Abstract
Background
Genetic susceptibility to insulin resistance is associated with lower adiposity in adults. Insulin resistance, and therefore adiposity, may alter sensitivity to Growth Hormone (GH). We aimed to determine the relationship between adiposity, genetic susceptibility to insulin resistance or insulin secretion, and response to GH treatment in short children born small for gestational age (SGA).
Methods
In 89 (55 boys) short prepubertal SGA children (age,6.2±1.6years) treated with GH for one year in a multicentre study, body fat percentage was estimated at baseline and 1-year using DXA. The main outcome measures were treatment-related changes in height, IGF-1 standard deviation scores (SDS), insulin sensitivity, insulin secretion and disposition index. Combined multiallele gene scores based on single nucleotide polymorphisms with known associations with lower insulin sensitivity (GS-InRes) and insulin secretion (GS-InsSec) were analysed for their relationships with adiposity.
Results
Mean percentage body fat at baseline was low compared to normative data (p=0.045), and decreased even further on GH treatment (baseline vs 1-year z-scores, -0.26±1.2 vs -1.23±1.54; p<0.0001). Baseline percentage body fat was positively associated with IGF-1 responses (p-trends=0.042), first-year height gains (B[95%CI]:0.61cm/year [0.28,0.95]; p<0.0001), insulin secretion at baseline (p-trends=0.020) and at 1-year (p-trends=0.004), and disposition index at 1-year (p-trends=0.024). GS-InRes was inversely associated with BMI (-0.13SDS per-allele [-0.26,-0.01]; p=0.040), body fat (-0.49% per-allele [-0.97,-0.007]; p=0.047), and limb fat (-0.81% per-allele [-1.62,0.00]; p=0.049) at baseline. During GH treatment, GS-InRes was related to a lesser decline in trunk fat (0.38% per-allele [0.16,0.59]; p=0.001) and a higher trunk-limb fat ratio at 1-year (0.04 per-allele [0.01,0.08]; p=0.008). GS-InSec was positively associated with truncal fat (0.36% per-allele [0.09, 0.63]; p=0.009).
Conclusions
Adiposity in SGA children has favourable effects on GH sensitivity and glucose metabolism. The associations with multiallele scores support a causal role of insulin resistance in linking lower body fat to reduced sensitivity to exogenous GH.
Keywords: Small for gestational age, growth hormone treatment, adiposity, insulin sensitivity, insulin secretion
Introduction
Increased body fat, in particular, central fat is thought to have a major role in the development of metabolic risk factors in children born small for gestational age (SGA) (1). However, in contrast to the majority of SGA children who undergo catch-up growth during infancy, short SGA children have significant deficits in body fat, mainly in the subcutaneous compartment compared with children born appropriate for gestation (AGA) (2,3). The phenotype of low adiposity is not an expected consequence of Growth Hormone (GH) deficiency or GH resistance (2) and therefore other mechanisms such as alterations in the neuroendocrine regulation of appetite and adipose tissue development may determine growth and body composition in these children (4). In short SGA children who fail to catch-up, GH treatment is licenced to improve linear growth (5). GH is a crucial regulator of substrate metabolism during fasting and its anabolic actions are tightly coupled with energy balance (6). Low adiposity in SGA children may reflect suboptimal energy balance and alter their sensitivity to GH.
Developmental programming of multiple endocrine axes has been hypothesised to underlie the increased risk for development of type 2 diabetes (T2D) in low birth-weight individuals (7). The close relationship between the actions of GH/IGF-1 axis and glucose metabolism may explain the link between reduced statural growth and metabolic abnormalities in SGA children(6,7). In addition, lower insulin sensitivity and insulin secretion are associated with reduced responses to GH treatment in SGA children(8,9). We recently employed a Mendelian randomisation approach to illustrate the likely causal link between insulin resistance and GH sensitivity in short SGA children: multiallele scores indicative of insulin resistance were associated with lower IGF-1 and height responses to GH treatment(10). In adults, the same multiallele score is associated with a lesser body fat, particularly in the gluteofemoral region and limbs(11). Furthermore, the multiallele score indicative of lower insulin secretion was associated with a reduced spontaneous growth in SGA children and higher android fat in adults (10). Therefore, insulin resistance and/or insulin secretion could potentially link adiposity to GH-treatment responses in short SGA children.
The aim of the study was to test the hypothesis that variations in adiposity in short SGA children could be related to sensitivity to GH, and to explore whether the gene polymorphisms indicative of insulin sensitivity or insulin secretion are also associated with body composition in these children.
Methods
Study Population
The subjects were from the North European Small for Gestational Age Study (NESGAS), a multi-centre study of GH treatment in short prepubertal SGA children involving 9 investigating centres in 4 North European countries (Denmark, Ireland, Sweden, and UK) and has been reported in detail previously (12). Briefly, the study population included prepubertal SGA children with persistent short stature at 4 years of age; the girls were aged between 4 and 9 years and the boys between 4 and 10 years. During the first year, children were treated with a uniform high dose of GH (67μg/kg/day) to induce catch-up growth. The study (NESGAS EudraCT 2005-001507-19) was approved by the relevant ethics committees, institutional review boards and national drug authorities at each study centre and performed according to the Helsinki II declaration. Written informed consent was obtained from parents of the children before any study activities.
Study assessments
The participants were assessed at study entry (baseline) and at every 3 months when anthropometry and pubertal assessments were undertaken and serum IGF-1 levels were measured. They also underwent a short intravenous glucose tolerance test (IVGTT) at baseline and at 1-year to evaluate insulin sensitivity and secretion (8).
DXA scans
Body composition was assessed by dual-energy X-ray absorptiometry (DXA) scans using Hologic QDR-1000/W scanner (Hologic Inc., Waltham, MA) (3 centres, n=39) or Lunar Prodigy DXA system (GE Medical Systems) (6 centres, n=50) at baseline and at 1-year. In one centre, the Hologic scanner was replaced with a Lunar Prodigy system during the study period and data from the children who were evaluated by two different scanners (n=7) were transformed to Lunar Prodigy DXA values using a published method (13). These children were excluded when the changes in body composition from the baseline to 1-year were analysed to avoid confounding by the type of scanner. Regional fat distribution was assessed using the default setting for segmental analysis in the scanners. The performance of the scanners was assessed using a phantom at the start of the study. The scanners showed a good level of agreement, and the difference in percentage body fat between centres were typically 1.5% with a maximum of 2.1%. Of the 110 children who participated in the study, data on body composition were available from 89 children at baseline (incomplete data: 4, scans not carried out: 17) and 85 children at 1-year (incomplete data: 1, scans not carried out: 24).
Genotyping method
The cohort was genotyped using the Metabochip, a custom Illumina iSelect genotyping array that assays nearly 200,000 single nucleotide polymorphisms (SNPs) chosen on the basis of genome-wide association study meta-analyses as previously described (10,11). In each individual, combined multiallele gene scores for insulin resistance (GS-InRes) or insulin secretion (GS-InSec) were generated as the count of the insulin sensitivity decreasing alleles at 10 variants and the insulin secretion decreasing alleles at 18 variants respectively (supplemental table-1a and1b) (10). Both combined multiallele scores have been validated in large population-based studies (11).
Assays
Serum levels of IGF-1, insulin and C-peptide were assayed centrally as previously reported (8). Plasma glucose and fasting lipid profile were measured locally.
Calculations
Standard deviation scores (SDS) for height, weight and BMI were derived using country-specific references (8). Insulin sensitivity was estimated from fasting glucose and C-peptide levels using the homeostatic model (HOMA) as previously reported (8). Acute insulin response (AIR) was calculated from the area under the curve of insulin response above the baseline during the first 10 minutes of IVGTT and provides a measure of the first-phase insulin secretion (14). The disposition index provides an estimate of insulin secretion adjusted for the degree of insulin sensitivity and was calculated as the product of the two (14).
To allow comparisons of adiposity of the subjects in relation to healthy children, we calculated z-scores of the percentage body fat using population based age- and gender-specific normative data on Caucasian children (z-scoresp) (15) after appropriate transformations to adjust for the scanner types (13,16). The limb fat was calculated as the sum of fat (in kilograms) in arms and legs, and the trunk-limb fat ratio by dividing the trunk fat by limb fat. We expressed the body fat as the percentage of total mass as it provided an estimate of adiposity independent of body size and calculated using the formula: percentage fat in a region = fat mass of the region (kg) x100/ total mass of the region (kg).
Statistics
The variables for insulin and C-peptide levels, insulin sensitivity, AIR and disposition index were log-transformed to normality. Although percentage body fat z-scoresp were derived using normative data, significant residual associations with age and gender were observed. Therefore, we derived ‘within-cohort’ z-scores of percentage body fat at baseline (z-scoresc) as an estimate of adiposity independent of these factors from a linear regression model with percentage body fat as the dependent variable, and age, gender and type of DXA scanner as covariants. To determine the associations of baseline adiposity, the children were categorised into tertiles of percentage body fat z-scoresc. The effect of baseline adiposity in predicting first-year height velocity was assessed by including percentage body fat z-scoresc in Ranke’s height prediction model for SGA children (17), which includes variables of age, weight SDS at start of treatment, GH dose and midparental height SDS. Associations between adiposity and multiallele scores were explored using regression models which also included age and gender to reduce the variability in the data. Statistical analyses were performed using the statistical package IBM SPSS statistics (version 20; SPSS Inc.). The data are presented as mean (SD) unless otherwise specified.
Results
The study included 89 Caucasian children (55 boys) with a mean age of 6.2±1.6 years.
Baseline adiposity
At baseline, the children had lower mean percentage body fat (z-scoresp, -0.26±1.2, p=0.045) and BMI (-1.29±1.37 SDS, p<0.0001) compared with healthy Caucasian children (12,15) (Table-1, Figure-1B). Although, percentage body fat z-scoresp were derived using age and gender-specific normative data, it showed residual associations with age (r=-0.21, p<0.05) and male vs. female gender (r=0.66, p<0.0001). Percentage body fat and the z-scoresp were not associated with height SDS. The tertile groups for baseline percentage body z-scoresc were similar in age and height SDS (Table-2); but the highest tertile group had greater BMI SDS (p-trends=0.04), percentage fat in trunk and limbs (all p-trends <0.0001), and trunk-limb fat ratio (p-trends=0.019). The tertile groups had similar levels of IGF-1, glucose, insulin and C-peptide, and insulin sensitivity, however, the highest tertile group had greater AIR (p-trends=0.02) (Figure-2E).
Table 1. Body composition and metabolism during first year of Growth Hormone treatment.
| Baseline | 1-year | P value | |
|---|---|---|---|
| Anthropometry | |||
| Height SDS | -3.35 (0.74) | -2.31 (0.69) | <0.0001 |
| Weight SDS | -3.10 (1.03) | -2.12 (1.00) | <0.0001 |
| BMI (kg/m2) | 14.16 (1.49) | 14.68 (1.62) | <0.0001 |
| BMI SDS | -1.34 (1.38) | -0.96 (1.29) | <0.0001 |
| Body Composition (DXA) | |||
| Total lean mass (kg) | 11.5 (2.66) | 15.6 (3.45) | <0.0001 |
| Bone Mineral Content (g) | 457 (166) | 606 (188) | <0.0001 |
| Total body fat mass (kg) | 2.26 (1.06) | 2.06 (1.12) | 0.007 |
| Trunk fat mass (kg) | 0.68 (0.37) | 0.72 (0.41) | 0.13 |
| Limbs fat mass (kg) | 1.10 (0.68) | 1.00 (0.67) | 0.0002 |
| Total body fat (%) | 15.8 (5.80) | 11.2 (4.70) | <0.0001 |
| total body fat % (z-score)* | -0.26 (1.21) | -1.23 (1.54) | <0.0001 |
| Trunk fat (%) | 10.6 (4.66) | 8.63 (4.03) | <0.0001 |
| Limb fat (%) | 23.1 (9.70) | 14.6 (7.70) | <0.0001 |
| Trunk-limb fat ratio | 0.61 (0.20) | 0.84 (0.32) | <0.0001 |
| Biochemistry | |||
| IGF- I (SDS) | -1.09 (1.28) | 2.88 (1.52) | <0.0001 |
| Glucose (mmol/l) | 4.32 (0.66) | 4.70 (0.55) | <0.0001 |
| Insulin (pmol/l) (log) | 1.19 (0.28) | 1.59 (0.22) | <0.0001 |
| C-peptide (pmol/l) (log) | 2.30 (0.24) | 2.61 (0.17) | <0.0001 |
| Insulin sensitivity (HOMA) (log) | 2.38 (0.25) | 2.06 (0.17) | <0.0001 |
| Acute Insulin Response (log) | 3.13 (0.24) | 3.39 (0.26) | <0.0001 |
| Disposition Index (log) | 5.51 (0.24) | 5.46 (0.23) | 0.11 |
| Total Cholesterol (mmol/L) | 3.94 (0.72) | 3.88 (0.70) | 0.38 |
| LDL Cholesterol (mmol/L) | 2.23 (0.63) | 2.15 (0.58) | 0.11 |
| HDL Cholesterol (mmol/L) | 1.47 (0.35) | 1.42 (0.33) | 0.070 |
| Triglycerides (mmol/L) | 0.64 (0.33) | 0.83 (0.40) | 0.001 |
Data presented as means (SD)
Z-scores for percentage body fat calculated based on normative data (z-scorep)
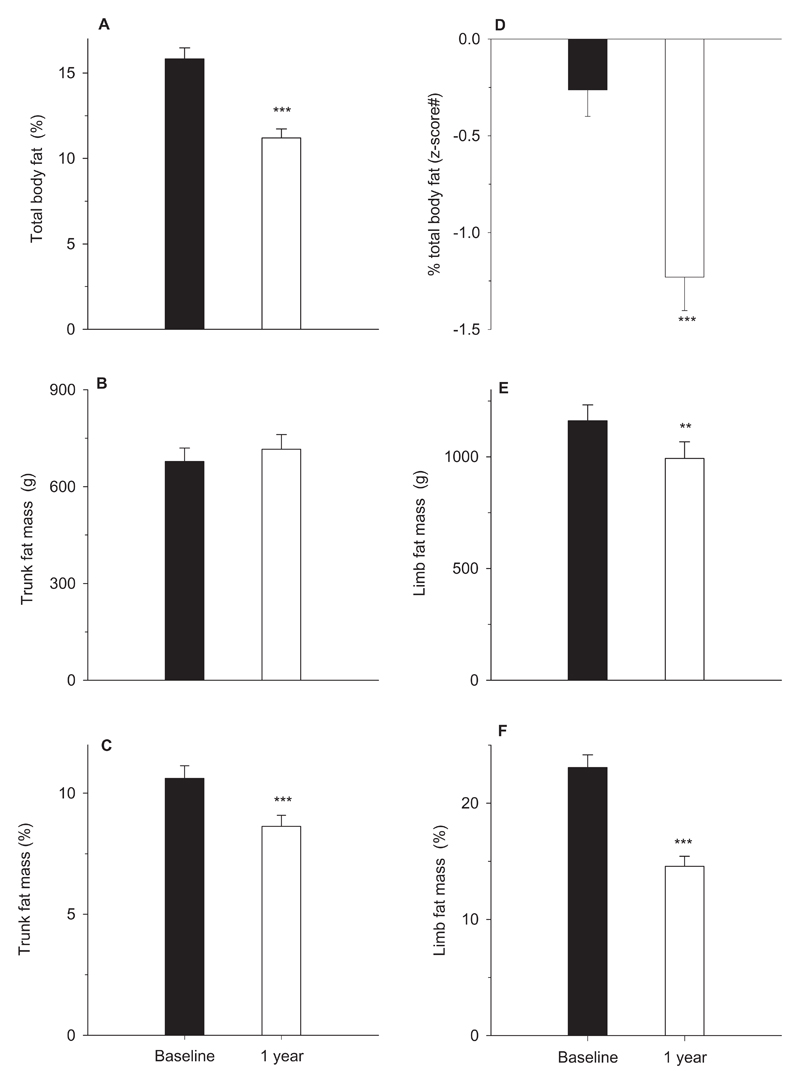
Figure-1. Changes in body fat during Growth Hormone treatment.
Total body fat percentage (A), z-scores for total body fat percentage (D), trunk fat mass in grams (B) and as percentage of total trunk mass (C), limb fat mass in grams (E) and as percentage of total limb mass (F). Bars represent means and error bars the standard error of means. Black and empty bars represent measurements at baseline and 1-year respectively; # z-scores for total body fat percentage are based on normative data (z-scoresp); p-values (*) are from the comparison between baseline and 1-year measurements; **, p<0.001 and ***, p<0.0001;
Table 2. Body composition, glucose metabolism and response to Growth Hormone treatment in patients categorised by tertiles of z-scores* for total body fat percentage at baseline.
| Tertiles of baseline total body fat | P Trends | |||
|---|---|---|---|---|
| Low | Middle | High | ||
| Baseline | ||||
| n (male) | 30 (19) | 29 (19) | 30 (17) | NS |
| Age (years) | 6.04 (1.53) | 5.95 (1.50) | 6.50 (1.72) | 0.36 |
| Height (SDS) | -3.30 (0.60) | -3.53 (0.85) | -3.26 (0.73) | 0.32 |
| Weight (SDS) | -3.41 (0.79) | -3.24 (0.93) | -2.71 (1.23) | 0.025 |
| BMI (kg/m2) | 13.65 (1.12) | 14.3 (0.91) | 14.66 (2.06) | 0.035 |
| BMI (SDS) | -1.73 (1.14) | -1.10 (0.91) | -0.89 (1.69) | 0.040 |
| Total body fat (%) | 11.9 (4.90) | 16.0 (4.20) | 20.5 (4.80) | <0.0001 |
| Total body fat % (z-score)* | -0.88 (0.36) | -0.17 (0.19) | 0.89 (0.51) | <0.0001 |
| Trunk fat (%) | 7.27 (2.51) | 9.72 (2.42) | 15.8 (4.26) | <0.0001 |
| Limb fat (%) | 17.8 (8.80) | 24.0 (8.60) | 28.0 (7.90) | <0.0001 |
| Trunk-limb fat ratio | 0.59 (0.18) | 0.56 (0.19) | 0.70 (0.20) | 0.019 |
| IGF-I (SDS) | -1.12 (1.09) | -1.18 (1.35) | -1.00 (1.40) | 0.87 |
| Glucose (mmol/L) | 4.17 (0.61) | 4.34 (0.64) | 4.47 (0.72) | 0.22 |
| Insulin (pmol/L) (log) | 1.15 (0.26) | 1.26 (0.23) | 1.27 (0.26) | 0.14 |
| C-peptide (pmol/L) (log) | 2.26 (0.24) | 2.35 (0.23) | 2.32 (0.23) | 0.41 |
| HOMA Insulin sensitivity (%) (log) | 2.42 (0.26) | 2.33 (0.24) | 2.36 (0.25) | 0.38 |
| Acute insulin response (log) | 3.04 (0.23) | 3.18 (0.20) | 3.21 (0.26) | 0.020 |
| Disposition index (log) | 5.46 (0.26) | 5.51 (0.21) | 5.57 (0.26) | 0.29 |
| 1-year | ||||
| Height (SDS) | -2.36 (0.56) | -2.42 (0.81) | -2.17 (0.70) | 0.36 |
| Weight (SDS) | -3.41 (0.79) | -3.24 (0.93) | -2.71 (1.23) | 0.010 |
| BMI (kg/m2) | 14.1 (1.20) | 14.6 (1.17) | 15.3 (2.13) | 0.017 |
| BMI (SDS) | -1.30 (1.16) | -1.04 (0.96) | -0.47 (1.69) | 0.12 |
| Total body fat (%) | 8.97 (4.06) | 11.1 (4.19) | 13.8 (4.77) | 0.001 |
| Trunk fat (%) | 6.13 (1.82) | 8.73 (4.41) | 11.1 (3.82) | <0.0001 |
| Limb fat (%) | 12.1 (7.60) | 14.6 (7.70) | 17.2 (7.30) | 0.057 |
| Trunk-limb fat ratio | 0.85 (0.38) | 0.81 (0.31) | 0.85 (0.26) | 0.87 |
| IGF-I (SDS) | 2.57 (1.34) | 2.63 (1.61) | 3.46 (1.47) | 0.036 |
| Glucose (mmol/L) | 4.62 (0.49) | 4.64 (0.60) | 4.78 (0.58) | 0.48 |
| Insulin (pmol/L) (log) | 1.54 (0.23) | 1.59 (0.20) | 1.65 (0.21) | 0.19 |
| C-peptide (pmol/L) (log) | 2.58 (0.16) | 2.62 (0.17) | 2.63 (0.19) | 0.59 |
| HOMA Insulin sensitivity (%) (log) | 2.09 (0.17) | 2.05 (0.17) | 2.03 (0.19) | 0.55 |
| Acute insulin response (log) | 3.27 (0.22) | 3.45 (0.22) | 3.48 (0.28) | 0.004 |
| Disposition index (log) | 5.36 (0.23) | 5.50 (0.22) | 5.52 (0.22) | 0.024 |
| Changes from baseline to 1-year | ||||
| Delta Height (SDS) | 0.94 (0.33) | 1.04 (0.22) | 1.14 (0.31) | 0.038 |
| Delta Weight (SDS) | 1.02 (0.40) | 1.03 (0.34) | 1.05 (0.49) | 0.96 |
| Delta BMI (kg/m2) | 0.46 (0.46) | 0.47 (0.80) | 0.72 (0.72) | 0.064 |
| Delta BMI (SDS) | 0.48 (0.45) | 0.45 (0.46) | 0.40 (0.54) | 0.84 |
| Delta total body fat (%) | -2.94 (1.38) | -3.88 (1.61) | -5.30 (2.99) | 0.001 |
| Delta trunk fat (%) | -0.90 (1.56) | -1.76 (1.79) | -3.61 (2.83) | <0.0001 |
| Delta limb fat (%) | -5.47 (2.90) | -7.80 (3.36) | -9.33 (4.88) | 0.003 |
| Delta trunk-limb fat ratio | 0.27 (0.33) | 0.18 (0.18) | 0.11 (0.18) | 0.19 |
| Delta IGF-I (SDS) | 3.69 (1.32) | 3.80 (1.45) | 4.17 (1.35) | 0.042 |
| Delta glucose (nmol/L) | 0.45 (0.55) | 0.30 (0.48) | 0.38 (0.46) | 0.49 |
| Delta insulin (pmol/L) (log) | 1.99 (0.09) | 1.98 (0.09) | 2.02 (0.08) | 0.27 |
| Delta C-peptide (pmol/L) (log) | 2.81 (0.15) | 2.73 (0.49) | 2.84 (0.09) | 0.47 |
| Delta HOMA-Insulin Sensitivity (%) (log) | 3.21 (0.10) | 3.24 (0.05) | 3.23 (0.04) | 0.35 |
| Delta Acute Insulin Response (Log) | 3.27 (0.29) | 3.40 (0.21) | 3.48 (0.18) | 0.014 |
| Delta Disposition Index (Log) | 5.74 (1.10) | 5.99 (0.08) | 5.96 (0.15) | 0.43 |
Data presented as means (SD). * within cohort z-scores (z-scorec) for total body fat percentage at baseline adjusted for age, gender and type of scanner
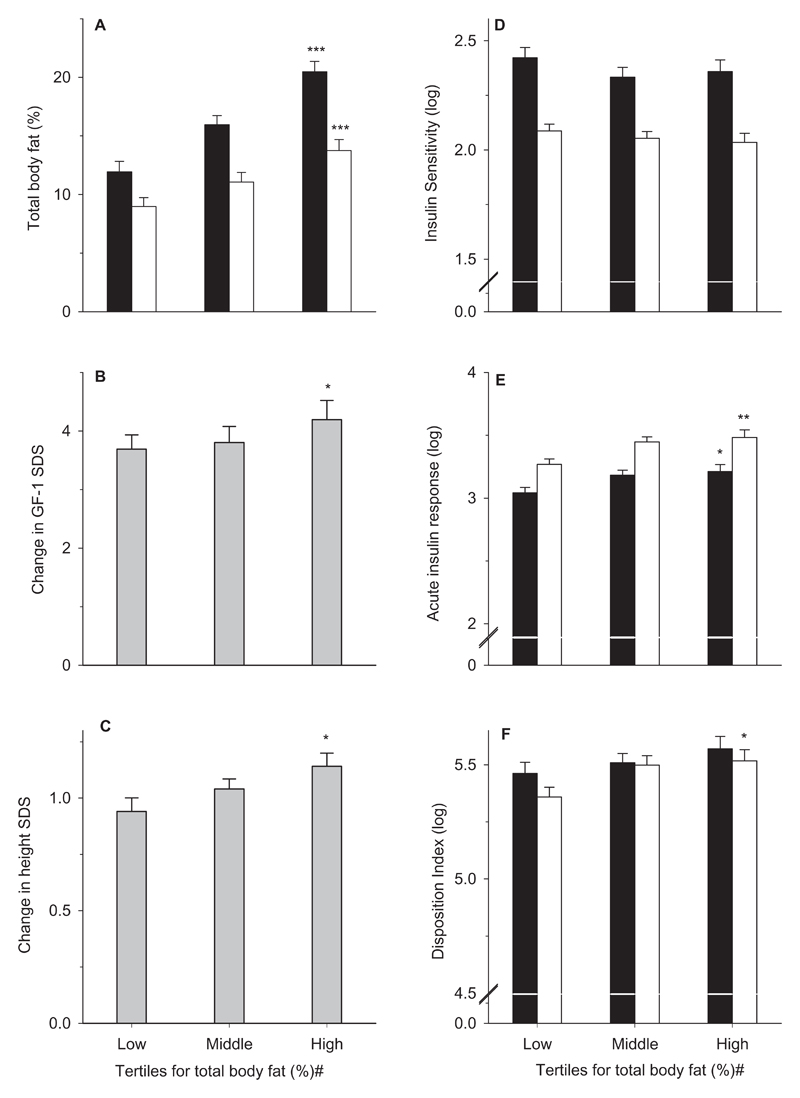
Figure-2. Changes in body fat, height, IGF-1 and measures of glucose metabolism in the tertile groups for percentage body fat z-scores at baseline during 1 year of Growth Hormone treatment.
Total body fat percentage (A), change in IGF-1 SDS (B) and height SDS (C), insulin sensitivity as HOMA % (log) (D), insulin secretion as log of acute insulin response (E) and Disposition Index (log) (F). Bars represent means and error bars the standard error of the means. Black and empty bars represent measurements at baseline and 1 year respectively; grey bars represent changes in measurements from baseline to 1 year. # tertiles of z-scores for total body fat percentage derived within the cohort (z-scorec), * represents p-trends across the tertile groups; *, p<0.05, **, p<0.01 and ***, p<0.001. In y-axes with log-transformed values, a break has been introduced (Figures E-F) to display the error bars and trends more clearly.
Changes in body composition and glucose metabolism during GH treatment
During the first year of GH treatment, catch-up growth was accompanied by increases in lean body mass (p<0.0001) and bone mineral content (p<0.0001) (Table-1). Conversely, total body fat mass and limb fat mass declined (both p<0.0001) whereas trunk fat mass remained unchanged resulting in an increased trunk-limb fat ratio at 1-year (Figure-1). The differential changes in fat mass compared to lean body mass and bone mineral content resulted in a markedly reduced percentage fat in the whole body, limbs and trunk (all p<0.0001) (Figure-1). GH treatment led to considerable increases in height SDS, BMI SDS, IGF-1 SDS, and fasting insulin and C-peptide levels (Table-1). Insulin sensitivity decreased substantially; however, a compensatory increase in insulin secretion resulted in an unchanged disposition index. Triglyceride levels also increased, but no changes in total, LDL or HDL- cholesterol were observed.
Adiposity and response to GH treatment
Body composition
Children in the highest tertiles of percentage body fat z-scoresc showed the greatest loss of percentage body fat in the whole body (p-trends=0.005), trunk (p-trends=0.0001) and limbs (p-trends=0.002) (Table-2, Figure-2). Nevertheless, the baseline differences in adiposity between the groups persisted at 1-year of treatment, with the highest tertile group still having the greatest fat percentage in the whole body (p-trends=0.001), trunk (p-trends<0.0001) and limbs (p-trends=0.057).
Height and IGF-1 response
Increase in height SDS was positively associated with baseline percentage body fat z-scoresc (p-trends=0.038). In this study, variance in the first-year height velocity on GH treatment predicted by Ranke’s model (R2=0.15) was relatively low because of the use of a fixed GH dose. The addition of percentage body fat z-scoresc explained a further 12% variance in the first-year height velocity (p<0.0001, R2=0.27) (Table-3). We evaluated the associations of regional fat distribution on first-year height velocity by deriving z-scores for trunk and limb fat percentages at baseline (adjusted for age, gender, and scanner type). The addition of percentage limb fat z-scores explained a higher variance in the first-year height velocity (B [95 %CI]: 0.77cm/year [0.37, 1.17], p<0.0001, R2= 0.25) compared with trunk fat z-scores (0.61cm/year [0.24, 0.98], p=0.001, R2= 0.22) in the Ranke’s model. Furthermore, percentage limb fat z-scores explained an additional 5% variance when added to the model with percentage trunk fat z-scores (R2 increased from 0.22 to 0.27, p [R2 change]=0.031). Higher total body percentage body fat z-scoresc were associated with greater IGF-1 responses (p-trends=0.042) and IGF-1 levels at 1-year (p-trends=0.036). The addition of changes in IGF-1 SDS from baseline to 1-year further increased the explained variance in the first-year height velocity from 27% to 33% (p [R2 change]=0.013) (Table-3), however, the effects of the baseline percentage body fat remained significant. Reductions in body fat percentage during GH treatment were strongly associated with increased height gains independent of the baseline body fat (r=0.47, p<0.0001), but they were not related to IGF-1 responses. Decreases in the limb fat percentage (r=0.41, p=0.001) were more strongly related to height gains compared with the decreases in the trunk fat percentage (r=0.25, p=0.053) independent of the corresponding fat percentages at baseline.
Table 3. Effect of baseline total body fat on Ranke’s Prediction model for the first-year height response in SGA children.
| Models | B | 95% CI | P value | Partial Correlation |
Collinearity (Tolerance) |
R2 | P value (R2 change) |
|
|---|---|---|---|---|---|---|---|---|
| 1 | Constant | 13.7 | 11.5, 15.8 | <0.0001 | ||||
| Age (year) | -0.31 | -0.54, -0.09 | 0.008 | -0.30 | 0.97 | |||
| Midparental height (SDS) | 0.46 | 0.07, 0.85 | 0.022 | 0.26 | 0.96 | |||
| Weight at baseline (SDS) | 0.12 | -0.26, 0.50 | 0.52 | 0.07 | 0.93 | 0.15 | 0.008 | |
| 2 | Constant | 13.04 | 11.1, 15.0 | <0.0001 | ||||
| Age (year) | -0.29 | -0.50, -0.08 | 0.008 | -0.31 | 0.97 | |||
| Midparental height (SDS) | 0.47 | 0.11, 0.84 | 0.012 | 0.29 | 0.96 | |||
| Weight at baseline (SDS) | -0.04 | -0.40, 0.33 | 0.84 | -0.02 | 0.88 | |||
| Baseline total body fat % (z-score)* | 0.61 | 0.28, 0.95 | <0.0001 | 0.39 | 0.94 | 0.27 | 0.001 | |
| 3 | Constant | 10.9 | 8.36, 13.5 | <0.0001 | ||||
| Age (year) | -0.19 | -0.41, 0.03 | 0.096 | -0.19 | 0.85 | |||
| Midparental height (SDS) | 0.45 | 0.10, 0.79 | 0.012 | 0.28 | 0.95 | |||
| Weight at baseline (SDS) | -0.13 | -0.49, 0.23 | 0.48 | -0.08 | 0.83 | |||
| Baseline total body fat % (z-score)* | 0.59 | 0.26, 0.92 | 0.001 | 0.38 | 0.91 | |||
| Delta IGF-1 SDS (0 to 1-yr) | 0.30 | 0.07, 0.54 | 0.013 | 0.28 | 0.82 | 0.33 | 0.013 | |
within cohort z-scores for total body fat percentage at baseline adjusted for age, gender and type of scanner (z-scorec) Dependent Variable: Height velocity (cm/year); B, unstandardized coefficient; CI, confidence interval
Model 1: Ranke’s Model for prediction of first-year height velocity in SGA children; Growth Hormone dose is not included in the model as a fixed dose was used in the study
Model 2: The effect of total body fat percentage on Ranke’s Prediction Model
Model 3: Effect of the addition of change in IGF-I SDS (0 to 1-year)
Glucose and lipid metabolism
During GH treatment, changes in glucose, insulin and C-peptide levels, and insulin sensitivity were similar across the tertile groups. However, children in the highest tertile group had greater increases in AIR during treatment (p-trends=0.014) resulting in higher AIR (p-trends=0.004) and disposition index (p=0.024) at 1-year (Figure-2E&2F). No differences were observed in the changes in fasting lipids between the tertile groups (data not shown).
Multiallele scores and body composition
Insulin sensitivity
At baseline GS-InRes was inversely related to BMI SDS (B [95 %CI]: -0.13 SDS per-allele [-0.26, -0.01], p=0.040) and percentage fat in the whole body (-0.49% per-allele [-0.97, -0.007], p=0.047) and limbs (-0.81% per-allele [-1.62, 0.00], p=0.049), but not in trunk (Table-4). During GH treatment, a higher GS-InRes was associated with lesser declines in total body fat (0.31% per-allele [0.10, 0.51], p=0.004) and trunk fat (0.38% per-allele [0.16, 0.59], p=0.001), and therefore increases in trunk-limb fat ratio (0.03 per-allele [0.01, 0.05], p=0.003). At 1-year, GS-InRes was still inversely associated with percentage fat in the limbs (-0.81% per-allele [-1.49, -0.13], p=0.020) and positively associated with trunk-limb fat ratio (0.04 per-allele [0.01, 0.08], p=0.008).
Table 4. Associations between multiallele scores for insulin sensitivity and body composition#.
| Effect size per allele (B) | 95 % CI | P value* | |
|---|---|---|---|
| Baseline | |||
| BMI (SDS) | -0.13 | -0.26, -0.01 | 0.040 |
| Body fat (%) | -0.49 | -0.97, -0.01 | 0.047 |
| Limb fat (%) | -0.81 | -1.62, 0.00 | 0.049 |
| Arm fat (%) | -1.19 | -2.31, -0.06 | 0.038 |
| Leg fat (%) | -0.76 | -1.55, 0.03 | 0.060 |
| Trunk fat (%) | -0.33 | -0.77, 0.12 | 0.16 |
| Trunk-limb fat ratio | 0.01 | -0.01, 0.03 | 0.49 |
| 1-year | |||
| BMI (SDS) | -0.07 | -0.22, 0.09 | 0.40 |
| Body fat (%) | -0.39 | -0.81, 0.02 | 0.064 |
| Limb fat (%) | -0.81 | -1.49, -0.13 | 0.020 |
| Arm fat (%) | -1.04 | -1.95, -0.13 | 0.026 |
| Leg fat (%) | -0.59 | -1.27, 0.09 | 0.087 |
| Trunk fat (%) | -0.03 | -0.43, 0.37 | 0.88 |
| Trunk-limb fat ratio | 0.04 | 0.01, 0.08 | 0.008 |
| Changes from baseline to 1-year | |||
| Delta body fat (%) | 0.31 | 0.10, 0.51 | 0.004 |
| Delta limb fat (%) | 0.28 | -0.11, 0.68 | 0.16 |
| Delta arm fat (%) | 0.18 | -0.41, 0.78 | 0.54 |
| Delta leg fat (%) | 0.27 | -0.16, 0.70 | 0.22 |
| Delta trunk fat (%) | 0.38 | 0.16, 0.59 | 0.001 |
| Delta trunk-limb fat ratio | 0.03 | 0.01, 0.05 | 0.003 |
higher scores associated with lower insulin sensitivity; B, unstandardized coefficient; CI, confidence interval
P-values and B are derived from regression models with age and gender as covariants
Insulin secretion
GS-InSec was positively associated with percentage trunk fat at baseline (0.36% per-allele [0.09, 0.63], p=0.009) and at 1-year (0.25% per-allele [0.01, 0.50], p=0.045) (Supplemental Table-2). However, it was not associated with percentage fat in the whole body or limbs.
Discussion
In this study of short SGA children, higher pre-treatment adiposity predicted greater height gains and IGF-1 response during GH treatment and increased β-cell function. Consideration of the baseline whole body and regional adiposity substantially improved the prediction of first-year height responses. Analysis of informative multiallele scores supported the likely causal role of insulin resistance in linking reduced body fat, particularly the peripheral body fat, to lower sensitivity to GH treatment.
In this large cohort, we confirmed the findings of reduced body fat in short SGA children (2,3,18). Previous studies using MRI scans (3,18) or skinfold thickness measurements (2,19) have reported deficits in subcutaneous fat both in the trunk and limbs, but similar visceral fat compared to AGA children (18). Alterations in adipose tissue development, adipokine signalling to the brain, and neuroendocrine regulation of appetite have been reported in animal models of intrauterine growth retardation associated with rapid catch-up growth (20,21). Conversely, similar mechanisms may be relevant in short SGA children with no catch-up growth, as they have a reduced appetite and food intake despite lower leptin levels compared with AGA controls (22). Nevertheless, the low adiposity reflects suboptimal energy stores and is consistent with the low levels of insulin and IGF-1 in short SGA children compared with weight-matched AGA controls (6). Anabolic actions of GH are closely linked to overall energy balance as shown by the increased IGF-1 responses in obesity and the low IGF-1 levels despite greater GH secretion during fasting (6,23). Our findings of lower IGF-1 and growth responses in children with lesser adiposity suggest that reduced sensitivity to exogenous GH related to suboptimal energy stores contributes to a poorer treatment effect. Alterations in GH/IGF-I axis ranging from relative GH deficiency to resistance may also explain these associations. However, overall leanness of these children as a group and that adiposity is unrelated to IGF-1 levels or insulin sensitivity suggest that they are less likely to have a primary role (2). Baseline adiposity predicted height gains independent of IGF-1 responses, which imply that pathways of GH action other than the hepatic IGF-1 generation are also influenced by the overall energy balance. The growth prediction models showed a substantial effect of baseline adiposity in promoting linear growth on GH treatment, however, the explained variance was insufficient (27%) for it to be used in clinical settings (24).
The energy balance is probably important in other childhood disorders treated with GH and may explain the inclusion of weight in the height prediction models for GHD patients (24). However, it is particularly relevant to SGA children who have low adiposity(17). Our observations of preferential loss of peripheral body fat during GH treatment support previous reports(2,19,25) and contrast the predominant effect on central fat in GHD patients (6,26). We postulate that the pattern of fat loss in SGA children results from further declines in energy stores as the limb depots are primarily related to long-term fat storage(27). A stronger relationship between growth response and limb fat at baseline compared to the trunk fat support this hypothesis. Furthermore, we found strong associations between first-year height gains and declines in body fat, particularly in the limbs, which suggests that rapid growth occurs at the expense of energy stores. The reduction of percentage body fat in our study (29%) on a higher GH dose (67μg/kg) was greater than that (21%) reported on the more common lower GH dose (35μg/kg), and is consistent with dose-dependent effects of GH on growth and lipolysis(6,17).
The findings of a relationship between lower adiposity, lesser insulin secretion and disposition index before and during GH treatment could reflect a physiological adaptation to prevent hypoglycaemia as seen during fasting and other suboptimal nutritional states (28,29). These associations may be mediated through alterations in the IGF-1 generation, which is important for maintaining β-cell function (30). The reduced β-cell function associated with lower adiposity could have long-term implications as thinness during childhood is related to an increased risk for T2D (31).
Following an initial marked decrease, body fat is reported to return to pre-treatment ranges in subsequent years when growth velocity declines(25). However, young SGA adults after stopping GH treatment have a tendency for a lesser limb fat percentage despite a higher total body fat percentage compared to AGA adults (32). Recently, fat depots in limbs and gluteofemoral region are shown to store triglycerides long-term more efficiently compared with the trunk fat and linked to favourable metabolic outcomes (11,27). The total number of adipocytes, which is fixed by late childhood, may also be a critical factor in determining the expandability of subcutaneous adipose tissue and metabolic decompensation in response to nutrient excess(21,33,34). Based on our findings of a positive relationship between adiposity, responses to GH treatment and β-cell function, conserving peripheral body fat could form the target for nutritional interventions to optimise energy balance in SGA children treated with GH.
Recent findings that common genetic variants for insulin resistance are related to lesser gluteofemoral and limb fat suggest an important role of expandability of regional subcutaneous adipose tissue in metabolic outcomes (11). We have observed for the first time the same relationship (with larger observed effect sizes) in a selected group of SGA children already present before GH treatment, which persisted at 1-year on treatment. The observed associations here, between lower adiposity and both genetic susceptibility to insulin resistance and lower growth response to GH treatment, complement our reported associations between the same alleles and lower growth and IGF-1 responses to GH treatment in the same cohort(10). Although Mendelian Randomisation analyses cannot formally model causal mediation, these findings support a causal role for insulin resistance in mediating the effects of lower adiposity on lesser GH action (Supplementary Figure-1). We speculate that these pathways could be linked to the hepatic IGF-1 generation and IGF-1 sensitivity. Reported effects of metformin treatment on improving linear growth despite lower IGF-1 levels in low birth-weight girls with premature adrenarche support this hypothesis (35,36). During treatment, the insulin resistance alleles were inversely related to reductions in body fat further suggesting reduced sensitivity to GH. However, the alleles were related to lesser reductions in the trunk fat and, therefore, an increased trunk-limb fat ratio at 1-year. We speculate that these changes could be due to the reduced function of peripheral adipose tissue and preferential fat storage centrally when lipid turnover is increased by GH treatment (6). The association between insulin secretion lowering alleles and higher trunk fat been reported in adults (11). Although its significance is not clear, this association could provide a link between a phenotype resulting from prenatal growth restraint with a tendency for central fat deposition and an increased risk for T2D (27,37).
Our study has some drawbacks. Although percentage body fat is a commonly used measure of adiposity in children, it is limited by the potential association with height(38). However, height was unrelated to adiposity in our selected group of lean subjects. The reasons for the residual associations between population derived z-scores for percentage body fat, and age and gender in the study were not clear(15). We speculate that comparisons to normative data from a different type of DXA scanner is an important reason and may underlie the higher pre-treatment body fat percentage in our study compared to previous reports (z-scores, -0.26 vs -0.6 to -1.2) (19,39). We used the within-cohort z-scores for percentage body fat in the calculations rather than further adjusting population derived z-scores for age and gender to avoid complex models in this modestly sized study. The associations between multiallele scores and body composition were modest; however, they were consistent when assessed at both baseline and at 1-year, and support similar findings in adults. Long-term illness may confound our observations, however, we excluded children with syndromes, severe learning difficulties or other disorders that may influence growth (8). We did not measure adipokines; further studies evaluating these and epigenetic changes in adipose tissue will be valuable to delineate the pathways underlying our findings.
In conclusion, our findings suggest that greater adiposity has beneficial effects on responses to GH treatment and glucose metabolism in short SGA children. Mechanisms associated with insulin resistance link lower adiposity and reduced response to GH treatment in these children. While the association between genetic susceptibility to insulin resistance and lower adiposity appears to be generalizable across adults and children, the conclusions linking these factors to GH treatment responses are limited to the population studied here.
Supplementary Material
Acknowledgements
We are extremely grateful to the study participants and their families. We thank the National Institute for Health Research, Cambridge BioResource, and the Institute of Metabolic Science, Cambridge Biomedical Campus, for collaboration. We thank the group of local investigators in the NESGAS group for collection of data. The NESGAS group includes the following investigators and study nurses: Birmingham—Mandy Aspinall, Tracey Kirkwood, Ellen Stone Kate Penny Thomas, Clare Williams; Cambridge—Catherine Fullah; Copenhagen—Niels Birkebaek, Peter Christiansen, Anni Ellerman, Kirsten Holm, Elise Snitker Jensen, Eva Mosfeldt-Jeppesen, Britta Kremke, Pawel Marcinski, Rune Naeraa, Claus Thøger Nielsen, Carsten Pedersen, Nina Saurbrey, Birgitte Scherling, Ebbe Thisted; Dublin—Elaine O’Mullane; Glasgow—Sheena McGowern; Malmoe—Helena Larsson, Carina Persson; Lund—Maria Elfving, Lena Rollof; and Stock-holm—Svante Norgren.
Funding: This study was funded by research grants from the Danish Council for Independent Research/Medical Sciences and Novo Nordisk A/S. The research work was also supported by International Center for Research and Research Training in Endocrine Disrupting Effects on Male Reproduction and Child Health (EDMaRC), Department of Growth and Reproduction, Rigshospitalet, University of Copenhagen, Denmark.
Footnotes
Disclosure Summary: The authors have nothing to declare
Clinical Trial Registration: NESGAS EudraCT 2005-001507-19
References
- 1.Meas T. Fetal origins of insulin resistance and the metabolic syndrome: a key role for adipose tissue? Diabetes & metabolism. 2010;36:11–20. doi: 10.1016/j.diabet.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.De Schepper J, Thomas M, Beckers D, Craen M, Maes M, de Zegher F. Growth hormone treatment and fat redistribution in children born small for gestational age. J Pediatr. 2008;152:327–330. doi: 10.1016/j.jpeds.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 3.Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV, Casano P, de Zegher F. Abdominal fat partitioning and high-molecular-weight adiponectin in short children born small for gestational age. J Clin Endocrinol Metab. 2009;94:1049–1052. doi: 10.1210/jc.2008-2176. [DOI] [PubMed] [Google Scholar]
- 4.Ojha S, Saroha V, Symonds ME, Budge H. Excess nutrient supply in early life and its later metabolic consequences. Clinical and experimental pharmacology & physiology. 2013;40:817–823. doi: 10.1111/1440-1681.12061. [DOI] [PubMed] [Google Scholar]
- 5.Kirk J. Indications for growth hormone therapy in children. Arch Dis Child. 2012;97:63–68. doi: 10.1136/adc.2010.186205. [DOI] [PubMed] [Google Scholar]
- 6.Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. The New England journal of medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen RB, Thankamony A, O'Connell SM, Salgin B, Kirk J, Donaldson M, Ivarsson SA, Soder O, Roche E, Hoey H, Dunger DB, et al. Baseline IGF-I levels determine insulin secretion and insulin sensitivity during the first year on growth hormone therapy in children born small for gestational age. Results from a North European Multicentre Study (NESGAS) Horm Res Paediatr. 2013;80:38–46. doi: 10.1159/000353438. [DOI] [PubMed] [Google Scholar]
- 9.Gies I, Thomas M, Tenoutasse S, De Waele K, Lebrethon MC, Beckers D, Francois I, Maes M, Rooman R, de Beaufort C, Massa G, et al. Insulin sensitivity modulates the growth response during the first year of high-dose growth hormone treatment in short prepubertal children born small for gestational age. Horm Res Paediatr. 2012;78:24–30. doi: 10.1159/000339829. [DOI] [PubMed] [Google Scholar]
- 10.Jensen RB, Thankamony A, Day F, Scott RA, Langenberg C, Kirk J, Donaldson M, Ivarsson SA, Soder O, Roche E, Hoey H, et al. Genetic markers of insulin sensitivity and insulin secretion are associated with spontaneous postnatal growth and response to growth hormone treatment in short SGA children: the North European SGA Study (NESGAS) J Clin Endocrinol Metab. 2015;100:E503–507. doi: 10.1210/jc.2014-3469. [DOI] [PubMed] [Google Scholar]
- 11.Scott RA, Fall T, Pasko D, Barker A, Sharp SJ, Arriola L, Balkau B, Barricarte A, Barroso I, Boeing H, Clavel-Chapelon F, et al. Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes. 2014;63:4378–4387. doi: 10.2337/db14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen RB, Thankamony A, O'Connell SM, Salgin B, Kirk J, Donaldson M, Ivarsson SA, Soder O, Roche E, Hoey H, Dunger DB, et al. Baseline IGF-I Levels Determine Insulin Secretion and Insulin Sensitivity during the First Year on Growth Hormone Therapy in Children Born Small for Gestational Age. Results from a North European Multicentre Study (NESGAS) Horm Res Paediatr. 2013;80:38–46. doi: 10.1159/000353438. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, Levine MA. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27:2208–2216. doi: 10.1002/jbmr.1654. [DOI] [PubMed] [Google Scholar]
- 14.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 15.van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM. Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch Dis Child. 2002;87:341–347. doi: 10.1136/adc.87.4.341. discussion 341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree NJ, Shaw NJ, Boivin CM, Oldroyd B, Truscott JG. Pediatric in vivo cross-calibration between the GE Lunar Prodigy and DPX-L bone densitometers. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16:2157–2167. doi: 10.1007/s00198-005-2021-2. [DOI] [PubMed] [Google Scholar]
- 17.Ranke MB, Lindberg A, Cowell CT, Wikland KA, Reiter EO, Wilton P, Price DA. Prediction of response to growth hormone treatment in short children born small for gestational age: analysis of data from KIGS (Pharmacia International Growth Database) J Clin Endocrinol Metab. 2003;88:125–131. doi: 10.1210/jc.2002-020867. [DOI] [PubMed] [Google Scholar]
- 18.Ibanez L, Lopez-Bermejo A, Diaz M, Jaramillo A, Marin S, de Zegher F. Growth hormone therapy in short children born small for gestational age: effects on abdominal fat partitioning and circulating follistatin and high-molecular-weight adiponectin. J Clin Endocrinol Metab. 2010;95:2234–2239. doi: 10.1210/jc.2009-2805. [DOI] [PubMed] [Google Scholar]
- 19.de Kort SW, Willemsen RH, van der Kaay DC, Duivenvoorden HJ, Hokken-Koelega AC. Does preterm birth influence the response to growth hormone treatment in short, small for gestational age children? Clin Endocrinol (Oxf) 2009;70:582–587. doi: 10.1111/j.1365-2265.2008.03484.x. [DOI] [PubMed] [Google Scholar]
- 20.Sarr O, Yang K, Regnault TR. In utero programming of later adiposity: the role of fetal growth restriction. Journal of pregnancy. 2012;2012 doi: 10.1155/2012/134758. 134758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultz NS, Broholm C, Gillberg L, Mortensen B, Jorgensen SW, Schultz HS, Scheele C, Wojtaszewski JF, Pedersen BK, Vaag A. Impaired leptin gene expression and release in cultured preadipocytes isolated from individuals born with low birth weight. Diabetes. 2014;63:111–121. doi: 10.2337/db13-0621. [DOI] [PubMed] [Google Scholar]
- 22.Boonstra VH, Arends NJ, Stijnen T, Blum WF, Akkerman O, Hokken-Koelega AC. Food intake of children with short stature born small for gestational age before and during a randomized GH trial. Horm Res. 2006;65:23–30. doi: 10.1159/000090376. [DOI] [PubMed] [Google Scholar]
- 23.Donzeau A, Bouhours-Nouet N, Fauchard M, Decrequy A, Mathieu E, Boux de Casson F, Gascoin G, Coutant R. Birth weight is associated with the IGF-1 response to GH in children: programming of the anabolic action of GH? J Clin Endocrinol Metab. 2015 doi: 10.1210/jc.2015-1603. jc20151603. [DOI] [PubMed] [Google Scholar]
- 24.Ranke MB, Lindberg A, Chatelain P, Wilton P, Cutfield W, Albertsson-Wikland K, Price DA. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999;84:1174–1183. doi: 10.1210/jcem.84.4.5634. [DOI] [PubMed] [Google Scholar]
- 25.Sas T, Mulder P, Hokken-Koelega A. Body composition, blood pressure, and lipid metabolism before and during long-term growth hormone (GH) treatment in children with short stature born small for gestational age either with or without GH deficiency. J Clin Endocrinol Metab. 2000;85:3786–3792. doi: 10.1210/jcem.85.10.6917. [DOI] [PubMed] [Google Scholar]
- 26.Norrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res. 2005;15:95–122. doi: 10.1016/j.ghir.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue[mdash]link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 28.Salgin B, Marcovecchio ML, Humphreys SM, Hill N, Chassin LJ, Lunn DJ, Hovorka R, Dunger DB. Effects of prolonged fasting and sustained lipolysis on insulin secretion and insulin sensitivity in normal subjects. Am J Physiol Endocrinol Metab. 2009;296:E454–461. doi: 10.1152/ajpendo.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hales C, Barker D. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. International Journal of Epidemiology. 2013;42:1215–1222. doi: 10.1093/ije/dyt133. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR. beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet. 2002;31:111–115. doi: 10.1038/ng872. [DOI] [PubMed] [Google Scholar]
- 31.Eriksson JG, Kajantie E, Lampl M, Osmond C. Trajectories of body mass index amongst children who develop type 2 diabetes as adults. J Intern Med. 2015 doi: 10.1111/joim.12354. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 32.Breukhoven PE, Kerkhof GF, van Dijk M, Hokken-Koelega AC. Long-term impact of GH treatment during childhood on body composition and fat distribution in young adults born SGA. J Clin Endocrinol Metab. 2011;96:3710–3716. doi: 10.1210/jc.2011-1658. [DOI] [PubMed] [Google Scholar]
- 33.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends in Endocrinology & Metabolism. 2015;26:193–200. doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, Bernard S, Arner P. Adipocyte Turnover: Relevance to Human Adipose Tissue Morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibanez L, Valls C, Ong K, Dunger DB, de Zegher F. Metformin therapy during puberty delays menarche, prolongs pubertal growth, and augments adult height: a randomized study in low-birth-weight girls with early-normal onset of puberty. J Clin Endocrinol Metab. 2006;91:2068–2073. doi: 10.1210/jc.2005-2329. [DOI] [PubMed] [Google Scholar]
- 36.Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F. Early metformin therapy to delay menarche and augment height in girls with precocious pubarche. Fertility and sterility. 2011;95:727–730. doi: 10.1016/j.fertnstert.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 37.Ong KK, Petry CJ, Emmett PM, Sandhu MS, Kiess W, Hales CN, Ness AR, Dunger DB, team As. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia. 2004;47:1064–1070. doi: 10.1007/s00125-004-1405-8. [DOI] [PubMed] [Google Scholar]
- 38.Weber DR, Leonard MB, Zemel BS. Body composition analysis in the pediatric population. Pediatric endocrinology reviews : PER. 2012;10:130–139. [PMC free article] [PubMed] [Google Scholar]
- 39.de Kort SW, Willemsen RH, van der Kaay DC, Hokken-Koelega AC. The effect of growth hormone treatment on metabolic and cardiovascular risk factors is similar in preterm and term short, small for gestational age children. Clin Endocrinol (Oxf) 2009;71:65–73. doi: 10.1111/j.1365-2265.2008.03504.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.