Abstract
Background
MET exon 14 alterations are actionable oncogenic drivers. Durable responses to MET inhibitors are observed in patients with advanced MET exon 14-altered lung cancers in prospective trials. In contrast, the activity of immunotherapy, PD-L1 expression and tumor mutational burden (TMB) of these tumors and are not well characterized.
Patients and methods
Patients with MET exon 14-altered lung cancers of any stage treated at two academic institutions were identified. A review of clinicopathologic and molecular features, and an analysis of response to single-agent or combination immune checkpoint inhibition were conducted. PD-L1 immunohistochemistry was carried out and TMB was calculated by estimation from targeted next-generation sequencing panels.
Results
We identified 147 patients with MET exon 14-altered lung cancers. PD-L1 expression of 0%, 1%–49%, and ≥50% were 37%, 22%, and 41%, respectively, in 111 evaluable tumor samples. The median TMB of MET exon 14-altered lung cancers was lower than that of unselected non-small-cell lung cancers (NSCLCs) in both independently evaluated cohorts: 3.8 versus 5.7 mutations/megabase (P < 0.001, n = 78 versus 1769, cohort A), and 7.3 versus 11.8 mutations/megabase (P < 0.001, n = 62 versus 1100, cohort B). There was no association between PD-L1 expression and TMB (Spearman’s rho=0.18, P = 0.069). In response-evaluable patients (n = 24), the objective response rate was 17% (95% CI 6% to 36%) and the median progression-free survival was 1.9 months (95% CI 1.7–2.7). Responses were not enriched in tumors with PD-L1 expression ≥50% nor high TMB.
Conclusion
A substantial proportion of MET exon 14-altered lung cancers express PD-L1, but the median TMB is lower compared with unselected NSCLCs. Occasional responses to PD-1 blockade can be achieved, but overall clinical efficacy is modest.
Keywords: non-small-cell lung cancer, MET exon 14, PD-L1, tumor mutational burden, immunotherapy
Key Message
MET exon 14 alterations are actionable oncogenic drivers and durable responses to MET inhibitors have been observed in prospective trials. A substantial proportion of MET exon 14-altered lung cancers express PD-L1, but the median TMB is lower compared with unselected NSCLCs. Occasional responses to PD-1 blockade can be achieved, but overall clinical efficacy appears to be modest.
Introduction
Targeted therapies have proven effective in patients whose advanced lung cancers harbor actionable driver alterations such as sensitizing EGFR mutations, ALK and ROS1 rearrangements, and BRAF V600E mutations; however, the development of acquired resistance to tyrosine kinase inhibition is nearly universal. Non-targeted approaches to systemic therapy, such as immunotherapy and chemotherapy, continue to play an important role in the management of these patients. The development of monoclonal antibodies targeting the programmed death 1 (PD-1) receptor and its ligand, program death ligand 1 (PD-L1), has led to significant improvements in overall survival (OS) in select patients with lung cancers and established new standards of care [1, 2]. An important question in the clinic is when to use immunotherapy in patients with driver-positive tumors.
In lung cancers harboring EGFR mutations or ALK rearrangements, objective response rates (ORRs) with PD-1/PD-L1 checkpoint blockade are modest, and do not appear to improve progression-free survival (PFS) and OS [3–5]. This may be related to lower tumor mutational burden compared with unselected lung cancers [6]. In contrast to immunotherapy, targeted therapy achieves ORRs of ∼60%–80% and thus remains the recommended standards of care in treatment-naïve patients with stage IV lung cancers harboring a sensitizing EGFR mutation, BRAF V600E mutation, or ALK or ROS1 rearrangements [7].
MET is a high-affinity proto-oncogene receptor tyrosine kinase that, upon activation, drives oncogenic pathways involved in cell proliferation, survival, and metastasis [8]. Select somatic alterations in MET lead to an alternatively spliced transcript that is a result of exon 14 skipping, leading to decreased MET degradation, enhanced signaling through the MET pathway, and downstream activation of the mitogen-activated protein kinase pathway [9]. MET exon 14 skipping alterations occur in 3%–4% of lung cancers, a frequency comparable to that of ALK-rearranged lung cancer, and are enriched to 8%–30% of sarcomatoid lung cancers [10–12]. The detection of MET exon 14 skipping alterations has only recently become more feasible in every day practice with the use of hybrid capture-based next-generation sequencing (NGS) platforms. MET inhibitors are active in patients with advanced MET exon 14-altered lung cancers [13–15]. In an expansion cohort of patients with MET exon 14 alterations on the phase I study of crizotinib (PROFILE 1001), an ORR of 39% and a median duration of response of 9.1 months were observed [16].
To date, the ideal treatment paradigm and sequencing of therapies for advanced stage lung cancers harboring a MET exon 14 skipping alteration is unknown and response to immunotherapy has not been well characterized. To shed light on this question, we conducted an analysis of patients with MET exon 14 skipping alterations, evaluating PD-L1 expression, tumor mutational burden, and response to immunotherapy.
Patients and methods
Study population
This study, composed of patients treated at Memorial Sloan Kettering Cancer Center (cohort A) and Dana Farber Cancer Institute (cohort B), was authorized by the institutional review board at each site. Patients with MET exon 14-altered lung cancers of any stage who were identified between 1 January 2014 and 1 May 2017 at either institution were eligible.
Next-generation sequencing
DNA isolated from tumor tissue was subjected to hybridization capture-based NGS to detect somatic alterations in 468 genes (cohort A, MSK-IMPACT) or 446 genes (cohort B, OncoPanel). The mean overall sequencing depth ranged from 500× to 1000× in both cohorts [17, 18]. Anchored multiplex RNA sequencing with the MSK-Fusion Solid panel, a custom RNAseq panel based on the Archer FusionPlex™ technology (ArcherDx, Boulder, CO) was carried out in select cases to identify or confirm MET exon 14 alterations (in cases where DNA-based NGS sequencing did not find an actionable driver) [19].
Tumor mutational burden
Tumor mutation burden (TMB), defined as the number of nonsynonymous coding mutations per megabase of genome covered by the respective NGS panel, was calculated for each patient in cohorts A and B. This strategy was employed as determining mutational signatures from clinical-grade targeted capture data were previously shown to be comparable with whole-exome sequencing [20]. TMB from cohorts A and B were analyzed separately as the NGS assays used differed between the two sites. In cohort A (MSK-IMPACT), germline variants identified by paired sequencing of matched normal blood were not included in the TMB calculation. To standardize comparisons, the TMB of patients with MET exon 14-altered lung cancers was compared with the TMB of patients with unselected non-small-cell lung cancers (NSCLCs), excluding MET exon 14-altered NSCLCs, at each institution (1769 patients in cohort A and 1100 patients in cohort B).
Immunohistochemistry
PD-L1 immunohistochemistry (IHC) was carried out using the PD-L1 antibody clone E1L3N (Cell Signaling Technology, Danvers, MA). IHC was scored as the percentage of tumor cells with positive staining for PD-L1 (0%, 1%–49%, and 50% or greater). In validation studies, E1L3N antibody was compared with the commercial pharmDx assay utilizing the 22C3 antibody, and was found to be comparable [21]. Testing was performed by a single pathologist at each institution who was not aware of other patient characteristics or outcomes.
Response to immunotherapy
Response to immunotherapy was assessed using RECIST in cohorts A and B. An additional exploratory assessment of immune-related response (irRECIST) [22] criteria was conducted in both cohorts. Duration of therapy, duration of response to therapy, PFS, and OS were assessed in all evaluable patients, and measured from the date of therapy initiation.
Statistical analysis
PD-L1 expression levels were compared between groups using the Wilcoxon rank sum test. Clinical characteristics were compared across the three PD-L1 expression groups using Fisher’s exact test. Using the Wilcoxon signed rank test, the TMB of MET exon 14-altered lung cancer samples was compared with the TMB unselected NSCLCs. This was done separately for cohorts A and B. The association between PD-L1 and TMB was assessed using the Spearman's rank correlation coefficient test. ORR was calculated along with an exact 95% confidence interval. ORR was compared between groups using Fisher’s exact test. PFS and OS were analyzed using the Kaplan–Meier method. All reported P values are two-sided.
Results
Patient characteristics
Of the 2869 patients with NSCLC whose tumors underwent NGS in cohorts A and B between 1 January 2014 and 1 May 2017, we identified 147 patients with MET exon 14 skipping alterations (5%). The clinical and pathologic characteristics of these patients are summarized in Table 1. The median age was 73 years (range 44–91 years). The median pack year history was 6 pack-years (range 0–125 pack-years). Adenocarcinoma was the most common histology, found in 74% (109/147) of cases, followed by sarcomatoid lung cancer in 19% (28/147) of cases. In total 69% (101/147) of patients had metastatic disease at diagnosis or later recurred and were eligible for systemic therapy.
Table 1.
Clinical and molecular characteristics of patients with lung cancers harboring MET exon 14 skipping alterations
| Clinical characteristics | No. (%) (N = 147) |
|---|---|
| Age, median (range), years | 73 (44–91) |
| Sex | |
| Male | 58 (39) |
| Female | 89 (61) |
| Smoking history, pack years | |
| 0 | 52 (35) |
| 1–10 | 30 (21) |
| >10 | 65 (44) |
| Pack years (median, range) | 6 (0–125) |
| Tumor histology | |
| Adenocarcinoma | 109 (74) |
| Sarcomatoid | 28 (19) |
| Adenosquamous | 5 (3.5) |
| Squamous | 5 (3.5) |
| Stage at diagnosis | |
| I | 39 (27) |
| II | 15 (10) |
| III | 25 (17) |
| IV | 68 (46) |
| Metastatic/recurrent disease | |
| Yes | 101 (69) |
| No | 46 (31) |
| Molecular characteristics | No. (%) (N=147) |
| METex14 splice site alteration | |
| 5′ splice donor site | 102 (69) |
| 3′ splice acceptor site | 38 (26) |
| Other | 7 (5) |
| Concurrent MET amplification (>2-fold change) | |
| Yes | 36 (25) |
| No | 105 (71) |
| Not available | 6 (4) |
Molecular characteristics
Most tumors [69% (102/147)] harbored a 5′ MET exon 14 donor splice site alteration and 26% (38/147) harbored a 3′ acceptor splice site alteration. The remaining 5% (7/147) of patients had other alterations including 3 patients with Y1003 substitutions, and 4 patients with whole exon deletions of MET exon 14. The most common MET alterations were D1010H (c.3028G>C), and D1010N (c.3028G>A). Concurrent MET amplification (≥2-fold change) was identified in 25% (36/147) of patients. Further molecular characteristics are summarized in supplementary Table S1, available at Annals of Oncology online.
PD-L1 expression
Of the 147 patients with MET exon 14 skipping alterations, tissue was available for PD-L1 IHC in 111 patients (76%). Tumors were grouped into PD-L1 0% (negative), PD-L1 1%–49% (intermediate), and PD-L1 ≥50% (high). Thirty-seven percent (41/111) were PD-L1 negative and 63% (57/111) were PD-L1 positive (PD-L1 expression of ≥1%). Age, gender, smoking status, stage, or alteration subtype did not significantly correlate with PD-L1 status. Fisher’s exact test for the simultaneous comparison of the three PD-L1 groups (<1, 1–49, ≥50%) revealed a statistically significant association between PD-L1 ≥50% and sarcomatoid histology P = 0.0041. Among those with MET exon 14 skipping alterations, patients with sarcomatoid histology had a higher PD-L1 expression [median 70% (range 0%–100%)] compared with adenocarcinoma [median 1% (range 0%–100%)], P = 0.021 by the Wilcoxon rank sum test. These results are summarized in Table 2.
Table 2.
PD-L1 expression of MET exon 14-altered lung cancers
| PD-L1 IHC,n = 111 | ||||
|---|---|---|---|---|
| 0% | 1%–49% | ≥50% | ||
| n (%) | 41 (37) | 24 (22) | 46 (41) | |
| Clinical and molecular features stratified by PD-L1 | P-value | |||
| Age | ||||
| <65 | 6 | 4 | 9 | 0.900 |
| ≥65 | 35 | 20 | 37 | |
| Sex | 0.460 | |||
| Women | 27 | 12 | 28 | |
| Men | 14 | 12 | 18 | |
| Smoking | 0.711 | |||
| Former/current | 29 | 15 | 29 | |
| Never | 12 | 9 | 17 | |
| Stage | 0.703 | |||
| I/II | 6 | 3 | 5 | |
| III | 7 | 4 | 4 | |
| IV | 28 | 17 | 37 | |
| Histology | 0.0041 | |||
| Adenocarcinoma | 36 | 16 | 29 | |
| Sarcomatoid | 5 | 3 | 14 | |
| Other | 0 | 5 | 3 | |
Stratified by patient and tumor characteristics.
Tumor mutational burden
TMB analysis was successfully carried out in tumors from 140 (95%) of the 147 patients with MET exon 14 skipping alterations (78 patients in cohort A, 62 patients in cohort B). TMB was lower in tumors with MET exon 14 alterations compared with unselected NSCLCs [median of 3.8 versus 5.7 mutations/megabase (n = 78 versus 1176) in cohort A; median of 7.3 versus 11.8 mutations/megabase (n = 62 versus 1100) in cohort B; P < 0.001 for both comparisons; Figure 1]. There was no correlation between PD-L1 and TMB (Spearman correlation coefficient = 0.18, P = 0.069; Figure 2).
Figure 1.
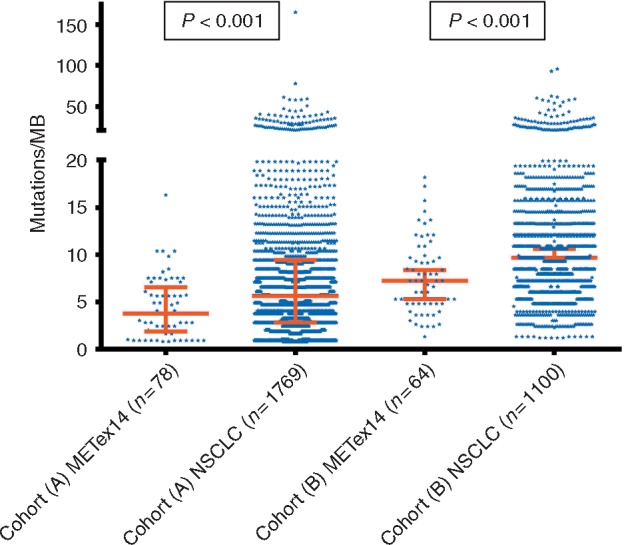
Tumor mutational burden in MET exon 14-altered lung cancers.
Figure 2.
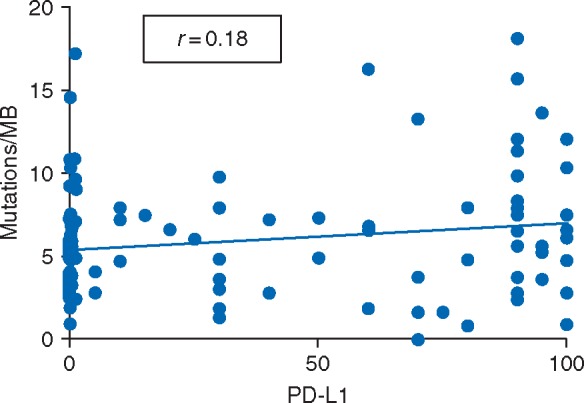
Correlation between PD-L1 expression by immunohistochemistry and tumor mutational burden by next-generation sequencing.
Response to immunotherapy
Twenty-four patients who received immunotherapy were evaluable for response: 22 patients received single agent anti-PD-1/PD-L1 therapy and 2 patients received combination anti-PD-1 and anti-CTLA-4 therapy. Immunotherapy was administered in 11 patients as first-line treatment, in 6 patients as second-line treatment, and in 7 patients as third-line treatment. Details regarding the immune checkpoint inhibitors used and the line of therapy these agents were administered are listed in supplementary Table S2, available at Annals of Oncology online.
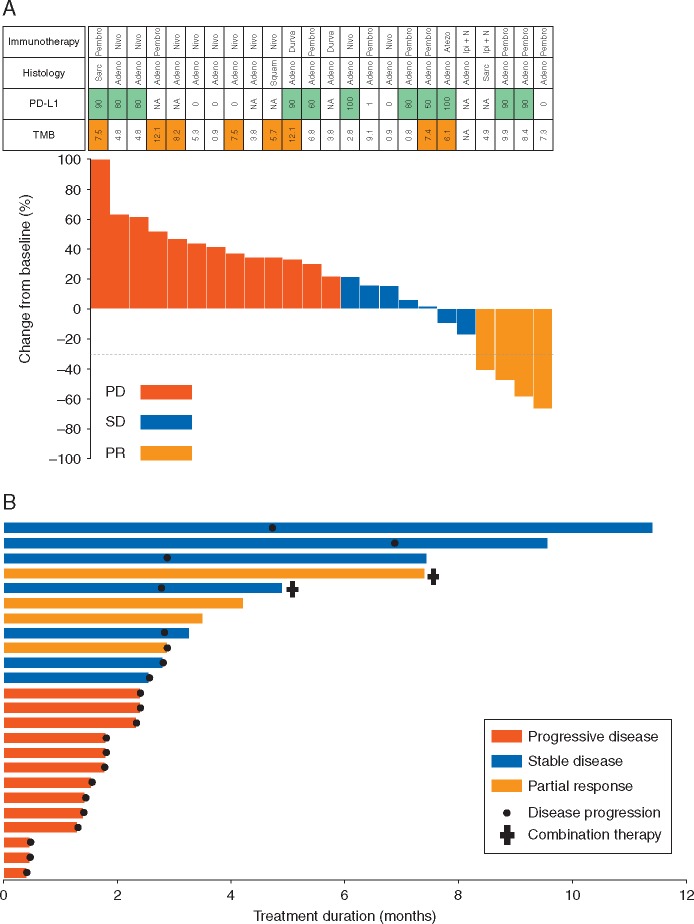
Four achieved a partial response (ORR 17%, 95% CI 6% to 36%): three patients who received single-agent therapy and one who received combination immune therapy (Figure 3A). An exploratory analysis of irRECIST was carried out in both cohorts. ORR to immunotherapy was concordant between RECIST and irRECIST, and pseudoprogression was not observed, consistent with previously published data in lung cancers [23]. The duration of therapy is shown in Figure 3B. The median PFS was 1.9 months (95% CI 1.7–2.7; Figure 4) for the 21 patients assessable for this end point. The median OS was 18.2 months (95% CI 12.9–NR; supplementary Figure S1, available at Annals of Oncology online) for all 24 patients. Responses were not enriched in those with either high PD-L1 expression (2/11, 18%) nor in those with high TMB (>median TMB of all NSCLCs in respective cohorts) (0/8, 0%).
Figure 3.
(A) Waterfall plot MET exon 14-altered lung cancers and response to single and combination immunotherapy. PD-L1 ≥50% in green; TMB≥median in cohorts A and B, respectively, in orange. Pembro, Pembrolizumab; Nivo, Nivolumab; Durva, Durvalumab; Atezo, Atezolizumab; Ipi+N, Ipilimumab+Nivolumab; Sarc, Sarcomatoid; Adeno, Adenocarcinoma; Squam, Squamous; NA, Not available. (B) Time on therapy.
Figure 4.
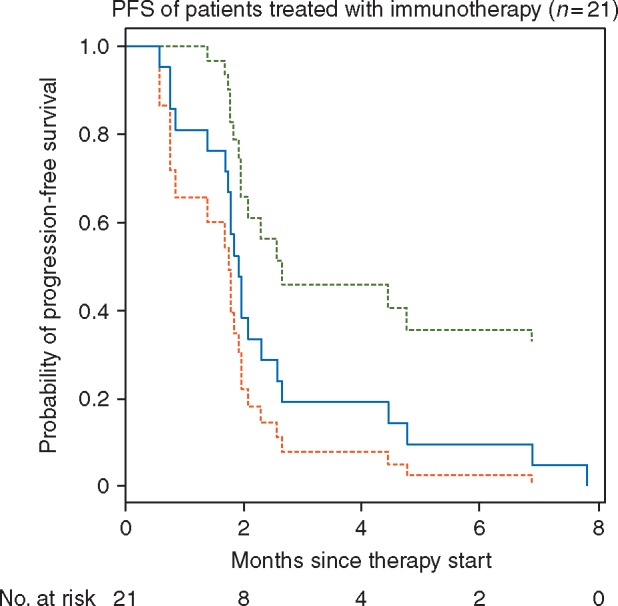
Kaplan–Meier survival curve, progression-free survival (PFS) of patients treated with immunotherapy.
Discussion
In this study, we evaluated the immunophenotype and response to immunotherapy of patients with MET exon 14-altered lung cancers. The ORR with immune checkpoint inhibition was low at 17%, although not distinctly different from efficacy in the unselected, second-line setting [24]; however, the duration of response and the PFS were short. These outcomes are modest compared with those achieved in the registrational trials of these agents in the first-line setting: an ORR of 44% and a median PFS of 10.3 months with pembrolizumab in treatment-naïve patients with EGFR and ALK wild-type lung cancers with PD-L1 expression ≥50% [1]. Of note, a prior series on sarcomatoid carcinomas included two patients with MET exon 14-altered lung cancers who did not respond to single-agent immunotherapy [12].
More importantly, the outcomes achieved with single-agent immunotherapy in this series are poor compared with the outcomes achieved with targeted therapy or platinum doublet therapy. The interim reported ORR of crizotinib on the PROFILE 1001 study in patients with MET exon 14-altered lung cancers was 39%, with a median PFS of 8 months [16]. The historical ORR with platinum doublet chemotherapy in treatment-naïve patients with unselected lung cancers is ∼30%, with a median PFS of 4–5 months.
The factors responsible for the modest outcomes achieved with single-agent immunotherapy in MET exon 14-altered lung cancers remain unclear. While we showed that a substantial proportion of these tumors express PD-L1, response was not associated with PD-L1 expression. Tumor mutational burden, a factor that has been associated with response to immunotherapy in other reports [6, 25–27], was lower overall in MET exon 14-altered lung cancers compared with unselected cases. TMB was slightly higher in cohort B than cohort A, and may be due to the exclusion of germline alterations by the assay used for cohort A. Similar to PD-L1 status, higher tumor mutational burden was not associated with response in this series.
Limitations of this retrospective analysis include the heterogeneity of immunotherapies received and the line of therapy in which immunotherapy was received. While the results of our retrospective series require further confirmation, these data appear to argue against the use of single-agent immunotherapy in patients with MET exon 14 alterations before administering targeted therapy or platinum doublet therapy. The role of combination strategies (i.e. immunotherapy and chemotherapy such as platinum, pemetrexed, and pembrolizumab, or combination immunotherapy) in this population deserves further investigation. In addition, it would not be unreasonable to consider a trial strategy administering combination targeted therapy and immune therapy, given that MET-directed therapy has been shown to augment the immune response, however, toxicity would represent a substantial concern.
Conclusion
Although a substantial proportion of MET exon 14-altered lung cancers express PD-L1, the overall response rate to PD-1/PD-L1-directed immune checkpoint inhibition was low and the median PFS was short. The median TMB was lower in these tumors compared with unselected lung cancers. Neither PD-L1 status nor tumor mutational burden correlated with response to therapy.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health [T32 CA009207 to JKS, R01 CA203636 and U0 1CA209414 to MN, P30 CA008747 to MDH, PKP, GJR, MGK, CMR, and AD] and Conquer Cancer Foundation to MM.
Disclosure
JKS, GCL, RU, JM, AN, RC, JD, CM, IB, WVL, MDO, KCA, AJP, DFH, LMS, and NR have declared no conflicts of interest. CAS is a consultant for Genentech Roche. PKP received research support from Celgene, AstraZeneca, and GlaxoSmithKline. BTL is a consultant for Genentech Roche, ThermoFischer Scientific and Guardant Health. GJR is a consultant for Genentech Roche, received travel support from Merck Sharp and Dohme, and research support from Novartis, Genentech Roche, Millennium, GlaxoSmithKline, Pfizer, Infinity Pharmaceuticals, and Ariad. MGK is a consultant for AstraZeneca. CMR is a consultant for Bristol-Myers Squibb, Abbvie, Seattle Genetics, Harpoon Therapeutics, Genentech Roche, and AstraZeneca. MN is a consultant for WorldCare Clinical, Toshiba, Daiichi Sankyo, received honoraria from Bayer Yakuhin, Roche Pharma AG, and received research support from Merck, Toshiba, and AstraZeneca. MDH is a consultant for Bristol-Myers Squibb, Merck, Genentech Roche, AstraZeneca/MedImmune, Novartis and Janssen, and received research support from Bristol-Myers Squibb. MMA is a consultant for Abbvie, Ariad, Astrazeneca/MedImmune, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Foundation Medicine, Genentech, Merck, Nektar, and Pfizer. AD is a consultant for Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech Roche, Takeda and has received research support from Foundation Medicine.
Supplementary Material
Footnotes
Note: This study has been presented as an Oral Presentation at ASCO 2017 Clinical Science Symposium: Old Targets, New Drugs: Her2 and MET; Sunday, 4 June 2017; Abstr 8512.
References
- 1. Reck M, Rodríguez-Abreu D, Robinson AG. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med 2016; 375(19): 1823–1833. [DOI] [PubMed] [Google Scholar]
- 2. Langer CJ, Gadgeel SM, Borghaei H. et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17(11): 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters S, Gettinger S, Johnson ML. et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol 2017; 35(24): 2781–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee C, Man J, Lord S. et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol 2018; 4(2): 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garassino MC, Cho B-C, Kim J-H. et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19(4): 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rizvi H, Sanchez-Vega F, La K. et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand (PD-L)-ligand 1 blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. JCO 2017; 75: 3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 6.2017). http://www,nccn.org/professional/physician_gls/pdf/nscl.pdf (1 August 2017, date last accessed).
- 8. Drilon A, Cappuzzo F, Ou S-HI. et al. Targeting MET in lung cancer: will expectations finally be MET? J Thorac Oncol 2017; 12(1): 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kong-Beltran M, Seshagiri S, Zha J. et al. Somatic mutations lead to an oncogenic deletion of MET in lung cancer. Cancer Res 2006; 66(1): 283–289. [DOI] [PubMed] [Google Scholar]
- 10. Frampton GM, Ali SM, Rosenzweig M. et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015; 5(8): 850–859. [DOI] [PubMed] [Google Scholar]
- 11. Awad MM, Oxnard GR, Jackman DM. et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol 2016; 34(7): 721–730. [DOI] [PubMed] [Google Scholar]
- 12. Schrock AB, Li SD, Frampton GM. et al. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thorac Oncol 2017; 12(6): 932–942. [DOI] [PubMed] [Google Scholar]
- 13. Paik PK, Drilon A, Fan P-D. et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015; 5(8): 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jenkins RW, Oxnard GR, Elkin S. et al. Response to crizotinib in a patient with lung adenocarcinoma harboring a MET splice site mutation. Clin Lung Cancer 2015; 16(5): e101–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee C, Usenko D, Frampton GM. et al. MET 14 deletion in sarcomatoid non-small-cell lung cancer detected by next-generation sequencing and successfully treated with a MET inhibitor. J Thoracic Oncol 2015; 10(12): e113–e114. [DOI] [PubMed] [Google Scholar]
- 16. Drilon A, Ou Sai-Hong K, Clark Jeffrey W. et al. Antitumor activity and safety of crizotinib in patients with advanced MET exon 14-altered non-small cell lung cancer IASLC 17th world conference on lung cancer. J Thorac Oncol 2016; 12: S225. [Google Scholar]
- 17. Cheng DT, Mitchell TN, Zehir A. et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015; 17(3): 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sholl LM, Do K, Shivdasani P. et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 2016; 1(19): e87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Archer. Archer FusionPlex. ArcherDx, 2017. http://archerdx.com/fusionplex-assays/.
- 20. Zehir A, Benayed R, Shah RH. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10, 000 patients. Nat Med 2017; 23(6): 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rimm DL, Han G, Taube JM. et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for pd-l1 expression in non-small cell lung cancer. JAMA Oncol 2017; 3(8): 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishino M, Gargano M, Suda M. et al. Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer 2014; 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishino M, Dahlberg SE, Adeni AE. et al. Tumor response dynamics of advanced non-small cell lung cancer patients treated with PD-1 inhibitors: imaging markers for treatment outcome. Clin Cancer Res 2017; 23(19): 5737–5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rittmeyer A, Barlesi F, Waterkamp D. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389(10066): 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rizvi NA, Hellmann MD, Snyder A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348(6230): 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carbone DP, Reck M, Paz-Ares L. et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376(25): 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hellmann MD, Ciuleanu T-E, Pluzanski A. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378(22): 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.