
Among the reported cases, patient age has varied between 20 months and 50 years, with the majority of patients being in their third or fourth decade. Female patients constituted 64% of cases (18/28), with some tendency to present at an older age than male patients. These results are in accordance with data from a large, multicentre case series including 237 patients with BCS, which showed that the median age was 35 (range: 13–76), and 67% of patients were female [25]. The authors of that study concluded that BCS is more common in females and usually presents in the third or fourth decade, although it may occur in young children, as in some of the cases reviewed herein [6]; thus CD-associated BCS does not appear to behave differently from isolated BCS.
Regarding the chronological presentation of CD and BCS, 12 patients were known to have CD and presented later with symptoms suggestive of BCS, like our patient. Eight had simultaneous presentation of CD and BCS, while eight more presented with a picture of BCS and were then found to have CD upon further investigation of symptoms suggestive of CD or endoscopic findings indicative of CD. Interestingly, in 16 out of 28 cases of CD-associated BCS, the diagnosis of CD was established upon investigating patients for symptoms of BCS in the absence of typical symptoms of CD, confirming that ′silent′ and atypical presentations of CD have become very common. On the other hand, hepatic vein thrombosis can be asymptomatic, especially in the setting of chronic occlusion with large collaterals, which explains the incidental finding of BCS in some patients known to have CD [25]. The clinical significance of this lies in the consideration of BCS in coeliac patients who present with unexplained ascites, right upper abdominal pain, jaundice, or hepatomegaly, similar to the case described herein. Physicians should therefore maintain a high index of suspicion for both conditions.
Among the reported cases, the clinical presentation of BCS has varied between fulminant and chronic, with the latter presentation being most common (14/28; 50%), and the former being least common (4/28; 14%). Our patient presented with sub-acute features, even though imaging studies and histopathological examination of the liver were consistent with chronic BCS. In a report of 163 patients with BCS, the disease was categorized as acute or fulminant (26%), sub-acute or chronic (20%), or both (50%) [26]. It thus appears that, when associated with CD, BCS tends to present more frequently in the chronic form; however the small number of reported cases does not allow us to draw valid conclusions in this regard.
The present review revealed that isolated hepatic vein thrombosis was reported in 24 cases (85.7%), while combined hepatic vein (HV) and suprahepatic inferior vena cava (IVC) thrombosis was found in three cases [17], while membranous obstruction of the suprahepatic IVC was detected in one case [14]. Similarly, the site of venous obstruction in BCS in the absence of CD is most commonly reported to be the hepatic vein, while combined hepatic vein and suprahepatic IVC thrombosis is less so, and isolated suprahepatic IVC thrombosis is the least common [14, 27]. It therefore appears that the site of venous obstruction in CD-associated BCS is no different from that in isolated BCS.
Interestingly, in the great majority of CD-associated BCS cases (17/28; 61%) no specific thrombotic aetiology was found, as in our case. Conversely, an underlying thrombotic condition can be detected in more than 80% of patients with isolated BCS [26]. Further, in a case series reported by Darwish et al. [27], 46% of patients had more than one risk factor for BCS; thus it is possible that CD per se may play a role in the thrombotic process leading to BCS. This hypothesis is supported by the association between extra-hepatic thrombotic disorders—both venous and arterial—and CD [28, 29]. Interestingly, other autoimmune digestive diseases, such as inflammatory bowel diseases (IBD), have been reported to be associated with venous thrombosis, including BCS [30]. It is possible that the commonly found hyposplenism in both CD and IBD patients could, through thrombocytosis, provide the basis for thrombosis in these autoimmune disorders; however, other mechanisms have been suggested as explaining the pro-thrombotic potential of CD, including (i) malabsorption of vitamin K causing protein C, S and antithrombin III deficiency, (ii) hyperhomocysteinemia secondary to folate deficiency and/or variants in the methylenetetrahydrofolic acid reductase (MTHFR) gene [31], (iii) an association with serum lupus anticoagulant [14], (iv) autoimmune vasculitis [32], (v) magnesium deficiency [33] and and (vi) myointimal proliferation leading to thrombosis/atheromasia [34].
It is arguable that multiple factors, both inherited and acquired, are needed to precipitate a thrombotic event in coeliac patients, given that only a minority of them experience clinical thrombosis; for instance, it is believed that gut-derived pro-thrombotic factors in patients with altered intestinal mucosa—such as in inflammatory bowel disease and CD—may contribute to the development of non-cirrhotic intrahepatic portal hypertension [35]. Therefore, additional studies are needed to confirm the relevance of the proposed thrombotic mechanisms and to identify other possible pro-thrombotic factors, including dietary, genetic or environmental aetiologies.
Finally, the effect of a GFD on the course of BCS in CD has not been frequently reported. In our patient, BCS had developed despite strict adherence to a GFD, as shown by the negative endoscopic examination of the duodenum, near-normal histopathology, and coeliac serology. In one study, seven out of nine patients (77%) with BCS-CD exhibited the disappearance of ascites and development of collateral porto-systemic circulations after 6 months on a GFD [17]. On the other hand, the two patients who did not adhere to a GFD had refractory ascites and developed extension of their thrombosis in the HV and the IVC. The findings in Afredji’s study are interesting but the small number of patients does not allow valid conclusions. We believe that factors other than gluten sensitivity per se could be involved in the pathogenesis of BCS in CD.
In conclusion, this case report highlights the intriguing association between BCS and CD, and sheds some light on the putative pathogenic mechanisms, although the limited number of cases reported raises the question of whether the CD–BCS association could be fortuitous. An underlying pro-thrombotic condition was detected in less than 50% of the patients, implying a possible thrombogenic role for CD. Factors usually implicated in the occurrence of BCS in CD—including hyperhomocysteinemia, geophagia, special diet, deficiency in proteins C and S, factor V Leyden mutation, and thrombocytosis are not well established. Other pathogenic mechanisms should be searched for in these patients. Although there is, thus far, no proven direct link between CD and BCS, clinicians should maintain a high index of suspicion for this association. We suggest that a diagnosis of CD should be pursued in the setting of BCS of undetermined aetiology. Similarly, CD patients with unexplained manifestations of acute or chronic liver injury should be assessed for BCS.
Conflict of interest statement: none declared.
References
- 1. Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology 2005;128(Suppl 1):S74–78. [DOI] [PubMed] [Google Scholar]
- 2. Catassi C, Kryszak D, Louis-Jacques O. et al. Detection of celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol 2007;102:1454–60. [DOI] [PubMed] [Google Scholar]
- 3. Rostami K, Malekzadeh R, Shahbazkhani B. et al. Coeliac disease in Middle Eastern countries: a challenge for the evolutionary history of this complex disorder? Dig Liver Dis 2004;36:694–97. [DOI] [PubMed] [Google Scholar]
- 4. Ludvigsson JF, Elfstrom P, Broom EU. et al. Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol 2007;5:63–69. [DOI] [PubMed] [Google Scholar]
- 5. Valla DC. The diagnosis and management of the Budd-Chiari syndrome: consensus and controversies. Hepatology 2003;38:793–803. [DOI] [PubMed] [Google Scholar]
- 6. Boudhina T, Ghram N, Ben Becher S. et al. Budd-Chiari syndrome and total villous atrophy in children: apropos of 3 case reports. Tunis Med 1990;68:59–62. [PubMed] [Google Scholar]
- 7. Hamdi A, Ayachi R, Saad H. et al. Hemiplegia revealing Budd-Chiari syndrome associated with celiac disease in an infant. Presse Med 1990; 19:1011–12. [PubMed] [Google Scholar]
- 8. Martheau P, Cadranel JF, Messing B. et al. Association of hepatic vein obstruction and celiac disease in North African subjects. J Hepatol 1994; 20:650–53. [DOI] [PubMed] [Google Scholar]
- 9. Aguirrebarrena G, Pulcinelli S, Giovannoni AG. et al. Celiac disease and Budd-Chiari syndrome: infrequent association. Rev Esp Enferm Dig 2001; 93:611. [PubMed] [Google Scholar]
- 10. Manzano ML, Garfia C, Manzanares J. et al. Celiac disease and Budd-Chiari syndrome: an uncommon association. Gastroenterol Hepatol 2002;25:159–61. [PubMed] [Google Scholar]
- 11. El Younsi S, Nassif T, Kuoch V. et al. Association of Budd-Chiari syndrome and celiac disease. Gastroenterol Clin Biol 2003;27:236–37. [PubMed] [Google Scholar]
- 12. Danalioğlu A, Poturoğlu S, Güngör Güllüoğlu M. et al. Budd-Chiari syndrome in a young patient with celiac sprue: a case report. Turk J Gastroenterol 2003;14:262–65. [PubMed] [Google Scholar]
- 13. Gelsi E, Ruitord F, Saint-Paul MC. et al. Association of Budd-Chiari syndrome with coeliac disease in a patient native from North Africa. Gastroenterol Clin Biol 2004;28:903–5. [DOI] [PubMed] [Google Scholar]
- 14. Martinez F, Berenguer M, Prieto M. et al. Budd-Chiari syndrome caused by membranous obstruction of the inferior vena cava associated with coeliac disease. Dig Liver Dis 2004;36:157–62. [DOI] [PubMed] [Google Scholar]
- 15. Ouakaa-Kchaou A, Ennaifer R, Belhadi N. et al. Celiac disease associated with Budd Chiari syndrome. Presse Med 2008;37:239–41. [DOI] [PubMed] [Google Scholar]
- 16. Kochhar R, Masoodi I, Dutta U. et al. Celiac disease and Budd Chiari syndrome: report of a case with review of literature. Eur J Gastroneterol Hepatol 2009;21:1092–94. [DOI] [PubMed] [Google Scholar]
- 17. Afredj N, Metatla S, Faraoun SA. et al. Association of Budd-Chiari syndrome and celiac disease. Gastroenterol Clin Biol 2010;34:621–24. [DOI] [PubMed] [Google Scholar]
- 18. Ben Hriz F, Habbassi H, Maamouri N. et al. Budd-Chiari syndrome associated with celiac disease. Rev Med Interne 2010;31:160–62. [DOI] [PubMed] [Google Scholar]
- 19. Hmami F, Chaouki S, Souilmi FZ. et al. Association of celiac disease and Budd-Chiari syndrome: a case report. Arch Pediatr 2011;18:102. [DOI] [PubMed] [Google Scholar]
- 20. Karim A, Hliwa W, Alaoui R.. Association of Budd-Chiari syndrome and celiac disease: A new observation. Journal Africain d’Hepato-gastroenterologie 2012;6:251–52. [Google Scholar]
- 21. Drastich P, Honsová E, Lodererová A. et al. Celiac disease markers in patients with liver diseases: A single center large scale screening study. World J Gastroenterol 2012;18:6255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Afredj N, Guessab N, Nani A. et al. Aetiological factors of Budd-Chiari syndrome in Algeria. World J Hepatol 2015;7: 903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maccini DM, Berg JC, Bell GA.. Budd-Chiari syndrome and Crohn’s disease. An unreported association. Dig Dis Sci 1989;34:1933–36. [DOI] [PubMed] [Google Scholar]
- 24. Catassi C, Rätsch IM, Gandolfi L. et al. Why is coeliac disease endemic in the people of the Sahara? Lancet 1999;354:647–48. [DOI] [PubMed] [Google Scholar]
- 25. Darwish Murad S, Valla DC, de Groen PC. et al. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology 2004;39:500–8. [DOI] [PubMed] [Google Scholar]
- 26. Hadengue A, Poliquin M, Vilgrain V. et al. The changing scene of hepatic vein thrombosis: recognition of asymptomatic cases. Gastroenterology 1994;106:1042–47. [DOI] [PubMed] [Google Scholar]
- 27. Darwish Murad S, Plessier A, Hernandez-Guerra M. et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med 2009;151:167–75. [DOI] [PubMed] [Google Scholar]
- 28. Zenjari T, Boruchowicz A, Desreumaux P. et al. Association of coeliac disease and portal venous thrombosis. Gastroenterol Clin Biol 1995;19:953–54. [PubMed] [Google Scholar]
- 29. Upadhyay R, Park RHR, Russel RI. et al. Acute mesenteric ischaemia: a presenting feature of coeliac disease? BMJ 1987;295: 958–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Solem CA, Loftus EV, Tremaine WJ. et al. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol 2004;99:97–101. [DOI] [PubMed] [Google Scholar]
- 31. Wilcox GM, Mattia AR.. Celiac sprue, hyperhomocysteinemia, and MTHFR gene variants. J Clin Gastroenterol 2006;40: 596–601. [DOI] [PubMed] [Google Scholar]
- 32. Vives MJ, Esteve M, Mariné M. et al. Prevalence and clinical relevance of enteropathy associated with systemic autoimmune diseases. Dig Liver Dis 2012;44:636–42. [DOI] [PubMed] [Google Scholar]
- 33. Durlach J. Celiac disease, magnesium deficiency and venous thrombosis. Presse Med 2001;30:904. [PubMed] [Google Scholar]
- 34. Merra G, Lago AD, Roccarina D. et al. Celiac disease and myointimal proliferation: a possible correlation? Case Rep Gastroenterol 2008;2:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eapen CE, Nightingale P, Hubscher SG. et al. Non-cirrhotic intrahepatic portal hypertension: associated gut diseases and prognostic factors. Dig Dis Sci 2011;56:227–35. [DOI] [PubMed] [Google Scholar]