Abstract
Restorative proctocolectomy with ileal pouch–anal anastomosis has become the surgical treatment of choice for patients with refractory ulcerative colitis, colitis-associated dysplasia or familial adenomatous polyposis. There are various pouch disorders and associated complications. Floppy pouch complex is defined as the presence of pouch prolapse, afferent limb syndrome, enterocele, redundant loop and folding pouch on pouchoscopy, gastrografin pouchogram or defecography. Common clinical presentation includes dyschezia, bloating, abdominal pain, straining or the sense of incomplete evacuation. Each disorder has its own unique endoscopic, radiographic and manometry findings. A range of therapeutic options are available for the management of the various causes of a pouch.
Keywords: Floppy pouch complex, ileal pouch, prolapse, afferent limb syndrome, efferent limb syndrome, ulcerative colitis
Introduction
Ulcerative colitis (UC) is an inflammatory disease of the colon and rectum limited to the mucosa up to superficial submucosa, and may vary in severity from a mild intermittent disease to an acute fulminant and potentially fatal disease requiring urgent surgery. Restorative proctocolectomy with ileal pouch–anal anastomosis (IPAA) is performed for patients with medically refractory UC or colitis-associated neoplasia [1] (Table 1). The goal of surgical therapy for UC is to remove the diseased bowel with as little alteration of normal physiological functions and lifestyle as possible [2].
Table 1.
Indications for surgery in ulcerative colitis
| Acute indications | Fulminant colitis |
| Toxic megacolon | |
| Perforation | |
| Massive hemorrhage | |
| Chronic indications | Unresponsive to medical management |
| Excessive steroid dose | |
| High grade dysplasia | |
| Dysplasia associated lesion or mass | |
| Carcinoma | |
| Fear of carcinoma | |
| Severe extra intestinal manifestation | |
| Growth retardation | |
| Chronic bleeding requiring transfusion | |
| Large bowel obstruction |
The J pouch is the most commonly constructed pouch, although the S pouch is used under certain circumstances discussed below. The J pouch is created by using two loops of small intestine, each measuring about 15–18 inches. The pouch is typically stapled to the top of the anorectal ring, which leaves 1–2 cm of mucosa termed the anal transitional zone (ATZ), as it can contain colonic as well as anal-canal lining tissue. The J pouch the commonly utilized pelvic pouch, as it can be constructed with staples (versus being hand-sewn) and long-term efferent limb complications are less frequent compared to the S pouch. The S pouch is made by utilizing three loops of small bowel about 10 cm each in length. It requires hand-sewing to construct and results in an efferent limb of 1–2 cm that is either stapled to the ATZ or sewn to the dentate line after a mucosectomy. The efferent limb has been shown over time to sometimes elongate, leading to evacuation difficulty. However, this pouch is used when the mesentery is short, as it allows the greatest reach of the small bowel into the pelvis. Sometimes, when the choice is an S pouch vs leaving a somewhat longer ATZ (really a rectal cuff), the difficult long-term evacuation problems with the S pouch sways the surgeon to choose the J/long ATZ method.
There are a variety of structural, inflammatory and functional disorders of the pouch. We previously proposed a classification system for ileal pouch disorders (Table 2). In the classification system, pouch prolapse and afferent limb syndrome (ALS) were briefly described as obstructive mechanical complications. In fact, pouch prolapse or pouchocele, ALS and efferent limb syndrome (ELS) share common features of redundant, elongated or a segment of floppy small bowel. Therefore, the term ‘floppy pouch complex’ (FPC) can be used to encompass all these dysfunctional conditions [3]. The different phenotypes in FPC may share similar etiology or risk factors. Studies have proposed several risk factors for FPC [4]. In our clinical practice, we have noticed that FPC was often associated with low body mass index (BMI), possible hysterectomy, general gastrointestinal dysmotility, possibly our migration to minimally invasive techniques (with less adhesions in the pelvis) and connective tissue disorders such as Ehlers Danlos syndrome.
Table 2.
Classification of ideal pouch disorders and associated complications
| Surgical and mechanical | Pelvic sepsis and abscess |
| Pouch sinuses | |
| Pouch fistula | |
| Strictures | |
| Afferent limb syndrome and afferent limb syndrome | |
| Infertility and sexual dysfunction | |
| Portal vein thrombi | |
| Pouch prolapse, twisted pouch bleeding, sphincter injury or dysfunction | |
| Inflammatory and infectious | Pouchitis |
| Cuffitis | |
| Crohn’s disease of the pouch | |
| Proximal small-bowel bacterial overgrowth | |
| Inflammatory polyps | |
| Functional | Irritable pouch syndrome |
| Anismus | |
| Pseudo-obstruction | |
| Dysplastic and neoplastic | Dysplasia or cancer of the pouch |
| Dysplasia or cancer of the anal transitional zone | |
| Systemic and metabolic | Anemia |
| Bone loss | |
| Vitamin B12 deficiency |
Pouch prolapse and pouchocele
Pouch prolapse is defined as excessive protrusion of the pouch wall into the lumen of the pouch body or anal canal. The prolapse can be mucosal or full-thickness. Even though there is a range from intussusception to overt prolapse, we term all forms of this type of pouch descent as pouch prolapse. In rare cases, it can protrude out of the anal canal—full-thickness overt pouch prolapse. Pouchoscopy, particularly when the patient is straining, could reveal redundant or bulging tissue above the anastomosis at the distal pouch or in the upper anal canal. It may not be the entire circumference of the pouch that descends and we have found that anterior descent appears to be more common (Figure 1). Our recent study showed that pouch prolapse is more common in female patients with low BMI and in those with lower peripouch fat measured on pelvic imaging [5, 6]. Clinical manifestations of any degree of prolapse of a pelvic pouch may include episodic abdominal colic, vomiting, palpable mass and the passage of blood per rectum. In severe cases, where the bowel becomes incarcerated, it can lead to bowel ischemia and necrosis.
Figure 1.
Pouch prolapse and adverse consequences. (A) Pouch inlet prolapse. (B) Distal anterior pouch prolapse. (C) Persistent cuff prolapse, later on led to formation of vaginal fistula that originated from its base (green arrow). (D) Perianal dermatitis from excessive straining.
Prolapse can be classified into (i) mucosal prolapse vs full-thickness prolapse (similar to rectal prolapse based on mucosal vs full-thickness descent), (ii) partial vs complete (based on the degree of lumen occupation), (iii) primary vs secondary (based on the etiology, e.g. paradoxical anal contraction during defecation leading to prolapse would be a secondary cause) and (iv) asymmetric vs circumferential [7].
Pouch prolapse may coexist with pouchocele or enterocele. The latter condition is defined as a peritoneum-lined sac herniating down between the vagina and rectum, encompassing the small intestine (Figure 2A). As with women who do not have a pelvic pouch, dynamic defecating proctogram (really pouchogram) is used to visualize this abnormality [8]. It is described as small bowel that impinges on the pouch of Douglas. The bowel is upstream from the bowel used for evacuation. During fecal evacuation, when a pouchocele is present, stool remains trapped in the protrusion [9]. A variant of pouchocele can be seen as the pouch protruding into the rectovaginal septum similar to a rectocele in a woman without a pelvic pouch [10]. Women with this condition may report symptoms experienced in non-pouch patients with a ‘classic’ rectotocele such as vaginal or perineal pressure and may need to use pressure with their fingers in the vagina or perineum to successfully expel stool [11].
Figure 2.
Other floppy pouch complex. (A) Pouchocele (green arrow). (B) Redundant afferent limb. (C) Pelvic descent (blue arrow).
Clinical presentation
The most common clinical presentation of this problem includes dyschezia, excessive straining or a sense of incomplete evacuation. Other reported symptoms are the patient noting protrusion of tissue outside of their anus, seepage of mucous or stool, fecal incontinence, anal pain and burning, anal itching, nausea or bloating. Patients with long-term excessive straining can present with perianal dermatitis (Figure 1D).
Endoscopic findings
Pouch prolapse can be evaluated with a careful pouchoscopy. The prolapse may be visualized while the patient is straining or induced by endoscopic suction with the tip of the scope being placed in the anal canal (Figure 1B). The common location of the prolapse is the anterior wall of the distal pouch body or cuff. Circumferential prolapse at the distal pouch, pouch inlet and the dome at the tip of the ‘J’ occasionally can be seen (Figure 3). Mucosal prolapse appears to be more common than full-thickness prolapse. Endoscopy may also reveal redundant tissue above or below the anastomosis, when the patient is asked to bear down. Solitary ulcers may be seen at the tip of the prolapsed bowel (Figure 4A), similar to those seen with ‘classic’ rectal prolapse in non-pouch patients. However, endoscopy does not seem to be a reliable diagnostic modality for the pouchocele or enterocele.
Figure 3.
Circumferential prolapse at the pouch inlet (A) and distal pouch body (B).
Figure 4.
Distal anterior pouch prolapse. (A) Ulcer at the tip of prolapse. (B) Prolapsed segment on gastrografin enema (green arrow).
Histology
Endoscopic biopsies taken from the tip of prolapse pouch or cuff may demonstrate elongation of the epithelium, intramucosal fibrosis, intercrypt smooth muscle fibers, disrupted and thickened muscularis mucosae and diamond-shaped crypts, with or without mild diffuse chronic inflammation in the lamina propria. In severe cases, erosion or ulceration can be seen [12] (Figure 5).
Figure 5.
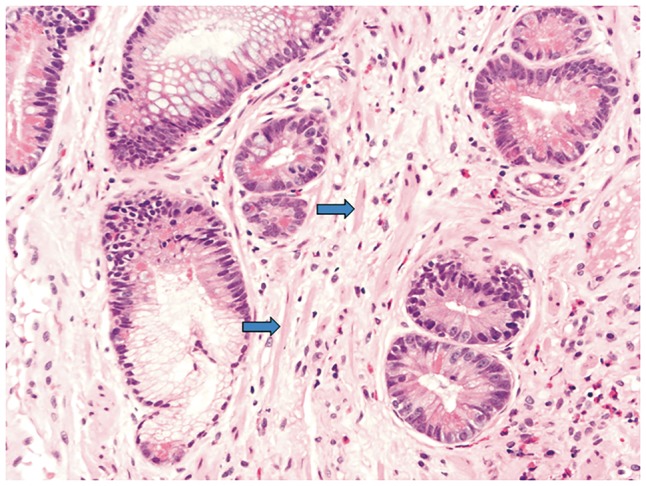
Rectal cuff with prolapse change (high power). Architectural distortion due to prolapse with strands of smooth muscle between glands (blue arrows).
Radiographic findings
Plain abdominal radiograph, small-bowel series, computerized tomography enterography (CTE) or magnetic resonance enterography (MRE) may show dilated bowel. However, dynamic pouch defecography appears to be one of the best tools to demonstrate abnormal pouch descent (Figure 2C) and protrusion. On barium defecography, we may notice bulging of the pouch wall towards the lumen (i.e. prolapse) (Figure 4B). We do not favor defeating MRI, since this test is typically done in the supine position. In patients with persistent pouch prolapse that leads to outlet obstruction, luminal dilation of the afferent limb proximal to the pouch inlet may be present [13].
Manometric findings
There are no specific manometric findings for prolapse but paradoxical contraction of the pelvic floor when the patient strains should always be investigated if outlet symptoms exist. Additionally a ‘sawtooth pattern’ when the anal probe is pulled back while assessing the resting pressure is abnormal and signals some form of internal anal spasm, which could contribute to functional outlet problems.
Treatment
Conservative management includes lifestyle changes, such as the avoidance of excessive toilet time and straining. This also includes strongly considering physical therapy retraining with biofeedback to reinforce coordination and relaxation during defecation. Female patients may apply vaginal maneuvers with supporting the posterior wall of vagina during defection. Endoscopic hot snare appears to be reasonable in the management of mucosal prolapse in the ileal pouch reservoir. Endoscopic mucosal banding (Figure 6) or local transanal excision of redundant mucosa (Figure 7) may be helpful in some patients. Some patients require endoscopic treatment with incision of the prolapsed pouch fold and nearby anastomosis (Figure 8). In patients with dyssynergic defecation, paradoxical or dis-coordinated defecation, intense biofeedback treatment as mentioned above is an important component of the treatment algorithm. In patients with a ‘sawtooth’ pattern of contractions, topical injection to the internal anal sphincter may be attempted [14, 15]. But this is typically steroids and not botox, as the incidence of fecal incontinence is very high in patients with a pelvic pouch.
Figure 6.
Endoscopic banding of distal pouch prolapse. (A) Distal pouch prolapse. (B) Intramucosal injection. (C and D) Banding ligation.
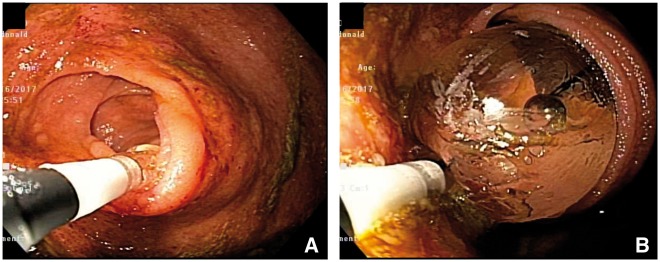
Figure 7.
Endoscopic therapy of pouch prolapse (A and B). A polypoid prolapse in the anterior wall of the cuff. The prolapse was removed with an endoscopic hot snare.
Figure 8.
Endoscopic therapy of prolapse. (A) Circumferential prolapse protruding to anastomosis leading to dyschezia. Endoscopic incision of anastomosis (B) and prolapse (C) followed by deployment of endoclips. (D) Resolution of prolapse on follow-up endoscopy 1 year later.
The management of mucosal or full-thickness ileal pouch prolapse is very challenging and no treatment algorithm has been established. Patients with symptomatic, refractory prolapse frequently require definitive surgical treatment. The reported surgical procedures include transanal attempts to reduce the prolapse tissue, pouchpexy with or without some form of mesh, redo pouch construction and pouch excision with permanent diversion [7, 9, 16, 17]. In one study of patients diagnosed with ileal pouch prolapse after restorative proctocolectomy, the overall incidence of ileal pouch prolapse in this study population was 0.3% (11 out of 3176 patients) [7]. Seven patients had full-thickness pouch prolapse and four were diagnosed with mucosal prolapse. Three patients with mucosal prolapse were successfully treated with stool-bulking agents and two required local transanal excision of redundant mucosa. Out of seven patients with full-thickness pouch prolapse, six were treated with pouch pexy. Three patients responded well but three others required pouch excision and formation of a continent ileostomy. One out of seven patients with full-thickness pouch prolapse required pouch pexy and biological mesh. The authors concluded that mucosal prolapse can be effectively treated with bulking agents or mucosal excision techniques. Full-thickness pouch prolapse required pouch fixation using an abdominal approach.
Afferent limb syndrome
ALS is defined as sharp angulation leading to symptoms of any segment of the afferent limb, from the pouch inlet to the previous loop ileostomy site, in the absence of intrinsic stricture. It has been described by our group as a distal small-bowel obstruction caused by acute angulation, prolapse or intussusception of the afferent limb at the junction to the pouch [18]. The pouch inlet just proximal to the pouch body is the most common location of ALS. ALS may be associated with a redundant loop of bowel or elongated mesentery in the area. Adhesions may also lead to conditions that set up the afferent limb for elongation and kinking (Figure 9).
Figure 9.
Afferent limb syndrome. (A) A sharp angulation at the pouch inlet on endoscopy, precluding easy passage of an endoscope (green arrow). (B) Plain X-ray showing dilated pouch body with air. (C) Gastrografin enema confirmed dilated pouch body without illumination of the afferent limb.
Clinical presentation
Common symptoms include dyschezia, straining, incomplete evacuation, recurrent intermittent abdominal pain, bloating, constipation or perianal pain. Partial small-bowel obstruction can be present in some cases. Literature search revealed a study that characterized 18 cases of ALS [19]. The mean age was 35.6 ± 14.3 years. Fifteen patients were diagnosed by pouch endoscopy with features of angulation of the pouch inlet and difficulty in intubating the afferent limb; 12 patients had kinking or narrowing of the pouch inlet identified with abdominal imaging. The median follow-up was 1.3 (range, 0.14–16.1) years. Nine patients required empiric balloon dilatation of the afferent limb/pouch inlet. Of nine, four underwent repeat dilatations. One patient with repeat dilatation ultimately underwent pouch excision. Eight patients underwent surgery: resection of angulated bowel (n = 3), pouch pexy (n = 2), pouch mobilization with small-bowel fixation (n = 1) and pouch excision (n = 2). One patient without symptoms did not receive any therapy despite the finding of ALS on pouchoscopy.
Endoscopic findings
One of the classical findings of ALS is difficulty in intubating the endoscopic afferent limb at any location from the pouch inlet to the previous ileostomy closure site due to the sharp angulated inlet (Figure 9A). The bowel lumen above the angulated segment may or may not be dilated and there should be no intrinsic strictures.
Radiographic findings
Retrograde gastrografin enema is the most reliable diagnostic modality for ALS. On gastrografin enema, it is helpful to alert the radiologist if you suspect this problem in order to obtain an optimal study. Typically, there is a minimum or a small quantity of contrast in the afferent limb, despite the pouch body being fully loaded, filled with the contrast material (Figure 9B and C). Barium defecography may show an incomplete emptying of contrast in the afferent limb (Figure 10). Antegrade imaging, such as small-bowel series, CTE and MRE, do not illustrate the problem, but may show proximal small-bowel dilation or even the air-fluid level [13].
Figure 10.
Afferent limb syndrome. Angulation at the pouch inlet during defecography (green arrows) (A & B).
Manometric findings
Manometric evaluation has a limited role in the diagnosis of ALS. However, long-term excessive straining may lead to secondary dyssynergic defecation, paradoxical contraction upon defecation and a dilated pelvic pouch.
Treatment
Conservative treatment includes the adjustment of diet with small, frequent, low-residual meals. Low FODMAP (fermentable oligo-, di- and monosaccharides and polyols) may be helpful [20]. Biofeedback treatment may be beneficial in some cases with concurrent dyscoordination and paradoxical contraction during defecation.
Since ALS is a mechanical problem, surgical intervention may be needed. Unfortunately, surgical treatment has been associated with suboptimal outcomes. Surgical options include resection of the angulated bowel with anastomosis, lysis of the adhesion, surgical pexy of the pouch to the pelvic sidewall, pouch mobilization and small-bowel fixation, mesh placement and pouch excision with end ileostomy. One published surgical study included 567 patients who underwent laparotomy for unresolved obstruction due to afferent limb obstruction [13]. Of 122 patients with one or more episodes of obstruction after IPAA, 48 underwent operative intervention. ALS was identified as the cause of obstruction in six patients (12%). The most common presentation was recurrent partial obstruction (four of six patients). Enemas and contrast small-bowel series were suggestive of obstruction in four of six patients. All patients underwent laparotomy for unresolved obstruction. The obstruction was bypassed utilizing a side-to-side anastomosis of the afferent limb to the pouch (entero-enterostomy) in five of six patients. One patient underwent ileostomy due to lack of other options. Two patients required re-exploration and pouch pexy to relieve recurrent afferent limb obstruction. Although the authors found that bypass of the obstructed segment from distal ileum to the pouch was safe and effective treatment, they recommended concurrent pouch pexy due to the risk of recurrent afferent limb angulation.
Since the surgical outcomes are poor, other treatment modalities have been explored in our Pouch Center at the Cleveland Clinic. For patients with persistent symptoms and not being a candidate for surgical therapy, or refractory to surgical treatment, endoscopic balloon dilation has been used (Figure 11), in addition to intraluminal or intrinsic strictures [21]. Balloon dilation, which is easy to perform, is mainly for diagnostic purposes, not for long-term therapy. Temporary relief from balloon dilation may suggest ALS as a source of the patient’s symptoms. In addition, endoscopic therapy with septectomy such as electroincision of the sharp angle may be attempted by an experienced endoscopist (Figures 12 and 13).
Figure 11.
Afferent limb syndrome with endoscopic balloon dilation (A & B).
Figure 12.
Endoscopic therapy of afferent limb syndrome. (A) A sharp angulation at the pouch inlet on the guide wire. (B & C) Needle knife therapy. (D) Placement of endoclips to maintain luminal patency and prevent bleeding and perforation.
Figure 13.
Endoscopic therapy of afferent limb syndrome. (A) A sharp angulation at the pouch inlet (green arrow). (B) Needle knife electroincision of the proximal pouch septum. (C & D) Placement of endoclips to maintain luminal patency and prevent bleeding and perforation.
Efferent limb syndrome
ELS is defined as a sharp angulation at the outlet of the pouch, which is almost exclusively seen in an S pouch versus a J pouch. The efferent limb of the S pouch can either be long or elongate with time and then kink, leading to ELS, when intra-abdominal pressure is exerted during defecation. Ideally, the length of the efferent limb should be 1.0–2.5 cm [22] (Figure 14A). ELS in patients with a J pouch is usually caused by a markedly long rectal stump, resulting in a sharp angulation between the pouch body and the rectal stump (Figure 14B). The excessively long rectal stump in patients with J pouches may be created intentionally to avoid anastomotic tension, especially in obese patients or those with a short mesentery. Both conditions lead to complete or partial obstruction of the pouch outlet due to a markedly long pouch outlet [4]. The etiology of ELS is mechanical, not from latency of the bowel in pouch prolapse or ALS. Therefore, we do not consider ELS a part of FPC.
Figure 14.
Efferent limb syndrome. (A) Long efferent limb in an S pouch (green arrow). (B) A long rectum stump in a J pouch (blue arrow).
Few studies have addressed ELS. A study evaluated six ELS patients who required surgical correction [23]. Reported characteristics were difficulty in catheterizing the anus to evacuate, fecal incontinence, stenosis of the efferent ileal limb, transanal prolapse of the efferent ileal limb and need to catheterize the reservoir. All patients were noted to have an excessively long efferent ileal limb of 8 cm or more, which was resected and re-anastomosed to the anal canal. The resection was performed endoanally in three patients but was successful in only one. In the two patients in whom endoanal excision was ineffective and in the remaining three, transabdominal resection of at least 5 cm was performed with endoanal re-anastomosis. Two patients still needed to catheterize while three out of five were converted from catheterizing the reservoir to spontaneous evacuation. All six patients reported improvement in continence or decreased frequency of catheterization. The findings suggest that excessive length of the efferent limb of an S-pouch reservoir could cause inadequate defecation and this might be improved by resection of the efferent limb and re-anastomosis.
Few studies are available in the literature addressing clinical characteristics and outcome of ELS. Our group has studied 11 symptomatic pelvic pouch patients with ELS after an S pouch and 15 patients with J-pouch–rectal anastomosis. A greater number of patients in the S-pouch ELS group had dyschezia than the pouch–rectal anastomosis group (90.9 vs 33.3%, P = 0.005). More patients in the S-pouch ELS group had a sense of incomplete evacuation versus the pouch–rectal anastomosis group (45.5 vs 13.3%, P = 0.10). Ten patients (90.9%) in the S-pouch ELS group and five patients in the pouch–rectal anastomosis group (33.3%) required surgical intervention (P = 0.005). After a mean follow-up of 3.4 ± 1.4 years, seven (87.5%) of the eight patients who underwent redo pouch construction with efferent limb/rectal stump excision maintained a functional pouch.
Clinical presentation
Symptoms of ELS include bloating, dyschezia, abdominal pain, a sense of incomplete evacuation, perianal burning and/or dermatitis. Patients sometimes require self-anal catheterization into the reservoir to evacuate [19].
Endoscopic findings
Endoscopic evaluation is valuable for the diagnosis ELS. The endoscopist may find an excessively long efferent limb (in the S pouch) or a long rectal stump (in the J pouch) with angulation between the segment and the pouch body above. The efferent limb or long rectal stump may be inflamed with ulceration and nodularity (Figure 15). The inflammation of the long rectal stump may mimic that seen in classic cuffitis. Biopsy may show acute and chronic inflammation and ischemic changes. For patients with long disease duration of ELS, diffuse, fecal stasis-associated diffuse mild pouchitis may be seen.
Figure 15.
Efferent limb syndrome. (A) Long efferent limb with nodular and prolapsed mucosa from excessive straining in an S pouch. (B) A long rectal stump in a J pouch with cuffitis/proctitis.
Radiographic findings
Gastrografin enema and a defecating pouchogram are an important part of the evaluation of ELS as well as ALS. Pouchograms may allow measurement of the length of the efferent limb in the S pouch and the length of the rectal stump in the J pouch along with the size of the pouch, as, in this group of patients, the pouch is frequently enlongated and dilated (Figure 14).
Manometric findings
Manometric evaluation may show a large-volume pouch, although we find the maximal total volume of the pouch measured in the physiology lab to be unreliable. With long-term excessive straining, a paradoxical contraction pattern may become an unconscious reflex with defecation.
Treatment
Conservative treatment includes adjustment of diet and topical therapy with anti-inflammatory or corticosteroid agents. Endoscopic treatment has a limited role, unless there is concurrent stricture, anastomotic sinus or fistula. The majority of patients require surgical intervention, with revision of the efferent limb (in the S pouch) or the rectal stump excision (in J pouch). Some patients require a complete pouch redo or pouch excision.
Folded pouch
Folding of the pouch body can also occur in patients with IPAA, which is defined as an angulation of the pouch body axis forming a ‘paper clip’ configuration on gastrografin enema or barium defecography (Figure 16). The pelvic pouch body may fold over, leading to fecal-evacuation problems. Classic symptoms of pouch folding are dyschezia, bloating or labor-type pains. Some patients may have partial small obstruction. It is possible that a surgically constructed long pouch body or pelvic adhesion may predispose the pouch to excessive movement around the long axis and folding. Pouch enema or MRI defecography is the diagnostic test of choice, although a careful pouchoscopy may show the anatomy.
Figure 16.
Pouch folding. (A) Folding of the pouch body on endoscopy. (B) Making a ‘paper clip’ configuration on a gastrografin enema (green arrow).
Surgery is the only option for patients with persistent and refractory symptoms. In most cases, patients require a revision of the pouch.
Floppy pouch complex
FPC is currently defined as the presence of pouch prolapse, ALS, redundant loop and folding pouch on pouchoscopy, gastrografin enema or in some cases MRI. Overall, the main symptoms of patients with FPC are dyschezia, incomplete evacuation or bloating. Each condition may have its own unique endoscopic, radiographic and manometry findings. There are overlapping clinical presentations and risk factors among the phenotypes of FPC. The etiology and pathogenesis of FPC and its phenotypes are largely unknown. Our recent studies showed that a lower BMI, lower peripouch fat area and female gender are risk factors of FPC [5, 6]. Higher incidence of pouch prolapse, ALS, perineal descent, folding pouch, redundant loop, predominant dyschezia symptoms and post-operative use of biologics has been found in FPC patients [24]. The finding of an increased use of post-operative biologics suggests that either the patient had been treated incorrectly for ‘pouchits’ with the biologic or that inflammation associated with pouchitis is a contributing factor. In fact, clinically, we often found patients with distal pouchitis and cuffitis that can be associated with prolapse. This mechanical factor-associated pouch or cuff inflammation typically does not respond to traditional antibiotic therapy or topical anti-inflammatory therapy. The patients may be mistaken as having refractory pouchitis, refractory cuffitis or even Crohn’s disease. Therefore, those patients may be treated with biological agents. Studies have suggested that the pouch itself possesses the ability to generate intense contractions in response to filling, and that probably aids in signaling the patient to seek appropriate conditions to defecate [25, 26]. Therefore, gastrointestinal dysmotility may play a role in the pathogenesis.
Summary and recommendations
Each of the diagnostic modalities has its advantages and limitations. Dynamic contrasted pelvic defecating studies (and in some instances maybe MRI defecography) are the diagnostic tests of choice for phenotypes of FPC. A high index of suspicion doing careful pouchoscopy is also important. Since those phenotypes of FPC often overlap, a combined assessment of clinical presentation, endoscopy, imaging and histology is needed. Patients with risk factors for FPC such as a lower BMI require close monitoring, although the efficacy of risk modification needs to be investigated. A multidisciplinary approach for the diagnosis and management is critical. Treatment options include lifestyle modification, physical therapy retraining, alterations in stool consistency and medical, endoscopic and surgical therapy. It is not unusual to recommend a combination of the above treatments, tailored to each individual patient in an effort to attain a functioning pouch with a reasonable quality of life.
Acknowledgements
Dr Bo Shen is supported by the Ed and Joey Story Endowed Chair.
Conflict of interest statement: none declared.
References
- 1. Shen B. Complications of IBD-related pouch surgery. Gastroenterol Hepatol 2007;3:678–80. [PMC free article] [PubMed] [Google Scholar]
- 2. Holzheimer RG, Mannick JA.. Surgical Treatment: Evidence-Based and Problem-Oriented. Munich: Zuckschwerdt, 2001. https://www.ncbi.nlm.nih.gov/books/NBK6880/ISBN-10: 3-88603-714-2. [PubMed] [Google Scholar]
- 3. Shen B, Remzi FH, Lavery IC. et al. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol 2008;6:145–58. [DOI] [PubMed] [Google Scholar]
- 4. Fazio VW, Tekkis PP, Remzi F. et al. Quantification of risk for pouch failure following ileal pouch anal anastomosis surgery. Ann Surg 2003;238:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan F, Gao XH, Shen B.. Retrospective study of predictive factors of pouch prolapse in ulcerative colitis patients. Am J Gastroenterol 2017;112:A1302. [Google Scholar]
- 6. Khan F, Gao XH, Shen B.. Lower peripouch fat area is related with increased incidence of pouch prolapse and floppy pouch complex. Gastroenterology Su1804. 2018;154:S-590. [DOI] [PubMed] [Google Scholar]
- 7. Joyce MR, Fazio VW, Hull TL. et al. Ileal pouch prolapse: prevalence, management, and outcomes. J Gastrointest Surg 2010;14:993–7. [DOI] [PubMed] [Google Scholar]
- 8. Felt-Bersma RJ, Tiersma ES, Cuesta MA.. Rectal prolapse, rectal intussusception, rectocele, solitary rectal ulcer syndrome, and enterocele. Gastroenterol Clin North Am 2008;37:645–68. [DOI] [PubMed] [Google Scholar]
- 9. Ehsan M, Isler JT, Kimmins MH. et al. Prevalence and management of prolapse of the ileoanal pouch. Dis Colon Rectum 2004;47:885–8. [DOI] [PubMed] [Google Scholar]
- 10. Shorvon PJ, McHugh S, Diamant NE. et al. Defecography in normal volunteers: results and implications. Gut 1989;30:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beck DE, Allen NL.. Rectocele. Clin Colon Rectal Surg 2010;23:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blazeby JM, Durdey P, Warren BF.. Polypoid mucosal prolapse in a pelvic ileal reservoir. Gut 1994;35:1668–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Read TE, Schoetz DJ Jr, Marcello PW. et al. Afferent limb obstruction complicating ileal pouch-anal anastomosis. Dis Colon Rectum 1997;40:566–9. [DOI] [PubMed] [Google Scholar]
- 14. Soffer EE, Hull TL.. Fecal incontinence: a practical approach to evaluation and treatment. Am J Gastroenterol 2000;95:1873–80. [DOI] [PubMed] [Google Scholar]
- 15. Quinn KP, Tse CS, Lightner AL. et al. Nonrelaxing pelvic floor dysfunction is an underestimated complication of ileal pouch–anal anastomosis. Clin Gastroenterol Hepatol 2017;15:1242–7. [DOI] [PubMed] [Google Scholar]
- 16. Williams NS, Giordano P, Dvorkin LS. et al. Full-thickness pouch prolapse after restorative proctocolectomy: a potential future problem treated by the new technique of external pelvic neorectal suspension (the express procedure). Dis Colon Rectum 2004;47:1415–9. [DOI] [PubMed] [Google Scholar]
- 17. Ragupathi M, Patel CB, Ramos-Valadez DI. et al. Robotic-assisted laparoscopic “salvage” rectopexy for recurrent ileoanal J-pouch prolapse. Gastroenterol Res Prac 2010:1–4. doi: 10.1155/2010/790462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institutes of Health/National Digestive Diseases Information Clearinghouse (NDDIC). Bowel Diversion Surgeries: Ileostomy, Colostomy, Ileoanal Reservoir, and Continent Ileostomy NIH Publication Number: 09-4641, 2009. http://sfsurgery.com/wp-content/uploads/2014/06/Bowel-Diversion.pdf (6 June 2014, date last accessed).
- 19. Kirat HT, Kiran RP, Remzi FH. et al. Diagnosis and management of afferent limb syndrome in patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis 2011;17:1287–90. [DOI] [PubMed] [Google Scholar]
- 20. Portincasa P, Bonfrate L, de Bari O. et al. Irritable bowel syndrome and diet. Gastroenterol Rep (Oxf) 2017;5:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tjandra JJ, Fazio VW.. Complications of the ileoanal pouch In: Mazier WP, Luchtefeld MA, Levien DH. et al. (eds). Surgery of the Colon, Rectum, and Anus. Philadelphia: WB Saunders, 1995, 893–903. [Google Scholar]
- 22. Baixauli J, Delaney CP, Wu JS. et al. Functional outcome and quality of life after repeat ileal pouch-anal anastomosis for complications of ileoanal surgery. Dis Colon Rectum 2004;47:2–11. [DOI] [PubMed] [Google Scholar]
- 23. Nicholls RJ, Gilbert JM.. Surgical correction of the efferent ileal limb for disordered defaecation following restorative proctocolectomy with the S ileal reservoir. Br J Surg 1990;77:152–4. [DOI] [PubMed] [Google Scholar]
- 24. Khan F, Gao XH, Hull TL. Risk factors of floppy pouch complex in ulcerative colitis patients with restorative proctocolectomy. Gastroenterology Su1781 2018;154:S-1314-S-1315. [Google Scholar]
- 25. Kmiot WA, Keighley MR.. Intussusception presenting as ileal reservoir ischaemia following restorative proctocolectomy. Br J Surg 1989;76:148.. [DOI] [PubMed] [Google Scholar]
- 26. Benezech A, Bouvier M, Grimaud JC. et al. Three-dimensional high-resolution anorectal manometry and diagnosis of excessive perineal descent: a comparative pilot study with defaecography. Colorectal Dis 2014;16:O170–5. [DOI] [PubMed] [Google Scholar]