Abstract
It is now well-established that the host’s adaptive immune system plays an important role in identifying and eliminating cancer cells in much the same way that intracellular pathogens are cleared during an adaptive immune response to infection. From a therapeutic standpoint, the adaptive immune system is unique in that it can co-evolve alongside a developing tumor. Tumor acquisition of immune evasive phenotypes, such as class-I MHC down-regulation, remains a major limitation of successful T-cell immunotherapy. Here, we consider a population dynamical model coupling tumor and adaptive immune compartments in order to study the dynamics and survival of an evolving threat when faced with adaptive immune pressure. We demonstrate that predicted optimal growth strategies depend on whether or not the threat may acquire an immune-evasive phenotype as well as the mode of immune detection. We parameterize adaptive immune functioning by T-cell turnover and repertoire diversity and predict that decreases in the latter quantity which occur in advanced age may substantially affect the ability to recognize, and therefore control, an immune evasive threat like cancer. This framework recapitulates general features of age-dependent AML incidence, thereby providing a probable association between cancer frequency and adaptive immune functioning. Lastly, we quantify therapeutic efficacy of adjuvant immunotherapeutic strategies, and predict their benefits and limitations with regard to handling immune evasion. Our model generates survival behavior consistent with known growth-dependent characteristics, and serves as a first attempt at modeling stochastic cancer evolution alongside an adaptive immune compartment.
Keywords: Cancer immunotherapy, Immune evasion, Applied probability, Stochastic processes
Graphical Abstract

1. Introduction
The lack of successful treatment options that lead to durable remission outcomes still remains an ongoing challenge for curing many malignancies. However, there have been significant advances made most recently in targeted and immune therapeutic strategies (Couzin-Frankel, 2013). It is now well-established that the CD8+ T-cell compartment of the human adaptive immune system plays an integral role in immunoediting, and tumor progression therefore necessarily requires successful evasion of adaptive immune surveillance (Fridman et al., 2011; Dunn et al., 2002). Immunotherapy is responsible for recent breakthroughs in cancer treatment and encompasses strategies aimed at enhancing the patient’s immune system, via tumor antigen vaccines (Fritsch et al., 2014; Ott et al., 2017; Hellmann and Snyder, 2017; Sahin et al., 2017), immune checkpoint inhibition (Alsaab et al., 2017; Leach et al., 1996), and introduction of tumor-specific immune cells, as is the case in CAR T-cell therapy (Sadelain et al., 2017; Wang and Rivière, 2016; Martin et al., 2016).
Despite these advances, tumors may acquire treatment- resistant clones during disease progression (Iwasa et al., 2006), thus limiting treatment efficacy. Cancer cells exploit a variety of strategies that facilitate CD8+ T-cell immune evasion (Bronte and Mocellin, 2009), including up-regulation of immune inhibitory genes like PD-L1 and HLA-G (Herbst et al., 2014; Driessens et al., 2009; Sheu and Shih, 2010; Lin and Yan, 2015), reduced immunoproteasome expression (Tripathi et al., 2016), and complete evasion of CD8+ recognition via loss of MHC-I (Garrido et al., 2016; Carretero et al., 2008; del Campo et al., 2014; Straten and Garrido, 2016). Such evasive tactics occur alongside active immunosurveillance by an adaptive T-cell repertoire. Naïve T-cell turnover can, in theory, lead to recognition of a dividing cancer cell population prior to acquisition of an immune-evasive phenotype. Previous studies have considered systems level interactions between the tumor and host adaptive immune system (Son-tag, 2017; Khailaie et al., 2013; Andrew et al., 2007; Nani and Ouztöreli, 1994; Kirschner and Panetta, 1998), while a separate effort has gone into understanding the acquisition of drug-resistant subpopulations during clonal evolution (Iwasa et al., 2006; Michor et al., 2004). At present, the temporal dynamics of acquired immune evasion on tumor development under adaptive immune pressure remains uncharacterized. Filling this gap is of fundamental importance to better understand tumor progression. Additionally, an improved quantitative framework for describing the successes, and failures, of adaptive immune targeting of cancer cells would enable more accurate predictions of treatment success rates.
Here, we propose a foundational model of adaptive immune system and tumor co-evolution wherein tumor cells may be recognized by the immune system, but may also acquire an immune-evasive phenotype. We account for key empirical behavior, including the growth-threshold conjecture which predicts initiation of an immune response depends on threat net growth rates instead of absolute threat size (Arias et al., 2015; Pradeu et al., 2013; Grossman and Paul, 1992; Johansen et al., 2008). For threats like cancer which may acquire an immune evasive phenotype, we show that the model supports the experimental observation of ‘sneak-through’ wherein threats with either large or small net growth rates have a preferential advantage over their intermediate-growth counterparts (Bocharov et al., 2004). We apply this model to study AML incidence as a function of immune turnover and repertoire diversity, and accurately characterize increased incidence as a result of an aging immune system in addition to chronic immunosuppression. We conclude our analysis by quantifying the benefit of certain types of T-cell immunotherapy and predicting treatment-specific advantages based on tumor growth rates and patient immune status.
2. Model Development
We conceptualize the dynamics between a foreign threat and the host immune system as a time-continuous birth process, where the state variable, X, represents the total number of cancerous or infected cells. In our model, these cells grow at net rate r per cell until either exceeding a critical size, M ≫ 1, or until the immune system mounts a response at a (possibly random) population size X = m and time S1. If recognized, the population undergoes net death at a per-cell rate of so that d characterizes per-cell immune system killing rate. We assume d is large enough to handle threats with a variety of growth rates so that r < d always. States 0 and M + 1 are absorbing. A more precise model would separately track birth and death rates both before and after treatment. We have opted for this simpler form to enable analytic investigations.
2.1. Static Threats
Static threats are those unable to acquire an immune evasive phenotype. These dynamics are most appropriate for investigating intracellular microbial pathogens that grow, divide, and infect host cells. In this case the process evolves according to the following transitions:
| (1) |
2.2. Dynamic Threats
Dynamic threats, including cancer, may randomly acquire an immune evasive phenotype that enables its members to avoid immune recognition targeting the initial population. We therefore consider two cell populations: a non-evasive population, X1, and an immune evasive population, X2 that broadly represents cells adopting a variety of CD8+ T-cell evasion strategies commonly observed during cancer progression. We assume that immune evasive cells are randomly acquired at rate μ ≪ 1 per cell division. Transitions are described by:
| (2) |
We assume that successful immune responses occur quickly and are not limited at large population sizes for both the static and dynamic cases.
2.3. Detection Limit
We assume that immune recognition at size X1 = m may only occur after the population reaches a lower detection limit, m0, based either on the population size or the population total net growth rate. We will refer to the former assumption as the size threshold or size-limited case (m0 = mc), and the latter as the growth-threshold or growth-limited case (m0 = m0(r) such that the total net growth rate first exceeds R), where appropriate. The growth-threshold conjecture is supported by empirical and theoretical observations (Sontag, 2017; Arias et al., 2015; Pradeu et al., 2013; Grossman and Paul, 1992; Johansen et al., 2008). We will argue later that a size-limited model is perhaps most appropriate for modeling CAR T-cell infusions, as CAR T self-activation is engineered directly in this case.
2.4. ·Recognition Mode
In the primary case of interest we model the response of an adaptive immune compartment whose present and future CD8+ T-cell repertoire may contain a T-cell capable of recognizing an immune susceptible threat X1. There is continual opportunity for the sensitive population to be recognized as its growth trajectory exceeds m0 and continues growing to final size M. We refer to this case hereafter as stochastic or adaptive recognition. Recognition depends on both the T-cell repertoire and turnover of new T-cell clones. The population at size m0 evades the current immune repertoire, due in part to a lack of sufficient levels of detectable antigen or recognizing T-cells, with a background escape probability pb, which we use to represent the clonal diversity of the TCR repertoire (larger pb implies lower diversity). We model the arrival of a recognizing T-cell clone as a Poisson process with rate k, corresponding to the thymic turnover rate of naïve CD8+ T-cells. It is also convenient to analyze a simpler process wherein a growing threat is (deterministically) recognized at threshold size m0, which applies for example to innate immune recognition of, or memory reactivation to, a previously encountered threat. We refer to this case as deterministic recognition.
2.5. Mathematical Analysis
Here we provide a broad outline of our general strategy, with the primary goal of calculating the probability that a threat either escapes immune detection, or acquires an evasive phenotype, or is controlled by the immune system. Complete derivations are included in the SI. We let Ee denote the event that a threat escapes recognition and exceeds size M with corresponding probability Pe. For dynamic threats, Ε𝜇 denotes the event that an immune evader arrives with probability Ρ𝜇. All events may be partitioned by: Ee,μ ≡ Ee ∩ Eμ, , and with corresponding probabilities Pe,μ, , and Pι (a threat loses if it neither escapes nor acquires an evader, see SI Sec. S5).
Pe is determined by relating the immune turnover and size parameters to recognition probabilities (Sec. 3.1). This may be obtained by solving for the probability that detection occurs at size m ≥ m0, denoted by pr (m; m0) (SI Sec. S3). The calculation of is more involved, and so we begin first by evaluating this quantity under deterministic recognition at fixed size m, denoted . We recall that S1 denotes type-1 arrival time. If τ2 is the type- 2 arrival time, and the type-1 extinction time, then the probability of interest is of the form
| (3) |
with both terms amenable to exact calculation by conditioning on the exponential inter-arrival times of each birth/death event (SI Sec. S5.1). The corresponding probability under adaptive recognition with variable detection size, denoted , may be calculated by taking weighted averages of each estimate conditioned on size m recognition via
| (4) |
provided m0 < M (SI Sec. S5.3). In all cases, Pι is calculated indirectly via
| (5) |
We obtain a solution in the adaptive recognition mode by weighting by the distribution of recognition sizes, calculated subsequently.
Under deterministic recognition, we also characterize the mean first time of type-2 arrival conditioned on acquired evasion, given by . Using the usual indicator random variable notation
| (6) |
This quantity may be calculated using the definition of conditional probability
| (7) |
and is derived to first order in μ following a similar strategy for characterizing , here instead assuming each inter-arrival time is well-characterized by its mean value (SI Sec. S5.2). This framework permits analytical comparisons between predicted evasion behavior for the various assumptions on detection limit and recognition mode. We apply this framework to study AML, a hematological malignancy, as our model disease due to its low mutation rate, relative immune accessibility, and rapid growth. We compare model predictions to AML incidence data, comparing our theory with the alternative ‘multi-stage’ theory of cancer incidence (Armitage and Doll, 1954), and conclude by expanding the analysis to predict post-immunotherapy treatment success probabilities.
3. Tumor Progression
The following sections present the main findings of our analysis. Full mathematical details are provided in the SI. In an effort to organize subsequent analyses, we subdivide outcomes of the process based on relevant events.
3.1. Adaptive repertoire and detection limit determine escape probability
This section focuses on static threats (Ρ𝜇 = 0). If recognition is deterministic, then Pe = 0 trivially. Adaptive recognition in contrast permits a threat to grow past minimal detection before recognition and subsequent elimination (Fig. 1), as long as m0 < M (for large simulation trajectories illustrating mean-variance behavior, see Fig. 1 in (George and Levine, 2018)). In this case a population’s survival probability is inversely related to its mean sojourn time on {m0,..., M}, given by
| (8) |
Recognition probability at size m is given by
| (9) |
From this formula, we observe that the escape probability to size m > m0 is
| (10) |
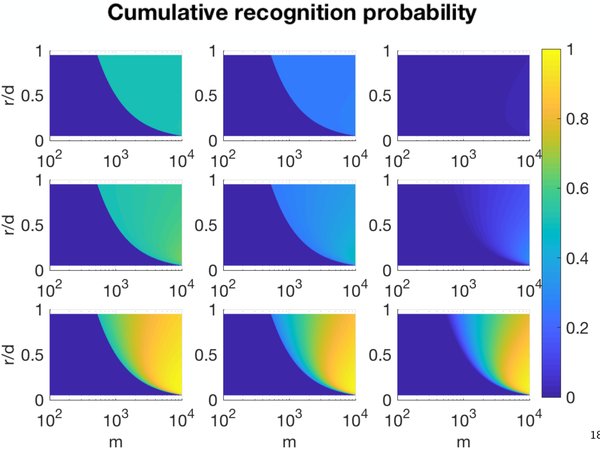
It is clear from the above that optimal growth rates of immune evaders balance the size of earliest detection with the amount of time spent under immunosurveillance. Faster growing threats always have higher escape probabilities if detection is size-limited. However, under growth-limited detection the existence of a slow-growth window r ϵ (0, eR/M) enables threats with slow growth rates to increase escape by minimizing surveillance time (Table S1). Thus, growth-limited detection of a static threat is consistent with the phenomena of ‘sneak-through’ (Arias et al., 2015; Pradeu et al., 2013; Grossman and Paul, 1992; Johansen et al., 2008; Bocharov et al., 2004). Analogously, if mc > M in the size-limited case, then clearly threats of all growth rates escape detection. Cumulative recognition probabilities, given by 1 - pe (m; m0), are presented for a variety of immune parameters (Fig. 2), indicating the relevance of repertoire diversity and T-cell turnover to tumor recognition.
Figure 1:
Dynamics under adaptive immune detection. Stochastic population trajectories obeying Eqs. 1 that are ultimately recognized and eliminated. Red trajectories illustrate recognition at detection limit m0 (dashed line) (r = 0.1, d = 0.2, m0 = 102, pb = 0.75, k = 0.1).
Figure 2:
Growth-limited cumulative recognition. Cumulative recognition probability 1 - pe (m; m0) as a function of population size m and relative growth rate r/d for various values of immune turnover (rows bottom-to-top: k={10−1, 10−2, 10−3}) and repertoire diversity (columns left-to-right: pb={0.5, 0.75,1.0}) (d = 0.2, R = 102).
3.2. Cancer ‘sneak-through’ predicted by detection limited by growth, not size
Non-escape occurs with certainty under deterministic recognition. However, a dynamic threat may acquire an immune evader . The probability of evader acquisition as a function of deterministic detection at size m is given by
| (11) |
This arrival probability is plotted in Fig. 3 alongside mean evader arrival times as a function of net growth rates r for size- and growth-limited detection assumptions (see SI for full details). Immune evaders rarely emerge for slow-growing threats under size-limited detection, and only do so on average over large time scales. This contrasts with growth-limited detection, for which immune evaders arrive with high probability for both large and small r, and do so with comparable time scales of arrival. Evasion behavior under the latter detection mode predicts for the first time a novel mechanism of sneak-through resulting exclusively from acquired immune evasion. A corollary to this implies that the enhancement of immune killing (via increased d) alone cannot eliminate the risk of immune evader arrival, and the effects of such immunomodulation are diminished for slow-growth cancers. This prediction corroborates known experimental observations linking growth-rate with immune resiliency, including slow-growth melanoma variants with known T-cell exclusion (Spranger et al., 2015), and are reminiscent of the state of reduced proliferation with concomitant survival phenotypes characteristic of drug tolerant persisters (Chisholm et al., 2015), as well as the observed concave relationship between viral growth rate and peak CD8+ T-cell response (Bocharov et al., 2004) . We focus next on the case of a dynamic threat under adaptive immune recognition in subsequent sections.
Figure 3:
‘Sneak-through’ via immune evasion under deterministic detection. (A) Immune evader arrival probability and (B) mean time of evader arrival for both growth-limited (blue, R = 102) and size-limited (red, mc = 103) detection assumptions (d = 0.2, μ = 10−6).
3.3. Acquired evasion and immune escape predicted with diminishing adaptive immune repertoire
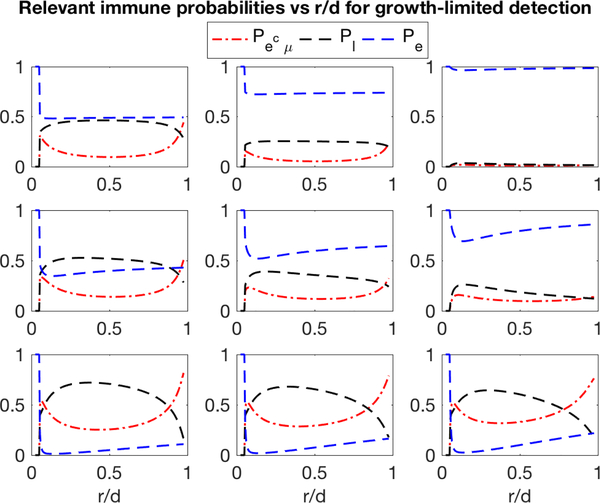
In contrast with the prior examples, a dynamic threat faced with an adaptive immune system has nontrivial likelihood of events Ee and Εμ. We focus here on escape Ee, immune evasion , and cancer loss (immune victory) with respective probabilities Pe, , and Pl. A similar analysis (Eq. S49) that marginalizes over the deterministic detection sizes in Eq. 11 is depicted for various immune parameters under growth-limited detection (Fig. 4). Under competent immune surveillance (high k, low pb) disease stems almost exclusively from acquired immune evasion, and the majority of threats are expected to be cleared. As turnover rate and repertoire diversity are compromised, immune escape is predicted to dominate over acquired evasion in its overall contribution to disease. Here, the overall predicted success probability of disease clearance, Pl, is again maximal for large and small r. In all cases, ‘sneak through’ is observed for slow growth threats, further supporting the growth-threshold conjecture under adaptive immune surveillance.
Figure 4:
Probability estimates of relevant immune outcomes. Dynamic threat escape, evasion, and loss probabilities as a function of relative net growth rate r/d for various values of immune turnover (rows bottom-to-top: k={10−1, 10−2,10−3}) and repertoire diversity (columns left-to-right: pb={0.5, 0.75, 1.0}) (d = 0.20, μ = 10−6, R = 104, M = 106).
The dependence of disease mechanism on immune status predicts an essential role of immune recognition in the ability to control adaptive threats. Motivated by this, we apply our model to predict cancer incidence as a function of an aging immune compartment. We select AML as a representative case due to the large amount of available incidence data, its low mutation burden, association with advanced age, and, unlike solid malignancies, relative accessibility by the adaptive immune compartment (Alexandrov et al., 2013). Empirically observed decreases in thymic output in middle age and T-cell repertoire diversity in advanced age (Naylor et al., 2005) are modeled by decreasing Hill functions of k and 1 - pb, respectively. While the absolute mapping between recognition probabilities via pr (m; m0) and TCR diversity and turnover rates remains an open problem, we may use the known relative decreases in each of these parameters as a function of age in order to estimate potential immune impairment. The model assumes equal cancer risk for every age and estimates escape probability by incorporating the total number of T-cell clones, Nt, and the probability of a single T-cell recognizing individual antigenic peptide, , via as done previously (George et al., 2017). Incidence (defined as 1–Pι) for a particular set of parameters explains empirical AML data well, outperforming the standard multi-hit model of cancer incidence (Fig. 5A).
Figure 5:
AML incidence vs immune function. (A) Bar plot of empirical data (gray) is compared to model-derived AML estimates (red dashed line) that assumes constant cancer incidence and decreasing immunity vs age. Thymic turnover rates and effective clone sizes are modeled as Hill functions. Escape probability is approximated using , where is the probability of a single TCR recognizing the immune sensitive population and Ns is the effective number of distinct clones (See SI for details). This estimate is compared to the least squares best-fit models of multi-stage incidence (solid lines), where incidence is assumed to be approximately proportional to tn, for age t and n number of hits.; (B) Predicted cumulative incidence over 10 years assuming chronic immunosuppression (1-pb and k scaled by α=0.9) and mean age of 43 prior to treatment.
The model agreement with age-specific incidence data (Cancer Research UK) would suggest that age-incidence may be partitioned into three categories: Early, unfortunate disease due to the (rare) arrival of immune evaders that escape immune detection, slightly increased incidence in middle age secondary to lower turnover rates, and dramatic, late-onset increases in risk as a result of decreasing TCR repertoire diversity. Our results are consistent with recent independent efforts to quantify immune-related cancer incidence (Palmer et al., 2018). The same parameter choice, assuming mild (~10%) decreases in T-cell recognition, is applied to characterize predicted AML incidence in the setting of chronic immunosuppression (Fig. 5B) and we find general agreement with large (n ~ 3 · 105) empirical datasets of heart and lung transplant recipients and healthy controls (Offman et al., 2004).
4. Applications to Immunotherapy
We now turn to the setting of adjuvant immunotherapy with the goal of estimating the therapeutic benefit of chimeric antigen receptor (CAR) T-cell and tumor antigen vaccination therapies. We introduce E to denote the event of overall disease elimination with corresponding probability equal to the sum of tumor loss probability due to immune system elimination (Pl above) and treatment probability, denoted by Pt . Treatment probability may be expanded by conditioning on relevant events:
| (12) |
where the necessary conditioning probabilities are given in SI. We assume that radio- (or chemo-) ablative therapy administered at time of detection TD prior to adjuvant immunotherapy reduces the population to a minimal residual level mmrd ≪ M.
4.1. CAR T-cell therapy
Chimeric Antigen Receptor (CAR) T-cells are a class of ex-vivo engineered T-cells with artificial receptors and co-stimulatory signals designed to target an epitope differentially expressed by cancer cells (Sadelain et al., 2017). Their use has been particularly effective against hematological malignancies, wherein a variety of B-cell lymphomas demonstrate response to anti-CD-19 CAR T-cells (Brudno and Kochenderfer, 2018). Importantly, the CAR T receptor is not limited to MHC-I recognition and can identify a variety of preferentially expressed tumor cell signatures, including surface proteins and carbohydrates (Yu et al., 2017). Thus, CAR T-cell therapy is effective in settings such as acute lymphoblastic leukemia, where mutation burden is low (Wang and Rivière, 2016; Martin et al., 2016). Its success in solid malignancies requires selection of preferentially over-expressed tumor-specific epitopes in the bulk tumor (Brown and Adusumilli, 2016; Newick et al., 2016).
Our analysis considers the case where a CAR T-cell clone has been engineered with recognition directed against a pre-identified tumor-specific epitope. We assume the CAR-T detection limit, mcar, is sufficiently low so that mcar ≤ mmrd (See SI for details). Deterministic detection therefore occurs immediately at size mmrd for some fixed dosage of CAR T-cells as a result of built-in co-stimulation that occurs automatically with receptor binding. We allow for the arrival of CAR epitope-evasive cells at rate 𝜇car following treatment, and neglect the event that an epitope-evasive clone is present prior to treatment. Since CAR T-cells target all cancer cells at detection, successful treatment proceeds identically independent of how the cancer population arrived at detection (escape vs. evasion). Therefore, the conditional probabilities of Eq. 12 reduce to deterministic recognition with detection limit at starting size mmrd, and may be written as a generalized version of Eq. 11.
Analytical estimates of CAR T-cell PE are plotted over all growth ranges for a variety of immune states in Fig. 6. 𝜇car~10−4 was chosen large to best illustrate CAR T efficacy vs growth rate. In reality, 𝜇car could be significantly less than μ depending on the CAR T target, so that Fig. 6 underestimates CAR T benefit. Intuitively, CAR T therapy is predicted to impart substantial treatment benefit independent of immune functioning because CAR T cancer elimination does not rely upon the host immune system. Detection at mmrd is effectively size-limited, and so this therapy is also less effective against threats with larger net growth rates.
Figure 6:
Predicted disease elimination probability (Pl+ Pe). CAR T (green) neoantigen vaccine (red) therapy benefit is compared to non-treatment survival predictions (black) as a function of relative net growth rate r/d for various values of immune turnover (rows bottom-to-top: k={10−1, 10−2,10−3}) and repertoire diversity (columns left-to-right: pb={0.5, 0.75, 1.0}) (d = 0.20, μ = 10−6, R = 104, M = 106, mmrd = 103, R = 0 for neoantigen vaccine case, μcαr = 10−4).
4.2. Autologous neoantigen vaccines
Autologous neoantigen vaccines are another type of immunotherapy which relies on the delivery of tumor antigens to the cell in order to augment or enhance the effect of neoantigen-specific CD8+ T-cells (Fritsch et al., 2014). Contrasting their reduced efficacy in tumors with lower mutation rates (Martin et al., 2016), neoantigen vaccines have been effective against highly mutagenic tumors, such as melanoma, where the neoantigen burden is significant (Ott et al., 2017; Hellmann and Snyder, 2017; Sahin et al., 2017). In our framework, successful immune priming occurs if sufficient levels of vaccine-delivered antigen suddenly become available for activating T-cells, effectively reducing the detection threshold R. For simplicity we assume that a sufficient level of tumor antigens are present so that R = 0 and any residual cancer size can be targeted.
Unlike CAR T treatment, vaccine therapy is only successful when there is an absence of immune evasive cells both prior to and following treatment , since type-2 cells can still evade vaccine-sensitized host T-cells. The only conditional probability in Eq. 12 that contributes to treatment success is , which is none other than Pl in the adaptive model with detection and initial population size both equal to mmrd (see SI). The final required quantity is the factor calculated as follows:
| (13) |
Vaccine treatment efficacy is plotted alongside CAR T therapy in Fig. 6. There is minimal predicted treatment benefit in immunocompromized patients, consistent with the fact that vaccine strategies are reliant upon proper immune recognition. The treatment benefit appears to be maximal for patients with preserved repertoire diversity and low T-cell turnover, presumably the result of allowing more time to pass, and hence immune turnover to occur, at low population sizes. Elimination of slow-growth threats that would normally sneak-through immune detection are increased, since there is no post-treatment detection limit.
5. Discussion
Our findings support the importance of the growth-threshold conjecture as an essential aspect of empirical recognition dynamics, since size-limited detection predicts an exclusive preference for fast-growing threats. In addition to static growth and recognition, ‘sneak-through’ is predicted by a new mechanism that involves preferential acquisition of immune evasion for slow and large growth rates.
Application of this relatively simple model to AML provides a quantification of the trade-off between T-cell turnover and repertoire size and control of malignancy. Moreover,the assumption that incidence carries equal risk through time places no mechanistic restrictions on the molecular generation of tumor development, and stands as a prediction of tumor generation largely orthogonal to the canonical ‘multi-hit’ hypothesis (Armitage and Doll, 1954). We find not only that this model agrees more closely with the data when compared to the best-fit multihit predictions, but that the immune incidence curve also accurately reproduces the sigmoid shape of AML incidence in a way that is impossible for the convex multi-hit model. It is also interesting that gender-specific differences in thymic output (favoring females) also correlate with a systematic reduction in AML incidence (Pido-Lopez et al., 2001). From a clinical standpoint, the prediction of maximizing immune health in order to mitigate incidence is broadly quantified for tumors that may adopt immune evasion strategies, as well as more general immune threats. In the case of treatment-refractory AML with subsequent hematopoietic stem cell transplant, our results would suggest that an emphasis be placed on maintaining repertoire diversity and, where possible, thymic turnover (Holländer et al., 2010).
In an attempt to provide an initial and straightforward presentation, we assumed pure birth in the recognition and death phase. Future analysis using the more complicated birth-death process would likely increase the rate of immune evasion generation during immune killing. We have also assumed immune killing ability occurs quickly and to a sufficient level for all threat sizes. We have also inherently assumed in this analysis that the threat net growth rate is fixed. We believe that this is a reasonable assumption for studying a rapidly growing threat such as AML, where reduced growth occurs only in advanced disease Akinduro et al. (2018). Other dynamic threats including many solid tumors, characterized by complicated, time-varying growth rates, are analytically intractable in this framework, necessitating a more extensive reliance on numerical simulation. In general, for sigmoid growth, the above results assuming mean exponential growth provide an upper-estimate on tumor escape, while evasion under growth-limited detection inherently depends on the functional form of the growth rate. Though an approximation, we believe these dynamics are reasonable as a naïve T-cell clone expands rapidly, though not instantaneously, to become a dominant clone during proper immune activation (Desponds et al., 2016).
We selected AML as a model disease for initial assessment of our model owing to several applicable features. Its rapid growth is well-approximated by a growth rate homogeneous in time and size. Unlike their solid counterparts, the immune cells have no difficulty interfacing with hematological cancer cells. Lastly, AML’s characteristically low mutational burden enables us to estimate immune evasion in the simplified framework above without the risk of ignoring possibly many intermediate phenotypes with mild evasion potential. It is likely however that the tumor-immune co-evolution described here applies broadly to many cancer types, and therefore warrants careful consideration of other relevant factors, such as mutational burden and immune infiltration, in future analyses (Blank et al., 2016).
The advantage of antigen vaccine strategies studied here resulted from augmented immune priming of the T-cell repertoire, thereby allowing recognition to overcome detection limits. Variations due to cancer subtype, including antigenicity and (for solid tumors) T-cell infiltration, may substantially affect immune priming, and hence vaccine efficacy, but are not considered here. In particular, the subclonal neoantigen landscape is an important consideration that warrants further analysis for a more complete understanding. Future studies will further distinguish between clones of differing fitnesses dependent upon their antigenicity and intermediate levels of immune evasion in an attempt to better characterize the co-evolution between a dynamic tumor and adaptive immune compartment.
Supplementary Material
Highlights.
Stochastic analysis of cancer growth in the face of adaptive immune pressure
Effects of immune recognition on tumor progression and acquired evasion are studied
Immune detection limited by size supports sneak- through of slow-growth threats
Cancer immune elimination and evasion probabilities are analytically estimated
Effects of decreased immune functioning explain AML age-incidence data
Acknowledgments
We would like to thank Jeffrey Molldrem for fruitful discussions on AML immunotherapy. J.T.G. is supported by the National Cancer Institute of the National Institutes of Health (F30CA213878). H.L. is supported by Cancer Prevention and Research Institute of Texas Scholars program (R1111).
Footnotes
Appendix
Supplementary information associated with this manuscript may be found in the attached document. Additional stochastic trajectories, data, and corresponding code for Fig. 1 may be found in the Data in Brief.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akinduro O, Weber T, Ang H, Haltalli M, Ruivo N, Duarte D, Rashidi N, Hawkins E, Duffy K, Celso CL, 2018. Proliferation dynamics of acute myeloid leukaemia and haematopoietic progenitors competing for bone marrow space. Nature communications 9, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen- Dale AL, et al. , 2013. Signatures of mutational processes in human cancer. Nature 500, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK, 2017. Pd-1 and pd-l1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Frontiers in pharmacology 8, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew SM, Baker CTH, Bocharov GA, 2007. Rival approaches to mathematical modelling in immunology. Journal of Computational and Applied Mathematics 205, 669–686. doi: 10.1016/j.cam.2006.03.035. [DOI] [Google Scholar]
- Arias CF, Herrero MA, Cuesta JA, Acosta FJ, Fernandez- Arias C, 2015. The growth threshold conjecture: a theoretical framework for understanding T-cell tolerance. Royal Society open science 2, 150016. doi: 10.1098/rsos.150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage P, Doll R, 1954. The age distribution of cancer and a multi-stage theory of carcinogenesis. British journal of cancer 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank CU, Haanen JB, Ribas A, Schumacher TN, 2016. The cancer immunogram. Science 352, 658–660. [DOI] [PubMed] [Google Scholar]
- Bocharov G, Ludewig B, Bertoletti A, Klenerman P, Junt T, Krebs P, Fraser C, Anderson RM, Luzyanina T, 2004. Underwhelming the Immune Response : Effect of Slow Virus Growth on CD8 + T-Lymphocyte Responses. Journal of virology 78, 2247–2254. doi: 10.1128/JVI.78.5.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Mocellin S, 2009. Suppressive influences in the immune response to cancer. Journal of immunotherapy 32, 1–11. [DOI] [PubMed] [Google Scholar]
- Brown CE, Adusumilli PS, 2016. Next frontiers in CAR T-cell therapy. Molecular Therapy - Oncolytics 3, 16028 URL: http: //dx.doi.org/10.1038/mto.2016.28, doi: 10.1038/mto.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudno JN, Kochenderfer JN, 2018. Chimeric antigen receptor t-cell therapies for lymphoma. Nature Reviews Clinical Oncology 15, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo AB, Kyte JA, Carretero J, Zinchencko S, Mendez R, González-Aseguinolaza G, Ruiz-Cabello F, Aamdal S, Gaudernack G, Garrido F, et al. , 2014. Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. International journal of cancer 134, 102–113. [DOI] [PubMed] [Google Scholar]
- Cancer Research UK, 2013. Acute myeloid leukaemia (aml) incidence by age. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/leukaemia-aml/incidence (accessed 2017–09-01).
- Carretero R, Romero JM, Ruiz-Cabello F, Maleno I, Rodriguez F, Camacho FM, Real LM, Garrido F, Cabrera T, 2008. Analysis of hla class i expression in progressing and regressing metastatic melanoma lesions after immunotherapy. Im- munogenetics 60, 439. [DOI] [PubMed] [Google Scholar]
- Chisholm RH, Lorenzi T, Lorz A, Larsen AK, Almeida L, Es- cargueil A, Clairambault J, 2015. Emergence of drug tolerance in cancer cell populations: an evolutionary outcome of selection, non-genetic instability and stress-induced adaptation. Cancer research, canres—2103. [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J, 2013. Cancer immunotherapy. Science 342, 1432–1433. [DOI] [PubMed] [Google Scholar]
- Desponds J, Mora T, Walczak AM, 2016. Fluctuating fitness shapes the clone-size distribution of immune repertoires. Proceedings of the National Academy of Sciences 113, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessens G, Kline J, Gajewski TF, 2009. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunological reviews 229, 126–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD, 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology 3, 991–998. URL: http://www.nature.com/doifinder/10.1038/ni1102-991, doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Fridman WH, Mlecnik B, Bindea G, Pages F, Galon J, 2011. Immunosurveillance in human non-viral cancers. Current Opinion in Immunology 23, 272–278. doi: 10.1016/j.coi.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Fritsch EF, Hacohen N, Wu CJ, 2014. Personal neoantigen cancer vaccines: the momentum builds. OncoImmunology 3, e29311. doi: 10.4161/onci.29311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido F, Aptsiauri N, Doorduijn EM, Lora AMG, van Hall T, 2016. The urgent need to recover mhc class i in cancers for effective immunotherapy. Current opinion in immunology 39, 4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JT, Kessler DA, Levine H, 2017. Effects of thymic selection on t cell recognition of foreign and tumor antigenic peptides. Proceedings of the National Academy of Sciences, 201708573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JT, Levine H, 2018. Stochastic trajectories and escape probabilities for immune evasion of a static threat. Data in brief, Submitted. [Google Scholar]
- Grossman Z, Paul WE, 1992. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proceedings of the National Academy of Sciences 89, 10365–10369. URL: http://www.pnas.org/cgi/doi/10.1073/pnas.89.21.10365, doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Snyder A, 2017. Making It Personal: Neoantigen Vaccines in Metastatic Melanoma. doi: 10.1016/j.immuni.2017.08.001. [DOI] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. , 2014. Predictive correlates of response to the anti-pd-l1 antibody mpdl3280a in cancer patients. Nature 515, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holländer GA, Krenger W, Blazar BR, 2010. Emerging strategies to boost thymic function. Current opinion in pharmacology 10, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa Y, Nowak MA, Michor F, 2006. Evolution of resistance during clonal expansion. Genetics 172, 2557–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen P, Storni T, Rettig L, Qiu Z, Der-Sarkissian A, Smith KA, Manolova V, Lang KS, Senti G, Mullhaupt B, Gerlach T, Speck RF, Bot A, Kundig TM, 2008. Antigen kinetics determines immune reactivity. Proceedings of the National Academy of Sciences 105, 5189–5194. URL: www.pnas.org/cgi/doi/10.1073/pnas.0706296105, doi: 10.1073/pnas.0706296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khailaie S, Bahrami F, Janahmadi M, Milanez-Almeida P, Huehn J, Meyer-Hermann M, 2013. A mathematical model of immune activation with a unified self-nonself concept. Frontiers in Immunology 4, 1–19. doi: 10.3389/fimmu.2013.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner D, Panetta JC, 1998. Modeling immunotherapy of the tumor - immune interaction. Journal of Mathematical Biology 37, 235–252. URL: http://link.springer.com/10.1007/s002850050127, doi: 10.1007/s002850050127. [DOI] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP, et al. , 1996. Enhancement of antitumor immunity by ctla-4 blockade. Science 271, 1734–1736. [DOI] [PubMed] [Google Scholar]
- Lin A, Yan WH, 2015. Human leukocyte antigen-g (hla-g) expression in cancers: roles in immune evasion, metastasis and target for therapy. Molecular Medicine 21, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SD, Brown SD, Wick DA, Nielsen JS, Kroeger DR, Twumasi-Boateng K, Holt RA, Nelson BH, 2016. Low mutation burden in ovarian cancer may limit the utility of neoantigen-targeted vaccines. PLoS ONE 11, e0155189. doi: 10.1371/journal.pone.0155189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michor F, Iwasa Y, Nowak MA, 2004. Dynamics of cancer progression. Nature reviews cancer 4, 197–205. [DOI] [PubMed] [Google Scholar]
- Nani FK, Ouztoäreli MN, 1994. Modelling and simulation of Rosenberg-type adoptive cellular immunotherapy. Mathematical Medicine and Biology 11, 107–147. doi: 10.1093/imammb/11.2.107. [DOI] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ, 2005. The influence of age on t cell generation and tcr diversity. The Journal of Immunology 174, 7446–7452. [DOI] [PubMed] [Google Scholar]
- Newick K, Moon E, Albelda SM, 2016. Chimeric antigen receptor T-cell therapy for solid tumors. Molecular Therapy - Oncolytics 3, 16006 URL: http://linkinghub.elsevier.com/retrieve/pii/S2372770516300456, doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offman J, Opelz G, Doehler B, Cummins D, Halil O, Banner NR, Burke MM, Sullivan D, Macpherson P, Kar- ran P, 2004. Defective dna mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood 104, 822–828. [DOI] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon H, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ, 2017. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Albergante L, Blackburn CC, Newman T, 2018. Thymic involution and rising disease incidence with age. Proceedings of the National Academy of Sciences 115, 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pido-Lopez J, Imami N, Aspinall R, 2001. Both age and gender affect thymic output : more recent thymic migrants in females than males as they age. Clin Exp Immunol , 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeu T, Jaeger S, Vivier E, 2013. The speed of change: towards a discontinuity theory of immunity? Nature Reviews Immunology 13, 764–769. URL: http://www.nature.com/oifinder/10.1038/nri3521, doi: 10.1038/nri3521. [DOI] [PubMed] [Google Scholar]
- Sadelain M, Riviere I, Riddell S, 2017. Therapeutic T cell engineering. Nature 545, 423–431. URL: 10.1038/nature22395, doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke B.p., Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Müller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Brück A.k., Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Höller C, Utikal J, Huber C, Loquai C, Türeci Ö, 2017. Personalized rna mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226. URL: 10.1038/nature23003, doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- Sheu J, Shih IM, 2010. Hla-g and immune evasion in cancer cells. Journal of the Formosan Medical Association 109, 248–257. [DOI] [PubMed] [Google Scholar]
- Sontag ED, 2017. A Dynamic Model of Immune Responses to Antigen Presentation Predicts Different Regions of Tumor or Pathogen Elimination. Cell Systems 4, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski TF, 2015. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231. [DOI] [PubMed] [Google Scholar]
- Straten TP, Garrido F, 2016. Targetless t cells in cancer immunotherapy. Journal for immunotherapy of cancer 4, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi SC, Peters HL, Taguchi A, Katayama H, Wang H, Momin A, Jolly MK, Celiktas M, Rodriguez-Canales J, Liu H, et al. , 2016. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proceedings of the National Academy of Sciences 113, E1555–E1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rivière I, 2016. Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Molecular Therapy - Oncolytics 3, 1–7. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Li A, Liu Q, Li T, Yuan X, Han X, Wu K, 2017. Chimeric antigen receptor t cells: a novel therapy for solid tumors. Journal of hematology & oncology 10, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.