Abstract
Background
Dysregulation of Rab18 has been implicated in human cancers. However, its clinical significance and biological function in gastric cancer have not been investigated.
Methods
We examined Rab18 expression in gastric cancer tissues using immunohistochemistry. We used SNU-1 and AGS cell lines for plasmid and siRNA transfection respectively. MTT, colony formation assay, cell cycle analysis, matrigel invasion, wound healing assay, AnnexinV/PI analysis and western blotting were used to examine the biological effect and mechanism of Rab18 in gastric cancer cell lines.
Results
Rab18 protein expression was upregulated in gastric cancer tissues and this correlated with advanced stage and poor prognosis. Rab18 overexpression promoted proliferation in vitro and in vivo. Cell cycle analysis showed that Rab18 overexpression upregulated, while its depletion downregulated S phase percentage. Matrigel invasion and wound healing assays indicated that Rab18 positively regulated SNU-1 cell invasion and migration while its knockdown inhibited AGS cell invasion and migration. Rab18 maintained cell viability and downregulated apoptosis after cisplatin treatment, with upregulated mitochondrial membrane potential and downregulated mitochondrial reactive oxygen species (ROS) production. Rab18 overexpression upregulated p-Rb, survivin while downregulated cytochrome c, cleaved caspase-3 and cleaved PARP.
Conclusion
In conclusion, our results indicate that Rab18 promoted gastric cancer growth and chemoresistance, possibly through regulation of mitochondrial function and survivin.
Keywords: Rab18, gastric cancer, survivin, proliferation, chemoresistance
Introduction
Gastric cancer is one of the most common malignant cancers worldwide. Although its incidence has been decreasing during the past decades, the prognosis remains poor for patients at advanced stage.1 The development of novel chemotherapeutic drugs helps to improve patient survival, but chemoresistance remains as an important obstacle during gastric cancer treatment.2 The mechanism behind gastric cancer progression and chemoresistance is quite complex, which involves genetic and epigenetic alterations. To improve the understanding of gastric cancer progression and chemoresistance, novel molecular mechanisms and therapeutic targets should be explored.
Rab18 belongs to the RAS superfamily of small G-proteins which are regulators of vesicular transport and signal transduction. Rab18 has been reported to localize to lipid droplets.3 Rab18 is involved in lipogenesis, lipolysis, and obesity.4 Rab18 binds to hepatitis C virus and promotes interaction between sites of viral replication and lipid droplets.5 Rab18 is important for normal endoplasmic reticulum structure and plays a critical role during brain and eye development; the loss-of-function mutations in Rab18 cause Warburg Micro syndrome.6 There is also evidence that Rab18 plays a key role during carcinogenesis. It has been reported that hepatitis B virus X protein upregulates Rab18, which leads to lipogenesis dysfunction and hepatoma proliferation.7 A study which performed screening of four medulloblastoma cDNAs indicated Rab18 as a novel tumor antigen.8 It has been reported that Rab18 promotes non-small-cell lung cancer cell proliferation,9 suggesting that Rab18 functions as an oncoprotein during human carcinogenesis. A recent study also showed that miR-455-5p acts as a tumor suppressor in gastric cancer by targeting Rab18,10 indicating the potential involvement of Rab18 in gastric cancer. However, this study did not validate the biological role of Rab18 and its clinical significance. To date, the expression pattern of Rab18 in human gastric cancers has not been explored. In addition, its biological roles and the potential biological mechanism need further investigation.
In the present study, we examined the expression pattern and biological roles of Rab18 in human gastric cancer in vitro and in vivo, and provide evidence that Rab18 serves as a prognostic indicator and oncoprotein in human gastric cancers. We also indicate that Rab18 regulates chemoresistance through survivin-mediated mitochondrial regulation.
Materials and methods
Patients and specimens
This study protocol was approved by the ethical review board of Chongqing Medical University. Primary tumor specimens were obtained from 91 patients diagnosed with gastric cancer between 2010 and 2015. Participants provided written informed consent, and the study was performed according to the principles of the Declaration of Helsinki. The histological examination was performed on sections stained with H&E according to the 2004 WHO classification guidelines. Fresh samples of gastric cancer tissues and corresponding adjacent normal tissues were obtained by surgical removal and stored at −80°C.
Immunohistochemistry
Paraffin tissue sections were made, and immunostaining was performed using Elivision kit from MaiXin (Fuzhou, China). After antigen retrieval in citrate buffer (pH 6.0) for 2 minutes in an autoclave, 0.3% hydrogen peroxide was added for 10 minutes. Sections were incubated with goat serum (ready-to-use; MaiXin) to reduce nonspecific binding. Then, sections were incubated with Rab18 antibody (1:300 dilution; Proteintech, Rosemont, IL, USA), p-Rb antibody (1:200 dilution; Cell Signaling Technology, Danvers, MA, USA), and survivin antibody (1:400 dilution; Cell Signaling Technology) at 4°C overnight. After incubation, horse radish peroxidase (HRP)-conjugated polymers were applied to sections. DAB-Plus kit (MaiXin) was used for staining.
Cell culture and transfection
GES-1, MGC-803, SNU-1, AGS, HGC-27, and NCI-N87 cell lines were purchased from Shanghai cell bank of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 with 10% FBS under the conditions of 37°C and 5% CO2. pCMV6 empty vector (EV) and pCMV6-Rab18 were obtained from OriGene (Rockville, MD, USA). Plasmid transfection was performed using Lipofectamine 3000 reagent. For stable Rab18 transfection, selection was performed using G418 for 10 days. Rab18 siRNA and negative control siRNA were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Dharmafect1 was used for siRNA transfection (GE Healthcare, Chicago, IL, USA).
Western blotting
Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) and incubated overnight at 4°C with antibody against Rab18 (1:1,000; Proteintech), p-Rb, survivin, cleaved caspase 3, cleaved PARP, cytochrome c (1:1,000; Cell Signaling Technology), Aurora B, Aurora B (phospho S227) (1:800; Abcam, Cambridge, UK), and GAPDH (1:3,000; Santa Cruz Biotechnology). After incubation with HRP-linked anti-mouse/rabbit IgG (1:3,000; Santa Cruz Biotechnology) at 37°C for 2 hours, proteins were visualized using enhanced chemiluminescence system (Thermo Fisher Scientific, Waltham, MA, USA) and DNR gel documentation system (DNR BioImaging Systems, Neve Yamin, Israel).
MTT and colony formation assays
For MTT assay, cells were plated in 96-well plates in medium containing 10% FBS at ~3,000 cells per well 24 hours after transfection. Then, 20 µL of 5 mg/mL MTT (thiazolyl blue) solution was added to each well and incubated for 4 hours at 37°C. The medium was removed from each well, and the resulting MTT formazan was solubilized in 150 µL of dimethyl sulfoxide, which was measured at 490 nm using a plate reader. To analyze colony formation, cells were seeded in culture dishes and cultured for 2 weeks. Then, these plates were stained using Giemsa.
Cell cycle analysis
Cells were fixed with 1% paraformaldehyde, and stained with propidium iodide (PI) in PBS with RNase A for 30 minutes. Cell cycle was analyzed using ACEA Flow Cytometer with NovoExpress software (ACEA, San Diego, CA, USA).
Annexin V/PI staining
Annexin V/PI staining kit from BD Biosciences was used to determine the rate of apoptosis according to the manufacturer’s protocol. Flow cytometry was performed using ACEA Flow Cytometer with NovoExpress software.
Matrigel invasion assay
Cell invasion assay was performed using a 24-well Transwell chamber coated with 20 µL Matrigel. Cell suspension with serum-free medium was transferred to the upper chamber. Medium supplemented with 10% FBS was added to the lower chamber. After 20–24 hours, invading cells that invaded through the membrane were fixed and stained with hematoxylin.
Wound healing assay
Gastric cells were seeded in a culture plate as a monolayer. Then, the cell monolayer was scratched with a pipette tip. Images of cell monolayer were taken using a microscope at 0- and 24-hour time points. The gap distance was measured, and the percentage of migration was calculated.
Cell adhesion assay
The cell adhesion assay was carried out using fibronectin-coated plates. Cells after transfection were plated and incubated for 2 hours at 37°C to adhere. The non-adherent cells were washed away and the remaining cells were fixed, stained, and counted under a microscope.
Mitochondrial membrane potential and ROS production
The mitochondrial membrane potential (MMP; Δψm) was detected using JC-1 staining method. Briefly, cells were harvested, washed with PBS, and incubated with 5 µM JC-1 (Cell Signaling Technology) for 30 minutes in the incubator. Then, cells were washed and analyzed using ACEA Flow Cytometer. Data were analyzed using NovoExpress software. ROS measurement was conducted using live cells with CellROX Deep Red Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. Cells were analyzed using ACEA Flow Cytometer. Data were analyzed using NovoExpress software.
Immunofluorescence
Cells on Lab-Tek Chamber Slides (Thermo Fisher Scientific) were fixed with 4% paraformaldehyde in PBS, permeabilized using 0.1% Triton X-100, blocked with serum, and then incubated with primary antibodies and AlexaFluro 488-conjugated secondary antibodies (Molecular Probes, Eugene, OR, USA). Photos were taken using Olympus BX53 microscope (Olympus, Tokyo, Japan).
Stable Rab18 knockdown shRNA sequence was designed based on the Rab18 sequence as follows: 5′-CCGGCATTAA CTCCCAGCTATTATACTCGAGTATAATAGCTGGGA GTTAATGTTTTTG-3′. The shRNA sequences were cloned into pLKO vector. The lentiviral vectors were transfected into cells at a multiplicity of infection of 30 in the presence of polybrene. At 72 hours after the infection, the infected cells were treated with 2 µg/mL puromycin for 10 days.
Nude mice xenograft
The Experimental Animal Ethics Committee of Chongqing Medical University approved the use of laboratory animals. BALB/c athymic nude mice (4 weeks old) were purchased from Shanghai Slac Laboratory Animals Ltd. (Shanghai, China). All animal experiments and procedures conformed to the institutional animal care guidelines. A xenograft model was established by subcutaneous right armpit injections of stable cell lines (8 million cells). Tumor size was measured every 5 days.
From day 10, cisplatin (CDDP) was administered at doses of 5 mg/kg every 5 days; three injections of CDDP were given in total. After 25 days, animals were sacrificed and xenograft tumors were removed.
Statistical analysis
SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Student’s t-test was carried out to compare data between control and experiment group. P<0.05 was regarded as statistically significant.
Results
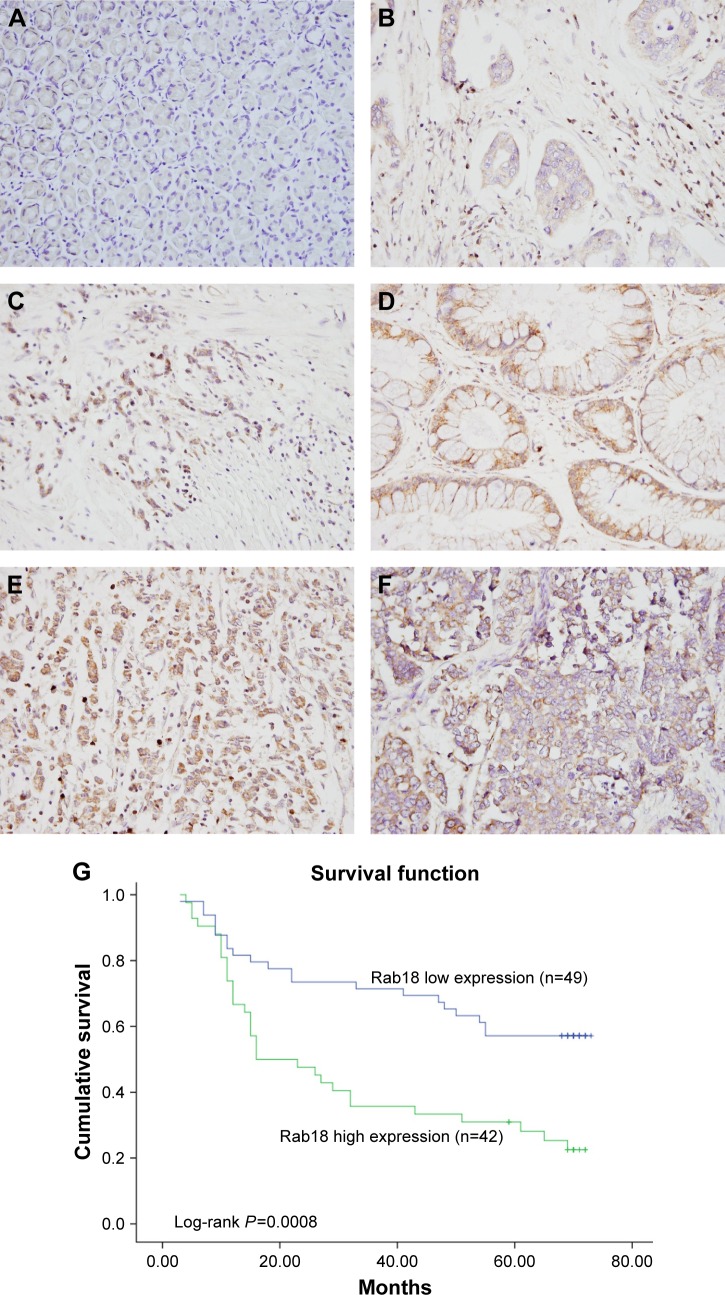
Rab18 was overexpressed in human gastric cancer and its overexpression was correlated with poor prognosis
To explore the expression pattern of Rab18 in human gastric cancer, 91 samples of gastric cancer tissues and 15 samples of normal gastric tissues were collected and Rab18 protein expression was examined using immunohistochemistry. As shown in Figure 1A, negative staining was observed in normal gastric tissues. Elevated cytoplasmic staining was observed in 42 of 91 (46.15%) samples of gastric cancer tissues (Figure 1B–F). The relationship between Rab18 expression and clinicopathological parameters was analyzed, and the results are listed in Table 1. We found that Rab18 overexpression was significantly associated with advanced TNM stage (P=0.002) and presence of nodal metastasis (P=0.0221). Kaplan–Meier analysis was performed to analyze its association with prognosis, and the results showed that patients with elevated Rab18 levels showed poorer overall survival than those with low Rab18 levels (log-rank test, P=0.0008) (Figure 1G). In addition, we validated Rab18 overexpression in fresh gastric cancer tissues and corresponding normal tissues using Western blots. As shown in Figure 2A, Rab18 protein expression in cancer tissues was higher than that in corresponding normal tissues. The specificity of antibody was validated using tissue samples and AGS cell proteins and AGS-siRab18 protein. As shown in Figure S1A, Western blot bands for Rab18 in tissues and AGS/AGS-siRab18 protein were of the same molecular weight, which validated the specificity of antibody for Western blot and immunohistochemistry.
Figure 1.
Expression of Rab18 in gastric cancers.
Notes: (A) Negative Rab18 staining in normal gastric tissues. (B) Weak cytoplasmic Rab18 staining in a sample of papillary gastric carcinoma. (C) Positive Rab18 staining in a sample of mucinous adenocarcinoma. (D) Positive Rab18 expression in a sample of tubular adenocarcinoma. (E) Positive staining in a sample of signet-ring cell carcinoma. (F) Rab18 staining in a sample of neuroendocrine carcinoma. (G) High Rab18 expression correlated with poor patient survival (log-rank test, P=0.0008).
Table 1.
Distribution of Rab18 status in gastric cancer according to clinicopathological characteristics
| Characteristics | Number of patients | Rab18 low expression | Rab18 high expression | P-value |
|---|---|---|---|---|
|
| ||||
| Age | 0.6047 | |||
| <60 | 45 | 23 | 22 | |
| ≥60 | 46 | 26 | 20 | |
| Gender | 0.2573 | |||
| Male | 71 | 36 | 35 | |
| Female | 20 | 13 | 7 | |
| Differentiation | 0.4164 | |||
| Poor | 41 | 24 | 17 | |
| Well–moderate | 50 | 25 | 25 | |
| Tumor invasion (T) | 0.7348 | |||
| T1+ T2 | 32 | 18 | 14 | |
| T3+ T4 | 59 | 31 | 28 | |
| Lymph node metastasis | 0.0221 | |||
| Absent | 33 | 23 | 10 | |
| Present | 58 | 26 | 32 | |
| TNM stage | 0.0020 | |||
| I | 16 | 10 | 6 | |
| II | 25 | 20 | 5 | |
| III | 50 | 19 | 31 | |
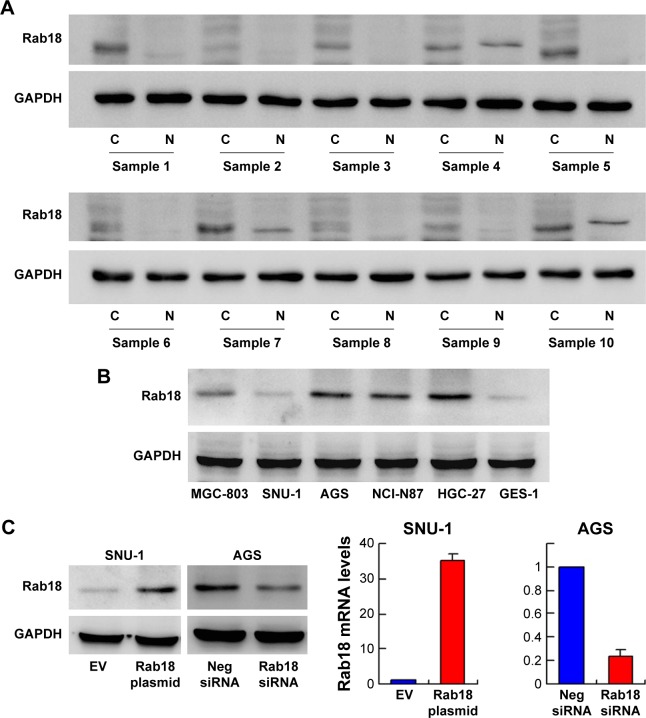
Figure 2.
Expression of Rab18 in gastric cancer cell lines.
Notes: (A) Western blot analysis of Rab18 protein in 10 samples of gastric cancer tissues with corresponding normal tissues. (B) Western blot analysis of Rab18 expression in gastric cancer cell lines (SNU-1, AGS, HGC-27, NCI-N87, MGC-803) and normal GES-1 cell line. (C) Plasmid transfection significantly upregulated Rab18 protein and mRNA levels in SNU-1 cells, and siRNA treatment downregulated Rab18 protein and mRNA expression in AGS cells.
Rab18 promoted cell proliferation and cell cycle progression
Rab18 protein expression was examined in five gastric cancer cell lines including SNU-1, AGS, HGC-27, NCI-N87, and MGC-803, and the normal cell line GES-1. As shown in Figure 2B, AGS cell line had relatively high endogenous Rab18 expression while SNU-1 had relatively low Rab18 expression. Immunofluorescence of Rab18 was performed in AGS and HGC-27 cell lines with higher Rab18 expression. Cytoplasmic localization was observed in both cell lines. Localization of Rab18 staining was observed around vesicles in AGS cells (Figure S1C), which was in accord with the fact that Rab18 was associated with vesicle transportation. SNU-1 was selected for plasmid transfection, and AGS was used for Rab18 siRNA knockdown. As shown in Figure 2C, transfection and siRNA knockdown efficiency were validated by quantitative real-time PCR and Western blotting, respectively.
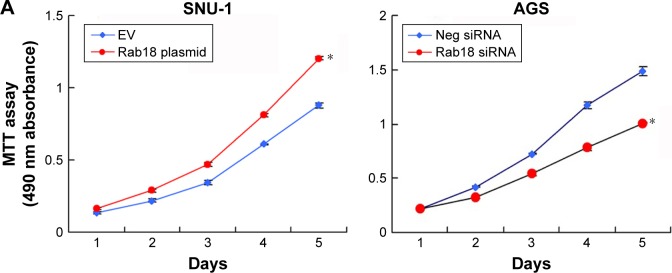
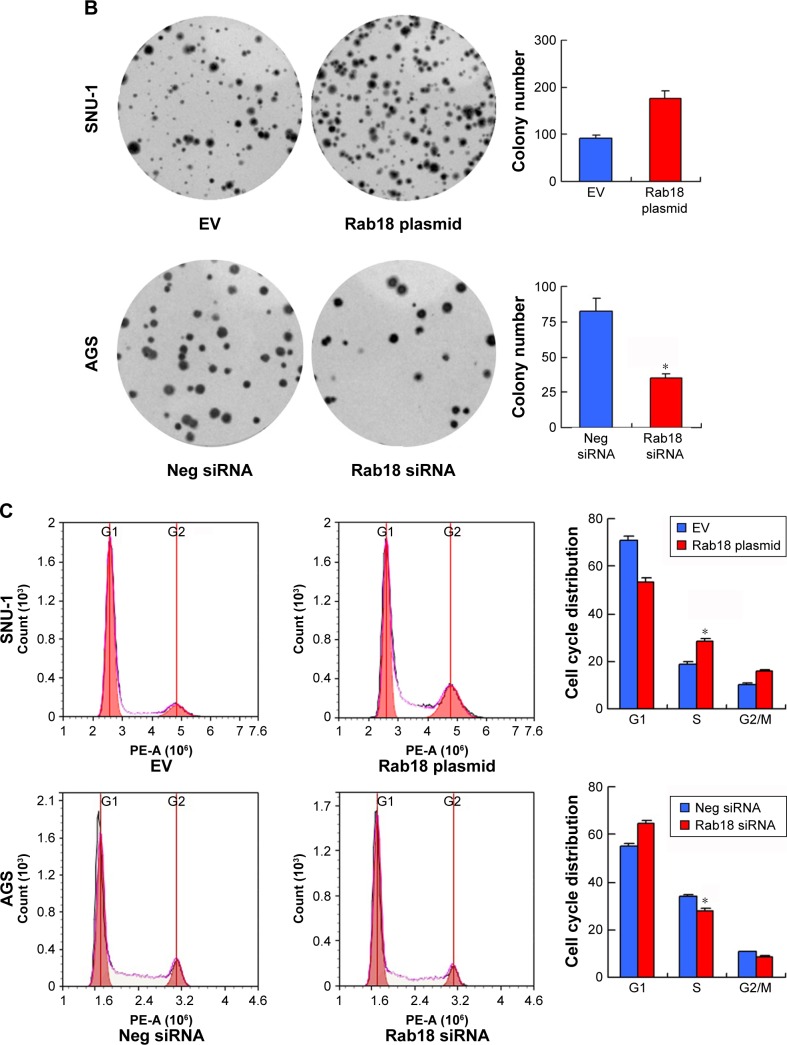
MTT and colony formation assays were carried out to analyze the role of Rab18 in proliferation. MTT assay showed that the proliferation rate of AGS cells decreased significantly after Rab18 depletion, while Rab18 overexpression promoted proliferation of SNU-1 cells (Figure 3A). Colony formation assay demonstrated that Rab18 positively regulated colony formation ability in gastric cancer cells (Figure 3B). In addition, cell cycle analysis demonstrated that Rab18 overexpression upregulated S phase percentage and promoted G1–S transition in SNU-1 cells, while Rab18 depletion inhibited G1–S cell cycle transition (Figure 3C).
Figure 3.
Rab18 regulates proliferation, colony formation, and cell cycle.
Notes: (A) MTT assay showed that Rab18 overexpression promoted proliferation in SNU-1 cell line while Rab18 depletion inhibited proliferation in AGS cell line. (B) Colony formation assay demonstrated that Rab18 overexpression upregulated colony number in SNU-1 cell line while Rab18 depletion downregulated colony number in AGS cell line. (C) Cell cycle analysis demonstrated that Rab18 overexpression upregulated S phase percentage and downregulated G1 phase percentage in SNU-1 cells while Rab18 depletion upregulated G1 phase percentage and downregulated S phase percentage in AGS cell line. *P<0.05.
Rab18 promoted gastric cancer cell invasion and migration and inhibited cell adhesion
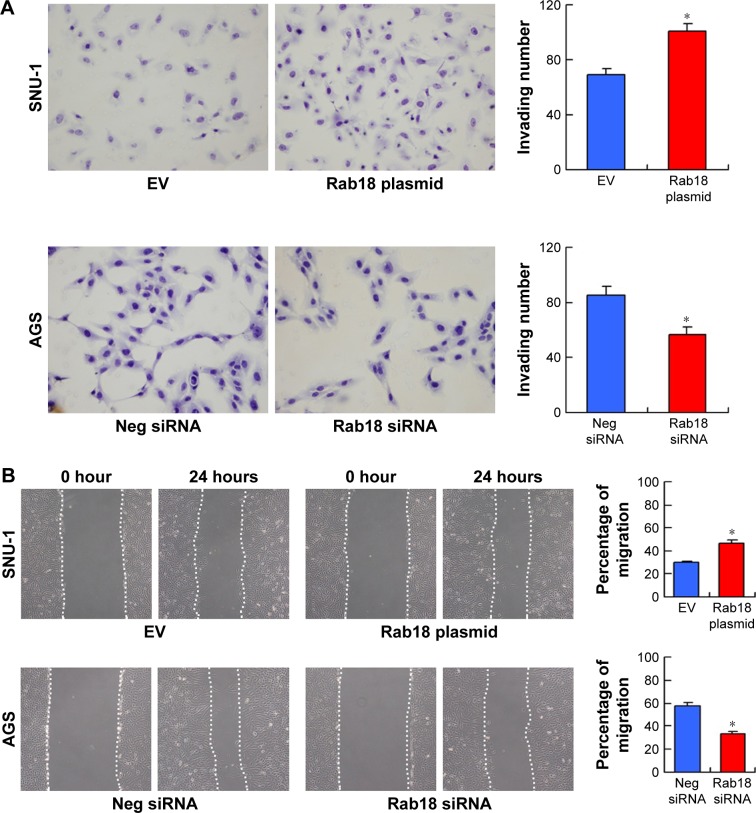
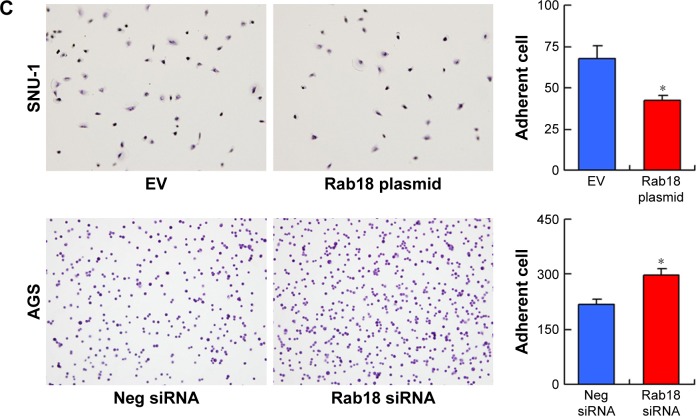
The correlation between Rab18 and nodal metastasis suggested the potential role of Rab18 in gastric cancer cell invasion, migration, and adhesion. To investigate this further, we performed Matrigel invasion assay, wound healing assay, and adhesion assay. As shown in Figure 4A, Rab18 overexpression upregulated the number of invading SNU-1 cells, while Rab18 depletion decreased the invasion of AGS cells. Wound healing assay was carried out to determine the effect of Rab18 on cell migration. As shown in Figure 4B, Rab18 overexpression elevated the migration speed of SNU-1 cells, while Rab18 knockdown inhibited AGS cell migration. Cell adhesion assay demonstrated that Rab18 overexpression downregulated cell adhesion ability in SNU-1 cells, while Rab18 depletion upregulated AGS cell adhesion (Figure 4C).
Figure 4.
Rab18 regulates invasion, migration, and adhesion.
Notes: (A) Matrigel invasion assay demonstrated that Rab18 overexpression upregulated invading cell number in SNU-1 cells while Rab18 depletion downregulated invading cell number in AGS cells. (B) Wound healing assay demonstrated that Rab18 overexpression facilitated cell migration in SNU-1 cells while Rab18 depletion inhibited AGS cell migration. (C) Cell adhesion assay demonstrated that Rab18 overexpression downregulated cell adhesion in SNU-1 cells while Rab18 depletion increased the number of adherent AGS cells. *P<0.05.
Rab18 enhanced chemoresistance in gastric cancer cells
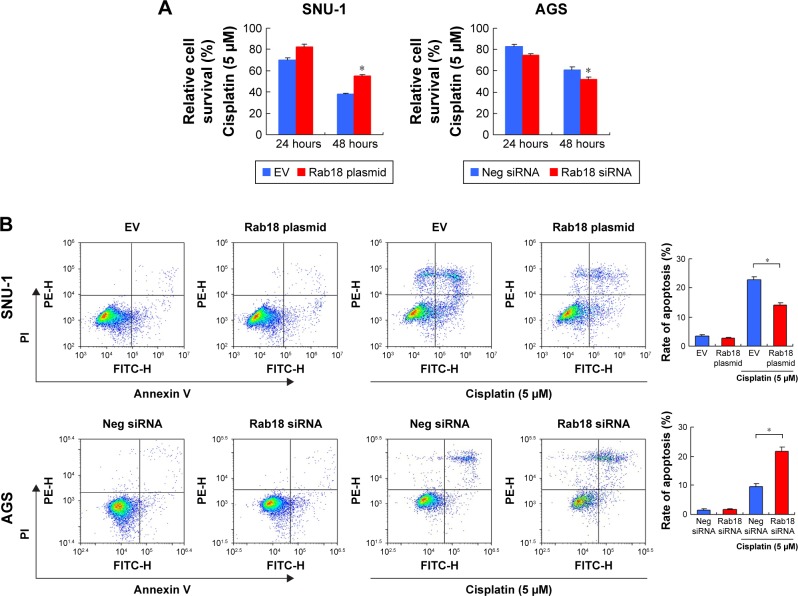
Next, we examined the role of Rab18 in CDDP resistance. First, 5 µM CDDP was used to treat Rab18-overexpressed and Rab18-depleted cells for 24 and 48 hours. Cell viability was determined by MTT assay. As shown in Figure 5A, Rab18 plasmid transfection in SNU-1 cells significantly maintained cell viability after 24 and 48 hours of CDDP treatment. On the other hand, Rab18 depletion in AGS cells reduced cell viability after the CDDP treatment, suggesting Rab18 could induce CDDP resistance in gastric cancer cells. Annexin V/PI analysis was also carried out to examine the change of apoptosis after CDDP treatment. As shown in Figure 5B, Rab18 overexpression reduced the rate of CDDP-induced apoptosis, while Rab18 knockdown upregulated CDDP-induced apoptosis in SNU-1 cell line. These results suggested that Rab18 overexpression conferred chemoresistance in gastric cancers.
Figure 5.
Rab18 regulates CDDP-induced apoptosis in gastric cancer cells.
Notes: (A) MTT assay showed that Rab18 overexpression upregulated cell viability in SNU-1 cells after 24 and 48 hours of CDDP treatment while Rab18 depletion downregulated cell viability in AGS cells. (B) Annexin V/PI analysis demonstrated that Rab18 overexpression inhibited CDDP-induced apoptosis in SNU-1 cells while Rab18 depletion upregulated CDDP-induced apoptosis in AGS cells. The change of apoptosis rate was not significant in non-treated cells. *P<0.05.
Abbreviations: CDDP, cisplatin; PI, propidium iodide.
Rab18 regulated MMP and ROS production
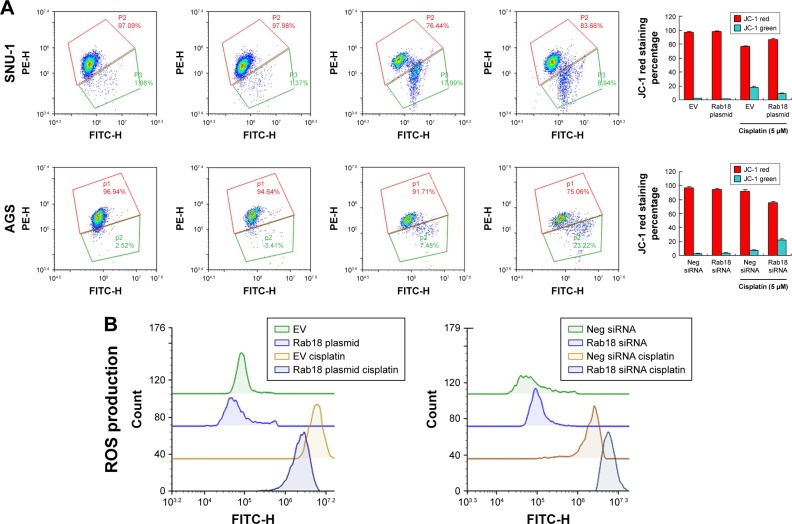
Because chemoresistance is closely related to mitochondrial function, we examined whether Rab18 could control MMP (Δψm). JC-1 staining was used to examine MMP, which shows red fluorescence under normal condition and turns into green when Δψm is decreased. As shown in Figure 6A, the percentage of green-stained cells was less among Rab18-overexpressed SNU-1 cells, suggesting Rab18 upregulated Δψm in CDDP-treated cells, while Rab18 siRNA showed the opposite effect in AGS cells. In cells without CDDP treatment, the change of apoptosis was not significant. ROS production is induced by chemotherapeutic agents leading to cell injury and apoptosis, which is closely associated with mitochondrial function. We examined ROS levels by flow cytometry using CellROX Deep Red Reagent. As shown in Figure 6B, Rab18 overexpression reduced the level of ROS accumulation, while Rab18 depletion increased ROS accumulation after the CDDP treatment.
Figure 6.
Rab18 regulates MMP and ROS production.
Notes: (A) JC-1 staining showed that Rab18 overexpression upregulated MMP with increased JC-1 red/green ratio in SNU-1 cells treated with CDDP, while Rab18 depletion downregulated MMP with decreased JC-1 red/green ratio in AGS cells treated with CDDP. (B) CellROX Deep Red staining showed that Rab18 overexpression reduced CDDP-induced ROS accumulation in SNU-1 cells while Rab18 depletion upregulated CDDP-induced ROS accumulation in AGS cells.
Abbreviations: CDDP, cisplatin; FITC, fluorescein isothiocyanate; MMP, mitochondrial membrane potential.
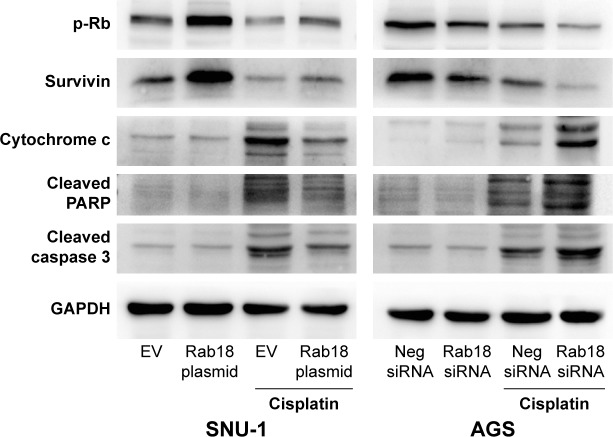
Rab18 regulated p-Rb, survivin, and apoptosis-related proteins
To further elucidate the association between Rab18 and chemoresistance and proliferation, we determined the changes of related proteins. As shown in Figure 7, Rab18 overexpression upregulated survivin and p-Rb protein levels, while Rab18 depletion reduced survivin and p-Rb expression in both CDDP-treated and CDDP-untreated groups. In addition, Rab18 downregulated cytochrome c, cleaved caspase 3, and cleaved PARP in CDDP-treated SNU-1 cells, whereas Rab18 depletion upregulated cytochrome c, cleaved caspase 3, and cleaved PARP in CDDP-treated AGS cells. We also examined protein expression of Aurora B and Aurora kinase B (S227), which act as upstream activators of survivin. As shown in Figure S1B, Rab18 did not cause significant change of these proteins.
Figure 7.
Rab18 regulates p-Rb, survivin, cytochrome c, cleaved PARP, and cleaved caspase 3.
Notes: Western blot showed that Rab18 overexpression upregulated the levels of p-Rb and survivin in SNU-1 cells while Rab18 depletion downregulated p-Rb and survivin levels in AGS cells. Rab18 overexpression downregulated cytochrome c, cleaved caspase 3, and cleaved PARP in CDDP-treated SNU-1 cells, while Rab18 depletion upregulated cytochrome c, cleaved caspase 3, and cleaved PARP in CDDP-treated AGS cells.
Abbreviation: CDDP, cisplatin.
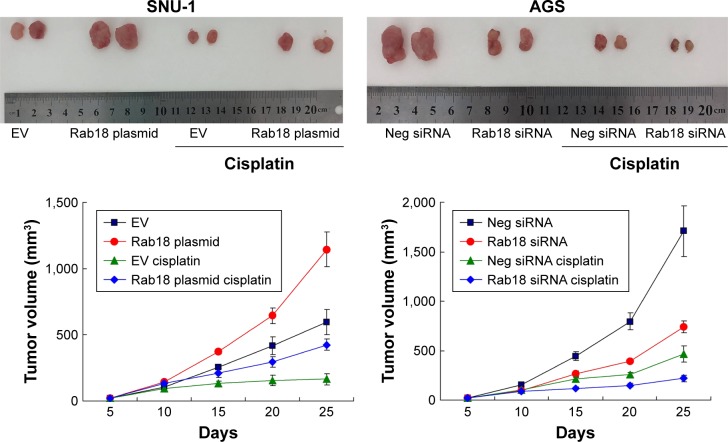
Rab18 regulated tumor growth in vivo
To examine the effect of Rab18 on tumor growth in vivo, we established Rab18-overexpressing SNU-1 cell line by G418 selection. We also identified AGS cells that were stably transfected with Rab18 shRNA using puromycin selection. These cells were injected subcutaneously into nude mice, and the mice were treated with CDDP after 10 days of injection. As shown in Figure 8, the in vivo growth rate and tumor sizes of Rab18-overexpressing SNU-1 cells were much larger than control cells. Tumors of Rab18-overexpressing cells were also larger than controls cells after cisplatin treatment, while Rab18 shRNA exhibited the opposite effects in AGS cells. In addition, H&E staining, TUNEL assay, and immunostaining for Rab18, p-Rb, and survivin were performed in tumor tissues (Figure S2). Rab18-overexpressing tumors showed higher expression of p-Rb and survivin in both CDDP-treated and CDDP-untreated groups. The level of TUNEL staining was lower in Rab18-overexpressing tumors than control tumors. Rab18 depletion showed the opposite effect on AGS and AGS-shRab18 cells.
Figure 8.
Rab18 promotes cancer cell growth in vivo.
Notes: SNU-1 and AGS cells were stably transfected with Rab18 plasmid and Rab18 shRNA, respectively. Cells were injected into nude mice. CDDP was administered at doses of 5 mg/kg every 5 days; three injections of CDDP were given in total. Representative images of tumors and growth curves are shown.
Abbreviation: CDDP, cisplatin.
Discussion
Rab18 was originally identified as a small G-protein that regulates lipid metabolism, and plays an important role during brain and eye development.3,4,6 Recent evidence indicated Rab18 as a cancer-associated protein. Rab18 could induce hepatoma and non-small-cell lung cancer cell proliferation.9 However, the clinical significance of Rab18 in human gastric cancer remains unexplored. To address this issue, we analyzed the protein expression pattern of Rab18 and found that Rab18 was overexpressed in gastric cancer tissues. High Rab18 status positively correlated with advanced TNM stage and presence of nodal metastasis. Importantly, Rab18 overexpression correlated with poor patient survival, making it a potential prognostic cancer marker in human gastric cancer.
The clinical data indicated that Rab18 is a potential oncoprotein. To validate this, we first screened gastric cancer cell lines for Rab18 expression and showed that the expression of Rab18 in gastric cancer cell lines was higher than that in normal GES-1 cell line. A previous report showed that Rab18 could positively regulate lung cancer cell proliferation.9 Hence, we examined the role of Rab18 in cell proliferation using MTT, colony formation assay, and cell cycle analysis. Our data demonstrated that Rab18 overexpression promoted cell growth and colony formation ability, with accelerated G1–S cell cycle transition, while Rab18 depletion showed the opposite effects. Importantly, our data showed that Rab18 also promoted cell proliferation in vivo. Accordingly, our results also showed that p-Rb expression was upregulated after Rab18 overexpression and downregulated after Rab18 depletion. The tumor suppressor protein Rb is dysregulated in most cancer, which prevents excessive cell growth by inhibiting cell cycle progression.11 When Rb is phosphorylated, it becomes inactive and allows cell cycle progression. Thus, our results indicated that Rab18 promoted gastric cancer proliferation in vitro and in vivo, possibly through facilitating cell cycle progression by positive regulation of p-Rb.
Because Rab18 protein status correlated with nodal metastasis, we assumed that it regulated gastric cancer cell invasion and migration. Matrigel invasion assay and wound healing assay were carried out to validate these functions. Our results demonstrated that Rab18 overexpression promoted cell invasion and migration. Decreased cell adhesion also contributed to cancer metastasis. These results indicated Rab18 as a promoter of cancer cell invasion. Our adhesion assay showed that Rab18 slightly decreased the adhesion of gastric cancer cells to fibronectin, which is not consistent with the fact of enhanced invasion. Cell invasion is a multistep process including attachment to the extracellular matrix (ECM) and proteolytic remodeling of the ECM, led by advancing protruding pseudopods and migration of the tumor cell body through the remodeled matrix. Change of each step could lead to enhanced cell invasion. Wound healing assay suggested that Rab18 could enhance the speed of cell migration, which may explain its role in cell invasion. In addition, no correlation between Rab18 and local invasion (T stage) has been identified. This discrepancy could be explained by the multiple genetic factors (loss of adhesion molecules and upregulation of ECM-degrading enzyme) contributing to the tumor invasion in clinical circumstance.
The role of Rab18 in apoptosis has not been reported. To clarify the role of Rab18 in cancer cell apoptosis and chemoresistance, we treated cells with CDDP and examined cell viability and apoptosis rate using Annexin V/PI staining. Our data demonstrated that Rab18 overexpression was able to maintain cell viability, which was downregulated by the CDDP treatment. In addition, the rate of CDDP-induced apoptosis was lower in Rab18-overexpressed cells than in the control cells, suggesting that Rab18 could induce chemoresistance and inhibit apoptosis in gastric cancer cells. The effect of Rab18 on gastric cancer survival was also validated by TUNEL assay in vivo.
Mitochondria play a pivotal role in apoptosis. Chemotherapeutic drugs, such as CDDP, induce apoptosis through mitochondria-dependent pathways.12–14 We first analyzed the role of Rab18 in mitochondrial function by examining MMP using JC-1 staining. We found that Rab18 overexpression was able to upregulate MMP while Rab18 depletion downregulated MMP. Downregulation of MMP could trigger apoptosis through mitochondria-dependent pathway, which releases cytochrome c with an increased membrane permeability.15 Thus, our results indicated that Rab18 might inhibit apoptosis through positive regulation of MMP.
Next, we examined the change of cytochrome c, cleaved caspase 3, and cleaved PARP. Cytochrome c is bound to the inner membrane by anionic phospholipids. Cytochrome c is released into the cytosol as the outer membrane of mitochondria becomes permeable with the downregulation of MMP, and this acts to trigger caspase and PARP cleavage/activation. Our results demonstrated that Rab18 inhibited the release of cytochrome c and the expression of cleaved caspase 3/PARP, supporting the role of Rab18 in apoptosis inhibition and MMP maintenance.
Apart from the induction of DNA damage, recent data have suggested that CDDP could induce the formation of ROS.16 ROS is a by-product of cell metabolism under normal conditions. However, excessive ROS triggers apoptosis pathway by altering the MMP and damaging the respiratory chain.17,18 Our data demonstrated that Rab18 had a protective role during CDDP-induced ROS formation. Considering the connection between ROS and MMP, it is possible that Rab18 inhibits CDDP-induced ROS production, which maintains MMP and reduces cytochrome c release, thus inhibiting CDDP-induced apoptosis. To identify the possible mechanism for the role of Rab18 in apoptosis, we screened related proteins and found that Rab18 was able to upregulate survivin in vitro and in vivo. Survivin, also known as BIRC5, is a member of the inhibitor of apoptosis family. Survivin localizes in the mitochondria and inhibits apoptosis.19,20 Previous reports indicate that survivin negatively regulates ROS production and protects cancer cells from drug-induced cell death.21 We also sought to explore the potential mechanism of survivin regulation by examining Aurora B, an upstream regulator of survivin. However, Rab18 did not cause a significant change in Aurora B expression. Thus, the above results suggest a link between Rab18, survivin, mitochondrial function, and chemoresistance.
In conclusion, the present study identified Rab18 as a cancer-related protein that was overexpressed in human gastric cancers. Its overexpression correlated with advanced stage and poor patient prognosis. Rab18 promoted gastric cancer cell proliferation in vitro and in vivo. Rab18 also inhibited apoptosis through the regulation of mitochondrial function and ROS production, possibly through survivin.
Supplementary materials
Validation of Rab18 antibody for Western blot and immunofluorescence.
Notes: (A) Western blot bands for Rab18 in cancer/normal tissues and AGS/AGS-siRab18 protein were of the same molecular weight. (B) Expression of Aurora B and Aurora B (phospho S227) in cells with Rab18 overexpression or knockdown. (C) Immunofluorescence of Rab18 in AGS and HGC-27 cells with relatively higher Rab18 expression. Cytoplasmic localization was observed in both cell lines. Localization of Rab18 staining could be observed around vesicles in AGS cells.
Immunohistochemistry for in vivo tumors formed by stably transfected cells.
Note: H&E staining, TUNEL assay, and immunostaining for Rab18, p-Rb, and survivin in tumor tissues formed by stably transfected SNU-1 and AGS cells.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Yoon C, Cho SJ, Aksoy BA, et al. Chemotherapy Resistance in Diffuse-Type Gastric Adenocarcinoma Is Mediated by RhoA Activation in Cancer Stem-Like Cells. Clin Cancer Res. 2016;22(4):971–983. doi: 10.1158/1078-0432.CCR-15-1356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Ozeki S, Cheng J, Tauchi-Sato K, Hatano N, Taniguchi H, Fujimoto T. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J Cell Sci. 2005;118(Pt 12):2601–2611. doi: 10.1242/jcs.02401. [DOI] [PubMed] [Google Scholar]
- 4.Pulido MR, Diaz-Ruiz A, Jiménez-Gómez Y, et al. Rab18 dynamics in adipocytes in relation to lipogenesis, lipolysis and obesity. PLoS One. 2011;6(7):e22931. doi: 10.1371/journal.pone.0022931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salloum S, Wang H, Ferguson C, Parton RG, Tai AW. Rab18 binds to hepatitis C virus NS5A and promotes interaction between sites of viral replication and lipid droplets. PLoS Pathog. 2013;9(8):e1003513. doi: 10.1371/journal.ppat.1003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bem D, Yoshimura S, Nunes-Bastos R, et al. Loss-of-function mutations in RAB18 cause Warburg micro syndrome. Am J Hum Genet. 2011;88(4):499–507. doi: 10.1016/j.ajhg.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.You X, Liu F, Zhang T, Li Y, Ye L, Zhang X. Hepatitis B virus X protein upregulates oncogene Rab18 to result in the dysregulation of lipogenesis and proliferation of hepatoma cells. Carcinogenesis. 2013;34(7):1644–1652. doi: 10.1093/carcin/bgt089. [DOI] [PubMed] [Google Scholar]
- 8.Behrends U, Schneider I, Rössler S, et al. Novel tumor antigens identified by autologous antibody screening of childhood medulloblastoma cDNA libraries. Int J Cancer. 2003;106(2):244–251. doi: 10.1002/ijc.11208. [DOI] [PubMed] [Google Scholar]
- 9.Zhong K, Chen K, Han L, Li B. MicroRNA-30b/c inhibits non-small cell lung cancer cell proliferation by targeting Rab18. BMC Cancer. 2014;14:703. doi: 10.1186/1471-2407-14-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Zhang J, Li Y, Wang L, Sui B, Dai D. MiR-455-5p acts as a novel tumor suppressor in gastric cancer by down-regulating RAB18. Gene. 2016;592(2):308–315. doi: 10.1016/j.gene.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Engel BE, Cress WD, Santiago-Cardona PG. The retinoblastoma protein: a master tumor suppressor acts as a link between cell cycle and cell adhesion. Cell Health Cytoskelet. 2015;7:1–10. doi: 10.2147/CHC.S28079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerra F, Arbini AA, Moro L. Mitochondria and cancer chemoresistance. Biochim Biophys Acta Bioenerg. 2017;1858(8):686–699. doi: 10.1016/j.bbabio.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Lee JM, Chwae YJ, et al. Cisplatin-induced apoptosis in Hep3B cells: mitochondria-dependent and -independent pathways. Biochem Pharmacol. 2004;67(8):1459–1468. doi: 10.1016/j.bcp.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhao W, You CC, Zhuang JP, et al. Viability inhibition effect of gambogic acid combined with cisplatin on osteosarcoma cells via mitochondria-independent apoptotic pathway. Mol Cell Biochem. 2013;382(1–2):243–252. doi: 10.1007/s11010-013-1740-5. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Wong JY, Wong P, Radany EH. Low-dose valproic acid enhances radiosensitivity of prostate cancer through acetylated p53-dependent modulation of mitochondrial membrane potential and apoptosis. Mol Cancer Res. 2011;9(4):448–461. doi: 10.1158/1541-7786.MCR-10-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JG, Lindup WE. Role of mitochondria in cisplatin-induced oxidative damage exhibited by rat renal cortical slices. Biochem Pharmacol. 1993;45(11):2215–2222. [PubMed] [Google Scholar]
- 17.Jing XB, Cai XB, Hu H, Chen SZ, Chen BM, Cai JY. Reactive oxygen species and mitochondrial membrane potential are modulated during CDDP-induced apoptosis in EC-109 cells. Biochem Cell Biol. 2007;85(2):265–271. doi: 10.1139/O07-014. [DOI] [PubMed] [Google Scholar]
- 18.Brozovic A, Ambriović-Ristov A, Osmak M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit Rev Toxicol. 2010;40(4):347–359. doi: 10.3109/10408441003601836. [DOI] [PubMed] [Google Scholar]
- 19.Waligórska-Stachura J, Jankowska A, Waśko R, et al. Survivin – prognostic tumor biomarker in human neoplasms – review. Ginekol Pol. 2012;83(7):537–540. [PubMed] [Google Scholar]
- 20.Liu JL, Gao W, Kang QM, Zhang XJ, Yang SG. Prognostic value of survivin in patients with gastric cancer: a systematic review with meta-analysis. PLoS One. 2013;8(8):e71930. doi: 10.1371/journal.pone.0071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattari M, Pazhang Y, Imani M. Calprotectin induces cell death in human prostate cancer cell (LNCaP) through survivin protein alteration. Cell Biol Int. 2014;38(11):1311–1320. doi: 10.1002/cbin.10328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of Rab18 antibody for Western blot and immunofluorescence.
Notes: (A) Western blot bands for Rab18 in cancer/normal tissues and AGS/AGS-siRab18 protein were of the same molecular weight. (B) Expression of Aurora B and Aurora B (phospho S227) in cells with Rab18 overexpression or knockdown. (C) Immunofluorescence of Rab18 in AGS and HGC-27 cells with relatively higher Rab18 expression. Cytoplasmic localization was observed in both cell lines. Localization of Rab18 staining could be observed around vesicles in AGS cells.
Immunohistochemistry for in vivo tumors formed by stably transfected cells.
Note: H&E staining, TUNEL assay, and immunostaining for Rab18, p-Rb, and survivin in tumor tissues formed by stably transfected SNU-1 and AGS cells.