Abstract
Abnormal fetal testis development has been proposed to underlie common disorders of the male reproductive system such as cryptorchidism, hypospadias, reduced semen quality and testicular germ cell tumour, which are regarded as components of a ‘testicular dysgenesis syndrome’. The increasing trends and geographical variation in their incidence have been suggested to result from in utero exposure to environmental chemicals acting as endocrine disruptors. In rodents, the anogenital distance (AGD), measured from the anus to the base of genital tubercle, is a sensitive biomarker of androgen exposure during a critical embryonic window of testis development. In humans, several epidemiological studies have shown alterations in AGD associated with prenatal exposure to several chemicals with potential endocrine disrupting activity. However, the link between AGD and androgen exposure in humans is not well defined. This review focuses on the current evidence for such a relationship. As in rodents, a clear gender difference is detected during fetal development of the AGD in humans which is maintained thereafter. Reduced AGD in association with clinically relevant outcomes of potential environmental exposures, such as cryptorchidism or hypospadias, is in keeping with AGD as a marker of fetal testicular function. Furthermore, AGD may reflect variations in prenatal androgen exposure in healthy children as shorter AGD at birth is associated with reduced masculine play behaviour in preschool boys. Several studies provide evidence linking shorter AGD with lower fertility, semen quality and testosterone levels in selected groups of adults attending andrology clinics. Overall, the observational data in humans are consistent with experimental studies in animals and support the use of AGD as a biomarker of fetal androgen exposure. Future studies evaluating AGD in relation to reproductive hormones in both infants and adults, and to gene polymorphisms, will help to further delineate the effect of prenatal and postnatal androgen exposures on AGD.
Introduction
Links between disorders of male reproductive system, such as reduced semen quality and testicular germ cell tumour (TGCC), as well as genital abnormalities at birth, such as cryptorchidism and hypospadias, are well established (Skakkebaek et al., 2001). These observations led to the hypothesis of ‘testicular dysgenesis syndrome’ (TDS) which proposes that abnormal testis development during fetal life is an important mechanism underlying common disorders of the male reproductive tract which manifest during infancy or adult life (Skakkebaek et al., 2001). Experimental animal studies, which used anti-androgens to alter fetal testis development, provide a compelling model to support the hypothesis (Dean and Sharpe, 2013). Although TGCC, one component of TDS, has not been replicated in animal models, this is perhaps due to species specificity (Juul et al., 2014). Rare disorders of sex development (DSD) due to a primary defect in testis determination, androgen secretion or androgen action manifest phenotypic features of TDS, supporting the relevance of the TDS model in humans (Hughes et al., 2007). The early origins of male reproductive disorders hypothesis is of relevance to public health as environmental exposure to potential endocrine disruptors in utero have been proposed to explain the increasing trends in the incidence and their marked geographical variation (Acerini and Hughes, 2006; Hauser et al., 2015). Estimating the burden of prenatal exposure to potential endocrine disruptors is a challenge, as congenital disorders of the male reproductive tract are relatively rare and disturbed reproductive function is likely to manifest a long time after the chemical exposure. In economic terms, an estimated annual cost to the European Union of 15 billion Euro has been calculated for the consequences of male reproductive disorders (e.g., treatments for infertility, orchidopexies, testis cancer) using the ‘Intergovernmental Panel on Climate Change’ weight-of-evidence characterization model for probability of causation (Hauser et al., 2015).
The anogenital distance (AGD) measured from the anus to the genital tubercle is a sensitive biomarker of prenatal androgen action in animals (McIntyre et al., 2001; Mylchreest et al., 2000; Wolf et al., 2004). AGD signifies perineal growth and androgen-dependent caudal migration of the genital tubercle in rodents (Bowman et al., 2003). It is influenced by exposure to anti-androgens during a critical period of testis development known as the ‘masculinisation programming window’ (MPW) (van den Driesche et al., 2012; Welsh et al., 2008). Consequently, measurement of AGD has been used to study the effects of prenatal exposure to a variety of chemicals with potential endocrine disrupting activity. Several epidemiological studies have shown reduced AGD in association with exposure to phthalates (Adibi et al., 2015; Bornehag et al., 2015; Suzuki et al., 2012; Swan et al., 2005), dioxins (Vafeiadi et al., 2013), bisphenol A (Miao et al., 2011) and high fat diet (Papadopoulou et al., 2013), the latter indicative of organic pollutant exposure. It is not possible to conduct experimental toxicological studies in humans and therefore, the evidence for a causal effect of prenatal androgen exposure on AGD in humans is indirect (Welsh et al., 2007). This review focuses on the evidence linking AGD and androgen exposure in humans.
Physiological aspects of androgen exposure in the male
Leydig cells differentiate at approximately 8 weeks of gestation and secrete testosterone to mediate differentiation of the internal and external genitalia (Grinspon et al., 2014). Testosterone production is initially regulated during the first trimester by human chorionic gonadotrophin (hCG), which reaches a peak concentration at 12-17 weeks and subsequently declines (Cole, 2010). Development of the male genitalia is completed by 14-16 weeks (Grinspon et al., 2014; Welsh et al., 2008). Fetal luteinising hormone (LH) regulates testosterone secretion from the second trimester which induces further growth of the phallus and scrotum, and together with insulin-like factor 3 (INSL-3), promotes testis descent (Asa et al., 1991; Bay et al., 2007). After a surge of LH and testosterone secretion following delivery, testosterone levels decline in the first week of life (Corbier et al., 1990). Further activation of the hypothalamic-pituitary-gonadal axis known as the mini-puberty, starts at the end of the first week, peaking at 1-3 months of age before declining to low or undetectable levels by 6 months of age (Bergada et al., 2006; Forest et al., 1974; Kuiri-Hanninen et al., 2014). Subsequently, the axis remains quiescent until the onset of puberty. The observation of a MPW in the rat (equivalent to 8-14 weeks of gestation in the human) suggests that alterations in androgen action during a critical window of fetal development results in TDS and permanent changes in AGD (Welsh et al., 2008).
Definition and measurement methods
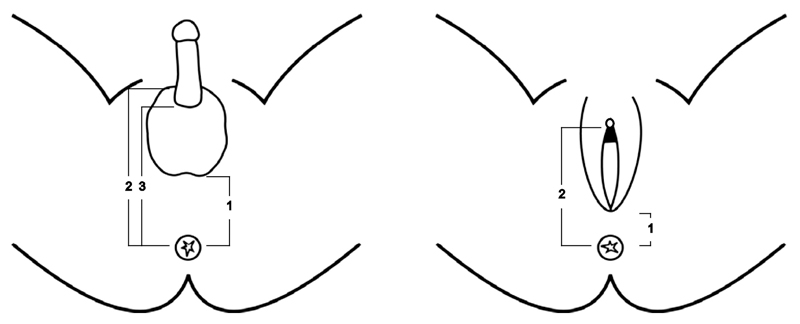
In rodents, AGD is measured from the anus to the posterior base of the genital tubercle (Gallavan et al., 1999). In contrast to rodents, the external genitalia are well developed at birth in humans with the genital tubercle transformed into the penis in males and clitoris in females. Investigators have used different landmarks to measure AGD in humans to replicate the measurement in rodents. In males, AGD has been measured from the anus to the perineoscrotal junction (anoscrotal distance) (Salazar-Martinez et al., 2004), to the posterior base or to the anterior base of the penis (Hsieh et al., 2008) (Fig.1). Measurements in females use the distance from the anus to the anterior fourchette (anofourchettal distance) (Salazar-Martinez et al., 2004) or to the base of the clitoris (anoclitoral distance) (Liu et al., 2014). The method described by Salazar-Martinez et al. is commonly used; it is more reliable and has a lower inter-observer variability (Dean and Sharpe, 2013; Papadopoulou et al., 2013; Salazar-Martinez et al., 2004). In this review, the term AGD describes ‘anoscrotal distance’ in males and ‘anofourchettal distance’ in females unless otherwise stated (Salazar-Martinez et al., 2004). Although AGD has been widely used as a marker of potential endocrine disruption in utero, its limitations include a lack of standardisation of methodology and information on reproducibility (Table-1) and insufficient data on normative references, including ethnic differences (Dean and Sharpe, 2013). AGD is associated with birth weight to a varying degree depending on the population studied (regression coefficient adjusted for gestation ranges from 1.5 to 3.0 mm/kg) (Papadopoulou et al., 2013; Romano-Riquer et al., 2007; Salazar-Martinez et al., 2004) and there is no consensus for adjusting AGD for the variations in body size. In addition, low birth weight is itself a risk factor for TDS as it is associated with hypospadias, cryptorchidism, male infertility and TGCC (Francois et al., 1997; Juul et al., 2014; Michos et al., 2007; Toppari et al., 2010).
Table 1. Normative data for anogenital Distance (mm) at birth showing gender differences.
| Reference* | Country | n | Male | Female | Male: Female Ratio |
|---|---|---|---|---|---|
| Salazar-Martinez et al., 2004 | Mexico | 87 | 21±3 | 11±2 | 1.9 |
| Thankamony et al., 2009 | UK | 564 | 19.8±6.1 | 9.1±2.8 | 2.2 |
| Huang et al., 2009 | Taiwan | 65 | 23 (10-36) | 16 (7-23) | 1.4 |
| Sathyanarayana et al., 2010 | USA | 169 | 23±4 | 15±3 | 1.5 |
| Papadopoulou et al., 2013 | Greece | 165 | 27.1±4.4 | 14.4±3.0 | 1.9 |
| Papadopoulou et al., 2013 | Spain | 187 | 23.6±5.1 | 13.8±2.5 | 1.7 |
| Sathyanarayana et al., 2015 | USA | 758 | 24.7±4.5 | 16.0±3.2 | 1.5 |
Means±SD or medians (range); The measurements correspond to the anoscrotal distance in boys and anofourchettal distance in girls.
studies reporting measurements in both genders at birth are included in the table
Associations between AGD, gender and age in healthy individuals
The AGD in rodents is approximately twice as long in males compared to females, and is routinely used to determine sex (Dean and Sharpe, 2013). We and others have reported that AGD is also sexually dimorphic in humans and displays a similar relative magnitude of difference (male: female ratio ranges from 1.4:1 to 2.2:1) (Table-1) (Huang et al., 2008; Papadopoulou et al., 2013; Salazar-Martinez et al., 2004; Sathyanarayana et al., 2010; Sathyanarayana et al., 2015; Thankamony et al., 2009). The sexual dimorphism is already present by gestation 11-13 weeks based on fetal imaging and, by weeks 17-20, it is of the same magnitude as that observed at birth, with a male: female ratio of 2:1 (Fowler et al., 2011). The latter time point corresponds to the completion of differentiation of the external genitalia in humans (Welsh et al., 2008).
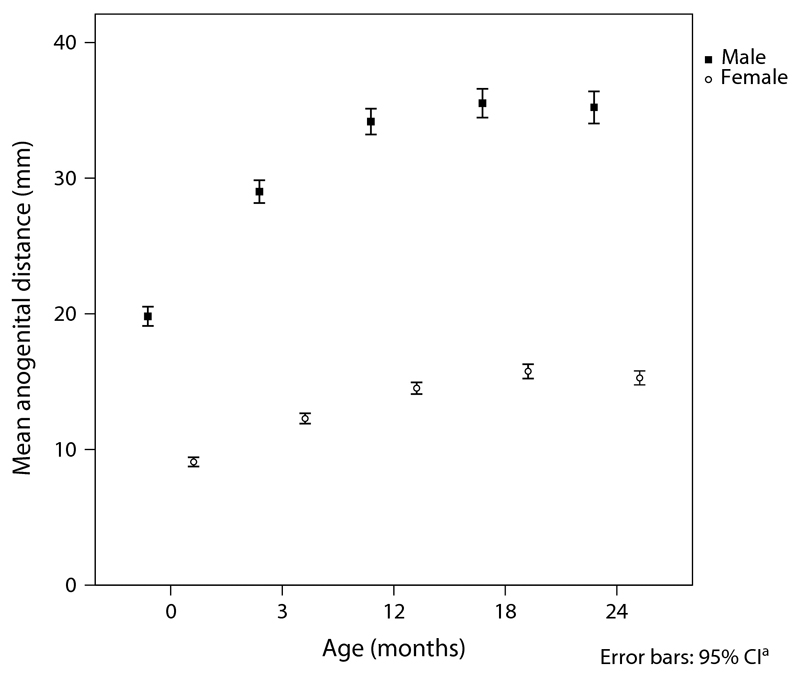
A large cohort (n=925) from the Cambridge Birth Growth Study (CBGS) had AGD measurements performed at birth and at ages 3, 12, 18 and 24 months (Thankamony et al., 2009). AGD increased rapidly during the first 3 months and plateaued after 1 year of age (Fig. 2). Using longitudinal and cross-sectional data from two birth cohorts in Greece and Spain, Papadopoulou et al. confirmed this pattern of growth. The sex dimorphism was maintained throughout to a similar degree to that observed at birth (male: female ratio; at birth, 2.2:1; age 24 months; 2.3:1) (Thankamony et al., 2009). The similar proportional increase in AGD in boys and girls during the first two years suggests the growth of perineum in proportion to overall body size. However, the period of rapid increase in AGD and penile length during the first three months of life corresponds to the mini-puberty (Kurtoglu and Bastug, 2014). We also found a modest association between increments in AGD and penile length during this period independent of the changes in body size, suggesting the postnatal surge in testosterone production may also contribute to changes in AGD (Thankamony et al., 2009). A positive association has been found between penile growth and serum testosterone levels during the first three months of life (Boas et al., 2006). This observation, coupled with animal data showing changes in AGD when postnatal androgen exposure is altered (Mitchell et al., 2015), supports the hypothesis that the mini-puberty plays a part in postnatal AGD development. Further studies are needed to delineate mini-puberty and its AGD component using detailed anthropometry, as well as more frequent hormone measurements which may require novel methods such as dried blood spot analytical technology (McDade et al., 2007).
Fig. 2.
Longitudinal measurements of AGD in males (n=463) and females (n=426) from birth to 2 years of age. Data presented as means and error bars represent 95% Confidence Intervals. Reproduced with permission from EHP (Thankamony et al., 2009).
Longitudinal data for AGD measurements from infancy to adulthood are not available. However, cross-sectional data in young adults show that large increases occur in later life and sexual dimorphism is maintained to a lesser degree (ranges; males, 48.3 - 51.3 mm; females, 34.8 - 37.7 mm; male: female ratio calculated from the means from different studies, 1.4:1) (Lee et al., 2015; Mendiola et al., 2012; Mendiola et al., 2011; Parra et al., 2015). When these changes occur is not known. We speculate that large increases in AGD occur during puberty in association with the development of the external genitalia. AGD remain unchanged in adult males, however, there are data to suggest that AGD decreases in females following the menopause (Eisenberg et al., 2013; Lee et al., 2015).
Cryptorchidism and hypospadias
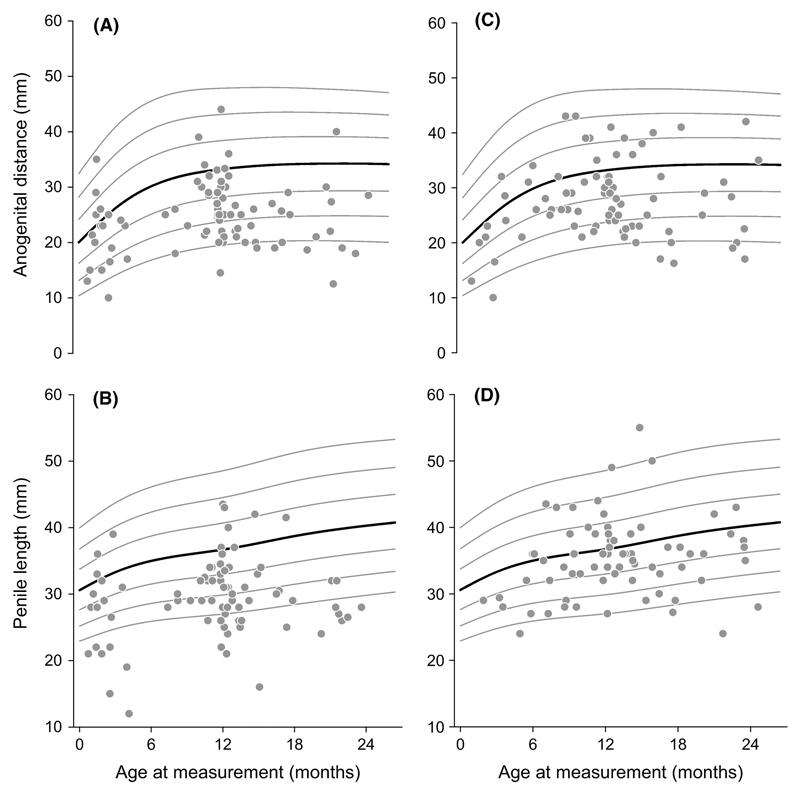
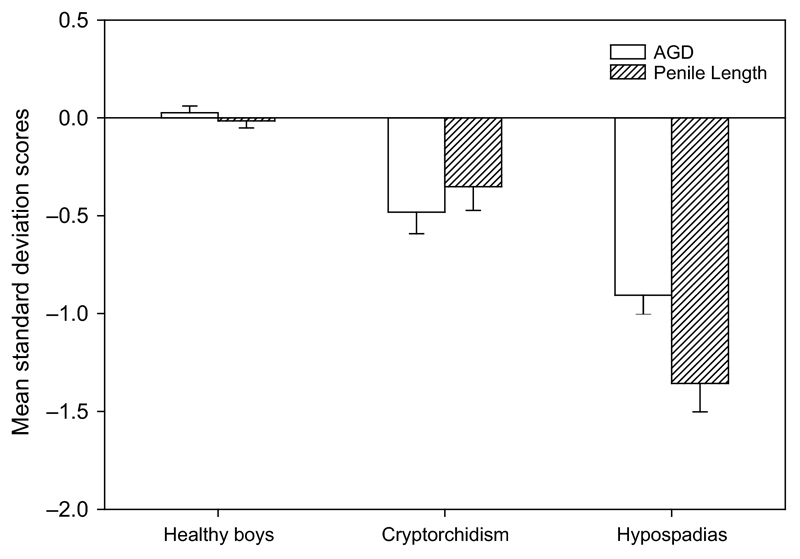
Cryptorchidism and hypospadias are the most common genital anomalies at birth with incidences of 2-9% and 0.2-1%, respectively, and provide an important outcome for studies of prenatal exposure to endocrine disrupting chemicals (Toppari et al., 2010). Establishing the relationship between AGD and congenital anomalies of the male reproductive tract at birth is key to determining whether AGD has a role as a biomarker of such prenatal exposure (Thankamony et al., 2014). A cross-sectional population study reported that boys with cryptorchidism have a shorter age-adjusted ‘anogenital index’ (a derivative of AGD adjusted for weight) (Swan et al., 2005). In boys undergoing surgery for hypospadias (n=26), AGD was measured under anaesthesia and found to be shorter compared with age-matched controls who had other urological conditions (Hsieh et al., 2012). In a larger study, we compared boys aged up to two years with cryptorchidism (n=71) or hypospadias (n=81) referred for surgical treatment with healthy controls from a birth cohort (n=482) by deriving age-specific standard deviation scores (SDS) of AGD and penile length (Thankamony et al., 2014). AGD measurements in boys with cryptorchidism (-0.48 SDS) or hypospadias (-0.90 SDS) were significantly lower compared with healthy controls (+0.03 SDS) (Fig. 3 & 4). They also had a shorter penile length (cryptorchidism, -0.35 SDS; hypospadias, -1.34 SDS) compared with healthy boys (-0.02 SDS). Boys with hypospadias also had smaller overall body size than controls, consistent with the well-documented prevalence of low birth weight in idiopathic hypospadias (Jensen et al., 2012). However, the reduction in AGD and penile length persisted following adjustment for body size in a multiple regression model. The observations of reduced AGD have also been reported in a large population-based study (boys with cryptorchidism, n=51; controls, n=534) from India involving consecutively born term male neonates evaluated at birth (Jain and Singal, 2013).
Hypospadias and cryptorchidism are relevant clinical disorders to evaluate the possible role of endocrine disruption, for which there is ample evidence from animal studies (van den Driesche et al., 2012; Welsh et al., 2008). The evidence to support a link between these disorders and altered testis function in utero in humans relies on epidemiological studies (Toppari et al., 2010). These common disorders are sometimes the manifestations of rare, but, well-defined disorders of androgen production or action such as androgen receptor mutations. However, in the majority of cases the cause is unknown (Toppari et al., 2010). The link between reduced semen quality and TGCC (Skakkebaek et al., 2001), and the association between cryptorchidism and lower INSL3 or higher gonadotrophin levels (Bay et al., 2007; Suomi et al., 2006), attest to underlying testis dysfunction. Cryptorchidism is considered to be the result of a milder defect in androgen function as most of the cases are due to impaired inguinoscrotal descent which normally occurs between 26 and 40 weeks of gestation, compared with hypospadias which occurs earlier during the MPW (between 8 and 20 weeks) (Thorup et al., 2010). Reported trends towards a shorter AGD and penile length with increasing severity of hypospadias (Thankamony et al., 2014), and higher testis position in cryptorchidism (Jain and Singal, 2013), further suggest that AGD is a marker of the severity of impairments in androgen production. It is now recognised that approximately half of all cases of cryptorchidism are “acquired” (i.e. following normal positioning of the testes at birth), hitherto referred to as ‘the ascending testis’ (Hack et al., 2012). It is possible that this form of cryptorchidism may be associated with suboptimal testosterone production during mini-puberty (Acerini et al., 2009; Wohlfahrt-Veje et al., 2009). Larger studies of boys with cryptorchidism may indicate whether AGD measurements sub-divided according to congenital versus acquired forms reflect altered androgen action occurring in utero or during the early postnatal period.
Gender-typical behaviour
A relationship between prenatal androgen exposure and gender-related behaviour is well established in animals and humans (Hines et al., 2015). The evidence to support this in humans is mainly derived from studies of girls with congenital adrenal hyperplasia (CAH) who have been exposed to higher androgen levels during intrauterine life. Females with CAH show greater preference for boys’ toys and activities during childhood, have lower heterosexual orientation and are less feminine in their gender identity (Berenbaum and Hines, 1992; Hines et al., 2004; Pasterski et al., 2011; Pasterski et al., 2007; Pasterski et al., 2005). In addition, androgen production during early infancy, as measured by urinary testosterone levels, is related to increased masculine play behaviour in healthy boys at 14 months of age (Lamminmaki et al., 2012). It is not clear, however, whether the observation is solely related to postnatal androgen exposure. We studied gender-typed play behavior in healthy boys at age 3-4 years (n=81) in relation to longitudinal measurements of AGD and penile length during their first two years of life (Pasterski et al., 2015). Gender- related play behaviour was evaluated using the ‘Preschool Activities Inventory’ which consists of a validated 24-item parent questionnaire that assesses gender-typed toy and activity preferences (Golombok and Rust, 1993). Both AGD at birth, and penile growth during the first 3 months of life, independently predicted masculine behavior in a regression model controlling for overall changes in body size. As expected, AGD was not related to gender type play behaviour in girls. These findings provide evidence that AGD in healthy boys at birth reflects prenatal androgen exposure, whereas the rate of penile growth in early infancy reflects more the effect of early postnatal androgen exposure during mini-puberty. Taken together, both parameters have potential use as biomarkers of endocrine disruption during pre- and early post-natal development.
Disorders of sex development (DSD)
Disorders of sex development are defined as congenital conditions in which development of chromosomal, gonadal, or anatomical sex is atypical and include several disorders with well-defined alterations in production or action of sex steroids (Hughes et al., 2006). Although such presentations provide opportunities to study AGD as part of a phenotype, there are difficulties in identifying the landmarks for measurement of AGD, particularly in the in severe forms where there are genital ambiguities. An increased AGD has been reported in a small study of girls with CAH (Callegari et al., 1987). The complete form of androgen insensitivity syndrome (CAIS) is an example of XY sex reversal due to mutations in the androgen receptor gene resulting in resistance to androgen action (Hughes et al., 2012). As expected, an androgen receptor knock-out mouse model (the ARKO mouse) showed a female phenotype in ARKO males, including AGD similar to female mice (Yeh et al., 2002). No data are available for AGD in women with CAIS using the commonly used method of measurement discussed in this review. However, in a heterogenous group of 19 women with a clinical diagnosis of CAIS examined during a routine clinic visit, clitoral length was reduced but the clitoral to urethral distance (measured from the base of the clitoris to the anterior aspect of the external urethral meatus) was similar to a control group of women undergoing a gynaecological examination under anaesthesia (Crouch et al., 2011). It is not clear whether the segment of perineum represented by this measurement truly reflects the AGD parameter which is accepted as being androgen dependent in both animal models and humans. Studies are required in the partial form of androgen insensitivity syndrome (PAIS) or hypogonadotrophic hypogonadism (where impaired androgen production occurs in late gestation) to determine if these, and other examples of DSD, provide information on assessing AGD as a quantitative measure of prenatal androgen exposure. Female rhesus monkeys exposed to androgens early in gestation exhibited hyperandrogenism, oligomenorrhea, large polyfollicular ovaries and other features consistent with the polycystic ovarian disease (PCOS) phenotype seen in humans (Abbott et al., 2005). The effects of such exposure in utero to higher androgens on AGD in female offspring of mothers with conditions such as CAH or PCOS merit study. A cross-sectional study of healthy young women showed greater follicular numbers were associated with longer AGD which, in turn, was also positively associated with higher serum testosterone levels (Mendiola et al., 2012; Mira-Escolano et al., 2014).
Testis function in adults and AGD
Evidence has been accumulating in several countries since it was first reported that sperm quality in men is in decline (Carlsen et al., 1992). Impaired semen quality, an important marker of testis function and component of TDS, is now reported in up to 20% of otherwise healthy young men (Andersson et al., 2008; Jorgensen et al., 2006). Shorter AGD is associated with lower sperm concentration, total sperm count, sperm motility and also testosterone levels in men attending andrology clinics (Eisenberg et al., 2011; Eisenberg and Lipshultz, 2015; Eisenberg et al., 2012; Mendiola et al., 2015). While a longer AGD is associated with a higher sperm count and better semen quality, the strength of association is insufficient to predict male fertility in an individual (Eisenberg and Lipshultz, 2015). A short AGD does distinguish men with non-obstructive causes of azoospermia (which is associated with TDS) from those with obstructive azoospermia (Eisenberg et al., 2012). These findings lend support for AGD as a biomarker for TDS and testis function in selected populations, but, few studies have explored the link between AGD and the larger range in testis size and semen quality in healthy males. A shorter AGD in association with lower semen quality has been reported in a US study of healthy young men (Mendiola et al., 2011); however, the results were not replicated in a similar study conducted in Spanish population (Parra et al., 2015). Report of a lower AGD measured from the anus to the anterior penile base in patients with prostate cancer compared to men with other causes of lower urinary tract symptoms (Castaño-Vinyals et al., 2012) suggest a possible link between alterations in the androgen-dependent fetal development of prostate (Wilson, 2011) and the cancer risk. However, anoscrotal distances were not different, and the findings of this small study need to be confirmed in future studies.
Although testis function is a key component of TDS, it relationship with AGD in adults is somewhat conflicting. Whereas studies in selected groups of men attending andrology clinics showed consistent associations between shorter AGD and lower semen quality or testosterone levels (Eisenberg et al., 2011; Eisenberg and Lipshultz, 2015; Eisenberg et al., 2012; Mendiola et al., 2015), these relations were inconsistent in healthy men with only one of the two studies showing an association with semen quality and none showed a relationship with testosterone levels (Mendiola et al., 2011; Parra et al., 2015). We speculate that the link between AGD and testis function is more robust in adults at the lower end of the distribution of reproductive health (possibly reflecting a high prevalence of testicular dysgenesis) compared to healthy men.
Genetic variations and AGD
As previously discussed, establishing a direct link between prenatal androgen exposure and AGD is difficult in humans, and the current evidence has relied mainly on data from observational epidemiological studies. Novel study designs using genes as instruments for causal inferences (i.e., Mendelian Randomisation) as has been applied to the study of cardiovascular disease (Thanassoulis, 2013) could provide additional strength to the evidence linking AGD and androgen exposure. Gene polymorphisms associated with TGCC, testosterone levels and male fertility and based on large genome-wide association studies (GWAS) are now available and may provide suitable instruments to test this hypothesis (Kosova et al., 2012; Litchfield et al., 2015; Ruth et al., 2015). A similar approach on the basis of candidate gene analysis has found a variant in the estrogen receptor alpha gene (ESR1) associated with shorter AGD (Sathyanarayana et al., 2012). Polymorphisms in ESR1 are associated with hypospadias, male infertility and alterations in semen parameters (Ban et al., 2008; Safarinejad et al., 2010). Measurement of the number of CAG repeats in the androgen receptor gene (AR) in a cohort of adult males attending a urology clinic showed that increased CAG lengths were associated with shorter AGD when the data were analysed in a stratified model (Eisenberg et al., 2013), but this finding was not replicated in another study (Sathyanarayana et al., 2012). There is an inverse association between the length of the CAG repeats and transcriptional activity of the androgen receptor as measured in vitro (Chamberlain et al., 1994), and when studied in relation to sperm quality, longer CAG repeats were associated with reduced sperm quality in some studies (Milatiner et al., 2004; Wallerand et al., 2001) but not in others (Dadze et al., 2000; Singh et al., 2006).
Conclusion
There is considerable observational evidence in humans that supports a link between AGD and exposure to androgens during fetal life. The findings are consistent with animal data that show a critical MPW during fetal male sex development and the programming of AGD. A plethora of studies are now reporting population data for AGD and applying the methods to assess testicular function and androgen action across a wide range of clinical disorders. AGD also appears to be a valid biomarker to assess the effects of an adverse environment on human reproductive development from fetal to adult life. Gathering such information will rely on epidemiological studies of birth cohorts followed longitudinally with detailed anthropometric measurements and analysis of targeted chemicals in appropriate biological samples.
Acknowledgements
The CBGS studies referred to in this review were supported by a European Union Framework V programme, the World Cancer Research Fund International, the Medical Research Council (UK), the Newlife Foundation, the Mothercare Foundation, the Evelyn Trust and the NIHR Cambridge Biomedical Research Centre.
Footnotes
Disclosure Summary: The authors have no conflicts of interest.
Authorship
AT wrote the original manuscript, IAH substantially modified and restructured the manuscript. VP, KKO and CA critically reviewed and substantially edited the manuscript.
References
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–74. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- Acerini CL, Hughes IA. Endocrine disrupting chemicals: a new and emerging public health problem? ArchDisChild. 2006;91:633–641. doi: 10.1136/adc.2005.088500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acerini CL, Miles HL, Dunger DB, Ong KK, Hughes IA. The descriptive epidemiology of congenital and acquired cryptorchidism in a UK infant cohort. Arch Dis Child. 2009;94:868–72. doi: 10.1136/adc.2008.150219. [DOI] [PubMed] [Google Scholar]
- Adibi JJ, Lee MK, Naimi AI, Barrett E, Nguyen RH, Sathyanarayana S, Zhao Y, Thiet MP, Redmon JB, Swan SH. Human Chorionic Gonadotropin Partially Mediates Phthalate Association With Male and Female Anogenital Distance. J Clin Endocrinol Metab. 2015;100:E1216–24. doi: 10.1210/jc.2015-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson AM, Jorgensen N, Main KM, Toppari J, Rajpert-De Meyts E, Leffers H, Juul A, Jensen TK, Skakkebaek NE. Adverse trends in male reproductive health: we may have reached a crucial 'tipping point'. Int J Androl. 2008;31:74–80. doi: 10.1111/j.1365-2605.2007.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asa SL, Kovacs K, Singer W. Human fetal adenohypophysis: morphologic and functional analysis in vitro. Neuroendocrinology. 1991;53:562–72. doi: 10.1159/000125775. [DOI] [PubMed] [Google Scholar]
- Ban S, Sata F, Kurahashi N, Kasai S, Moriya K, Kakizaki H, Nonomura K, Kishi R. Genetic polymorphisms of ESR1 and ESR2 that may influence estrogen activity and the risk of hypospadias. Hum Reprod. 2008;23:1466–71. doi: 10.1093/humrep/den098. [DOI] [PubMed] [Google Scholar]
- Bay K, Virtanen HE, Hartung S, Ivell R, Main KM, Skakkebaek NE, Andersson AM, Nordic Cryptorchidism Study G. Toppari J. Insulin-like factor 3 levels in cord blood and serum from children: effects of age, postnatal hypothalamic-pituitary-gonadal axis activation, and cryptorchidism. J Clin Endocrinol Metab. 2007;92:4020–7. doi: 10.1210/jc.2007-0974. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Hines M. Early androgens are related to childhood sex-typed toy preferences. Psychological Science. 1992;3:203–206. [Google Scholar]
- Bergada I, Milani C, Bedecarras P, Andreone L, Ropelato MG, Gottlieb S, Bergada C, Campo S, Rey RA. Time course of the serum gonadotropin surge, inhibins, and anti-Mullerian hormone in normal newborn males during the first month of life. J Clin Endocrinol Metab. 2006;91:4092–8. doi: 10.1210/jc.2006-1079. [DOI] [PubMed] [Google Scholar]
- Boas M, Boisen KA, Virtanen HE, Kaleva M, Suomi AM, Schmidt IM, Damgaard IN, Kai CM, Chellakooty M, Skakkebaek NE, Toppari J, et al. Postnatal penile length and growth rate correlate to serum testosterone levels: a longitudinal study of 1962 normal boys. Eur J Endocrinol. 2006;154:125–9. doi: 10.1530/eje.1.02066. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Carlstedt F, Jonsson BA, Lindh CH, Jensen TK, Bodin A, Jonsson C, Janson S, Swan SH. Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ Health Perspect. 2015;123:101–7. doi: 10.1289/ehp.1408163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CJ, Barlow NJ, Turner KJ, Wallace DG, Foster PM. Effects of in utero exposure to finasteride on androgen-dependent reproductive development in the male rat. Toxicol Sci. 2003;74:393–406. doi: 10.1093/toxsci/kfg128. [DOI] [PubMed] [Google Scholar]
- Callegari C, Everett S, Ross M, Brasel JA. Anogenital ratio: measure of fetal virilization in premature and full-term newborn infants. J Pediatr. 1987;111:240–3. doi: 10.1016/s0022-3476(87)80075-6. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebæk NE. Evidence for decreasing quality of semen during past 50 years. Bmj. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño-Vinyals G, Carrasco E, Lorente JA, Sabaté Y, Cirac-Claveras J, Pollán M, Kogevinas M. Anogenital distance and the risk of prostate cancer. BJU International. 2012;110:E707–E710. doi: 10.1111/j.1464-410X.2012.11516.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Research. 1994;22:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbier P, Dehennin L, Castanier M, Mebazaa A, Edwards DA, Roffi J. Sex differences in serum luteinizing hormone and testosterone in the human neonate during the first few hours after birth. J Clin Endocrinol Metab. 1990;71:1344–8. doi: 10.1210/jcem-71-5-1344. [DOI] [PubMed] [Google Scholar]
- Crouch NS, Michala L, Creighton SM, Conway GS. Androgen-dependent measurements of female genitalia in women with complete androgen insensitivity syndrome. BJOG: An International Journal of Obstetrics & Gynaecology. 2011;118:84–87. doi: 10.1111/j.1471-0528.2010.02778.x. [DOI] [PubMed] [Google Scholar]
- Dadze S, Wieland C, Jakubiczka S, Funke K, Schroder E, Royer-Pokora B, Willers R, Wieacker PF. The size of the CAG repeat in exon 1 of the androgen receptor gene shows no significant relationship to impaired spermatogenesis in an infertile Caucasoid sample of German origin. Mol Hum Reprod. 2000;6:207–14. doi: 10.1093/molehr/6.3.207. [DOI] [PubMed] [Google Scholar]
- Dean A, Sharpe RM. Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab. 2013;98:2230–8. doi: 10.1210/jc.2012-4057. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS One. 2011;6:0018973. doi: 10.1371/journal.pone.0018973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Hsieh TC, Lipshultz LI. The relationship between anogenital distance and age. Andrology. 2013;1:90–3. doi: 10.1111/j.2047-2927.2012.00019.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg ML, Hsieh TC, Pastuszak AW, McIntyre MG, Walters RC, Lamb DJ, Lipshultz LI. The relationship between anogenital distance and the androgen receptor CAG repeat length. Asian J Androl. 2013;15:286–9. doi: 10.1038/aja.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Lipshultz LI. Anogenital distance as a measure of human male fertility. J Assist Reprod Genet. 2015;32:479–84. doi: 10.1007/s10815-014-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Shy M, Walters RC, Lipshultz LI. The relationship between anogenital distance and azoospermia in adult men. Int J Androl. 2012;35:726–30. doi: 10.1111/j.1365-2605.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- Forest MG, Sizonenko PC, Cathiard AM, Bertrand J. Hypophyso-gonadal function in humans during the first year of life. 1. Evidence for testicular activity in early infancy. J Clin Invest. 1974;53:819–28. doi: 10.1172/JCI107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler PA, Bhattacharya S, Flannigan S, Drake AJ, O'Shaughnessy PJ. Maternal cigarette smoking and effects on androgen action in male offspring: unexpected effects on second-trimester anogenital distance. J Clin Endocrinol Metab. 2011;96:E1502–6. doi: 10.1210/jc.2011-1100. [DOI] [PubMed] [Google Scholar]
- Francois I, de Zegher F, Spiessens C, D'Hooghe T, Vanderschueren D. Low birth weight and subsequent male subfertility. Pediatr Res. 1997;42:899–901. doi: 10.1203/00006450-199712000-00029. [DOI] [PubMed] [Google Scholar]
- Gallavan RH, Jr, Holson JF, Stump DG, Knapp JF, Reynolds VL. Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. ReprodToxicol. 1999;13:383–390. doi: 10.1016/s0890-6238(99)00036-2. [DOI] [PubMed] [Google Scholar]
- Golombok S, Rust J. The measurement of gender role behaviour in pre-school children: a research note. J Child Psychol Psychiatry. 1993;34:805–11. doi: 10.1111/j.1469-7610.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Grinspon RP, Loreti N, Braslavsky D, Valeri C, Schteingart H, Ballerini MG, Bedecarras P, Ambao V, Gottlieb S, Ropelato MG, Bergada I, et al. Spreading the clinical window for diagnosing fetal-onset hypogonadism in boys. Front Endocrinol (Lausanne) 2014;5:51. doi: 10.3389/fendo.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack WWM, Goede J, van der Voort-Doedens LM, Meijer RW. Acquired undescended testis: putting the pieces together. International Journal of Andrology. 2012;35:41–45. doi: 10.1111/j.1365-2605.2011.01155.x. [DOI] [PubMed] [Google Scholar]
- Hauser R, Skakkebaek NE, Hass U, Toppari J, Juul A, Andersson AM, Kortenkamp A, Heindel JJ, Trasande L. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100:1267–77. doi: 10.1210/jc.2014-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Brook C, Conway GS. Androgen and psychosexual development: core gender identity, sexual orientation and recalled childhood gender role behavior in women and men with congenital adrenal hyperplasia (CAH) J Sex Res. 2004;41:75–81. doi: 10.1080/00224490409552215. [DOI] [PubMed] [Google Scholar]
- Hines M, Constantinescu M, Spencer D. Early androgen exposure and human gender development. Biol Sex Differ. 2015;6:3. doi: 10.1186/s13293-015-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh MH, Breyer BN, Eisenberg ML, Baskin LS. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. CurrUrolRep. 2008;9:137–142. doi: 10.1007/s11934-008-0025-0. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Eisenberg ML, Hittelman AB, Wilson JM, Tasian GE, Baskin LS. Caucasian male infants and boys with hypospadias exhibit reduced anogenital distance. Hum Reprod. 2012;27:1577–80. doi: 10.1093/humrep/des087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Chou YY, Lin SJ, L CC. Association between prenatal exposure to phthalates and the health of newborns. EnvironInt. 2008 doi: 10.1016/j.envint.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, MacDougall J. Androgen insensitivity syndrome. Lancet. 2012;380:1419–28. doi: 10.1016/S0140-6736(12)60071-3. [DOI] [PubMed] [Google Scholar]
- Hughes IA, Houk C, Ahmed SF, Lee PA, Group LC, Group EC Consensus statement on management of intersex disorders. Arch Dis Child. 2006;91:554–63. doi: 10.1136/adc.2006.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IA, Nihoul-Fékété C, Thomas B, Cohen-Kettenis PT. Consequences of the ESPE/LWPES guidelines for diagnosis and treatment of disorders of sex development. Best Practice & Research Clinical Endocrinology & Metabolism. 2007;21:351–365. doi: 10.1016/j.beem.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Jain VG, Singal AK. Shorter anogenital distance correlates with undescended testis: a detailed genital anthropometric analysis in human newborns. Hum Reprod. 2013;28:2343–9. doi: 10.1093/humrep/det286. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Wilcox AJ, Olsen J, Bonde JP, Thulstrup AM, Ramlau-Hansen CH, Henriksen TB. Cryptorchidism and hypospadias in a cohort of 934,538 Danish boys: the role of birth weight, gestational age, body dimensions, and fetal growth. Am J Epidemiol. 2012;175:917–25. doi: 10.1093/aje/kwr421. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Asklund C, Carlsen E, Skakkebaek NE. Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int J Androl. 2006;29:54–61. doi: 10.1111/j.1365-2605.2005.00635.x. discussion 105-8. [DOI] [PubMed] [Google Scholar]
- Juul A, Almstrup K, Andersson A-M, Jensen TK, Jorgensen N, Main KM, Meyts ER-D, Toppari J, Skakkebaek NE. Possible fetal determinants of male infertility. Nat Rev Endocrinol. 2014;10:553–562. doi: 10.1038/nrendo.2014.97. [DOI] [PubMed] [Google Scholar]
- Kosova G, Scott NM, Niederberger C, Prins GS, Ober C. Genome-wide association study identifies candidate genes for male fertility traits in humans. Am J Hum Genet. 2012;90:950–61. doi: 10.1016/j.ajhg.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiri-Hanninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82:73–80. doi: 10.1159/000362414. [DOI] [PubMed] [Google Scholar]
- Kurtoglu S, Bastug O. Mini puberty and its interpretation. Turk Pediatri Ars. 2014;49:186–91. doi: 10.5152/tpa.2014.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamminmaki A, Hines M, Kuiri-Hanninen T, Kilpelainen L, Dunkel L, Sankilampi U. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm Behav. 2012;61:611–6. doi: 10.1016/j.yhbeh.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Lee D, Kim TH, Lee HH, Kim JM, Jeon DS, Kim YS. A pilot study of the impacts of menopause on the anogenital distance. J Menopausal Med. 2015;21:41–6. doi: 10.6118/jmm.2015.21.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield K, Shipley J, Turnbull C. Common variants identified in genome-wide association studies of testicular germ cell tumour: an update, biological insights and clinical application. Andrology. 2015;3:34–46. doi: 10.1111/andr.304. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu X, Huo X. Anogenital distance and its application in environmental health research. Environmental Science and Pollution Research. 2014;21:5457–5464. doi: 10.1007/s11356-014-2570-z. [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams SA, Snodgrass JJ. What a Drop Can Do: Dried Blood Spots as a Minimally Invasive Method for Integrating Biomarkers Into Population-Based Research. Demography. 2007;44:899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Foster PM. Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. ToxicolSci. 2001;62:236–249. doi: 10.1093/toxsci/62.2.236. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Melgarejo M, Monino-Garcia M, Cutillas-Tolin A, Noguera-Velasco JA, Torres-Cantero AM. Is anogenital distance associated with semen quality in male partners of subfertile couples? Andrology. 2015;3:672–6. doi: 10.1111/andr.12059. [DOI] [PubMed] [Google Scholar]
- Mendiola J, Roca M, Minguez-Alarcon L, Mira-Escolano MP, Lopez-Espin JJ, Barrett ES, Swan SH, Torres-Cantero AM. Anogenital distance is related to ovarian follicular number in young Spanish women: a cross-sectional study. Environ Health. 2012;11:90. doi: 10.1186/1476-069X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Stahlhut RW, Jorgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011;119:958–63. doi: 10.1289/ehp.1103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao M, Yuan W, He Y, Zhou Z, Wang J, Gao E, Li G, Li DK. In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res A Clin Mol Teratol. 2011;91:867–72. doi: 10.1002/bdra.22845. [DOI] [PubMed] [Google Scholar]
- Michos A, Xue F, Michels KB. Birth weight and the risk of testicular cancer: a meta-analysis. Int J Cancer. 2007;121:1123–31. doi: 10.1002/ijc.22771. [DOI] [PubMed] [Google Scholar]
- Milatiner D, Halle D, Huerta M, Margalioth EJ, Cohen Y, Ben-Chetrit A, Gal M, Mimoni T, Eldar-Geva T. Associations between androgen receptor CAG repeat length and sperm morphology. Hum Reprod. 2004;19:1426–30. doi: 10.1093/humrep/deh251. [DOI] [PubMed] [Google Scholar]
- Mira-Escolano MP, Mendiola J, Minguez-Alarcon L, Melgarejo M, Cutillas-Tolin A, Roca M, Lopez-Espin JJ, Noguera-Velasco JA, Torres-Cantero AM. Longer anogenital distance is associated with higher testosterone levels in women: a cross-sectional study. Bjog. 2014;121:1359–64. doi: 10.1111/1471-0528.12627. [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Mungall W, McKinnell C, Sharpe RM, Cruickshanks L, Milne L, Smith LB. Anogenital distance plasticity in adulthood: implications for its use as a biomarker of fetal androgen action. Endocrinology. 2015;156:24–31. doi: 10.1210/en.2014-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, Cattley RC, Foster PM. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. ToxicolSci. 2000;55:143–151. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Vafeiadi M, Agramunt S, Basagana X, Mathianaki K, Karakosta P, Spanaki A, Koutis A, Chatzi L, Vrijheid M, Kogevinas M. Anogenital distances in newborns and children from Spain and Greece: predictors, tracking and reliability. Paediatr Perinat Epidemiol. 2013;27:89–99. doi: 10.1111/ppe.12022. [DOI] [PubMed] [Google Scholar]
- Papadopoulou E, Vafeiadi M, Agramunt S, Mathianaki K, Karakosta P, Spanaki A, Besselink H, Kiviranta H, Rantakokko P, KaterinaSarri, Koutis A, et al. Maternal diet, prenatal exposure to dioxins and other persistent organic pollutants and anogenital distance in children. Sci Total Environ. 2013;461–462:222–9. doi: 10.1016/j.scitotenv.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Parra MD, Mendiola J, Jorgensen N, Swan SH, Torres-Cantero AM. Anogenital distance and reproductive parameters in young men. Andrologia. 2015 doi: 10.1111/and.12403. [DOI] [PubMed] [Google Scholar]
- Pasterski V, Acerini CL, Dunger DB, Ong KK, Hughes IA, Thankamony A, Hines M. Postnatal penile growth concurrent with mini-puberty predicts later sex-typed play behavior: Evidence for neurobehavioral effects of the postnatal androgen surge in typically developing boys. Horm Behav. 2015;69:98–105. doi: 10.1016/j.yhbeh.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Pasterski V, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and childhood sex segregation: playmate and play style preferences in girls with congenital adrenal hyperplasia. Horm Behav. 2011;59:549–55. doi: 10.1016/j.yhbeh.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterski V, Hindmarsh P, Geffner M, Brook C, Brain C, Hines M. Increased aggression and activity level in 3- to 11-year-old girls with congenital adrenal hyperplasia (CAH) Horm Behav. 2007;52:368–74. doi: 10.1016/j.yhbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterski VL, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child development. 2005;76:264–278. doi: 10.1111/j.1467-8624.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- Romano-Riquer SP, Hernandez-Avila M, Gladen BC, Cupul-Uicab LA, Longnecker MP. Reliability and determinants of anogenital distance and penis dimensions in male newborns from Chiapas, Mexico. PaediatrPerinatEpidemiol. 2007;21:219–228. doi: 10.1111/j.1365-3016.2007.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth KS, Campbell PJ, Chew S, Lim EM, Hadlow N, Stuckey BG, Brown SJ, Feenstra B, Joseph J, Surdulescu GL, Zheng HF, et al. Genome-wide association study with 1000 genomes imputation identifies signals for nine sex hormone-related phenotypes. Eur J Hum Genet. 2015 doi: 10.1038/ejhg.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safarinejad MR, Shafiei N, Safarinejad S. Association of polymorphisms in the estrogen receptors alpha, and beta (ESR1, ESR2) with the occurrence of male infertility and semen parameters. J Steroid Biochem Mol Biol. 2010;122:193–203. doi: 10.1016/j.jsbmb.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Salazar-Martinez E, Romano-Riquer P, Yanez-Marquez E, Longnecker MP, Hernandez-Avila M. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environ Health. 2004;3:8. doi: 10.1186/1476-069X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Beard L, Zhou C, Grady R. Measurement and correlates of ano-genital distance in healthy, newborn infants. International Journal of Andrology. 2010;33:317–323. doi: 10.1111/j.1365-2605.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Grady R, Redmon JB, Ivicek K, Barrett E, Janssen S, Nguyen R, Swan SH, Team TS. Anogenital distance and penile width measurements in The Infant Development and the Environment Study (TIDES): methods and predictors. J Pediatr Urol. 2015;11:76 e1–6. doi: 10.1016/j.jpurol.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Swan SH, Farin FM, Wilkerson HW, Bamshad M, Grady R, Zhou C, Schwartz SM. A pilot study of the association between genetic polymorphisms involved in estrogen signaling and infant male genital phenotypes. Asian J Androl. 2012;14:766–72. doi: 10.1038/aja.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Deepa SR, Madhavi S, Gupta NJ, Chakravarty B, Singh L, Thangaraj K. Male infertility: no evidence of involvement of androgen receptor gene among Indian men. J Androl. 2006;27:102–5. doi: 10.2164/jandrol.05095. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De ME, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects 17. HumReprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Suomi AM, Main KM, Kaleva M, Schmidt IM, Chellakooty M, Virtanen HE, Boisen KA, Damgaard IN, Kai CM, Skakkebaek NE, Toppari J. Hormonal changes in 3-month-old cryptorchid boys. J Clin Endocrinol Metab. 2006;91:953–8. doi: 10.1210/jc.2004-2318. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2012;35:236–44. doi: 10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. EnvironHealth Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassoulis G. Mendelian randomization: how genetics is pushing the boundaries of epidemiology to identify new causes of heart disease. Can J Cardiol. 2013;29:30–6. doi: 10.1016/j.cjca.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Thankamony A, Lek N, Carroll D, Williams M, Dunger DB, Acerini CL, Ong KK, Hughes IA. Anogenital distance and penile length in infants with hypospadias or cryptorchidism: comparison with normative data. Environ Health Perspect. 2014;122:207–11. doi: 10.1289/ehp.1307178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankamony A, Ong KK, Dunger DB, Acerini CL, Hughes IA. Anogenital distance from birth to 2 years: a population study. Environ Health Perspect. 2009;117:1786–90. doi: 10.1289/ehp.0900881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup J, McLachlan R, Cortes D, Nation TR, Balic A, Southwell BR, Hutson JM. What is new in cryptorchidism and hypospadias--a critical review on the testicular dysgenesis hypothesis. J Pediatr Surg. 2010;45:2074–86. doi: 10.1016/j.jpedsurg.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Toppari J, Virtanen HE, Main KM, Skakkebaek NE. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res A Clin Mol Teratol. 2010;88:910–9. doi: 10.1002/bdra.20707. [DOI] [PubMed] [Google Scholar]
- Vafeiadi M, Agramunt S, Papadopoulou E, Besselink H, Mathianaki K, Karakosta P, Spanaki A, Koutis A, Chatzi L, Vrijheid M, Kogevinas M. In utero exposure to dioxins and dioxin-like compounds and anogenital distance in newborns and infants. Environ Health Perspect. 2013;121:125–30. doi: 10.1289/ehp.1205221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S, Kolovos P, Platts S, Drake AJ, Sharpe RM. Inter-relationship between testicular dysgenesis and Leydig cell function in the masculinization programming window in the rat. PLoS One. 2012;7:11. doi: 10.1371/journal.pone.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerand H, Remy-Martin A, Chabannes E, Bermont L, Adessi GL, Bittard H. Relationship between expansion of the CAG repeat in exon 1 of the androgen receptor gene and idiopathic male infertility. Fertil Steril. 2001;76:769–74. doi: 10.1016/s0015-0282(01)01987-2. [DOI] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, Smith LB, Sharpe RM. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118:1479–90. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Sharpe RM. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology. 2007;148:3185–3195. doi: 10.1210/en.2007-0028. [DOI] [PubMed] [Google Scholar]
- Wilson JD. The Critical Role of Androgens in Prostate Development. Endocrinology and Metabolism Clinics of North America. 2011;40:577–590. doi: 10.1016/j.ecl.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, Boisen KA, Boas M, Damgaard IN, Kai CM, Schmidt IM, Chellakooty M, Suomi AM, Toppari J, Skakkebaek NE, Main KM. Acquired cryptorchidism is frequent in infancy and childhood. Int J Androl. 2009;32:423–8. doi: 10.1111/j.1365-2605.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, LeBlanc GA, Gray LE., Jr Interactive effects of vinclozolin and testosterone propionate on pregnancy and sexual differentiation of the male and female SD rat. ToxicolSci. 2004;78:135–143. doi: 10.1093/toxsci/kfh018. [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai M-Y, Xu Q, Mu X-M, Lardy H, Huang K-E, Lin H, Yeh S-D, Altuwaijri S, Zhou X, Xing L, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: An in vivo model for the study of androgen functions in selective tissues. Proceedings of the National Academy of Sciences. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]