Summary
Infectious disease (ID) consultations were associated with significantly lower mortality, more lumbar punctures, and treatment with amphotericin and flucytosine. This suggests that ID consultations should be included in management of cryptococcosis.
Keywords: Cryptococcus, infectious disease consult, prognosis, mortality, therapy.
Abstract
Background.
An infectious disease (ID) consultation (consult) is often obtained to treat patients with cryptococcosis due to the complex nature of the disease, but has never been demonstrated to impact outcomes.
Methods.
We assembled a retrospective cohort of 147 consecutive cases of cryptococcosis in patients without human immunodeficiency virus. Patients who were diagnosed <24 hours prior to death were excluded. Survival analysis was performed with Cox regression with survival censored past 90 days.
Results.
The patients with an ID consult had a higher fungal burden but a lower 90-day mortality compared with patients without ID involvement (27% vs 45%; P < .001), with an adjusted hazard ratio of not receiving an ID consult of 4.2 (95% confidence interval, 2.2–7.6). The ID consult group was more likely to receive an indicated lumbar puncture (86% vs 32%; P < .001), and more likely to be treated with amphotericin B (AmB) (87% vs 24%; P < .001) and flucytosine (5-FC) (57% vs 16%; P < .001) when indicated. The duration of therapy with AmB (14 vs 11 days; P = .05) and 5-FC (7.5 days vs 1 day; P < .001) was longer in the ID consult group.
Conclusions.
Patients who received an ID consult were significantly less likely to die in the 90 days following diagnosis. Patients seen by ID physicians were more likely to be managed according to evidence-based practice established by randomized controlled trials and published in Infectious Diseases Society of America guidelines. These data suggest that an ID consult should be an integral part of clinical care of patients with cryptococcosis.
Cryptococcus is one of the most common opportunistic fungal infections in humans. In the United States, cryptococcal disease is more common in patients without human immunodeficiency virus (HIV) infection [1, 2]. Several studies have shown a predisposition toward lower mortality in cryptococcal patients with HIV [1–3], and worse outcomes in cryptococcal non–solid organ transplant (SOT) patients who are HIV-uninfected [1, 2]. Treatment consistent with Infectious Diseases Society of America (IDSA) guidelines has been associated with improved outcomes in cryptococcal patients, both in selection of induction treatment [4] and management of opening pressure [5]. An important determinant of outcomes in patients with cryptococcosis is the presence of disseminated disease, defined as cryptococcemia (positive blood cultures) and/or meningitis [6]. In addition, the recommendation for treatment of severe pulmonary disease is similar to disseminated disease [6]. Early and aggressive therapy for disseminated and moderate to severe pulmonary cryptococcosis included amphotericin B (AmB) plus flucytosine (5-FC) in several studies [4, 6–15]. Patients who have mild to moderate pulmonary infection, such as solitary nodule or pneumonia with mild symptoms, can be treated with fluconazole alone [6].
Infectious disease (ID) consultation (hereafter, “consult”) has been associated with lower mortality in patients with Staphylococcus aureus bacteremia [16–18] and candidemia [19]. Given the complexity and rarity of cryptococcal infection, we were interested in evaluating whether ID consultation was associated with a difference in outcomes in patients with cryptococcal infection. We also evaluated whether management recommended by ID compared to other physicians was more likely to follow evidence-based guidelines [6].
METHODS
Cohort Construction
Data were collected from patients with cryptococcal infection admitted to Barnes-Jewish hospital, a 1315-bed tertiary care academic hospital located in an urban environment, with a significant suburban and rural referral base. The study was approved by the Washington University (St Louis, Missouri) Human Research Protection Office with a waiver of informed consent.
Patients diagnosed with cryptococcosis from 1 January 2002 to 1 February 2015 were identified by either a positive cryptococcal antigen (CrAg) or culture positive for Cryptococcus species. Latex agglutination was used exclusively for all serum and central nervous system (CSF) CrAg tests. Histopathology, if available, was used to define the site of disease. A standardized data collection tool was used to collect data on demographics, survival, predisposing factors, presentation, laboratory values, treatment, and whether an ID consult was obtained.
Patients in whom the diagnosis of cryptococcosis was made posthumously or <24 hours before death were excluded from analysis, as an ID consult could not have been reasonably obtained. In addition, patients who lacked sufficient clinical data in the medical record, had infection with non-neoformans species of Cryptococcus, or had a false-positive CrAg result (titer <1:8 that did not confirm on repeat test, and patient improved without treatment) were excluded from analysis. Because all HIV-infected patients in our hospital received an ID consult, and their outcomes, presentation and management were significantly different, they were excluded from the analysis. The remaining patients were classified into 2 groups, those who received an ID consult and those that did not.
Sites of infection and associated predisposing factors were defined as previously described [2]. The Elixhauser comorbidity classification was used to control for underlying health conditions, as it has been shown to be a good predictor of death in acute illness [20].
Indications for a lumbar puncture (LP) and recommended treatment with AmB and 5-FC were defined based on the IDSA cryptococcal guidelines and recent review [6, 21]. LP was deemed appropriate if the patient had any of the following: (1) evidence of disseminated disease such as skin lesions, cryptococcemia, or Cryptococcus isolated from a normally sterile source; (2) positive serum CrAg with titer of ≥1:8; (3) symptoms indicative of central nervous system (CNS) disease (ie, headache, stiff neck, altered mental status, visual changes, or cranial nerve palsy); or (4) evidence of significant immunocompromise, specifically SOT, bone marrow transplant, hematologic malignancy, recent receipt of cytotoxic chemotherapy, end-stage liver disease, and receipt of immuncompromising medications.
Induction therapy with AmB and 5-FC was deemed appropriate in the presence of any of the following: (1) severe immunocompromise, defined as the same immunocompromising conditions indicating an LP; (2) severe pulmonary disease, defined as need for supplement oxygenation; (3) disseminated disease, as defined by CNS disease, cryptococcemia, or other extrapulmonary sites of infection; or (4) a high cryptococcal burden, defined by a serum CrAg titer of ≥1:512 [6].
Dates of death were extracted from the hospital consortium’s Medical Informatics database and supplemented with information from the Social Security Death Index (SSDI). Patients diagnosed with cryptococcal infection <90 days prior to the last available date in the SSDI (28 February 2014) who were not observed in our institution after the 90-day postdiagnosis period were censored at the date of last visit.
Attributable mortality was defined as the presence of 1 or more of the following: (1) an autopsy where the pathologist concluded the cause of death to be cryptococcus; (2) documented ongoing cryptococcal CNS disease and evidence of herniation; (3) multiorgan failure in the setting of ongoing cryptococcal infection within 14 days after diagnosis, and without evidence of another infection; (4) respiratory failure in the setting of cryptococcal pneumonia.
Statistical Analysis
Statistical analysis was performed using SPSS software version 23 (IBM, Armonk, New York). Fisher exact and Student t test or Mann-Whitney U test were used for descriptive statistics. A Cox proportional hazards model was used to evaluate the association of predisposing factors, comorbidities, location of disease, and antigen levels on all-cause and attributable mortality. Censoring was performed at 90 days, as mortality beyond 90 days was deemed less likely to be related to cryptococcal infection. For the initial multivariable Cox model, all variables with P < .2 were considered for entry in the model. The proportional hazards assumption for the multivariable model was assessed visually. Where this assumption was violated, we used a heavy-side step function to model the time interaction [22]. The final model was built with the significant variables in a parsimonious manner, and the c-statistic was computed. All statistical tests were 2-tailed and significance was set at α = .05.
RESULTS
Cohort
Two hundred fifty-eight patients met the initial inclusion criteria; 91 patients were excluded because they were HIV infected. An additional 20 patients were excluded because they lacked sufficient clinical data in the medical record (n = 5); had false-positive antigen tests (n = 3), died within 24 hours of diagnosis or were diagnosed postmortem (n = 5); or were infected by a non-neoformans species of Cryptococcus (n = 7). This included 2 cases of C. laurentii, and 1 case each of C. luteolus, C. albidus, C. uniguttulatus, C. humicolus, and 1 unidentifiable non-neoformans Cryptococcus species.
One hundred (68%) patients with an ID consult and 47 (32%) patients without an ID consult were included in subsequent analyses. Patients with an ID consult had a similar age as those who did not receive an ID consult (59.1 vs 59.6 years, respectively; P = .87). Most patients were male, and sex, race, and time to diagnosis were similar in the 2 groups (Table 1). The distributions of predisposing factors were comparable in the ID and no consult groups, in all categories other than SOT, which was more common in the non-ID consult group (Table 1).
Table 1.
Baseline Characteristics of 147 Human Immunodeficiency Virus-Uninfected Patients With Cryptococcosis by Receipt of Infectious Disease Consultation, 2002–2015
| Characteristic | ID Consult (n = 100) |
No ID Consult (n = 47) |
P Value | Total Cohort (N = 147) |
|---|---|---|---|---|
| Age, y, mean (SD) | 59.1 (13.8) | 59.6 (13.8) | .87 | 59.2 (15.5) |
| Time from admission to diagnosis, d, mean (SD) | 6.2 (11.6) | 4.8 (10.6) | .48 | 5.8 (11.2) |
| Male sex | 68 (68) | 33 (70) | .79 | 101 (69) |
| Race | ||||
| White | 78 (78) | 40 (85) | b | 118 (80) |
| African American | 12 (12) | 6 (13) | .13 | 18 (12) |
| Other | 10 (10) | 1 (2) | .17 | 11 (75) |
| Site of infection | ||||
| CNS | 57 (57) | 5 (11) | <.001 | 62 (42) |
| Pulmonary | 27 (27) | 30 (64) | <.001 | 57 (39) |
| Bloodstream | 41 (41) | 17 (36) | .58 | 58 (40) |
| Other | 8 (8) | 2 (4) | .78 | 10 (7) |
| Disseminated disease | 79 (79) | 23 (49) | <.001 | 102 (69) |
| Positive serum CrAg | 86 (86) | 22 (47) | <.001 | 108 (74) |
| Serum CrAg titer | ||||
| 0 | 19 (19) | 24 (51) | b | 39 (27) |
| 1:2–1:8 | 6 (6) | 8 (17) | .001 | 14 (10) |
| 1:16–1:512 | 60 (60) | 13 (28) | .093 | 73 (50) |
| ≥1:1204 | 18 (18) | 2 (4) | .22 | 20 (14) |
| Positive CSF CrAga | 60/79 (75) | 5/12 (42) | .002 | 65 (71) |
| CSF CrAg titera | ||||
| 0 | 19/79 (24) | 7/12 (58) | b | 28 (31) |
| 1:2–1:8 | 18/79 (23) | 2/12 (17) | .99 | 19 (21) |
| 1:16–1:512 | 26/79 (33) | 3/12 (25) | .99 | 28 (31) |
| ≥1:1024 | 16/79 (20) | 0/12 (0) | .99 | 16 (18) |
| Associated predisposing factors | ||||
| None | 24 (24) | 10 (21) | .72 | 34 (23) |
| Chemotherapy | 22 (22) | 9 (19) | .69 | 31 (21) |
| Hematologic malignancy | 18 (18) | 7 (15) | .64 | 25 (17) |
| Diabetes mellitus | 22 (22) | 8 (17) | .49 | 30 (20) |
| End-stage renal disease | 6 (6) | 3 (6) | .93 | 9 (6) |
| End-stage liver disease | 12 (12) | 10 (21) | .14 | 22 (15) |
| Solid organ transplant | 12 (12) | 12 (26) | .038 | 24 (16) |
| Glucocorticoid therapy | 33 (33) | 14 (30) | .70 | 47 (32) |
| Biologic therapy | 7 (7) | 5 (11) | .45 | 12 (8) |
| Other immunosuppressants | 15 (15) | 10 (21) | .35 | 25 (17) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CNS, central nervous system; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; ID, infectious disease; SD, standard deviation.
Percentages were calculated out of 91 that received lumbar puncture, 79 with and 12 without ID consult.
Reference category.
Patients receiving ID consult appeared to have more-severe forms of cryptococcosis. Patients with an ID consult were significantly less likely to have pulmonary disease (27% vs 64%), but more likely to have cryptococcal meningitis (57% vs 11%). The presence of cryptococcemia and infection at other sites were similar between the 2 groups. The ID consult patients were more likely to have a positive CrAg in both serum and CNS, and also had higher antigen titers than patients in the non-ID consult group (Table 1), consistent with a higher burden of disease.
Management
Patients receiving ID consult were significantly more likely to have appropriate performance of an initial diagnostic LP (86% vs 32%), but were there was no difference between the 2 groups in inappropriate performance of an LP (25% vs 30%) (Table 2).
Table 2.
Management of 147 Patients With Cryptococcosis by Receipt of Infectious Disease Consultation, 2002–2015
| Treatment | ID Consult (n = 100) |
No ID Consult (n = 47) |
P Value | Total Cohort (N = 147) |
|---|---|---|---|---|
| LP performed when indicated | 79/92 (86) | 12/37 (33) | <.001 | 91/129 (71) |
| LP performed when not indicated | 2/8 (25) | 3/10 (30) | .81 | 5/18 (27.8) |
| No. of LPs performed among those who had at least 1, median (range) | 2 (1–18) | 1 (1–3) | .048 | 2 (1–18) |
| Neurosurgical intervention for ICP managementa | 8 (8) | 0 (0) | .042 | 8 (5.4) |
| AmB administered when indicated | 81/93 (87) | 11/45 (24) | <.001 | 76/138 (55) |
| Duration of AmB therapy when indicated, d, median (IQR) | 14 (16) | 11 (9) | .050 | 14 (14.5) |
| 5-FC administered when indicated | 53/93 (57) | 7/45 (16) | <.001 | 60/138 (44) |
| Duration of 5-FC therapy when indicated, d, median (IQR) | 7.5 (13) | 1 (1) | <.001 | 4 (14) |
Data are presented as No. (%) unless otherwise indicated. Among the patients without an indication for AmB and 5-FC, none received either AmB or 5-FC.
Abbreviations: 5-FC, flucytosine; AmB, amphotericin B; ICP, intracranial pressure; ID, infectious disease; IQR, interquartile range; LP, lumbar puncture.
Includes ventriculoperitoneal shuts and external drains.
ID consult patients were also significantly more likely to be started on appropriate AmB therapy (87% vs 24%). The duration of AmB therapy was significantly longer in the ID consult than non-ID consult group (14 vs 11 days). ID consult patients were also more significantly likely to receive 5-FC when indicated (57% vs 16%) and for a longer duration than the comparison group (7.5 days vs 1 day). No patient in either group received AmB or 5-FC if they could have been treated with fluconazole.
The ID consult group had more aggressive management of intracranial pressure (ICP), through the use of repeat LPs and neurosurgical interventions for ICP management (eg, lumbar drains or ventriculoperitoneal shunts) (Table 2).
Mortality
By 90 days, 27 patients (27%) had died in the ID consult group, compared with 21 (45%) in the group without ID consult. The hazard ratio (HR) for mortality in the ID consult group was 0.49 (95% confidence interval [CI], .28–.87) (Table 3). Only 3 out-of-state patients had a survival status that could not be definitively assessed at 90 days. All 3 were in the ID consult group, and their survival was censored at 23, 39, and 41 days. Several other factors were significantly associated with mortality in univariate analysis (Table 3). The variables from the Elixhauser comorbidity index are reported in Supplementary Table 1.
Table 3.
Univariate Analysis of Mortality Within 90 Days in 147 Human Immunodeficiency Virus-Uninfected Patients With Cryptococcosis
| Variable | No. (%) of Patients Who Died (n = 48) | No. (%) of Patients Who Survived (n = 99) | Hazard Ratio (95% CI) |
P Value |
|---|---|---|---|---|
| ID consultation | 27 (56) | 73 (73) | 0.49 (.28–.87) | .014 |
| Age >55 y | 40 (83) | 48 (49) | 3.9 (1.9–8.5) | <.001 |
| Male sex | 38 (79) | 63 (64) | 1.8 (.9–3.7) | .086 |
| Race | ||||
| White | 39 (81) | 79 (80) | b | |
| African American | 8 (17) | 10 (10) | 1.6 (.7–3.4) | .23 |
| Other | 1 (2) | 10 (10) | 0.25 (.4–1.8) | .170 |
| Site of infection | ||||
| Central nervous system | 17 (35) | 45 (46) | 0.70 (.4–1.2) | .25 |
| Pulmonary | 17 (35) | 40 (4) | 0.9 (.5–1.6) | .62 |
| Bloodstream | 27 (56) | 31 (31) | 2.4 (1.4–4.3) | .003 |
| Other | 3 (6) | 7 (7) | 0.9 (.3–2.8) | .82 |
| Disseminated disease | 39 (81) | 63 (64) | 2.2 (1.1–4.5) | .036 |
| Positive serum CrAg | 43 (90) | 65 (66) | 3.6 (1.4–9.2) | .006 |
| Serum CrAg titer | ||||
| 0 | 3 (6) | 34 (34) | b | |
| 1:1–1:8 | 4 (8) | 7 (7) | 5.3 (1.2–23.8) | .028 |
| 1:16–1:512 | 31 (65) | 48 (49) | 5.9 (1.8–19.2) | .003 |
| ≥1:1204 | 10 (21) | 10 (10) | 8.5 (2.3–30.8) | .001 |
| Positive CSF CrAg | 16 (64) | 44 (69) | 0.9 (.4–2.0) | .80 |
| CSF CrAg titera | ||||
| 0 | 9 (36) | 20 (30) | b | .44 |
| 1:2–1:8 | 4 (16) | 16 (24) | 0.7 (.2–2.3) | .57 |
| 1:16–1:512 | 6 (24) | 22 (33) | 0.7 (.3–2.0) | .51 |
| ≥1:1024 | 6 (24) | 8 (12) | 1.7 (.6–4.0) | .34 |
| Predisposing factors | ||||
| None | 6 (13) | 28 (28) | 0.41 (.17–.96) | .039 |
| Chemotherapy | 18 (38) | 13 (13) | 2.7 (1.5–4.8) | .001 |
| Hematologic malignancy | 12 (25) | 13 (13) | 1.9 (.99–3.6) | .055 |
| End-stage renal disease | 3 (6) | 6 (6) | 0.97 (.30–3.1) | .97 |
| End-stage liver disease | 13 (27) | 9 (9) | 3.0 (1.6–5.8) | .001 |
| Solid organ transplant | 4 (8) | 20 (20) | 0.41 (.14–1.1) | .088 |
| Glucocorticoid therapy | 12 (25) | 35 (35) | 0.66 (.34–1.3) | .21 |
| Biologic therapy | 3 (6) | 9 (9) | 0.65 (.20–2.1) | .47 |
| Other immunosuppressive therapy | 7 (15) | 18 (18) | 0.77 (.35–1.7) | .52 |
Abbreviations: CI, confidence interval; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; ID, infectious disease; y, year.
Percentages were calculated of 91 that received lumbar punctures.
Reference category.
The association between age and mortality did not follow a linear relationship and was included in the multivariable model as a dichotomous variable. CSF CrAg levels were not significantly associated with mortality in univariate analysis, but serum levels were significantly associated with mortality, although the linearity of this association could not be assessed due to wide confidence intervals. A category of “disseminated disease,” defined as patients who had any disease other than localized pneumonia, was associated with significantly increased risk of mortality (Table 3).
In the final multivariable model, 5 factors were significantly associated with 90-day mortality (Table 4). ID consult was associated with significantly lower mortality, with a HR of 0.24 (95% CI, .13–.45). Age >55 years, end-stage liver disease, positive cryptococcal antigen, and recent receipt of chemotherapy were also significantly associated with increased mortality in the multivariable model. When age was modeled in 5-year increments to assess for residual confounding, the HR for ID consult changed by <10% (HR, 0.23 [95% CI, .12–.45]). The C statistic of the final multivariable model was 0.785.
Table 4.
Multivariable Analysis of All-Cause Mortality Within 90 Days in 147 Human Immunodeficiency Virus-Uninfected Patients With Cryptococcosis
| Variable | Hazard Ratio | (95% CI) | P Value |
|---|---|---|---|
| ID consultation | 0.24 | (.13–.45) | <.001 |
| Age >55 y | 3.2 | (1.5–7.2) | .003 |
| Positive serum CrAg | 5.7 | (2.1–15.5) | .001 |
| End-stage liver disease | 2.4 | (1.2–4.6) | .011 |
| Receipt of chemotherapya | 2.1 | (1.1–3.9) | .020 |
Abbreviations: CI, confidence interval; CrAg, cryptococcal antigen; ID, infectious disease.
Receipt of chemotherapeutic agent within 90 days.
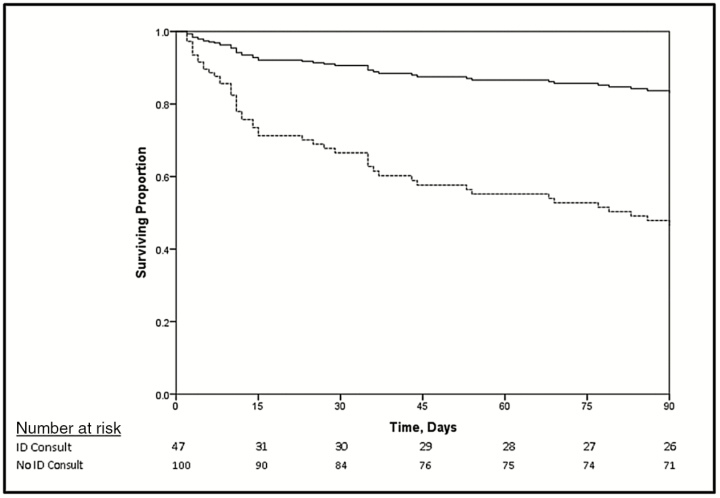
The difference in survival by ID consult status was most dramatic in the first 15 days, but persisted up until day 90 (Figure 1). In a Cox model with an interaction of ID consult and time, ID consult was significantly associated with lower mortality through day 15 after diagnosis (HR, 0.15 [95% CI, .07–.34]) but was no longer associated with mortality after that (HR, 0.49 [95% CI, .17–1.38] from day 16 through day 90). This translates to a 6.5-fold protective association of an ID consult in the first 15 days, and nonsignificant 2.0-fold effect from days 16 to 90.
Figure 1.
Survival curve of 147 Human Immunodeficiency Virus-uninfected patients with cryptococcosis by receipt of infectious disease (ID) consultation adjusted for a positive cryptococcal antigen test, age >55 years, end-stage liver disease, and receipt of chemotherapy. Patients without an ID consultation had a higher mortality than those who did (P < .001). This was most pronounced in the first 15 days. Mortality was censored after 90 days as it was less likely to be related to cryptococcosis. Solid line indicates ID consultation; dashed line indicates no ID consultation.
The cryptococcal-attributable mortality was 38 of 48 (79%) in the cohort; 20 of 27 (74%) in patients with an ID consult, and 18 of 21 (86%) in those without consult (P = .33). Patients who died of other causes included 7 (5 in the ID consult group) who died outside the hospital and the cause of death was not known, and 3 (1 in ID consult group) who had care withdrawn for their underlying condition, with a relatively well-controlled cryptococcal infection. Death occurred within 14 days of discharge in all patients with observed outpatient mortality.
The univariate and multivariable analyses were repeated with mortality attributable to cryptococcal infection, as defined above, as the dependent variable. In both univariate and multivariate analysis, the association of ID consult with cryptococcal morality was virtually the same as with all-cause mortality (HR, 0.45 [95% CI, .24–.84]; adjusted HR, 0.22 [95% CI, .12–.43]).
DISCUSSION
ID consult was associated with an adjusted HR of 0.24 for mortality, translating to a 4.2-fold high risk of mortality in patients who did not receive an ID consult. Despite the finding of a protective effect, patients with an ID consult had a higher fungal burden (as measured by higher CrAg levels in both CSF and serum), which has previously been associated with higher mortality rates [23, 24].
The ID consult group was less likely to have had SOT (12% vs 26%), although SOT was marginally associated with lower mortality in our study, as well as others [1], as has been the receipt of tacrolimus [25], which the majority of the SOT patients in our population received.
Patients in the ID consult group were more likely to have meningitis (57% vs 11%), and less likely to have pulmonary disease (27% vs 64%). This, however, may reflect some ascertainment bias, as more patients with an ID consult received an appropriate LP. This was not as a result of an indiscriminate increase in LPs on the part of the consultants, as the proportion of LPs performed without an indication was similar. This finding raises the possibility that meningitis may have been missed in the no-consult group. This is important because the early and aggressive management of meningitis has also been associated with lower complication rates and improved outcomes in patients with cryptococcal meningitis [5, 26]. The lower use of LPs, leading to an increased likelihood of missed diagnoses of meningitis, could potentially explain the higher mortality in the group of patients who did not receive an ID consult.
Similarly, to the finding of increased performance of LPs, the treatment for cryptococcal infection was more often adherent to evidence-based guidelines in patients with an ID consult compared to the no-consult group. Patients who had an indication for AmB (89% vs 24%) and 5-FC (71% vs 24%) received the antifungal therapy more frequently if they had an ID consult. In addition, when the appropriate induction therapy was given, the duration was also longer in the ID consult compared to the no-consult group.
The ID consult group also received more interventions for their ICP management, including more repeat LPs and neurosurgical interventions. This could have contributed to decreased mortality, as successful management of ICP has been associated with improved outcomes [27]. However, opening pressure was not recorded in a consistent manner, so we cannot make any inferences regarding differences in opening pressure between groups.
Several other factors that were significantly associated with mortality in univariate analysis have been reported previously in the literature. Increased mortality has been observed in patients with cryptococcemia [1], cirrhosis [2], and advanced age [23]. Older age has previously been associated with increased 2-week mortality in cryptococcal meningitis [23]. Elevations of both serum cryptococcal antigen [12, 13, 28], and another marker of cryptococcal burden, the quantitative cryptococcal culture [23], have been associated with higher 2-week mortality. Due to our small sample size, we could not detect a dose response of cryptococcal burden, as assessed by CrAg levels, with mortality.
The variables associated with mortality in our final multivariable model included ID consult (protective), age >55 years, positive CrAg, end-stage liver disease, and receipt of chemotherapy within the past 90 days. Cryptococcemia was not included in the model as it was very highly correlated with a positive CrAg, and did not contribute significantly to the model. The receipt of chemotherapy was associated with 2.1-fold increased risk of mortality, although solid organ tumor was not, suggesting that it is not the metastatic cancer but rather the recent receipt of chemotherapy that appears to be associated with increased mortality in patients with cryptococcal infection.
We also found that the patients who received an ID consult underwent a diagnostic workup for meningitis more consistent with evidence based recommendations, and that they were more likely to receive AmB and 5-FC when indicated and to have more appropriate diagnostic LPs as well as more aggressive ICP management. It is likely that better diagnosis of the severity of cryptococcal infection with more appropriate antifungal management explains why an ID consult was beneficial in our cohort; however, the retrospective nature of this study does not allow us to prove causation.
This study is limited by its retrospective nature, a single site, and the relatively small number of patients. However, despite the limited sample size, the receipt of an ID consult appears to be significantly associated with improved survival. Furthermore, we were unable to directly assess ICP in patients due to missing data. However, we were able to assess the number of LPs performed and use of neurosurgical interventions in the management of ICP. This is the first study that has examined the impact of ID consultation on the outcomes of patients with cryptococcosis. However, our findings parallel those from studies evaluating the effect of ID consultation in Staphylococcus aureus bacteremia. In that setting, ID consultation was associated with improved outcome, largely because of greater likelihood of diagnosing more complicated infections than initially realized, and greater likelihood of adherence to evidence-based guidelines for treatment.
In conclusion, in our cohort of 147 patients with cryptococcosis over 13 years, 32% of the patients had an opportunity for an ID consult but did not receive one, and this group of patients had significantly increased risk of death compared with patients who were seen by an ID physician. There was significantly lower all-cause and attributable mortality in the ID consult group, despite the fact that these patients had higher organism burden, and more evidence of disseminated cryptococcal disease. The lower mortality in the ID consult group may be due at least in part to increased adherence to management based on evidence-based recommendations, as patients seen by an ID physician were more likely to receive an appropriate LP and to be treated with AmB and 5-FC when indicated. Our results suggest that an ID consult is appropriate in all patients with cryptococcosis to improve outcomes and reduce mortality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. A. S. and W. G. P. conceived of the study and M. A. O. contributed significantly to study design. All authors contributed to data collection and analysis. A. S. wrote the manuscript and performed the statistical analysis. The final manuscript was approved by all authors.
Potential conflicts of interest. A. S. has received research funding and consults for Astellas and Scynexis. M. A. O. has received research funding and consults for Pfizer and Sanofi Pasteur, and has consulted previously for Merck. W. G. P. has received research funding from MiraVista and Scynexis and consults for Merck and Gilead. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS One 2013; 8:e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spec A, Raval K, Powderly WG. End-stage liver disease is a strong predictor of early mortality in cryptococcosis. Open Forum Infect Dis 2016; 3:ofv197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jongwutiwes U, Sungkanuparph S, Kiertiburanakul S. Comparison of clinical features and survival between cryptococcosis in human immunodeficiency virus (HIV)-positive and HIV-negative patients. Jpn J Infect Dis 2008; 61:111–5. [PubMed] [Google Scholar]
- 4. Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O; French Cryptococcosis Study Group Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS One 2008; 3:e2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shoham S, Cover C, Donegan N, Fulnecky E, Kumar P. Cryptococcus neoformans meningitis at 2 hospitals in Washington, D.C.: adherence of health care providers to published practice guidelines for the management of cryptococcal disease. Clin Infect Dis 2005; 40:477–9. [DOI] [PubMed] [Google Scholar]
- 6. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmutzhard E, Vejajjiva A. Treatment of cryptococcal meningitis with high-dose, long-term combination amphotericin B and flucytosine. Am J Med 1988; 85:737–8. [DOI] [PubMed] [Google Scholar]
- 8. Bicanic T, Wood R, Meintjes G, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis 2008; 47:123–30. [DOI] [PubMed] [Google Scholar]
- 9. Larsen RA, Leal MA, Chan LS. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Ann Intern Med 1990; 113:183–7. [DOI] [PubMed] [Google Scholar]
- 10. Sharkey PK, Graybill JR, Johnson ES, et al. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clin Infect Dis 1996; 22:315–21. [PubMed] [Google Scholar]
- 11. Chotmongkol V, Jitpimolmard S. Treatment of cryptococcal meningitis with triple combination of amphotericin B, flucytosine and itraconazole. Southeast Asian J Trop Med Public Health 1995; 26:381–3. [PubMed] [Google Scholar]
- 12. Bennett JE, Dismukes WE, Duma RJ, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis. N Engl J Med 1979; 301:126–31. [DOI] [PubMed] [Google Scholar]
- 13. Dismukes WE, Cloud G, Gallis HA, et al. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N Engl J Med 1987; 317:334–41. [DOI] [PubMed] [Google Scholar]
- 14. Saag MS, Powderly WG, Cloud GA, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med 1992; 326:83–9. [DOI] [PubMed] [Google Scholar]
- 15. Day JN, Chau TT, Wolbers M, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 2013; 368:1291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015; 60:1451–61. [DOI] [PubMed] [Google Scholar]
- 17. Honda H, Krauss MJ, Jones JC, Olsen MA, Warren DK. The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore) 2009; 88:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel M, Kunz DF, Trivedi VM, Jones MG, Moser SA, Baddley JW. Initial management of candidemia at an academic medical center: evaluation of the IDSA guidelines. Diagn Microbiol Infect Dis 2005; 52:29–34. [DOI] [PubMed] [Google Scholar]
- 20. Johnston JA, Wagner DP, Timmons S, Welsh D, Tsevat J, Render ML. Impact of different measures of comorbid disease on predicted mortality of intensive care unit patients. Med Care 2002; 40:929–40. [DOI] [PubMed] [Google Scholar]
- 21. Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am 2016; 30:179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kleinbaum DG. Survival analysis: a self-learning text. New York: Springer, 1996. [Google Scholar]
- 23. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anekthananon T, Manosuthi W, Chetchotisakd P, et al. ; BAMSG 3-01 Study Team Predictors of poor clinical outcome of cryptococcal meningitis in HIV-infected patients. Int J STD AIDS 2011; 22:665–70. [DOI] [PubMed] [Google Scholar]
- 25. Husain S, Wagener MM, Singh N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg Infect Dis 2001; 7:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trachtenberg JD, Kambugu AD, McKellar M, et al. The medical management of central nervous system infections in Uganda and the potential impact of an algorithm-based approach to improve outcomes. Int J Infect Dis 2007; 11:524–30. [DOI] [PubMed] [Google Scholar]
- 27. Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis 2000; 30:47–54. [DOI] [PubMed] [Google Scholar]
- 28. Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med 1974; 80:176–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.