Abstract
Background and objective
The benefit from adjuvant chemotherapy for patients treated with neoadjuvant chemoradiotherapy (NCRT) and curative surgery remains controversial, particularly among those responding well to NCRT. This retrospective study aimed to clarify the benefits of adjuvant chemotherapy in terms of the oncological outcomes of patients with ypT0–2N0 rectal cancer after NCRT and curative surgery.
Methods
All patients with ypT0–2N0 rectal cancer after NCRT and curative resection between 2005 and 2014 were examined. The oncological outcomes between patients treated with adjuvant chemotherapy and those without any chemotherapy were compared.
Results
The clinicopathological characteristics of 110 patients were reviewed in this study; one patient was excluded due to lack of follow-up. Of the 109 patients included, 58 (53.2%) underwent adjuvant chemotherapy (chemo group), whereas the remaining 51 (46.8%) did not receive any chemotherapy (non-chemo group). After a median follow-up of 50 months, there were no significant differences in the 5-year overall survival (OS) or recurrence-free survival (RFS) rates between the groups (OS: 92.1 vs 86.3%, P = 0.375; RFS: 80.9 vs 74.7%, P = 0.534). Subgroup analysis also demonstrated no significant differences in 5-year OS and RFS rates between patients with ypT0N0 rectal cancer (P = 0.712 and P = 0.599, respectively) and those with ypT1–2N0 disease (P = 0.255 and P = 0.278, respectively).
Conclusions
These results indicate that patients with ypT0–2N0 rectal cancer after NCRT followed by curative surgery may not derive significant benefit from adjuvant chemotherapy. However, further prospective randomized trials, with larger sample sizes, are warranted to confirm this conclusion.
Keywords: Rectal cancer, adjuvant chemotherapy, neoadjuvant chemoradiotherapy, survival
Background
For patients with locally advanced rectal cancer (LARC), the introduction of a total mesorectal excision and neoadjuvant chemoradiotherapy (NCRT) have significantly decreased local recurrence rates and prolonged overall survival (OS) rates; however, distant recurrences still eventually occur in 25–35% of patients [1–5]. Adjuvant chemotherapy is thought to prevent or eradicate circulating tumor cells and micro-metastases, decreasing distant recurrence. In principle, adjuvant chemotherapy is recommended for all patients who have completed NCRT and curative surgery, irrespective of final post-operative pathological stage [6]. However, recent studies have highlighted the paucity of evidence supporting the benefit from adjuvant chemotherapy for patients treated with NCRT and curative surgery [7], particularly among those responding well to NCRT [8–11].
Currently, the recommendations to provide adjuvant chemotherapy for patients treated with NCRT and surgery are based on clinical stage; however, given the imprecision of assignment of pre-operative clinical stage and the ease with which pathological T and N classifications are established, some researchers suggest that adjuvant chemotherapy should be used selectively, depending on the final pathological stage. Moreover, the final pathological stage is reported to be a superior predictor of oncological outcomes compared with clinical stage or tumor-regression grade [12–14]. Among patients treated with NCRT and surgery, those with stage ypT0–2N0 disease had favorable oncological outcomes, including 5-year disease-free survival (DFS) rates of 83–95% and are considered a subgroup who respond well to NCRT [15–18]. Nevertheless, there is no clear consensus on whether to provide or omit adjuvant chemotherapy for good responders. Not all patients with ypT0–2N0 rectal cancer benefit from adjuvant chemotherapy after NCRT and surgery; however, studies investigating this have reported somewhat inconsistent results [15, 16, 19, 20].
In this study, we aimed to assess the role of post-operative chemotherapy on the oncological outcomes of patients with ypT0–2N0 rectal cancer who were treated with NCRT and curative surgery.
Methods
Patients and pre-treatment evaluation
All patients with resectable LARC who received NCRT and surgery between January 2005 and December 2014 were identified retrospectively. Eligible patients were selected according to the following inclusion criteria: (i) the tumor was diagnosed as mid-to-low rectal adenocarcinoma (i.e. the distance between the tumor and the anal verge was up to 10 cm); (ii) the tumors were evaluated before treatment as clinical stage II and III diseases; (iii) the patients had no clinical evidence of distant metastases; (iv) the patients underwent R0 resection; (v) the tumors were classified on pathological examination as ypT0–2N0 diseases after NCRT and curative resection; and (vi) the patients completed neoadjuvant and adjuvant therapy. Exclusion criteria included: (i) of the patients with LARC had other malignancies; (ii) the patients had a history of malignant disease or recurrence; (iii) the patients received a ‘watch and wait’ strategy after NCRT; or (iv) the patients had other pathological types of tumors, such as mucinous adenocarcinoma and malignant melanoma. The study was approved by the Institutional Review Board committee of the Cancer Hospital, Chinese Academy of Medical Sciences.
Colonoscopic biopsy was performed for all patients before treatment to confirm pathological diagnosis. All patients received a digital rectal examination, endorectal ultrasound (EUS) and/or pulmonary and abdominopelvic contrast-enhanced computed tomography (CT) scans and rectal magnetic resonance imaging (MRI) for clinical staging. Clinicopathological classification and staging were determined according to the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system.
Treatment
The NCRT regimen included a total of 42–50-Gy radiation in 21–25 fractions, with concurrent chemotherapy including single agent capecitabine regimen (n = 50) and capecitabine plus oxaliplatin regimen (CAPOX; n = 59).
The median interval between the completion of NCRT and surgery was 7.7 weeks (range 2.1–13.6 weeks). An experienced colorectal surgical team performed all surgery, using the total mesorectal excision technique.
Fifty-eight (53.2%) patients received post-operative chemotherapy; the regimens were as follows: (i) single agent capecitabine for at least 6 months (n = 14); (ii) 4–9 cycles of CAPOX (n = 39); (iii) 6–10 cycles of a combination of 5-fluorouracil, leucovorin and oxaliplatin (n = 4); and (iv) 4 cycles of oxaliplatin + S-1 (n = 1). Fifty-one (46.8%) patients did not receive adjuvant chemotherapy. Documented reasons for not receiving adjuvant chemotherapy included physician discretion because of favorable pathology (n = 27), poor performance status (n = 11), patient choice (n = 10) and post-operative complications (n = 1). The reasons for not receiving adjuvant chemotherapy were not documented for two patients.
Follow-up
All patients were followed at 3-month intervals for 2 years, 6-month intervals for the next 3 years and annually thereafter. Follow-up examinations included a clinical history, physical examination, serum carcinoembryonic antigen, stool occult blood text, chest X-ray, colonoscopy, abdominopelvic CT or MRI and positron emission tomography (PET) scanning, if available. Recurrence was determined based on the results of clinical and radiological examination or histological confirmation.
Statistical methods
Categorical variables are presented as number (frequency) and quantitative variables as median followed by interquartile range (IQR) or mean ± standard deviation. Categorical variables were compared by chi-square test or Fisher’s exact test and quantitative variables by the t-test or the Wilcoxon rank sum test. Recurrence-free survival (RFS) was defined as the time between the date of surgery and the first tumor recurrence (local or distant metastasis). OS was defined as the time between the date of surgery and the date of death from any cause or the last follow-up. Kaplan–Meier survival analysis was used to determine the 5-year RFS and OS rates, and RFS and OS rates were compared using the log-rank test. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS software (Version 22.0; IBM Corp., New York, USA).
Results
Patient demographics
A total of 110 patients were enrolled in this study, one of whom was excluded due to lack of follow-up. Of the remaining 109 patients, 67 (61.5%) were males and 42 (38.5%) were females, with a median age of 52 (range, 23–79) years; 58 (53.2%) completed adjuvant chemotherapy (chemo group) and 51 (46.8%) did not receive any chemotherapy (non-chemo group). All patients underwent R0 resection with negative distal and circumferential margins. Fifty-one (46.8%) patients achieved pathological complete response (ypT0N0) and 58 (53.2%) had residual tumors (6 ypT1N0 and 52 ypT2N0). During surgery, 69 patients (63.3%) underwent abdominoperineal resection (APR) and 40 (36.7%) received low anterior resection (LAR). Of those who underwent LAR, 14 (35%) patients received temporary enterostomy (5 in the chemo group and 9 in the non-chemo group). The demographic and clinicopathological characteristics of the patients did not differ significantly between the chemo and the non-chemo groups, except for age and duration of enterostomy (Table 1). The chemo group was significantly younger than the non-chemo group (50.4 vs 59.4 years, P < 0.001). The duration of enterostomy was significantly greater in the chemo than the non-chemo group (28.4 vs 7.6 months, P = 0.023). Two patients in the chemo group experienced parastomal hernia, whereas none in the non-chemo group suffered this condition.
Table 1.
Demographic and clinicopathological characteristics of patients with ypT0–2N0 rectal cancer
| Variable | Chemo group (n = 58) | Non-chemo group (n = 51) | P-value |
|---|---|---|---|
| Agea (years) | 50.4 ± 10.5 | 59.4 ± 12.3 | <0.001 |
| Sex | 0.891 | ||
| Male | 36 (62.1) | 31 (60.8) | |
| Female | 22 (37.9) | 20 (39.2) | |
| BMIa (kg/m2) | 24.3 ± 3.5 | 24.6 ± 3.0 | 0.636 |
| Distance between tumor and the anal vergea (cm) | 4.5 ± 2.0 | 3.9 ± 1.9 | 0.071 |
| CEA (ng/mL) | 0.815 | ||
| ≤5 | 41 (70.7) | 35 (68.6) | |
| >5 | 17 (29.3) | 16 (31.4) | |
| Clinical T stage | 0.982 | ||
| cT2 | 3 (5.2) | 3 (5.9) | |
| cT3 | 44 (75.9) | 38 (74.5) | |
| cT4 | 11 (18.9) | 10 (19.6) | |
| Clinical N stage | 0.409 | ||
| cN0 | 12 (20.7) | 14 (27.5) | |
| cN1–2 | 46 (79.3) | 37 (72.5) | |
| Pre-operative chemotherapy | 0.536 | ||
| Capecitabine | 25 (43.1) | 25 (49.0) | |
| CAPOX | 33 (56.9) | 26 (51.0) | |
| Time interval (weeks) | 0.295 | ||
| <6 | 14 (24.1) | 10 (19.6) | |
| 6–8 | 23 (39.7) | 15 (29.4) | |
| >8 | 21 (36.2) | 26 (51.0) | |
| ASA class | 0.074 | ||
| I | 5 (8.6) | 1 (2.0) | |
| II | 50 (86.2) | 42 (82.3) | |
| III | 3 (5.2) | 8 (15.7) | |
| Type of surgery | 0.139 | ||
| APR | 33 (56.9) | 36 (70.6) | |
| LAR | 25 (43.1) | 15 (29.4) | |
| Laparoscopic vs open surgery | 0.436 | ||
| Fully laparoscopic | 35 (60.3) | 27 (52.9) | |
| Open from the beginning | 23 (39.7) | 24 (47.1) | |
| Post-operative complication | 0.827 | ||
| Yes | 10 (17.2) | 8 (15.7) | |
| No | 48 (82.8) | 43 (84.3) | |
| Tumor differentiation | 0.440 | ||
| Well differentiated | 2 (3.5) | 2 (3.9) | |
| Moderately differentiated | 33 (56.9) | 29 (56.9) | |
| Poorly differentiated | 10 (17.2) | 4 (7.8) | |
| Unknown | 13 (22.4) | 16 (31.4) | |
| Post-operative T stage | 0.138 | ||
| ypT0 | 22 (37.9) | 29 (56.9) | |
| ypT1 | 4 (6.9) | 2 (3.9) | |
| ypT2 | 32 (55.2) | 20 (39.2) | |
| Tumor response | 0.179 | ||
| Severe response | 35 (60.3) | 37 (72.5) | |
| Moderate response | 23 (39.7) | 14 (27.5) | |
| Perineural invasion | 3 (5.2) | 0 (0) | 0.246 |
| Number of LN retrievala | 13.6 ± 7.4 | 11.4 ± 7.4 | 0.129 |
| Duration of enterostomya,c (months) | 28.4 ± 17.5 | 7.6 ± 1.7 | 0.023 |
| Follow-upb (months) | |||
| 51.5 (37.0–70.3) | 50.0 (37.0–68.0) | 0.642 |
aThese values are presented as mean ± standard deviation. bthis value is presented as median followed by range in parentheses; other values are presented as number of patients followed by percentage in parentheses. cFourteen patients had temporary enterostomy, of which five and nine were in the chemo and non-chemo groups, respectively.
BMI, body mass index; CEA, carcinoembryonic antigen; CAPOX, capecitabine and oxaliplatin; ASA, American Society of Anesthesiologists; APR, abdominoperineal resection; LAR, low anterior resection; LN, lymph nodes.
Oncological outcomes
The median follow-up was 50.0 (IQR, 37.0–69.5) months for all patients and did not differ significantly between the chemo (51.5; IQR, 37.0–70.3 months) and the non-chemo (50.0; IQR, 37.0–68.0 months) groups (P = 0.642). During follow-up, 21 patients (19.3%) relapsed a median duration of 15 (range, 7–81) months after surgery: one patient (4.8%) in the non-chemo group had only local recurrence; 19 (90.6%), including 10 in the chemo group and 9 in the non-chemo group, had distant metastases; and one (4.8%) in the non-chemo group had concomitant local and distant recurrences. Ten (four in the chemo group and six in the non-chemo group) patients (9.2%) died a median duration of 43.5 (range, 4–71) months after surgery. The causes of the 10 deaths were related to cancer (n = 8) and post-operative complications (n = 1). The reasons for death were not documented for one patient.
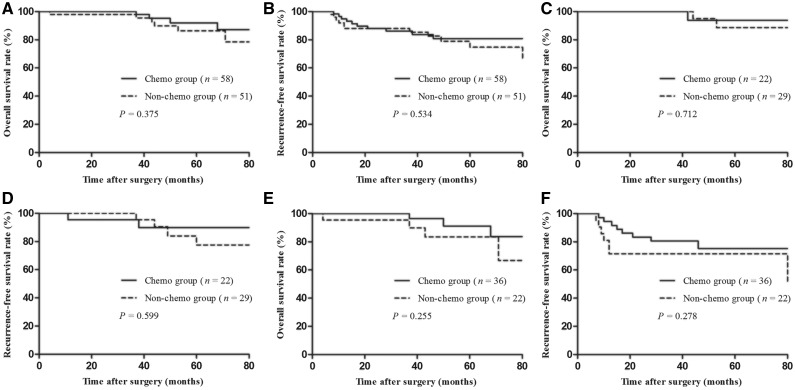
Survival was analysed using Kaplan–Meier survival curves; no significant differences between the two groups were detected. The 5-year OS rate was 89.3% for all patients, 92.1% for the chemo group and 86.3% for the non-chemo group (P = 0.375). The 5-year RFS rate was 78.1% for all patients, 80.9% for the chemo group and 74.7% for the non-chemo group (P = 0.534; Figure 1A and B).
Figure 1.
Oncological outcomes of 109 patients with ypT0–2N0 rectal cancer. (A) Overall survival rate of all patients with ypT0–2N0 between chemo and non-chemo groups. (B) Recurrence-free survival rate of all patients with ypT0–2N0 between chemo and non-chemo groups. (C) Overall survival rate of the patients with ypT0N0 between chemo and non-chemo groups. (D) Recurrence-free survival rate of the patients with ypT0N0 between chemo and non-chemo groups. (E) Overall survival rate of the patients with ypT1–2N0 between chemo and non-chemo groups. (F) Recurrence-free survival rate of the patients with ypT1–2N0 between chemo and non-chemo groups
Subgroup analyses showed that, in patients with stage ypT0N0 disease, the 5-year OS and RFS rates were 90.9 and 82.7%, respectively. The 5-year OS and RFS rates of the chemo group did not show any significant difference with those of the non-chemo group (OS: 93.8 vs 88.7%, P = 0.712; RFS: 89.8 vs 77.5%, P = 0.599; Figure 1C and D).
In patients with stage ypT1–2N0 disease, the 5-year OS and RFS rates were 88.1 and 74.1%, respectively. The 5-year OS and RFS rates in the chemo group were not significantly different to those of the non-chemo group (OS: 91.2 vs 83.4%, P = 0.255; RFS: 75.2 vs 71.4%, P = 0.278; Figure 1E and F).
Discussion
For patients with clinical stage T3 or T4 rectal cancer or node-positive mid-to-low rectal cancer who have received NCRT and curative surgery, 4–6 months of post-operative chemotherapy is still recommended to improve DFS and OS rates, irrespective of the pathological stage. The current advice to administer adjuvant chemotherapy to patients with LARC who underwent NCRT and surgery is primarily based on data from colon cancer [21–23]; however, the optimal duration of adjuvant chemotherapy for colon cancer is controversial. The International Duration Evaluation of Adjuvant Chemotherapy (IDEA) collaboration assessed whether 3 months of adjuvant chemotherapy was non-inferior to 6 months of treatment and the results failed to confirm non-inferiority; however, for low-risk patients with T1–3N1 tumors, 3-year DFS was non-inferior after 3 months of adjuvant chemotherapy compared with 6 months [24]. These interesting results raised the possibility that a shorter duration of adjuvant therapy for rectal cancer, or omission of adjuvant therapy for patients who have responded well to NCRT, may be reasonable.
Several randomized clinical trials have been conducted to investigate the benefits of adjuvant chemotherapy in LARC patients who were treated with NCRT and curative surgery; however, none has shown a significant survival benefit of receiving adjuvant chemotherapy [4, 5, 25, 26]. Subsequently, a systematic review conducted by Breugom et al. [7] of 1196 cases included in four European randomized trials also determined that adjuvant fluorouracil-based chemotherapy did not influence the oncological outcomes of patients with (y)pTNM II or III rectal cancer. However, another systematic review including more retrospective studies showed that adjuvant chemotherapy conferred a survival benefit [19]. Some recent retrospective studies also indicated that adjuvant chemotherapy is associated with increased DFS rate and prolonged OS time [27, 28]. Unfortunately, these studies have some important flaws. First, although patients had already been randomized to receive adjuvant chemotherapy, the rate of adherence to adjuvant chemotherapy was only 43–73.6% [4, 5, 25, 26] and two studies ended prior to completion owing to high dropout and poor accrual rates [5, 25]. Second, the chemotherapy regimens used in these studies were not identical. In addition, the number of patients who did not receive adjuvant chemotherapy in the retrospective studies was small, limiting their statistical power. Moreover, there was no investigation of whether adjuvant chemotherapy can be omitted for specific patient subgroups.
Currently, adjuvant chemotherapy is recommended for all patients after NCRT and curative surgery, based on clinical stage and independent of the final pathological stage; however, final pathological stage has superior predictive value for oncological outcomes compared with clinical stage [12–14]. For patients with LARC who underwent NCRT followed by curative surgery, ypN stage is regarded as the strongest independent prognostic factor influencing oncological outcomes [29]. Several studies analysed the prognostic value of adjuvant chemotherapy for patients with ypN– and ypN+ tumors and found that adjuvant chemotherapy may be unnecessary for those with ypN– disease and that only patients with ypN+ tumors were candidates for adjuvant chemotherapy [14, 18]. Pathological T stage is also a powerful prognostic predictor. A retrospective study conducted by Govindarajan et al. [30] in 2011 reported that, for ypN0 rectal cancer, patients with ypT3–4 tumors had a higher risk of recurrence than those with ypT0–2 malignancies. In 2015, Lee et al. [17] conducted another retrospective study of patients with ypN0 rectal cancer and found that advanced pathological T stage was associated with decreased DFS and OS rates and that ypT3–4 was a strong independent factor influencing oncological outcomes. Considering the findings of the studies mentioned above, we may safely conclude that the patients with ypT3–4 or ypN+ rectal cancers who underwent NCRT and surgery have worse oncological outcomes and may require intensive adjuvant chemotherapy, whereas patients with ypT0–2N0 tumors, who have excellent survival outcomes, could be spared adjuvant therapy. The phase II study, ADORE, confirmed our conclusion and suggested that patients with ypTNM II and III rectal cancer treated with FOLFOX had improved 3-year DFS rate compared with those receiving 5-FU/leucovorin, although patients with ypT0–2 tumors were excluded from this trial [31].
In the present study, we analysed the oncological benefits of adjuvant chemotherapy for patients with ypT0–2N0 tumors. The results showed that the 5-year RFS and OS rates were not significantly different in patients with and without adjuvant chemotherapy. These results are in accordance with the results of previous studies. In 2009, Huh and Kim [15] showed that patients with ypT0–2N0 rectal cancer did not receive any further benefit from post-operative adjuvant chemotherapy. A multicenter analysis of 1016 patients with ypT0–2N0 rectal cancer after NCRT and surgery conducted by Park et al. [16] in 2014 determined that 5-year RFS was not affected by the addition of adjuvant chemotherapy. In contrast, Lichthardt et al. [20] found that patients who received adjuvant chemotherapy exhibited a significant improvement in OS rate, especially those with lower pathological stage tumors. They suggested that good responses to NCRT could predict the response of recurrence; therefore, they advised that patients who showed a good response to NCRT should receive a personalized adjuvant chemotherapy regimen.
Subgroup analyses in the present study also showed that, in patients with ypT0N0 or ypT1–2N0 disease, the 5-year DFS and OS rates did not differ significantly between the chemo and non-chemo groups, consistently with the results of the majority of previous reports [8–11].
The present study demonstrated excellent prognosis for patients with ypT0–2N0 rectal cancer after NCRT and surgery, with identical 5-year RFS and OS rates, independently of the addition of adjuvant chemotherapy; however, approximately 20% of patients still eventually experienced recurrence, 66.7% of recurrences occurred within the first 3 years after surgery and 80% of documented reasons for death were related to distant metastases. Therefore, except for intensified observation during the first 3 years after surgery, the prognostic factors in patients with ypT0–2N0 rectal cancer remain to be illuminated; no significant prognostic factors were identified in our study (data not shown). In addition, given the poor compliance associated with adjuvant chemotherapy, the use of a course of initial neoadjuvant chemotherapy before NCRT and surgery to improve the tolerance and completion rates of chemotherapy and eradicate micro-metastases beforehand, or adding neoadjuvant chemotherapy after NCRT and before surgery to increase pathological complete response (pCR) rates, are potentially relevant options to control distant metastases [32–34]. Nevertheless, issues regarding the antedisplacement of adjuvant chemotherapy should not be ignored. First, there is no clear consensus on whether to select a full course of chemotherapy or a semi-course of chemotherapy. Second, we could be misguided by excessive pursuit of higher rates of pCR at the expense of the primary aim of systematic chemotherapy. Moreover, the feasibility of this method is only supported by the findings of a few phase II trials and more phase III trials are needed to test the safety and validity of this method.
Beyond the potential implications in terms of financial costs, substantial toxicity and poor compliance associated with adjuvant chemotherapy, our study also revealed a longer duration of enterostomy in the chemo group, similar to previous reports [10, 35]. Although a delay in enterostomy closure does not exert adverse impact on post-operative complications, extended duration of enterostomy is associated with a higher occurrence rate of parastomal hernia, which is the most common long-term complication. Parastomal hernia may have significant impacts on quality of life, including skin irritation, stoma site discomfort, intestinal obstruction and even disturbance of fluid and electrolyte balance. In the present study, two patients in the chemo group experienced parastomal hernia, whereas none in the non-chemo group suffered from the condition.
Due to its retrospective design, the present study has several limitations. First, it had a limited sample size and was based at a single-center setting, which could have led to selection bias, potentially hampering the elimination of confounding factors. Second, owing to the low recurrence rate and mortality, particularly among patients in the ypT0N0 group, the percentage of censored data was relatively high, which could limit the statistical power. In addition, patients who did not receive adjuvant chemotherapy were older and included a higher proportion of pCR cases than those receiving adjuvant chemotherapy, suggesting that the non-chemo group may have more morbidities and more responsive tumors. Finally, although all patients in the chemo group completed adjuvant chemotherapy, different cytotoxic regimens and cycles were used.
In conclusion, based on the results of this study, patients with ypT0–2N0 rectal cancer after NCRT followed by curative surgery may not benefit from adjuvant chemotherapy. However, given that this and other similar studies have been retrospective analyses, prospective randomized trials with larger sample sizes are warranted to further justify the oncological benefits of adjuvant chemotherapy.
Conflict of interest statement: none declared.
Acknowledgements
This work was supported by National Key R&D Program of China (No. 2017YFC0908203) and CAMS Initiative for Innovative Medicine (No. CAMS-I2M-003).
References
- 1. Fleming FJ, Påhlman L, Monson JR.. Neoadjuvant therapy in rectal cancer. Dis Colon Rectum 2011;54:901–12. [DOI] [PubMed] [Google Scholar]
- 2. van Gijn W, Marijnen CAM, Nagtegaal ID. et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–82. [DOI] [PubMed] [Google Scholar]
- 3. Sauer R, Liersch T, Merkel S. et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–33. [DOI] [PubMed] [Google Scholar]
- 4. Bosset J-F, Calais G, Mineur L. et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184–90. [DOI] [PubMed] [Google Scholar]
- 5. Breugom AJ, van Gijn W, Muller EW. et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 2015;26:696–701. [DOI] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network. Rectal Cancer (Version 1. 2017) 2017. http://www.nccnorg/professionals/physician_gls/pdf/rectalpdf (23 November 2016, date last accessed).
- 7. Breugom AJ, Swets M, Bosset J-F. et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200–7. [DOI] [PubMed] [Google Scholar]
- 8. Geva R, Itzkovich E, Shamai S. et al. Is there a role for adjuvant chemotherapy in pathological complete response rectal cancer tumors following neoadjuvant chemoradiotherapy? J Cancer Res Clin Oncol 2014;140:1489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou J, Qiu H, Lin G. et al. Is adjuvant chemotherapy necessary for patients with pathological complete response after neoadjuvant chemoradiotherapy and radical surgery in locally advanced rectal cancer? Long-term analysis of 40 ypCR patients at a single center. Int J Colorectal Dis 2016;31:1163–8. [DOI] [PubMed] [Google Scholar]
- 10. Gamaleldin M, Church JM, Stocchi L. et al. Is routine use of adjuvant chemotherapy for rectal cancer with complete pathological response justified? Am J Surg 2017;213:478–83. [DOI] [PubMed] [Google Scholar]
- 11. Kuan F-C, Lai C-H, Ku H-Y. et al. The survival impact of delayed surgery and adjuvant chemotherapy on stage II/III rectal cancer with pathological complete response after neoadjuvant chemoradiation. Int J Cancer 2017;140:1662–9. [DOI] [PubMed] [Google Scholar]
- 12. Kuo L-J, Liu M-C, Jian JJ-M. et al. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol 2007;14:2766–72. [DOI] [PubMed] [Google Scholar]
- 13. Quah H-M, Chou JF, Gonen M. et al. Pathologic stage is most prognostic of disease-free survival in locally advanced rectal cancer patients after preoperative chemoradiation. Cancer 2008;113:57–64. [DOI] [PubMed] [Google Scholar]
- 14. De Stefano A, Moretto R, Bucci L. et al. Adjuvant treatment for locally advanced rectal cancer patients after preoperative chemoradiotherapy: when, and for whom? Clin Colorectal Cancer 2014;13:185–91. [DOI] [PubMed] [Google Scholar]
- 15. Huh JW, Kim HR.. Postoperative chemotherapy after neoadjuvant chemoradiation and surgery for rectal cancer: is it essential for patients with ypT0-2N0? J Surg Oncol 2009;100:387–91. [DOI] [PubMed] [Google Scholar]
- 16. Park IJ, Kim DY, Kim HC. et al. Role of adjuvant chemotherapy in ypT0-2N0 patients treated with preoperative chemoradiation therapy and radical resection for rectal cancer. Int J Radiat Oncol Biol Phys 2015;92:540–7. [DOI] [PubMed] [Google Scholar]
- 17. Lee K-H, Kim J-C, Kim J-Y. et al. Oncologic results and prognostic predictors of patients with locally advanced rectal cancer showing ypN0 after radical surgery following neoadjuvant chemoradiotherapy. Int J Colorectal Dis 2015;30:1041–50. [DOI] [PubMed] [Google Scholar]
- 18. Chen P, Yao Y, Gu J.. Rectal cancer patients after neoadjuvant radiotherapy (30Gy/10f) with negative lymph node may not benefit from postoperative adjuvant chemotherapy: a retrospective study. Int J Colorectal Dis 2015;30:1695–704. [DOI] [PubMed] [Google Scholar]
- 19. Petrelli F, Coinu A, Lonati V. et al. A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorectal Dis 2015;30:447–57. [DOI] [PubMed] [Google Scholar]
- 20. Lichthardt S, Zenorini L, Wagner J. et al. Impact of adjuvant chemotherapy after neoadjuvant radio- or radiochemotherapy for patients with locally advanced rectal cancer. J Cancer Res Clin Oncol 2017;143:2363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taal BG, Van Tinteren H, Zoetmulder FA.. Adjuvant 5FU plus levamisole in colonic or rectal cancer: improved survival in stage II and III. Br J Cancer 2001;85:1437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Twelves C, Wong A, Nowacki MP. et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704. [DOI] [PubMed] [Google Scholar]
- 23. André T, Boni C, Navarro M. et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16. [DOI] [PubMed] [Google Scholar]
- 24. Shi Q, Sobrero A, Shields A. et al. Prospective pooled analysis of six phase III trials investigating duration of adjuvant (adjuv) oxaliplatin-based therapy (3 vs 6 months) for patients (pts) with stage III colon cancer (CC): the IDEA (International Duration Evaluation of Adjuvant chemotherapy) collaboration. J Clin Oncol 2017;35:abstr LBA1. [Google Scholar]
- 25. Glynne-Jones R, Counsell N, Quirke P. et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 2014;25:1356–62. [DOI] [PubMed] [Google Scholar]
- 26. Sainato A, Cernusco Luna Nunzia V, Valentini V. et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol 2014;113:223–9. [DOI] [PubMed] [Google Scholar]
- 27. Jung KU, Kim HC, Park JO. et al. Adjuvant chemotherapy after neoadjuvant chemoradiation and curative resection for rectal cancer: is it necessary for all patients? J Surg Oncol 2015;111:439–44. [DOI] [PubMed] [Google Scholar]
- 28. Ahn DH, Wu C, Wei L. et al. The efficacy of adjuvant chemotherapy in patients with stage II/III resected rectal cancer treated with neoadjuvant chemoradiation therapy. Am J Clin Onco 2017;40:531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim TH, Chang HJ, Kim DY. et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys 2010;77:1158–65. [DOI] [PubMed] [Google Scholar]
- 30. Govindarajan A, Reidy D, Weiser MR. et al. Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Surg Oncol 2011;18:3666–72. [DOI] [PubMed] [Google Scholar]
- 31. Hong YS, Nam B-H, Kim K-P. et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol 2014;15:1245–53. [DOI] [PubMed] [Google Scholar]
- 32. Fernández-Martos C, Pericay C, Aparicio J. et al. Phase II, randomized study of Concomitant Chemoradiotherapy Followed by Surgery and Adjuvant Capecitabine Plus Oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging–defined, locally advanced rectal cancer: Grupo Cáncer de Recto 3 Study. J Clin Oncol 2010;28:859–65. [DOI] [PubMed] [Google Scholar]
- 33. Fernandez-Martos C, Garcia-Albeniz X, Pericay C. et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol 2015;26:1722–8. [DOI] [PubMed] [Google Scholar]
- 34. Garcia-Aguilar J, Chow OS, Smith DD. et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waterland P, Goonetilleke K, Naumann DN. et al. Defunctioning ileostomy reversal rates and reasons for delayed reversal: does delay impact on complications of ileostomy reversal? A study of 170 defunctioning ileostomies. J Clin Med Res 2015;7:685–9. [DOI] [PMC free article] [PubMed] [Google Scholar]