Abstract
Objective:
In 2016, North Carolina blood lead level (BLL) surveillance activities identified elevated BLLs among 3 children exposed to take-home lead by household members employed at a lead oxide manufacturing facility. We characterized BLLs among employees and associated children and identified risk factors for occupational and take-home lead exposure.
Methods:
We reviewed BLL surveillance data for 2012-2016 to identify facility employees and associated children. We considered a BLL ≥5 μg/dL elevated for adults and children and compared adult BLLs with regulatory limits and recommended health-based thresholds. We also conducted an environmental investigation and interviewed current employees about exposure controls and cleanup procedures.
Results:
During 2012-2016, 5 children associated with facility employees had a confirmed BLL ≥5 μg/dL. Among 77 people employed during 2012-2016, median BLLs increased from 22 μg/dL (range, 4-45 μg/dL) in 2012 to 37 μg/dL (range, 16-54 μg/dL) in 2016. All employee BLLs were <60 μg/dL, the national regulatory threshold for immediate medical removal from lead exposure; however, 55 (71%) had a BLL ≥20 μg/dL, a recommended health-based threshold for removal from lead exposure. Because of inadequate controls in the facility, areas considered clean were visibly contaminated with lead dust. Employees reported bringing personal items to work and then into their cars and homes, resulting in take-home lead exposure.
Conclusions:
Integration of child and adult BLL surveillance activities identified an occupational source of lead exposure among workers and associated children. Our findings support recent recommendations that implementation of updated lead standards will support better control of lead in the workplace and prevent lead from being carried home.
Keywords: lead, occupational health, environmental exposure, epidemiology
Lead exposure is associated with acute and chronic adverse health effects ranging from subclinical changes to acute lead intoxication, which can be life threatening.1,2 Even at low levels of lead exposure, children can have developmental delays, reduced IQ scores, behavioral problems, and nervous system damage. Adults are at increased risk for hypertension, neurological problems, and reproductive problems.3
In the United States, lead is used in multiple industries, and occupational exposures account for most reported elevated blood lead levels (BLLs) among adults.4 In 1978, the Occupational Safety and Health Administration (OSHA) adopted a lead standard for general industry to prevent overt lead poisoning of workers.5 The standard dictates an airborne lead action level (level at which companies must take action to reduce employee exposure), permissible exposure limit (PEL), BLL monitoring, and medical removal requirements (ie, BLL at which employees must be removed from lead exposure until their BLL is 40 μg/dL) that drive legal compliance. However, other governmental agencies and expert panels have concluded that OSHA’s lead standard does not reflect current scientific knowledge and does not sufficiently protect worker health.1,2
When lead is not well controlled in the workplace, workers can unintentionally carry lead dust on themselves and their personal items, potentially contaminating their cars and homes and resulting in take-home lead exposure among household members.6 Although residential lead-based paint and lead-contaminated dust and soil are the most common sources of childhood lead exposure, take-home lead is a preventable source of childhood lead exposure associated with multiple industries.6-13
To reduce lead exposure among children, the North Carolina Division of Public Health (DPH) conducts blood lead surveillance and case management.14 Local health departments investigate sources of exposure for children identified with an elevated BLL and provide recommendations to eliminate the source; depending on the BLL and county, investigations can include an interview of the child’s guardian(s), home assessment, and environmental sampling. In May 2016, surveillance activities identified 3 children living in 1 county with BLLs ≥5 μg/dL, the current reference level set by the Centers for Disease Control and Prevention (CDC).4 Routine investigations conducted by the local health department found no sources of lead exposure in these children’s homes; however, each child had a household member employed at a local lead oxide manufacturing facility. Lead dust was found on clothes and shoes worn by the household member employed at the facility, in cars, and near the home entrance, indicating take-home exposure. The BLLs reported to the North Carolina DPH from the company biomonitoring program showed persistently high BLLs among facility employees during 2012-2016 despite a history of notifications and education provided by local and state health departments.
Local and state public health staff members worked together to characterize BLLs among facility employees, identify and describe BLLs among associated children, and identify risk factors for occupational and take-home lead exposure.
Methods
We immediately notified facility management of elevated BLLs among employees and their household members and requested that the facility provide a current roster of employees with contact information. The local health department hosted educational outreach meetings to explain the public health concern and provide guidance to facility employees about reducing and preventing occupational and take-home lead exposure.
Characterization of BLLs and Case Finding
We reviewed data from 2 surveillance systems—the North Carolina Electronic Lead surveillance system (NC LEAD; Conduent Public Health Solutions, Maven Software, Austin, Texas) and the North Carolina Adult Blood Lead Epidemiology and Surveillance (NC ABLES)15 program—to identify facility employees and associated children with at least 1 BLL reported during 2012-2016.
Children
All children are recommended to have a blood lead test at well-child visits at 12 and 24 months of age; testing is a requirement for children enrolled in Medicaid, Health Choice, or the Special Supplemental Nutrition Program for Women, Infants, and Children.14 North Carolina laboratories are required to report all BLLs for children aged <6 years and typically report BLLs ≥5 μg/dL for children aged 7-17 to public health.14 We used NC LEAD to identify children meeting 1 of the following criteria:
During an investigation into an elevated BLL source, an environmental history or home investigation indicated a household member worked at the lead oxide manufacturing facility.
Had a last name and address recorded at the time of blood lead specimen collection for a BLL check that matched a known employee’s last name and address recorded in NC ABLES.
Had a current address matching a known employee’s address from the June 2016 employee roster provided by the facility.
To identify additional cases, we asked current employees to report the names and birth dates of children spending time in their households, and we searched NC LEAD for matches. Local health departments also offered free BLL testing to all household members (aged <18) of current facility employees. We categorized children according to North Carolina DPH guidance into BLL categories of 5-9 μg/dL, 10-19 μg/dL, or ≥20 μg/dL, based on the lower of 2 consecutive BLLs within a 6-month period.14
Adults
Laboratories report BLLs for adults to the North Carolina DPH. Employees at the lead oxide facility receive monthly BLL tests through the company’s biomonitoring program, which is required under the OSHA lead standard.5 We calculated each employee’s mean, median, and maximum BLLs by year, during 2012-2016, and calculated the number and proportion of employees with at least 1 BLL record greater than or equal to the following thresholds:
60 μg/dL: the maximum BLL at which OSHA requires temporary removal from lead exposure (referred to subsequently as the maximum medical removal requirement)5
40 μg/dL: BLL at which OSHA allows workers to return to work after temporary medical removal from lead exposure5
20 μg/dL: a health-based recommendation for removal of a worker from sources of lead exposure supported by the Council of State and Territorial Epidemiologists and other medical and public health organizations2,16
5 μg/dL: National Institute for Occupational Safety and Health (NIOSH) ABLES reference BLL for adults (adopted in November 2015)4
Environmental Investigation and Employee Interviews
We reviewed company-provided personal (ie, sampled in a worker’s airspace) airborne lead levels collected during 12-hour shifts in March 2016 (n = 14). These levels included at least 1 sample for each shift, job classification, and work area, consistent with OSHA requirements. We compared these levels with the OSHA action level (30 μg/m3 8-hour time-weighted average [TWA]) and PEL (50 μg/m3 8-hour TWA). Because company air samples were collected during 12-hour shifts, we applied the OSHA reduction adjustment to the PEL (maximum permissible limit [in ug/m3] = 400 divided by hours worked in the day).5 We also reviewed the facility’s written standard operating procedures, including those guiding the use of personal protective equipment (PPE) and personal hygiene procedures. We toured the facility and observed employees at work.
Finally, using a structured questionnaire, we attempted to interview all current employees to collect information about employment history, PPE use, personal hygiene practices, and symptoms. The company allowed workers to participate in interviews at the facility during their shifts. We calculated the frequencies of employee responses to interview questions. We tabulated results for employees working in the manufacturing area for whom PPE and hygiene requirements apply. CDC reviewed this investigation for human subjects protection and deemed it to be non-research.
Results
During 2012-2016, 77 facility employees (mean of 33 employees per year) and 17 associated children had at least 1 BLL reported to a surveillance system. Five of the 17 associated children had a confirmed BLL ≥5 μg/dL during the study period; all were reported during or after 2014. Of these 5 children, 3 had BLLs confirmed at 5-9 μg/dL and lived in separate households, and 2 had BLLs confirmed at 10-19 μg/dL and were living in the same household. The homes of all 5 children were investigated, and take-home exposure was confirmed as the likely source. Three household members from 1 home opted for BLL testing at the local health department; no additional children with elevated BLLs were identified through the health department.
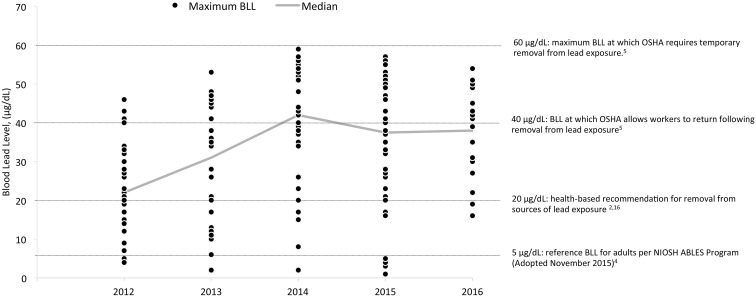
During 2012-2016, maximum BLLs remained below 60 μg/dL (Figure). The median of these values increased from 22 μg/dL (range, 4-45 μg/dL) in 2012 to 42 μg/dL (range, 2-59 μg/dL) in 2014; in 2016, the median of these values was 37 μg/dL (range, 16-54 μg/dL). Of the 77 employees, 33 (43%) had at least 1 BLL ≥40 μg/dL, 54 (70%) had at least 1 BLL ≥20 μg/dL, and 70 (91%) had at least 1 BLL ≥5 μg/dL.
Figure.
Distribution of blood lead level (BLL) measurements (n = 172) for 77 employees at a lead oxide manufacturing facility in North Carolina in relation to regulatory and health-based thresholds, 2012-2016. Each dot represents the maximum BLL for a single employee for that year; some employees have a BLL in more than 1 year. Abbreviations: ABLES, Adult Blood Lead Epidemiology and Surveillance; CSTE, Council of State and Territorial Epidemiologists; NIOSH, National Institute for Occupational Safety and Health; OSHA, Occupational Safety and Health Administration.
We found little variation in BLLs among employees with the same job title. However, average maximum BLLs for managers, foremen, and operators who worked in the manufacturing area consistently ranged from 40 to 59 μg/dL. Average maximum BLLs for truck drivers and others who did not regularly work in the manufacturing area were lower (10-39 μg/dL).
Environmental Investigation and Employee Interviews
Facility description
The facility included areas considered clean, meaning free from contamination (ie, administrative offices, break room, and clean locker room), and areas considered dirty (ie, dirty locker room, quality assurance laboratory, manufacturing area, and baghouse). The manufacturing area was a single open room containing 3 manufacturing lines. Employees operated the lines 24 hours per day, 7 days per week, with no shutdown for routine maintenance. We observed multiple open sources of exposure to lead fumes and dust, including open oven doors and no ventilated enclosures for dust- or fume-generating tasks.
Air sampling results
Samples of personal airborne lead levels ranged from 5 to 218 μg/m3 per 12-hour TWA; the comparable PEL for a 12-hour shift is 33.3 μg/m3 TWA. Of the 14 samples, 10 exceeded OSHA’s action level and PEL, requiring the company to provide additional controls, including PPE and personal hygiene protocols and facilities, to reduce worker exposure down to the PEL.
PPE use and personal hygiene
We observed that the company provides employees with air-purifying respirators and NIOSH-approved high-efficiency particulate air (HEPA) P-100 cartridges, uniforms and steel-toed boots, safety glasses or goggles, and multiple types of gloves depending on the job and worker preference. The facility is equipped with showers and separate dirty and clean locker rooms. In addition, the company provides off-site uniform laundering. However, employees were unaware of written instructions guiding the use and maintenance of PPE or hygiene procedures, which resulted in inconsistencies in adherence to exposure controls.
At the time of interview, 26 people were employed at the facility. Of 21 employees working in the manufacturing area, we interviewed 18 employees. The median age of participating employees was 43 years (range, 20-57 y), and the median length of employment was 1 year (range, <1-20 y); 7 employees had worked at the facility for >5 years. All 18 interviewed employees reported receiving respirator use training and fit testing; however, only 17 reported always wearing their respirator, 11 reported cleaning their respirator at least daily, and 5 reported changing their respirator cartridges at least daily. During interviews, we observed facial hair on multiple employees, which could interfere with a proper respirator seal (Table).
Table.
Number of manufacturing employees reporting adherence to occupational and take-home exposure control measures at a lead oxide manufacturing facility in North Carolina, 2016
| Control Measures | No. of Respondents (n = 18) |
|---|---|
| Occupational exposure control | |
| Always wears a respirator | 17 |
| Cleans respirator at least daily | 11 |
| Changes respirator cartridges at least daily | 5 |
| Personal protective equipment (always use) | |
| Uniform pants | 18 |
| Boots | 18 |
| Uniform shirt (sleeves rolled down) | 12 |
| Gloves | 12 |
| Glasses | 11 |
| During breaks, before entering clean areas of the facility, do you always…? | |
| Wash hands | 18 |
| Remove dust from clothes with high-efficiency particulate air (HEPA) vacuum | 16 |
| Clean boots | 15 |
| Wear booties | 7 |
| Shower | 4 |
| Change clothes | 4 |
| Take-home exposure controls | |
| Before leaving work to go home, do you always…? | |
| Shower | 18 |
| Change clothes | 18 |
| Change boots | 18 |
| Leave work clothes at work | 18 |
| Change into clean shoes before getting in car | 12 |
| Refrain from carrying personal items home from work | 0 |
Of the 18 employees interviewed, 17 reported always wearing a company-provided uniform and boots while working; however, only 12 reported always wearing their sleeves rolled down, 12 reported always wearing gloves, and 11 reported always wearing glasses while working (Table). Interviewees explained that high temperatures in the manufacturing area were the main barrier to wearing PPE consistently and correctly.
Before entering clean areas of the facility during breaks, all interviewed employees reported washing their hands, whereas 16 reported removing dust from clothes with a HEPA vacuum, 15 reported using a boot cleaner, 7 reported donning disposable booties, 4 reported showering, and 4 reported changing clothes (Table). While visiting the facility, we observed employees enter areas considered clean directly from the manufacturing area without completing cleanup activities. We also observed employees exiting the facility during breaks to visit their cars without cleaning up.
High levels of airborne lead and dust in the manufacturing area and poor adherence to hygiene practices resulted in visible contamination in areas considered clean, including the break room, administrative offices, and clean locker room. All interviewed employees reported carrying personal items such as cell phones and bags into work and then carrying them home (Table). Only 12 employees reported changing out of shoes they wore in contaminated areas of the facility before getting into their cars after work.
All interviewed employees reported at least 1 symptom that is associated with lead exposure; however, symptoms were nonspecific and could not be linked with lead exposure.
Discussion
By using data collected from adult and child statewide BLL surveillance systems, we identified a preventable source of lead exposure among lead oxide manufacturing facility employees and children living in their households. High levels of airborne lead and multiple deficiencies in exposure controls at the facility resulted in lead overexposure among employees and visible contamination of facility areas that were considered clean. These conditions provided opportunities for unintentionally carrying lead dust to employees’ homes and exposing household members to lead. Our findings serve as an additional reminder that occupational lead exposure is an important and preventable public health concern, and additional action is needed to protect workers and their families.
During the past few decades, environmental sources of lead have been reduced in the United States. As a result, BLLs among the US population have declined dramatically. For example, the percentage of children aged <6 years with a BLL ≥5 μg/dL decreased from 26% during 1988-1994 to 2% during 2007-2014.17 Despite these improvements, occupational sources of lead exposure are an important public health problem18; 95% of all elevated BLLs among adults in the United States have a work-related source.18,19 Research has associated take-home lead exposure among children with workers in multiple industries, including lead smelting,6 battery production and recycling,7,11 boatyard work,8 work in radiator shops,12 construction site work,9 oil field work,13 and work in electronic scrap recycling locations.10
In this investigation, we found that a lead oxide manufacturer, although in regulatory compliance with airborne lead and BLL monitoring requirements dictated by the OSHA lead standard, lacked adequate exposure controls to protect employees from lead overexposure. The company relied heavily on PPE and personal hygiene practices to limit exposure; however, those methods were inconsistently adhered to and less effective than engineering controls, such as improvements in ventilation and covering of open lead sources. They also placed responsibility for reducing exposure primarily on the worker and less on the employer.20 Over time, a lack of effective controls resulted in visible contamination of facility areas that were considered clean, providing the opportunity for contamination of workers’ clothes, skin, hair, and personal items after shifts and cleanup. Currently, the OSHA lead standard does not specify a regulatory limit for surface lead dust; therefore, routine monitoring of surface contamination is not required.
Although the prevalence of elevated BLLs has declined in recent years,19 regulatory standards have remained the same since the development of the OSHA lead standard in the 1970s.2 When the standard was developed, the geometric mean BLL among adults in the general US population was 12.8 μg/dL, and the health effects of low-level lead exposure were not well understood.1 Today, the geometric mean BLL among adults in the United States is 0.967 μg/dL, and research continues to find associations between BLLs as low as 5 μg/dL and harmful health effects.2,3 Updated science has led NIOSH’s ABLES program to reduce adult reference levels, most recently from 10 μg/dL to 5 μg/dL in November 2015.4 In addition, multiple public health and medical organizations have recommended alternative medical-management criteria for lead-exposed workers. These recommendations call for removal of workers from lead exposure based on their BLL (medical removal) at lower BLLs (≥20 μg/dL) than the OSHA standard dictates (≥50-60 μg/dL) and encourage OSHA to review and revise the standard to reflect today’s science.1,2,16 Specifically, recommendations stipulate that a revised standard include more protective action levels; PELs for both airborne and surface lead; more stringent requirements for PPE, personal hygiene, and training; more stringent medical removal protection requirements; and enhanced medical surveillance.1,2 Implementation of an updated lead standard or industry-driven initiatives to protect employees’ health that address these recommendations could have reduced the burden of lead exposure among employees and children identified in this investigation.
Limitations
Our investigation had several limitations. First, only employee BLLs are consistently monitored by the company per the OSHA lead standard requirements, resulting in challenges to quantifying the true burden of lead exposure among household members. The 5 children with confirmed elevated BLLs are likely an underestimate of those exposed to take-home lead. Furthermore, we were unable to document take-home lead exposure in adult household members because BLL testing is conducted infrequently among adults not employed in a lead-related industry. Take-home lead exposure among pregnant or breastfeeding women would be of greatest concern,1 and at least 1 employee reported a pregnant household member. In addition, some degree of recall and reporting bias might have influenced information reported during employee interviews, potentially resulting in an overestimate of the proportion of workers adhering to protective behaviors. We were also unable to interview former employees. If former employees had different exposure experiences than current employees, we might have missed identifying other areas for improvement.
Public Health Implications
This investigation illustrates the utility of BLL surveillance activities in identifying nonresidential point sources of lead exposure. In addition to detecting this source of exposure, BLL surveillance data allowed rapid characterization of the magnitude and duration of exposure among facility employees and identification of associated children. Together, these surveillance data provided the necessary evidence to support additional public health action to prevent continued exposure. Because of this investigation, the North Carolina DPH improved its ability to identify and respond to occupational and take-home lead exposure. To more systematically identify and track occupational sources of child lead exposure, in 2017 we added dedicated data fields to the NC LEAD surveillance system that allow for an occupational source to be uniformly documented. These fields alert the program to potential point source clusters that require investigation. North Carolina DPH adult and child BLL surveillance coordinators now communicate regularly about potential occupational exposure sources and created a standard operating procedure to guide responses to take-home lead exposure.
Because of our findings, the North Carolina DPH provided extensive verbal and written recommendations to the manufacturing facility. The department continues to provide technical assistance to facility employees and management. It also assisted the company in requesting technical consultation from the North Carolina Department of Labor Consultative Unit and in requesting support from the company’s corporate office to create a safer work environment. Finally, the North Carolina DPH and the local health department continue to track the company’s employee and child BLLs quarterly and communicate with management and regulatory partners about BLL trends over time.
Conclusions
Our findings illustrate that when lead exposures are not well controlled in the workplace, workers and their families can be overexposed to lead, placing them at higher risk for adverse health outcomes. The integration of adult and child BLL surveillance can identify occupational sources of lead exposure and guide public health actions. Furthermore, current regulatory standards for occupational lead exposure should be reviewed in the context of current science to enhance protections for workers and their families.
Acknowledgments
We thank all local health department staff members who assisted with this investigation, especially Mr. Marlon Hunter and the Forsyth County Environmental Health staff. We also thank the following people for their assistance: Stacey Bosch, Gregory Dang, Tena Hand, Kate Koehler, David Lipton, Ed Norman, Mina Shehee, Kelly Squires, and Lauren Thie.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kosnett MJ, Wedeen RP, Rothenberg SJ, et al. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115(3):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holland MG, Cawthon D; ACOEM Task Force on Blood Lead Levels. Workplace lead exposure. J Occup Environ Med. 2016;58(12):e371–e374. [DOI] [PubMed] [Google Scholar]
- 3. National Toxicology Program. NTP Monograph: Health Effects of Low-Level Lead. Research Triangle Park, NC: National Institute of Environmental Health Sciences, National Toxicology Program; 2012. [Google Scholar]
- 4. Centers for Disease Control and Prevention. Adult Blood Lead Epidemiology and Surveillance (ABLES). Updated May 2018 https://www.cdc.gov/niosh/topics/ables/description.html. Accessed August 12, 2018.
- 5. Occupational Safety and Health Act of 1970. 29 CFR 1910.1025–Lead. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1025. Accessed September 5, 2018.
- 6. Roscoe RJ, Gittleman JL, Deddens JA, Petersen MR, Halperin WE. Blood lead levels among children of lead-exposed workers: a meta-analysis. Am J Ind Med. 1999;36(4):475–481. [DOI] [PubMed] [Google Scholar]
- 7. Garcia BR, Rullan J, O’Neill M, et al. Take-home lead exposure among children with relatives employed at a battery recycling facility—Puerto Rico, 2011 [published erratum appears in MMWR Morb Mortal Wkly Rep. 2012;61(49):1012]. MMWR Morb Mortal Wkly Rep. 2012;61(47):967–970. [PubMed] [Google Scholar]
- 8. Thanapop C, Geater AF, Robson MG, Phakthongsuk P, Viroonudomphol D. Exposure to lead of boatyard workers in southern Thailand. J Occup Health. 2007;49(5):345–352. [DOI] [PubMed] [Google Scholar]
- 9. Whelan EA, Piacitelli GM, Gerwel B, et al. Elevated blood lead levels in children of construction workers. Am J Public Health. 1997;87(8):1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newman N, Jones C, Page E, Ceballos D, Oza A. Investigation of childhood lead poisoning from parental take-home exposure from an electronic scrap recycling facility—Ohio, 2012. MMWR Morb Mortal Wkly Rep. 2015;64(27):743–745. [PMC free article] [PubMed] [Google Scholar]
- 11. Daniell WE, Van Tung L, Wallace RM, et al. Childhood lead exposure from battery recycling in Vietnam. Biomed Res Int. 2015;2015: 193715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dykeman R, Aguilar-Madrid G, Smith T, et al. Lead exposure in Mexican radiator repair workers. Am J Ind Med. 2002;41(3):179–187. [DOI] [PubMed] [Google Scholar]
- 13. Khan F. Take home lead exposure in children of oil field workers. J Okla State Med Assoc. 2011;104(6):252–253. [PubMed] [Google Scholar]
- 14. North Carolina Childhood LEAD Surveillance System. Children’s environmental health. Updated July 2017 http://ehs.ncpublichealth.com/hhccehb/cehu/lead/nclead.htm. Accessed August 12, 2018.
- 15. North Carolina Department of Health and Human Services. Occupational & environmental epidemiology: adult blood lead. Updated August 2018 http://epi.publichealth.nc.gov/oee/programs/ables.html. Accessed August 12, 2018.
- 16. Council of State and Territorial Epidemiologists. Management guidelines for blood lead levels in adults. 2013. http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/OccupationalHealth/ManagementGuidelinesforAdult.pdf. Accessed August 12, 2018.
- 17. Franco SJ, Koehrn K, Axelrad D. QuickStats: percentage of children aged 1-5 years with elevated blood lead levels, by race/ethnicity—National Health and Nutrition Examination Survey, United States, 1988-1994, 1999-2006, and 2007-2014. MMWR Morb Mortal Wkly Rep. 2016;65(39):1089. [DOI] [PubMed] [Google Scholar]
- 18. Alarcon WA, Graydon JR, Calvert GM. Adult blood lead epidemiology and surveillance—United States, 2008-2009. MMWR Morb Mortal Wkly Rep. 2011;60(25):841–845. [PubMed] [Google Scholar]
- 19. Alarcon WA. Elevated blood lead levels among employed adults—United States, 1994-2012. MMWR Morb Mortal Wkly Rep. 2015;62(54):52–75. [DOI] [PubMed] [Google Scholar]
- 20. National Institute for Occupational Safety and Health. Hierarchy of controls. Updated May 2018 https://www.cdc.gov/niosh/topics/hierarchy. Accessed August 12, 2018.