Abstract
The production of reactive oxygen species (ROS) is a prominent response to infection among innate immune cells such as macrophages and neutrophils. To better understand the relationship between antimicrobial and regulatory functions of blood cell ROS, we have characterized the ROS response to infection in Drosophila hemocytes. Using fluorescent probes, we find a biphasic hemocyte ROS response to bacterial infection. In the first hour, virtually all hemocytes generate a transient ROS signal, with nonphagocytic cells including prohemocytes and crystal cells displaying exceptionally strong responses. A distinct, and more delayed ROS response starting at 90 minutes is primarily within cells that have engulfed bacteria, and is sustained for several hours. The early response has a clear regulatory function, as dampening or intensifying the intracellular ROS level has profound effects on plasmatocyte activation. In addition, ROS are necessary and sufficient to activate JNK signaling in crystal cells, and to promote JNK-dependent crystal cell rupture. These findings indicate that Drosophila will be a promising model in which to dissect the mechanisms of ROS stimulation of immune activation.
Keywords: Drosophila, hemocyte, ROS, adhesion, cellular immune response, crystal cell
1. Introduction
Reactive oxygen species (ROS) are compounds such as superoxide anion (O2−), hydroxyl radical (·OH), peroxynitrite anion (ONOO−) and hydrogen peroxide (H2O2) that are short-lived and potent oxidants of a wide number of biological macromolecules. ROS are generated both as byproducts of mitochondrial electron transport chain respiration, as well as by dedicated enzymes.
Phagocytic uptake of microbes by mammalian phagocytes such as neutrophils and macrophages is accompanied by an energetically costly “respiratory burst” of O2 consumption that is converted into O2− and other ROS. The importance of this process to immune defense is evident in the severe immunodeficiency of Chronic Granulomatous Disease, caused by defects in the NADPH oxidase that generates the bulk of O2− produced during phagocytosis [1]. Indeed, O2− and other ROS are delivered to microbe-containing phagosomes where they wield strong microbicidal activity [2]. Additionally, it has become clear that ROS have regulatory roles in immune cells, apart from their antimicrobial effects [3, 4]. However, the relative importance of antimicrobial vs. signalling roles of ROS in immune cells is poorly understood, and major questions include the sources and molecular identities of ROS, the mechanisms regulating ROS production, and how ROS exert their regulatory effects.
The fruit fly Drosophila relies solely on innate immune mechanisms for antimicrobial defense [5], including a well-characterized population of blood cells, or hemocytes, that carries out such evolutionarily conserved immune defenses as phagocytosis, encapsulation, and wound healing [6, 7]. Specification of hemocyte lineages from multipotent progenitors is regulated by evolutionarily conserved hematopoietic factors [8], with ROS recently found to provide additional regulation [9, 10]. However, the production and function of ROS by circulating hemocytes during infection has not been described.
Here we undertook a systematic characterization of the Drosophila blood cell ROS response in the early hours following bacterial infection, and describe two important aspects of cellular immune activation that are promoted by intracellular ROS.
2. Materials and methods
2.1. Drosophila stocks
These Drosophila strains were obtained from the Bloomington Stock Center: He-GAL4 (RRID:BDSC_8699); HmlΔ-GAL4 (RRID:BDSC_30141); Lz-GAL4; UAS-GFP (RRID:BDSC_6313); UAS-GFP (RRID:BDSC_6874); UAS-TRE-Red (RRID:BDSC_59012). Pxn-GAL4 (FBtp0022490) was kindly donated by Utpal Banerjee. All “wild-type” strains were relevant GAL4 driver outcrossed to either UAS-GFP or to w; P(w+).
2.2. Infection
Larvae were infected with E. coli expressing tdTomato (RFP) or GFP, directly injected using pulled capillary needles and Picospritzer injection pump.
2.3. Hemocyte Extraction and Treatments
Hemocytes were extracted from late 3rd instar larvae by tearing open the cuticle and bathing the carcass in media for 5-10 seconds. For live imaging (DHE, DCFDA, TRE-Red), larvae were bled into chambered coverslips, and centrifuged 60 seconds at 50g. For fixed samples (phalloidin, DAPI, crystal morphology) and melanization assay, larvae were bled directly onto slides into 50 – 100 μL media, and hemocytes allowed to settle before fixation (2% paraformaldehyde, 20 mins) or melanization assessments. Prior to bleeding, larvae were surface sterilized by dipping 5-10 seconds in 70% Ethanol, and then kept on wet paper towel for 20 mins to remove residual surface yeast.
Solutions of menadione (Cayman 15950), SP600125 (Cayman 10010466), and NAC (Cayman 20261) were prepared in Schneider’s media.
For DHE, larvae were bled into 30μM DHE (Thermofisher D11347) in PBS, immediately centrifuged 60 seconds at 50g; incubated 15 mins in the dark; re-centrifuged 60 seconds X 50g; DHE/PBS was replaced with PBS, followed by a final 50g centrifugation and immediate imaging. For DCFDA, larvae were bled into 10μM CM-H2-DCFDA (Thermofisher C6827), and subjected to the same 15 min incubation, wash, and centrifugation steps as DHE, but resuspended in media for imaging. DHE intensities were defined according to fluorescence values: none < 350; weak: 351-500; moderate: 501-1000; strong: 1000-2000; intense: >2001.
Actin was visualized following fixation with 2 hour staining in 400 nM TRITC-phalloidin. Samples mounted in Vectashield.
2.4. Image Collection and Analysis
Phase-contrast and epifluorescence images were collected on an Olympus IX83 inverted microscope. Quantifications of fluorescence intensity and cell diameters were done with Olympus CellSens software.
2.5. Adherent Cell Counts
Using 10x lens, images were collected of 10 random fields per sample. The average number of hemocytes per field (.56 mm2) was multiplied by 143 to approximate the number of hemocytes in entire 1 cm diameter (79 mm2) sample. This number was divided by the number of larvae to arrive at # of hemocytes/larva retained.
3. Results and Discussion
3.1. Bacterial Infection Induces Both Rapid and Delayed Hemocyte ROS Responses
Hemocyte production of ROS was visualized using two cell-permeant redox probes: dihydroethidium (DHE) is oxidized by superoxide and other ROS to ethidium and 2-hydroxyethidium, which fluoresce red upon binding DNA [11]; and dichlorodihydrofluorescein diacetate (DCFDA) is oxidized to green fluorescein by a variety of cellular oxidants including H2O2 [12].
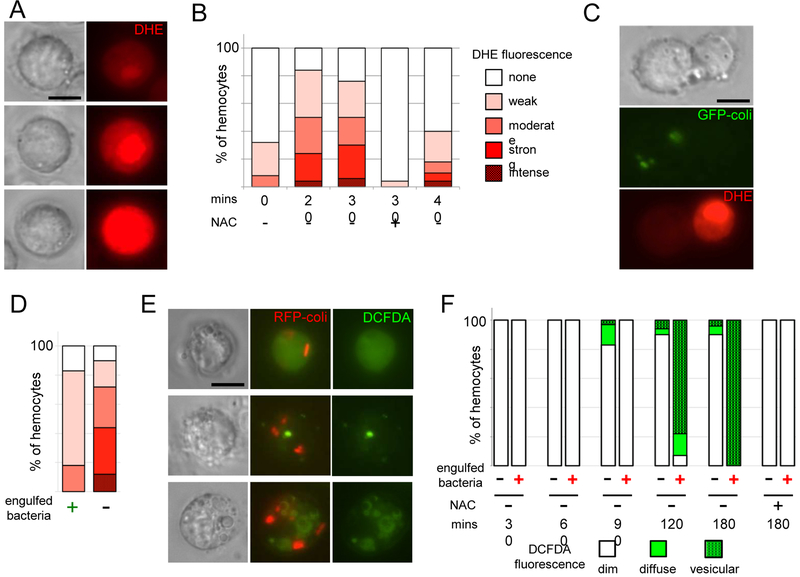
Bacterial infection triggers a rapid but heterogeneous ROS response in larval hemocytes. At 20 minutes, DHE fluorescence is visible in over 80% of hemocytes, ranging considerably in intensity (Fig 1 a, b). The percentage of DHE-positive hemocytes then declines rapidly between 30 and 40 minutes. Incubation of hemocytes in in N-acetyl cysteine (NAC), which replenishes cellular antioxidants [13], virtually extinguishes DHE fluorescence (Fig 1b), confirming the ROS-dependence of the DHE signal.
Fig. 1.
Biphasic ROS production by larval hemocytes following bacterial infection. (A) Examples of DHE fluorescence intensity in hemocytes 30 mins after infection. Top: moderate; Middle: strong; Bottom: intense. (B) Quantification of DHE fluorescence intensity frequencies in hemocytes analysed at time points following bacterial infection (see Methods). (C) 30 mins after infection with GFP expressing E. coli, left cell has engulfed bacteria, and has weak DHE fluorescence; right cell has not engulfed bacteria and has strong DHE fluorescence. (D) Quantification of DHE fluorescence intensities among hemocytes that have (left) and have not (right) engulfed bacteria at 30 mins postinfection, as in (C). 80 hemocytes were examined; about 50% of hemocytes have visible bacteria at this time point. Scale as in (B). (E) Examples of DCFDA (green) fluorescence in hemocytes 2 hours following infection with RFP-expressing E. coli. Top: diffuse fluorescence; Middle & Bottom: vesicular fluorescence. (F) Quantification of DCFDA fluorescence patterns in hemocytes analysed at time points following bacterial infection. NAC: 10mM. Results are representative of at least 3 experiments. Percentages in B, D, F derived from counts of ≥ 50 hemocytes from single experiment. Scale bars: 5 μm.
Larval hemocytes rapidly phagocytose bacteria, and we expected that phagocytosis would trigger a strong ROS response. However, hemocytes with engulfed bacteria generally displayed quite dim DHE fluorescence (Fig. 1 c, d). The hemocytes with the highest intensities of DHE fluorescence were never found to have engulfed bacteria.
DCFDA fluorescence revealed a more delayed hemocyte ROS response to infection. DCFDA fluorescence was first detected at 90 minutes after infection, with the strongest signal found at 2-3 hours (Fig. 1 e, f). Unlike DHE, DCFDA fluorescence was strongly associated with phagocytosis; 90%-100% of hemocytes with engulfed bacteria were also DCFDApositive. Although about 10% of hemocytes without detectable internalized bacteria were also DCFDApositive, it is possible that those hemocytes had engulfed bacteria that were no longer detectable. Hemocytes showed two patterns of DCFDA fluorescence: diffuse staining throughout the cell, and more commonly, fluorescence in vesicles in a range of sizes (Fig. 1e, f). These DCFDA-positive vesicles were generally not directly localized to bacteria-containing phagosomes (Fig. 1e, f).
It is not possible to definitively establish that the early response (seen with DHE) is superoxide, and that the later response (DCFDA) is H2O2, because the probes are only somewhat selective for ROS [11, 12]; nevertheless the ROS predominating at the two stages are clearly distinct. Additionally, the strength of the DHE signal in non-phagocytic cells indicates that the early ROS are likely to have a primarily regulatory rather than microbicidal function.
3.2. Strongest Rapid ROS Responses in Crystal Cells and Immature Prohemocytes
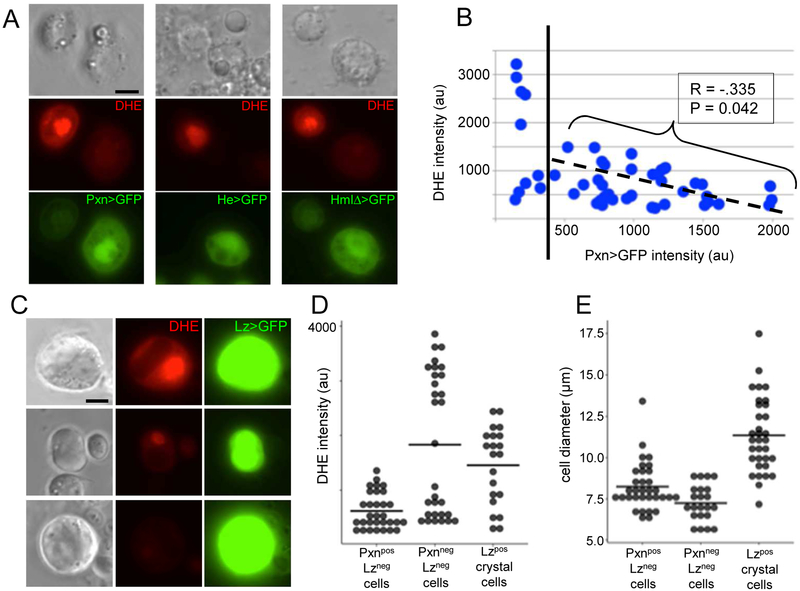
The two major larval hemocyte subtypes are the macrophage-like plasmatocytes (95%) and the crystal cells (5%), which rupture to release a proenzyme in the humoral melanization response [7]. Lamellocytes, huge flattened hemocytes, comprise a third subtype generally only seen during sustained infection [14, 15]. Additionally, small undifferentiated prohemocytes are found in low numbers in circulation [14]. To characterize the nonphagocytic hemocytes generating the rapid high ROS response, we used the GAL4/UAS system [16] and a panel of hemocyte GAL4 drivers driving GFP expression. Peroxidasin-GAL4 (Pxn; [17]), Hemese-GAL4 (He; [18]), and HemolectinΔ-GAL4 (HmlΔ; [19]) all drive transgene expression in the majority of hemocytes [20]. To our surprise, the hemocytes with the highest DHE intensity expressed generally low levels of all three plasmatocyte GAL4 drivers; this along with their small size indicates they are prohemocytes (Fig 2a,e; [14, 15]).
Fig. 2.
DHE intensity is strongest in undifferentiated prohemocytes and in crystal cells. (A) Examples of hemocyte pairs showing inverse correlation between DHE fluorescence intensity and expression level of hemocyte GAL4 drivers Peroxidasin (Pxn), Hemese (He), and HemolectinΔ (HmlΔ). (B) Quantification of relationship between DHE fluorescence intensity and level of Pxn>GFP expression. Pearson correlation coefficient and P value calculated using values to the right of dotted line (Pxn-GFP > 400). (C) Examples of DHE fluorescence in crystal cells expressing Lozenge (Lz)-GAL4 and UAS-GFP (crystal morphology less distinct in aqueous media used for live imaging). (D,E) Comparison of DHE intensities (D) and cell body diameters (E) among Pxnpos hemocytes, Pxnneg hemocytes, and Lzpos crystal cells. Scale bars: 5μm.
We analysed the relationship between ROS level and GAL4 expression more closely for Pxn. Among plasmatocytes with detectable (>350) GFP fluorescence driven by Pxn-GAL4 there was a striking negative correlation between DHE fluorescence intensity and strength of Pxn>GFP expression (Fig 2b). Although Pxn proteins catalyse the reaction of H2O2 to hypohalous acids [21], we do not anticipate that Pxn proteins themselves influence intracellular ROS levels since Pxn is a secreted protein (Fig 2a). Perhaps when plasmatocytes get activated by infection, they temporarily downregulate genes such as Pxn that primarily functions in basement membrane maintenance [21]. The prohemocytes with the lowest levels of Pxn expression (Pxnneg; <350) display a bimodal distribution of DHE fluorescence, with either very low or very high levels of intracellular ROS (Fig 2b).
The crystal cell driver Lozenge-GAL4 (Lz; [22]) revealed high levels of intracellular ROS in some crystal cells, with others showing moderate and low levels of DHE fluorescence (Fig 2 c, e). There was no correlation between the strength of Lz-GAL4 expression and either cell size or DHE fluorescence (Fig 2 c-e).
In summary, our results show that the rapid hemocyte ROS response to infection is heterogeneous, with the highest production of intracellular ROS among the undifferentiated prohemocytes and the crystal cells.
3.3. Hemocyte ROS production activates plasmatocyte spreading and adhesion
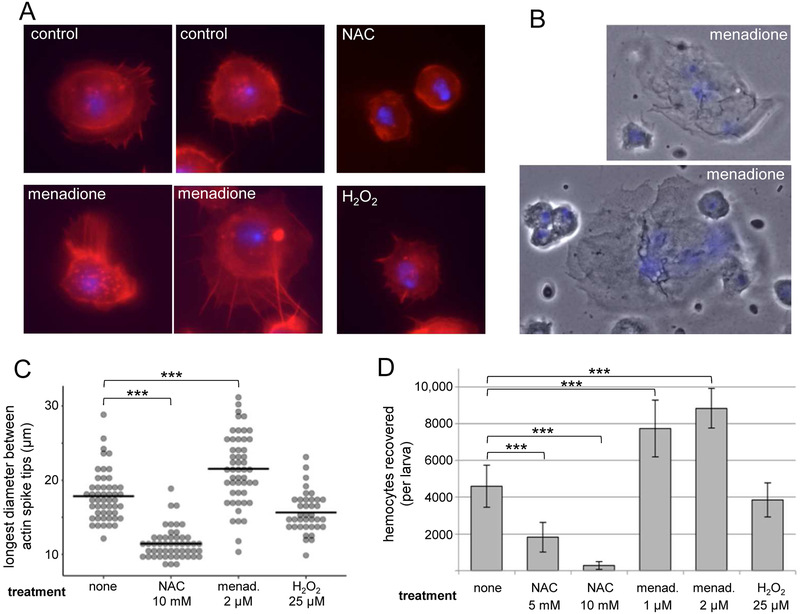
ROS are required for the correct specification of blood cell progenitors during hematopoiesis, and genetic manipulation of ROS production using existing hemocyte GAL4 drivers results in aberrant hemocyte differentiation and numbers ([9] and data not shown). Therefore, we took an ex vivo pharmacological approach to investigate the post-hematopoietic immune function of hemocyte ROS. To determine the effect of rapid ROS production, we examined the behavior of hemocytes from uninfected animals 30 minutes after exposure to modulators of ROS: to boost intracellular ROS, we used low concentrations (1-2 μM) of menadione, which provokes redox cycling and ROS generation [23], and we used the antioxidant NAC [13] to dampen intracellular ROS.
When plasmatocytes settle on a glass slide, they adhere and spread in a regulated actin-dependent activation response that is a proxy for in vivo processes of spreading and clotting at wounds, as well as phagocytosis ([6, 24-26] Fig 3a). When bled into media containing NAC, instead of spreading and extending actin-based lamellae, hemocytes remain rounded up, maintaining a small diameter and actin constrained to a cortical ring (Fig 3a,c). Conversely, hemocytes bled into menadione elaborate much more extensive actin protrusions than controls, either radially or polarized in a single direction, and additionally display exuberant membrane ruffling (Fig 3a, c and Suppl Fig 1).
Fig. 3.
ROS promote plasmatocyte adhesion and spreading. (A) Examples of hemocyte morphology 30 minutes after bleeding into Schneider’s media (control), 2μM menadione, 5 mM NAC, 25 μM H2O2. DAPI: blue. Phalloidin: red. (B) Examples of lamellocytes recovered from uninfected larvae 30 minutes after bleeding into 2 μM menadione. (C) Quantification of cell spreading seen in (A); cell measurements made of longest diameter between actin spike tips. (D) Quantification of hemocytes recovered after 30 minutes on glass slide in indicated media, followed by fixation and washes. Scale bars: 5μm in (A) and 20μm in (B). Arrowhead indicates membrane ruffling. *** P< 0.001.
This increased spreading is also associated with increased adhesion to the glass substrate; the number of hemocytes firmly adhered following 30 minute incubation and remaining after fixation and washing steps, was 68% - 92% higher in menadione than in control media, and 60% - 94% lower in NAC (Fig 3d). In addition to the lower number of hemocytes recovered in NAC, the lower adhesion strength of those remaining was evident upon close observation: many of the hemocytes were floating in the mounting media above the plane of the glass, apparently tethered at a single point (Suppl Fig 2).
Also striking was the recovery of multinucleate lamellocytes after 30 minutes incubation in menadione, even from uninfected larvae, where they are reported to be very rare ([14] Fig 3b). It is unlikely that plasmatocytes transdifferentiate into lamellocytes during the very short incubation in menadione, thus we favor the hypothesis that lamellocytes are present in low numbers in uninfected larvae, but are not activated to adhere and thus rarely recovered in hemocyte preps from uninfected controls, but that ROS is sufficient to activate their adhesion.
Because H2O2 is an ROS that can cross the plasma membrane [27], and acts as macrophage chemoattractant to wound sites in Drosophila and zebrafish [28, 29], we tested whether the enhanced cellular protrusions and adhesion caused by menadione treatment could be replicated by exposure to H2O2. However, we found that neither 5 μM, 25 μM, nor 125 μM H2O2 enhanced the actin extensions or adhesion of hemocytes to the slide (Fig 3a, c, d, and data not shown).
Menadione and NAC both function intracellularly to modulate intracellular ROS levels. This, along with the failure of exogenous H2O2 to replicate the activating effects of menadione, as well as the short half-life and inability to cross the plasma membrane of most ROS, lead us to favor a model whereby ROS function as cell-intrinsic inflammatory mediators that activate plasmatocytes, a mechanism with some precedence from mammalian systems [3]. The function of ROS in the prohemocytes is not clear; notably only about half of them generating the strong ROS response (Fig 2a, d), and those that do remain rounded instead of flattening and spreading (Fig 1c, 2a). Future investigations may reveal a different response to ROS among these putative progenitor cells; in the hematopoietic niche, ROS in prohemocytes can prime them to either proliferate or differentiate [9, 15, 30].
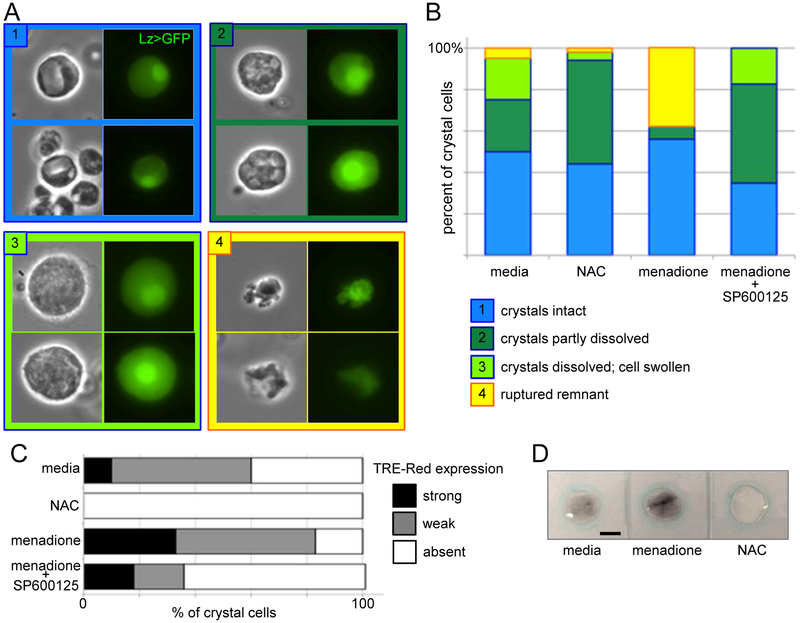
3.4. Hemocyte ROS activate crystal cell rupture and melanization
Crystal cells are platelet-like cells that rupture to release enzymes that promote clotting and melanization, an immune response critical for the encapsulation and killing of invaders [31, 32]. Because high levels of intracellular ROS were observed in crystal cells (Fig 2c, d), we assessed the effects of intensifying and dampening the ROS response using menadione and NAC, respectively. Upon immune stimulation, first the intracellular prophenoloxidase crystals of crystal cells begin to dissolve, then the cell swells and finally bursts, releasing the melanization enzymes into the hemolymph ([31]; Fig 4a). Thirty minutes after bleeding into control media, about 50% of Lzpos crystal cells have intact crystals with sharp edges; another 25% show partially dissolved crystals with rounded surfaces; 20% are swollen and have fully dissolved crystals; and about 5% are ruptured (Fig 4b). It can be difficult to identify the ruptured remains of crystal cells, which are also sometimes engulfed by plasmatocytes (data not shown), and so the 5% ruptured is likely an undercount. In the antioxidant NAC, although the percentage of cells with intact crystals is similar to controls, there is a marked expansion in the population of cells with partially dissolved crystals, and a concomitant reduction in the number of swollen cells with fully dissolved crystals that are ready to burst (Fig 4b). Conversely, the presence of menadione is associated with very low recovery of cells with partially and fully dissolved crystals, but many more cells (38%) that are fully ruptured (again, likely to be an undercount). The mechanisms coupling crystal dissolution with cell rupturing are not known, but the two processes are separately regulated [31]. Our results suggest that ROS may not be involved in the initiation of crystal dissolution, but rather promote the subsequent swelling and rupturing of the cell.
Fig. 4.
ROS promote JNK-dependent crystal cell rupture and melanization. (A) Examples of crystal cells at 4 stages according to key. (B) Crystal cell stages 30 minutes after bleeding into 10 mM NAC, 2μM menadione, and 2μM menadione + 50μM SP600125. (C) Comparison of crystal cell expression of JNK transcriptional reporter TRE-Red after 30 mins incubation in 10 mM NAC, 2μM menadione, and 2μM menadione + 50μM SP600125. (D) Melanization (black) 40 minutes after 10 larvae each bled into 60 μM of Schneider’s media, 2 μM menadione, or 5 mM NAC. Scale bars: 10μm in (A) and 500μm in (D).
Similarly, the JNK pathway is required for crystal cell rupture, but not the earlier crystal dissolution step [31]. Since ROS activate JNK signalling in many systems, including Drosophila hematopoiesis [9], we analysed crystal cell morphology following incubation in menadione plus SP600125, a JNK inhibitor [33]. Addition of SP600125 dramatically increased the accumulation of cells with partially or fully dissolved crystals compared to menadione alone, but appeared to block the final rupturing step (Fig 4b). In further confirmation of the role of ROS in crystal cell JNK activation, we found that expression of the JNK pathway transcriptional reporter TRE-Red [34] was completely blocked after incubation in NAC, but significantly strengthened after incubation in menadione (Fig 4c). Consistent with the ROS role in promoting crystal cell rupture, melanization was increased in the presence of menadione and completely blocked in the presence of NAC (Fig. 4d). These data indicate that, in addition to activating plasmatocytes, intracellular ROS are necessary and sufficient to activate the JNK-mediated rupture of crystal cells.
3.5. SUMMARY
We have established a biphasic pattern of ROS production in the early hours of bacterial infection, and found a regulatory role for the rapid ROS response in activating two important aspects of the cellular immune response: plasmatocyte adhesion and spreading, and crystal cell rupture. Fly hemocytes have been shown to respond to extracellular H2O2 generated by distressed tissues [29, 35]. Our work establishes that hemocyte responses to intracellular and extracellular ROS signals are distinct, but more work will be necessary to understand how they are sensed differently.
Since the early ROS pulse is detected with the DHE probe, which senses O2−, and the later response visualized by DCFDA, which fluoresces upon exposure to H2O2 and other oxidants, it is tempting to speculate that the two probes are identifying two phases of the same response, with an early burst of superoxide that then dismutates to H2O2. However, this is unlikely since the DHE signal is primarily in nonphagocytic cells, whereas DCFDA is mostly in hemocytes that have taken up bacteria. Rather, we propose that these represent distinct cellular ROS responses that differ not only in timing, cell type, and the ROS compounds generated, but also in how the ROS generation is activated, as well as in the function of the ROS. One possibility is that the rapid DHE-positive ROS response is generated by mitochondria, as is true of hematopoietic ROS production [9], and the later DCFDA phagocyte response by dedicated enzymes such as NADPH oxidase. Further work dissecting these two responses will clarify the mechanisms by which they are generated, any regulatory interactions between them, and how they function differently within the cell. In short, our findings lay the groundwork for development of Drosophila as a model to investigate inflammatory roles of ROS in immune responses.
Supplementary Material
More examples of hemocyte and actin morphology 30 minutes after bleeding into (A) Schneider’s media, or (B) 2μM menadione. Red = phalloidin; Blue = DAPI. Arrowheads indicate membrane ruffling. Scale bars: 5μm.
Images taken at different focal planes of fixed and mounted hemocytes following 30 mins incubation in 5 mM NAC. Many cells are floating above the plane of the slide.
HIGHLIGHTS.
biphasic hemocyte ROS response to infection in flies
early response high in non-phagocytic prohemocytes and crystal cells
delayed ROS in phagocytic cells
ROS activate plasmatocyte spreading and adhesion
ROS promote JNK-dependent crystal cell rupture
4. Acknowledgements
This work was supported by the NIH (SC2-AI133653), and the CSU Program for Education and Research in Biotechnology. We are grateful for the assistance of Sean Walker with RStudio, Doug Green for comments on the manuscript, and Uli Theopold for advice on crystal cell assays.
Abbreviations
- NAC
N-acetyl cysteine
- JNK
N-terminal Jun kinase
- RFP-coli
E. coli expressing Red Fluorescent Protein
- GFP-coli
E. coli expressing Green Fluorescent Protein
- Pxn
Peroxidasin
- He
Hemese
- Lz
Lozenge
- HmlΔ
HemolectinΔ
- ROS
Reactive Oxygen Species
- DHE
Dihydroethidium
- DCFDA
Chloromethyl-dichlorodihydrofluorescein diacetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].Babior BM, NADPH oxidase. Curr. Opin. Immunol 16 (2004) 42–47. [DOI] [PubMed] [Google Scholar]
- [2].Slauch JM, How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol 80 (2011) 580–583. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dupre-Crochet S, Erard M, Nubetae O, ROS production in phagocytes: why, when, and where? J. Leukoc. Biol 94 (2013) 657–670. doi: 10.1189/jlb.1012544. [DOI] [PubMed] [Google Scholar]
- [4].Nathan C, Cunningham-Bussel A, Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol 13 (2013) 349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brennan CA, Anderson KV, Drosophila: the genetics of innate immune recognition and response. Annu. Rev. Immunol 22 (2004) 457–483. doi: 10.1146/annurev.immunol.22.012703.104626. [DOI] [PubMed] [Google Scholar]
- [6].Fauvarque MO, Williams MJ, Drosophila cellular immunity: a story of migration and adhesion. J. Cell. Sci 124 (2011) 1373–1382. doi: 10.1242/jcs.064592. [DOI] [PubMed] [Google Scholar]
- [7].Gold KS, Bruckner K, Drosophila as a model for the two myeloid blood cell systems in vertebrates. Exp. Hematol 42 (2014) 717–727. doi: 10.1016/j.exphem.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Evans CJ, Hartenstein V, Banerjee U, Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell. 5 (2003) 673–690. [DOI] [PubMed] [Google Scholar]
- [9].Owusu-Ansah E, Banerjee U, Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 461 (2009) 537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Small C, Ramroop J, Otazo M, et al. An unexpected link between notch signaling and ROS in restricting the differentiation of hematopoietic progenitors in Drosophila. Genetics. 197 (2014) 471–483. doi: 10.1534/genetics.113.159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zielonka J, Vasquez-Vivar J, Kalyanaraman B, Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc 3 (2008) 8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- [12].Oparka M, Walczak J, Malinska D, et al. Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods. 109 (2016) 3–11. doi: 10.1016/j.ymeth.2016.06.008. [DOI] [PubMed] [Google Scholar]
- [13].Zafarullah M, Li WQ, Sylvester J, Ahmad M, Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci 60 (2003) 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lanot R, Zachary D, Holder F, Meister M, Postembryonic hematopoiesis in Drosophila. Dev. Biol 230 (2001) 243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- [15].Sinenko SA, Hung T, Moroz T, et al. Genetic manipulation of AML1-ETO-induced expansion of hematopoietic precursors in a Drosophila model. Blood. 116 (2010) 4612–4620. doi: 10.1182/blood-2010-03-276998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brand AH, Perrimon N, Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118 (1993) 401–415. [DOI] [PubMed] [Google Scholar]
- [17].Stramer B, Wood W, Galko MJ, et al. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J. Cell Biol 168 (2005) 567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zettervall CJ, Anderl I, Williams MJ, et al. A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. U S A 101 (2004) 14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sinenko SA, Mathey-Prevot B, Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene. 23 (2004) 9120–9128. doi: 10.1038/sj.onc.1208156. [DOI] [PubMed] [Google Scholar]
- [20].Makhijani K, Alexander B, Tanaka T, Rulifson E, Bruckner K, The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 138 (2011) 5379–5391. doi: 10.1242/dev.067322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bhave G, Cummings CF, Vanacore RM, et al. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat. Chem. Biol 8 (2012) 784–790. doi: 10.1038/nchembio.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lebestky T, Chang T, Hartenstein V, Banerjee U, Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 288 (2000) 146–149. [DOI] [PubMed] [Google Scholar]
- [23].Thomas NO, Shay KP, Kelley AR, Butler JA, Hagen TM, Glutathione maintenance mitigates age-related susceptibility to redox cycling agents. Redox Biol 10 (2016) 45–52. doi: 10.1016/j.redox.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pearson AM, Baksa K, Ramet M, et al. Identification of cytoskeletal regulatory proteins required for efficient phagocytosis in Drosophila. Microbes Infect. 5 (2003) 815–824. [DOI] [PubMed] [Google Scholar]
- [25].Williams MJ, Wiklund ML, Wikman S, Hultmark D, Rac1 signalling in the Drosophila larval cellular immune response. J. Cell Sci 119 (2006) 2015–2024. doi: 10.1242/jcs.02920. [DOI] [PubMed] [Google Scholar]
- [26].Babcock DT, Brock AR, Fish GS, et al. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc. Natl. Acad. Sci. U S A 105 (2008) 10017–10022. doi: 10.1073/pnas.0709951105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bienert GP, Schjoerring JK, Jahn TP, Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 1758 (2006) 994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- [28].Niethammer P, Grabher C, Look AT, Mitchison TJ, A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 459 (2009) 996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moreira S, Stramer B, Evans I, Wood W, Martin P P, Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr. Biol 20 (2010) 464–470. doi: 10.1016/j.cub.2010.01.047. [DOI] [PubMed] [Google Scholar]
- [30].Dragojlovic-Munther M, Martinez-Agosto JA, Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development. 139 (2012) 3752–3763. doi: 10.1242/dev.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bidla G, Dushay MS, Theopold U, Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J. Cell Sci 120 (2007) 1209–1215. doi: 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- [32].Binggeli O, Neyen C, Poidevin M, Lemaitre B B, Prophenoloxidase activation is required for survival to microbial infections in Drosophila. PLoS. Pathog 10 (2014) e1004067. doi: 10.1371/journal.ppat.1004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U S A 98 (2001) 13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chatterjee N, Bohmann D, A versatile PhiC31 based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS. One 7 (2012) e34063. doi: 10.1371/journal.pone.0034063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fogarty CE, Diwanji N, Lindblad JL, et al. Extracellular Reactive Oxygen Species Drive Apoptosis-Induced Proliferation via Drosophila Macrophages. Curr. Biol 26 (2016) 575–584. doi: 10.1016/j.cub.2015.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
More examples of hemocyte and actin morphology 30 minutes after bleeding into (A) Schneider’s media, or (B) 2μM menadione. Red = phalloidin; Blue = DAPI. Arrowheads indicate membrane ruffling. Scale bars: 5μm.
Images taken at different focal planes of fixed and mounted hemocytes following 30 mins incubation in 5 mM NAC. Many cells are floating above the plane of the slide.