Cancer has remained one of the biggest challenges for human health during the past few decades despite major advances in medicine [1]. Poor prognosis of cancer is mainly caused by a lack of effective treatment. Moreover, many patients cannot receive appropriate treatment despite early diagnosis. High heterogeneity is a tumor characteristic and different types of cancer usually vary in clinical features and tumorigenic mechanisms, which results in different responses to drugs [2]. How to predict accurately the patient’s response to drugs and choose personalized treatment is one of the main concerns of doctors.
Cancer cell lines and patient-derived xenograft (PDX) models are widely used as preclinical tumor models for screening drugs and assessing drug responses. Cancer cell lines are derived from primary patient tumors and have contributed greatly to cancer research. Cancer cell lines are able to expand rapidly and are often used for high-throughput drug screening. Moreover, they are amenable to genetic modification, which is necessary for research on mechanisms of cancer development. However, it is clear that histological and genetic features in cancer cell lines have changed a lot compared with those in native tumors [3]. As a result, many drugs that perform well in cancer cell lines finally fail in clinical trials. PDX models are generated by transplanting fresh tumor tissue from patients subcutaneously or orthotopically into immunodeficient mice. They mimic the biological characteristics of the primary tumors better than do cancer cell lines. Although PDX models can better predict drug responses, their application in medicine is limited because they are expensive and time- and resource-consuming [4]. It is necessary to develop a new model that has the advantages of the two models mentioned above.
Tumor organoids may be superior models to identify and test novel anticancer drugs. Organoids are 3D cultured multicellular clusters. They are derived from pluripotent stem cells or isolated organ progenitors that differentiate to form an organ-like tissue exhibiting multiple cell types. Organoids have self-renewal and self-organization capabilities and retain the characteristics of the physiological structure and function of their source tumor. Sato et al. [5] placed intestinal stem cells into a gel matrix to mimic the extracellular matrix microenvironment, and finally the stem cells began to divide, differentiate and form structures resembling intestinal villi and crypts. Since then, organoids have been widely studied. Upon embedment into a 3D matrix, tissue-derived tumors from patients can also grow into organoids, termed tumor organoids (Figure 1) [6]. So far, 12 tumor organoids have been established (Table 1) [7–16]. In a recent study, Vlachogiannis and colleagues [7] developed patient-derived organoids (PDOs) derived from 110 metastatic tumor samples from 71 patients with colorectal or gastroesophageal cancer enrolled in phase I/II clinical trials. Phenotypic and genotypic profiling of PDOs was similar to that of the original tumors and they had the same gene-mutation spectrum. Besides, molecular profiling of the tumor organoids was matched to drug-screening results. More importantly, PDOs had 88% accuracy for predicting whether they would respond to some drugs and 100% accuracy for predicting whether they would not. The outcomes of PDOs for predicting treatment are encouraging and suggest that PDOs may be an ideal platform to identify and assess the efficacy of anticancer drugs.
Figure 1.
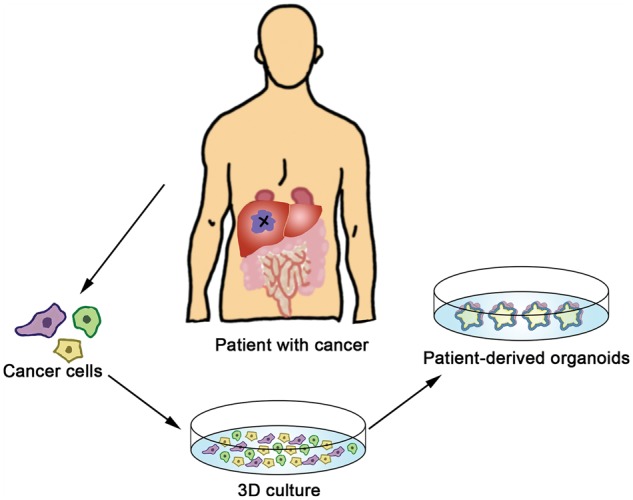
Generation of patient-derived organoids. Cancer cells from patients are embedded into a matrix for 3D culture in vitro and they can grow into tumor organoids. Patient-derived organoids mimic the biological characteristics of the primary tumors.
Table 1.
Summary of patient-derived organoids
| System | Tumor organoid | Tissue source | Reference(s) |
|---|---|---|---|
| Digestion | Gastrointestinal cancer | Primary tumor and metastases | [7] |
| Pancreatic cancer | Metastases and circulating tumor cells | [8] | |
| Colorectal cancer | Primary tumor and metastases | [9] | |
| Hepatocellular carcinoma | Primary tumor | [10] | |
| Cholangiocarcinoma | Primary tumor | [10] | |
| Combined hepatocellular | |||
| cholangiocarcinoma | Primary tumor | [10] | |
| Barrett’s oesophagus | Primary tumor | [11] | |
| Intestinal carcinoma | Primary tumor | [12] | |
| Urinary | Prostate cancer | Primary tumor and metastases | [13] |
| Bladder cancer | Primary tumor | [14] | |
| Reproduction | Breast cancer | Primary tumor | [15] |
| Nerve | Glioblastoma | Primary tumor and metastases | [16] |
PDOs are 3D in vitro models; many studies have shown that they can recapitulate basic features of primary tumors, including histological complexity and genetic heterogeneity of human cancer [7–16]. Hematoxylin and eosin staining shows that PDOs share morphological features with the primary tumor and immunohistochemistry markers used in the diagnosis of specific tumors show that the expression pattern of the tumor is maintained in PDOs [7]. Moreover, PDOs preserve the genomic and transcriptomic characteristics of their primary tumors [7]. For example, mutation status, copy number alteration and identification of fusion genes and transcriptional landscape are similar to those of the primary tumor. These genomic alterations persist even after several months of subsequent culture. Compared with 2D culture models, the 3D models have advantages in gene expression, mutation status, metastatic potential and drug response. PDOs can also be expanded long-term in vitro and used for high-throughput drug screening. These characteristics of PDOs discussed above allow a wide range of potential applications in medicine (Figure 2). First, PDOs could promote personalized cancer treatment. Individual PDOs share histological and genetic characteristics with the corresponding patient, so each PDO can serve like a small patient trial. A good supply of organoids derived from cancer patients can be generated in the short term and then effective drugs can be confirmed quickly. Each patient has a chance to receive the most suitable treatment with the help of their own PDOs. More importantly, the heterogeneity of cancer subtypes can be captured using individual PDOs and the results based on individual PDOs are more consistent with the real patients’ response to drugs. PDOs can help to optimize treatment plans. When combining tumor organoids and healthy organoids from one patient for assessing therapeutic effects, we can choose the best therapy that selectively kills tumor cells while leaving healthy cells unharmed. Second, PDOs are an ideal platform for high-throughput anticancer drug screening. They have great potential for disease modeling for cancer research and living biobanks of different types of cancer organoids can be established. An increasing number of researchers are choosing organoids instead of 2D culture models. Organoids can be easily manipulated using CRISPR/Cas9 approaches; therefore, PDOs are useful in cancer modeling and in identifying key driver mutations involved in cancer development [17].
Figure 2.
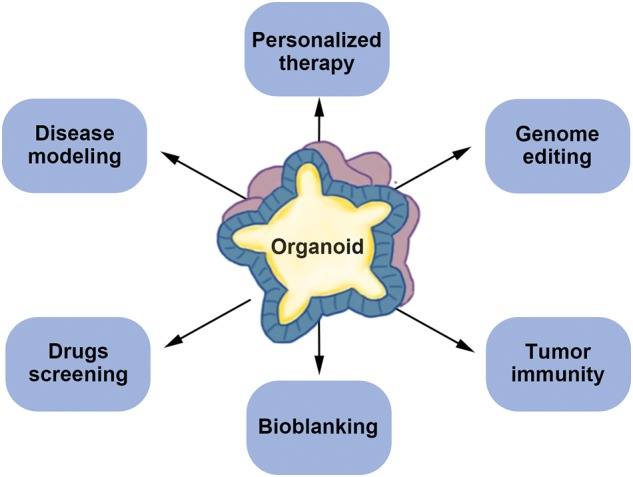
Application of patient-derived organoids (PDOs). PDOs have a wide range of applications in medicine and individual PDOs can serve as a small patient trial and predict drug response. Gene editing can be used in PDOs for disease models and basic research. Besides, PDOs are an ideal platform for disease modeling, drugs screening, bioblanking and tumor immunity.
Some recent studies have explored the potential of PDOs in tumor immunity and liquid biopsy. Circulating tumor cells (CTCs) are an alternative to invasive biopsies. CTC-derived organoids could help to acquire relevant genetic and epigenetic information about tumors [18]. Dijkstra et al. [19] succeeded in generating tumor-reactive T cells by co-culture of peripheral blood lymphocytes and gastrointestinal tumor organoids. Induced T-cell populations do not recognize healthy organoids or tissues. This indicates that PDOs can be used to assess the efficiency of T-cell-mediated tumor killing.
However, there is still much left to be improved for PDOs. The PDOs generated in published studies have mostly lacked essential elements, such as blood vessels, immune cells and other stromal cells. It is well known that angiogenesis is one of the most important biological characteristics of cancer. Cells in PDOs exchange material mainly through slow infiltration rather than blood vessels, which may have an effect on growth and drug response. Recently, tumor immunotherapy, particularly blockade of immune checkpoints programmed death-1/programmed death ligand-1 (PD-1/PD-L1), has yielded a breakthrough for cancer treatment [20]. How to predict anti-PD-1/PD-L1 efficacy is a current research focus. The lack of immune components in PDOs seems to limit their value in tumor immunotherapy. In fact, some studies are trying to establish culture systems where immune cells are expanded in vitro in co-cultures with organoids, which will help to improve PDOs [21].
PDOs are an alternative model for personalized cancer treatment and drug development because of the above-mentioned advantages. The research on and application of PDOs are at the initial stage. In the future, researchers should try to improve the tumor microenvironment in PDOs and more clinical trials are needed.
Funding
This study was supported by grants from the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2016-I2M-1–001) and the National High-tech Research and Development Projects (863) (No. 2015AA020303).
Acknowledgments
We thank Cathel Kerr, PhD, for editing the English text of a draft of this manuscript. Y.M. proposed the study and revised the manuscript. H.Y. and L.S. drafted the first manuscript. M.L. made charts and illustrations. All authors contributed to the design and interpretation of the study and to further drafts. Y.M. is the guarantor. All authors read and confirm the final version of this paper.
Conflict of interest statement: The authors disclose no conflicts.
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Weiskirchen R. Intratumor heterogeneity, variability and plasticity: questioning the current concepts in classification and treatment of hepatocellular carcinoma. Hepatobiliary Surg Nutr 2016;5:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drost J, Clevers H.. Organoids in cancer research. Nat Rev Cancer 2018;18:407–18. [DOI] [PubMed] [Google Scholar]
- 4. Moro M, Casanova M, Roz L.. Patient-derived xenografts, a multi-faceted in vivo model enlightening research on rare liver cancer biology. Hepatobiliary Surg Nutr 2017;6:344.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sato T, Vries RG, Snippert HJ. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262–5. [DOI] [PubMed] [Google Scholar]
- 6. Aberle MR, Burkhart RA, Tiriac H. et al. Patient-derived organoid models help define personalized management of gastrointestinal cancer. Br J Surg 2018;105:e48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vlachogiannis G, Hedayat S, Vatsiou A. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018;359:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boj SF, Hwang CI, Baker LA. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015;160:324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van de Wetering M, Francies HE, Francis JM. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015;161:933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Broutier L, Mastrogiovanni G, Verstegen MMA. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med 2017;23:1424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sato T, Stange DE, Ferrante M. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011;141:1762–72. [DOI] [PubMed] [Google Scholar]
- 12. Yin X, Farin HF, van Es JH. et al. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 2014;11:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao D, Vela I, Sboner A. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014;159:176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SH, Hu W, Matulay JT. et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 2018;173:515–28.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sachs N, de Ligt J, Kopper O. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 2018;172:373–86.e10. [DOI] [PubMed] [Google Scholar]
- 16. Hubert CG, Rivera M, Spangler LC. et al. A three-dimensional organoid culture system derived from human glioblastomas recapitulates the hypoxic gradients and cancer stem cell heterogeneity of tumors found in vivo. Cancer Res 2016;76:2465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lannagan TRM, Lee YK, Wang T. et al. Genetic editing of colonic organoids provides a molecularly distinct and orthotopic preclinical model of serrated carcinogenesis. Gut 2018;0:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Praharaj PP, Bhutia SK, Nagrath S. et al. Circulating tumor cell-derived organoids: current challenges and promises in medical research and precision medicine. Biochim Biophys Acta Rev Cancer 2018;1869:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dijkstra KK, Cattaneo CM, Weeber F. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 2018;174:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El-Khoueiry AB, Sangro B, Yau T. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nozaki K, Mochizuki W, Matsumoto Y. et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J Gastroenterol 2016;51:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


