Abstract
Background and Aims
Adalimumab has been shown to be more effective than placebo in healing fistulae in adults with moderately to severely active Crohn’s disease. The efficacy and safety of adalimumab in healing fistulae in children/adolescents with Crohn’s disease from the 52-week IMAgINE 1 clinical trial, and its open-label extension IMAgINE 2, are reported.
Methods
Children/adolescents with perianal fistulae at baseline of IMAgINE 1 were assessed for fistula closure and improvement during IMAgINE 1 [Weeks 0–52] and from Week 0 of IMAgINE 2 [Week 52 of IMAgINE 1] through to Week 240 of IMAgINE 2 using non-responder imputation.
Results
A total of 36 children/adolescents had fistulae at baseline of IMAgINE 1 and were included in the analysis. Fistula closure and improvement were observed in 44.4% and 52.8%, respectively, at Week 12. Rates of closure and improvement were maintained throughout the analysis period to Week 292. No new safety signals were identified.
Conclusions
In children/adolescents with moderately to severely active, fistulizing Crohn’s disease, adalimumab induced perianal fistula closure and improvement within 12 weeks of treatment, with rates that were sustained for more than 5 years. The safety profile of adalimumab in patients with fistulae at baseline was similar to that of the overall population in IMAgINE 1/2. ClinicalTrials.gov identifiers: IMAgINE 1 (NCT00409682); IMAgINE 2 (NCT00686374).
Keywords: Anti-TNF, adalimumab, fistula
1. Introduction
Crohn’s disease [CD] is a chronic inflammatory disorder, principally of the gastrointestinal tract, associated with characteristic mural and often transmural granulomatous inflammation.1,2 Population-based studies have estimated that the cumulative incidence of perianal fistulae in patients with CD ranges from 23% to 38%.3 In a defined paediatric cohort, the cumulative incidence of perianal fistula was 9.4% at 10 years after diagnosis of CD.4 Severe perianal fistulizing disease is a potential predictor of poor disease outcome in paediatric CD patients.5 Complex perianal CD, commonly featuring perianal fistula, has a considerable negative impact on quality of life.6
Anti-tumour necrosis factor [anti-TNF] biologic therapies infliximab7,8 and adalimumab9 have been reported to be effective treatments in healing fistulae in adult patients with CD, but data about fistulae healing with anti-TNF therapy in children/adolescents are limited.5
IMAgINE 1 was a 52-week, randomized, double-blind Phase III trial to evaluate the efficacy and safety of adalimumab in children/adolescents with moderately to severely active CD.10 Patients who completed IMAgINE 1 and who had responded at any time were eligible to enter the open-label extension study, IMAgINE 2. The long-term efficacy and safety of adalimumab for treatment of IMAgINE 1/2 patients with CD have been reported recently.11
In the current analysis of IMAgINE 1/2, we examined the efficacy of adalimumab for fistula healing over time, covering a total period of 292 weeks of adalimumab exposure.
2. Methods
2.1. Study design and population
IMAgINE 1 [NCT00409682]10 and IMAgINE 2 [NCT00686374]11 methods have been described previously. Patients from the overall intention-to-treat population with fistulae at screening and baseline of IMAgINE 1 were assessed in the current analysis. Fistulae were defined by draining cutaneous fistulae upon gentle compression during physical examination, without use of pelvic magnetic resonance imaging. All fistulae were perianal. Further details are provided in the Supplementary Methods.
2.2. Efficacy and safety assessments
Fistula closure and fistula improvement were defined as closure of all IMAgINE 1 baseline fistulae or a decrease in number by ≥50%, respectively, for at least two consecutive visits. These end points were assessed at pre-specified time points in IMAgINE 1/2 and data were pooled, thereby capturing efficacy data over 292 weeks of adalimumab treatment. In addition, maintenance of fistula closure was analysed to Week 240 of IMAgINE 2 in patients with fistula closure at Week 0 of IMAgINE 2. Serum samples were obtained at selected time points for measurement of adalimumab concentration.
In IMAgINE 1, subgroup analyses of fistula closure in patients were performed for key potential predictors of response: randomized dose of adalimumab (high dose [HD] or low dose [LD]), prior infliximab therapy [yes versus no], corticosteroid use at baseline [yes versus no], immunomodulator use at baseline [yes versus no] and C-reactive protein [CRP] levels [<1 vs ≥1 mg/dL at baseline].
Safety was monitored in the subgroup of patients with fistulae, as described in the Supplementary Methods.
2.3. Data analyses and statistical methods
IMAgINE 1 data were analysed using non-responder imputation [NRI] and as-observed methods. For NRI, patients with missing data and those who escalated to open-label every week [ew] adalimumab dosing were imputed as non-responders. Patients who moved to blinded weekly dosing were not imputed and were counted according to their observed response.
IMAgINE 2 data were analysed using a hybrid non-responder imputation [hNRI] method as used previously in IMAgINE 2 analysis,11 and as-observed methods. For hNRI, missing data were treated as follows: patients who discontinued from the study owing to study-site closure due to approval of adalimumab in the respective country [n = 10] were analysed using the last observation carried forward from that time point onwards; patients with missing data, or who discontinued for other reasons, were imputed as non-responders using the NRI method. Fisher’s exact test and one-way analysis of variance were used to compare fistula closure in subgroup analyses. See the Supplementary Methods for details.
3. Results
3.1. Patient disposition, demographics and baseline characteristics
IMAgINE 1 baseline characteristics for this analysis are shown in Table 1. Of the 188 patients randomized in IMAgINE 1,10 36 [19.1%] patients [mean age 14.4 years] had at least one fistula; 23 of the 36 patients had a single fistula. All fistulae were draining enterocutaneous perianal fistulae. Baseline characteristics were similar between randomized groups receiving LD or HD adalimumab in IMAgINE 1, except for antibiotic use [Table 1].
Table 1.
Baseline demographics and clinical characteristics of patients with fistulae at IMAgINE 1 baseline.
| LD adalimumab 20/10 mg [n = 21] |
HD adalimumab 40/20 mg [n = 15] |
All patients [n = 36] |
|
|---|---|---|---|
| Male, n [%] | 17 [81.0] | 7 [46.7] | 24 [66.7] |
| Mean age ± SD, years | 14.3 ± 2.1 | 14.5 ± 2.3 | 14.4 ± 2.2 |
| ≥13 years, n [%] | 14 [66.7] | 11 [73.3] | 25 [69.4] |
| Caucasian, n [%] | 18 [85.7] | 14 [93.3] | 32 [88.9] |
| Mean weight ± SD, kg | 46.1 ± 9.8 | 45.7 ± 12.1 | 45.9 ± 10.6 |
| ≥40 kg, n [%] | 15 [71.4] | 11 [73.3] | 26 [72.2] |
| Fistulae per patient [all perianal], n | |||
| 1 | 14 | 9 | 23 |
| 2 | 3 | 5 | 8 |
| 3 | 2 | 0 | 2 |
| ≥4 | 2 | 1 | 3 |
| Baseline median CRP [range], mg/dLa | 2.04 [0–7.8] | 1.33 [0.2–6.8] | 1.95 [0–7.8] |
| ≥1 mg/dL, n [%] | 11 [55.0] | 9 [60.0] | 20 [57.1] |
| Baseline median PCDAI [range] | 42.5 [30.0–60.0] | 45.0 [32.5–62.5] | 42.5 [30.0–62.5] |
| Median disease duration [range], years | 2.1 [0.3–9.2] | 2.8 [0.3–7.0] | 2.5 [0.3–9.2] |
| Baseline medication use, n [%] | |||
| Systemic corticosteroids | 10 [47.6] | 3 [20.0] | 13 [36.1] |
| IMMs | 13 [61.9] | 12 [80.0] | 25 [69.4] |
| Thiopurinesb | 10 [47.6] | 9 [60.0] | 19 [52.8] |
| Methotrexate | 3 [14.3] | 3 [20.0] | 6 [16.7] |
| Antibioticsc | 6 [28.6] | 0 | 6 [16.7] |
| Metronidazole | 4 [19.1] | 0 | 4 [11.1] |
| Ciprofloxacin | 2 [9.5] | 0 | 2 [5.6] |
| Prior infliximab use, n [%] | 7 [33.3] | 6 [40.0] | 13 [36.1] |
CRP, C-reactive protein; HD, high dose; IMM, immunomodulator; LD, low dose; PCDAI, Paediatric Crohn’s Disease Activity Index; SD, standard deviation.
aOne CRP measurement missing from the LD adalimumab group. bAzathioprine, 6-mercaptopurine.
Comparisons between LD and HD subgroups were calculated by using Fisher’s exact test. cMore patients in the HD group were receiving antibiotics compared with the LD group [P = 0.03], otherwise P > 0.05.
3.2. Fistula closure and improvement
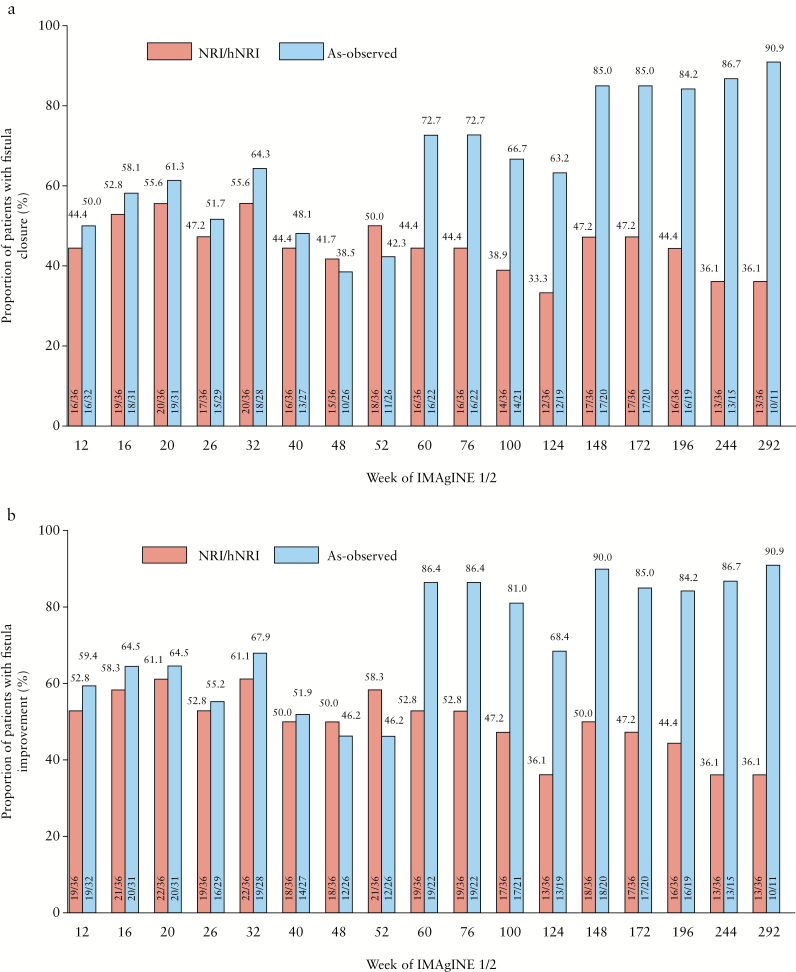
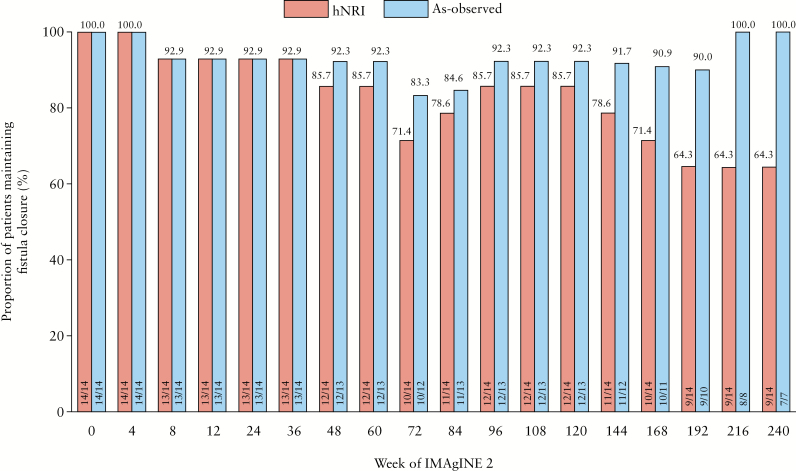
In IMAgINE 1, fistula closure and improvement were achieved in 44.4% and 52.8% of patients, respectively, at Week 12 by NRI analysis, with similar results in as-observed analysis [Figure 1a and b]. Rates of fistula closure and improvement were sustained to Week 52 in both NRI [50.0% and 58.3%, respectively] and as-observed analyses [42.3% and 46.2%, respectively]. Serum concentration of adalimumab in patients with fistula closure trended slightly higher than those not achieving fistula closure [Supplementary Table 1]. In IMAgINE 2, fistula closure and improvement rates were generally maintained with long-term [in total 292 weeks] adalimumab treatment, although hNRI and as-observed rates diverged as expected due to patient discontinuation from the study [Figure 1a and b]. At Week 292, 36.1% of patients by hNRI analysis and 90.9% of patients by as-observed analysis had fistula closure, and the same proportions of patients had documented fistula improvement. The majority of patients [64% hNRI, 100% as observed] who entered IMAgINE 2 with healed fistulae maintained fistula closure up to Week 240 of IMAgINE 2 [Figure 2].
Figure 1.
Fistula closure rates [a] and fistula improvement rates [b] with adalimumab treatment from Week 12 to Week 292 in IMAgINE 1 and IMAgINE 2 in patients with fistulae at baseline (non-responder imputation [NRI] in IMAgINE1, hybrid non-responder imputation [hNRI] in IMAgINE2 and as-observed analyses).
Figure 2.
Long-term maintenance of fistula closure through to Week 240 of IMAgINE 2 in patients with fistula closure at IMAgINE 2 baseline (hybrid non-responder imputation [hNRI] and as-observed analyses).
IMAgINE 2 baseline [Week 0 data] was derived from the IMAgINE 1 study Week 52 dataset.
In subgroup analyses, patients who were randomized to HD [versus LD] adalimumab, those who were naïve to infliximab [versus experienced], and those taking systemic corticosteroids or immunomodulators at IMAgINE 1 baseline [versus not taking] experienced generally higher rates of fistula closure, although no statistical significance was demonstrated at measured time points [Supplementary Table 2]. Interestingly, patients with CRP ≥ 1 mg/dL at IMAgINE 1 baseline consistently showed higher fistula closure rates than patients with CRP < 1 mg/dL, with statistical significance demonstrated at two time points [Supplementary Table 2]. Other covariates explored, including gender, age, weight, baseline antibiotic use, CD disease duration, CD disease severity, CD location, and number of fistulae, were not associated with fistula closure or improvement [data not shown].
3.3. Safety and tolerability
The safety profile of adalimumab in children/adolescents enrolled in the IMAgINE 1 and IMAgINE 2 clinical trials has been reported previously.10,11 Treatment-emergent adverse events [TEAEs] during IMAgINE 1 and IMAgINE 2 for patients with fistulae are presented in Supplementary Table 3. The safety profile and rates of TEAEs were consistent with those of the overall IMAgINE 1/2 study population.
4. Discussion
In IMAgINE 1/2, adalimumab treatment of children/adolescents with moderately to severely active CD complicated by perianal fistulae was efficacious in achieving fistula closure as early as Week 12 in approximately half of the patients, and rates of closure were sustained for more than 5 years. Furthermore, the majority of patients who entered IMAgINE 2 with healed fistulae maintained fistula closure up to Week 240 of IMAgINE 2. Fistula improvement rates were generally slightly higher than closure rates across both studies, indicating that patients who do not achieve complete fistula closure may still derive clinically meaningful benefit from treatment. Our results complement a previous evaluation of the efficacy of adalimumab in inducing and maintaining fistula closure in 117 adults with fistulizing CD.9
In subgroup analyses of fistulae closure during IMAgINE 1 evaluating possible associations with randomized dose of adalimumab [HD or LD] or prior [infliximab] or concomitant [corticosteroids or immunomodulators] medication covariates, no statistically significant differences were demonstrated between these subgroups. However, higher fistula closure rates in patients with higher baseline serum CRP levels [≥1 mg/dL] were observed. Indeed, CRP has been recognized as a marker for detection and follow-up of disease activity in patients with CD,12 and trials with anti-TNFs have shown that an elevated CRP level predicts better response in patients with CD.12
Despite the increasing number of treatment options, medical management of perianal fistula in patients with CD remains challenging.13,14 Antibiotics provide a first-line management option, but are not feasible for long-term use.13 Immunomodulators such as thiopurines may offer long-term improvements, but achievement of clinical efficacy may require >3 months of treatment13 and may increase lymphoma risk.15,16 Anti-α4 integrin-targeting agents natalizumab and vedolizumab are approved for use in the treatment of CD in adults,17 although evidence of efficacy in fistula healing is currently modest.18,19 ECCO guidelines recommended anti-TNF therapy for induction and maintenance therapy in treatment of fistulae in paediatric patients following appropriate antibiotic and surgical management of lesions.5 Our findings add to previous evidence with infliximab, supporting the use of anti-TNFs in the treatment of children/adolescents with fistulae.20–26
Our analysis has several limitations. Patients were not randomly assigned to treatment according to the presence of a fistula at baseline, the study was not powered to detect statistical differences between treatment groups, the study was not placebo controlled, and an imbalance in baseline antibiotic therapy between dosing groups was present. Fistula assessment was based on physical examination only and did not include imaging. Furthermore, recruitment into IMAgINE 2 may have constituted a selection for responders, as is typical for extension studies. Finally, the analysis population was relatively small, although to our knowledge the present analysis included the largest number of paediatric CD patients with fistulae from a clinical trial as well as the longest follow-up yet reported.
In conclusion, adalimumab treatment led to clinically meaningful rates of fistula closure and improvement within 12 weeks in children/adolescents with moderately to severely active fistulizing CD. Fistula closure and improvement rates were stable over time and sustained for more than 5 years. The safety profile was similar to that previously reported for the overall IMAgINE 1/2 study population.
Funding
AbbVie Inc. funded the study and the analysis, provided financial support for medical writing, and reviewed and approved the final draft.
Conflict of Interest
FR has received speaker fees from Shering-Plough, Nestlé, MeadJohnson, Ferring, MSD, Johnson & Johnson, Centocor and AbbVie; serves as a board member for: SAC:DEVELOP (Johnson & Johnson), CAPE (AbbVie) and LEA (AbbVie), and has been invited to MSD France, Nestlé Nutrition Institute, Nestlé Health Science, Danone, MeadJohnson, Takeda, Celgene, Biogen, Shire, Pfizer and Therakos.
JR has received consultancy fees from AbbVie, Janssen, Luitpold and Pfizer; is a board member for GI Health Foundation; and has received financial support for research from AbbVie and Janssen.
WAF has received consultancy fees from Connecticut Children’s Medical Center—Safety Office as part of a subcontracted NIH clinical trial award; serves as a board member [no personal compensation] for AbbVie and UCB; and serves as a consultant [no personal compensation] for AbbVie, Boehringer Ingelheim Pharma, Janssen Research & Development, Celgene Corporation, Genentech and Shire Development.
MCD has received consultancy fees from AbbVie, Janssen, Takeda, Pfizer, Celgene, Boehringer Ingelheim, Prometheus Labs and UCB; and has received research support from Janssen.
DT has received consultation fees, research grants, royalties or honoraria from Janssen, Pfizer, Toronto Hospital for Sick Children, Ferring, MegaPharm, AstraZeneca, AbbVie, Takeda, Rafa, Boehringer Ingelheim, Biogen, Atlantic Health and Shire, during the last 3 years.
JSH has received consultancy fees from Janssen Ortho Biotech, AbbVie, Celgene, Entera Health, Pfizer, Soligenix, Takeda, Lilly, Genentech, Boehringer Ingelheim and AstraZeneca; has provided expert testimony on behalf of Janssen Ortho Biotech; has received speaker fees from Janssen Ortho Biotech; and has received payment for development of educational presentations from Janssen Ortho Biotech.
AL, SE, J-FM, GA and AMR are employees of AbbVie, and may own AbbVie stock and/or options.
Supplementary Material
Acknowledgments
Medical writing support was provided by Kevin Hudson, 2 the Nth, funded by AbbVie Inc.
Author Contributions
All authors were involved in the conception and design of the study, analysed and interpreted the study data, critically reviewed the content of this manuscript, and approved the final version for submission.
References
- 1. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 2. Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 2010;139:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marzo M, Felice C, Pugliese D, et al. Management of perianal fistulas in Crohn’s disease: an up-to-date review. World J Gastroenterol 2015;21:1394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta N, Bostrom AG, Kirschner BS, et al. Incidence of stricturing and penetrating complications of Crohn’s disease diagnosed in pediatric patients. Inflamm Bowel Dis 2010;16:638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 2014;8:1179–207. [DOI] [PubMed] [Google Scholar]
- 6. Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, et al. Management of complex perianal Crohn’s disease. Ann Gastroenterol 2017;30:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340:1398–405. [DOI] [PubMed] [Google Scholar]
- 8. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85. [DOI] [PubMed] [Google Scholar]
- 9. Colombel JF, Schwartz DA, Sandborn WJ, et al. Adalimumab for the treatment of fistulas in patients with Crohn’s disease. Gut 2009;58:940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hyams JS, Griffiths A, Markowitz J, et al. Safety and efficacy of adalimumab for moderate to severe Crohn’s disease in children. Gastroenterology 2012;143:365–74.e2. [DOI] [PubMed] [Google Scholar]
- 11. Faubion WA, Dubinsky M, Ruemmele FM, et al. Long-term efficacy and safety of adalimumab in pediatric patients with Crohn’s disease. Inflamm Bowel Dis 2017;23:453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 2004;10:661–5. [DOI] [PubMed] [Google Scholar]
- 13. Klag T, Goetz M, Stange EF, et al. Medical therapy of perianal Crohn’s disease. Viszeralmedizin 2015;31:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gomollón F, Dignass A, Annese V, et al. ; ECCO. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 15. O’Connor A, Qasim A, O’Moráin CA. The long-term risk of continuous immunosuppression using thioguanides in inflammatory bowel disease. Ther Adv Chronic Dis 2010;1:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol 2009;7:874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chandar AK, Singh S, Murad MH, Peyrin-Biroulet L, Loftus EV Jr. Efficacy and safety of natalizumab and vedolizumab for the management of Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis 2015;21:1695–708. [DOI] [PubMed] [Google Scholar]
- 18. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 19. Raine T. Vedolizumab for inflammatory bowel disease: changing the game, or more of the same?United European Gastroenterol J 2014;2:333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruemmele FM, Lachaux A, Cezard JP, et al. Efficacy of infliximab in pediatric Crohn’s disease: a randomized multicenter open-label trial comparing scheduled to on demand maintenance. Inflamm Bowel Dis 2009;15:388–94. [DOI] [PubMed] [Google Scholar]
- 21. Cezard JP, Nouaili N, Talbotec C, et al. A prospective study of the efficacy and tolerance of a chimeric antibody to tumor necrosis factors (Remicade) in severe pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2003;36:632–6. [DOI] [PubMed] [Google Scholar]
- 22. Lionetti P, Bronzini F, Salvestrini C, et al. Response to infliximab is related to disease duration in paediatric Crohn’s disease. Aliment Pharmacol Ther 2003;18:425–31. [DOI] [PubMed] [Google Scholar]
- 23. de Ridder L, Escher JC, Bouquet J, et al. Infliximab therapy in 30 patients with refractory pediatric Crohn disease with and without fistulas in The Netherlands. J Pediatr Gastroenterol Nutr 2004;39:46–52. [DOI] [PubMed] [Google Scholar]
- 24. Crandall W, Hyams J, Kugathasan S, et al. Infliximab therapy in children with concurrent perianal Crohn disease: observations from REACH. J Pediatr Gastroenterol Nutr 2009;49:183–90. [DOI] [PubMed] [Google Scholar]
- 25. Teitelbaum JE, Saeed S, Triantafyllopoulou M, Daum F. Infliximab in pediatric Crohn disease patients with enterovesicular fistulas. J Pediatr Gastroenterol Nutr 2007;44:279–82. [DOI] [PubMed] [Google Scholar]
- 26. Dupont-Lucas C, Dabadie A, Alberti C, et al. Predictors of response to infliximab in paediatric perianal Crohn’s disease. Aliment Pharmacol Ther 2014;40:917–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.