Abstract
Background:
Secondhand smoke (SHS) exposures have been linked to asthma related outcomes but quantitative dose-responses using biomarkers of exposure have not been widely reported.
Objectives:
Assess dose-response relationships between plasma cotinine-determined SHS exposure and asthma outcomes in minority children, a vulnerable population exposed to higher levels of SHS and underrepresented in the literature.
Methods:
We performed analyses in 1172 Latino and African American children with asthma from the mainland US and Puerto Rico. We used logistic regression to assess relationships of cotinine levels ≥0.05 ng/mL with asthma exacerbations (defined as asthma related hospitalizations, emergency room visits or oral steroid prescription) in the previous year and asthma control. The shape of dose-response relationships was assessed using a continuous exposure variable in generalized additive logistic models with penalized splines.
Results:
The odds ratio (OR) for experiencing asthma exacerbations in the previous year for cotinine levels ≥0.05 ng/mL, compared to <0.05 ng/mL was 1.40 (95% confidence interval (CI): 1.03 – 1.89), while the OR for poor asthma control was 1.53 (95% CI: 1.12 – 2.13). Analyses for dose-response relationships indicated increasing odds of asthma outcomes related with increasing exposure, even at cotinine levels associated with light SHS exposures.
Conclusions:
Exposure to SHS was associated with higher odds of asthma exacerbations and having poorly controlled asthma with an increasing dose-response even at low levels of exposure. Our results support the conclusion that there are no safe levels of SHS exposures.
INTRODUCTION
Exposure to secondhand smoke (SHS) has been linked to multiple adverse health effects, including cardiovascular disease and respiratory diseases such as lung cancer and asthma.[1–4] Despite public health awareness efforts including legislation aimed at protecting non-smokers from SHS exposure, and an overall decreasing trend in exposures, SHS exposure remains a prevalent risk factor worldwide, contributing to a large portion of overall disease burden and mortality in adults and children.[4–7] In the US, more than 40% of children aged 3–11 are estimated to be exposed to some SHS.[7] Disparities exist in the prevalence of SHS exposures based on race/ethnicity and socioeconomic status, with non-Hispanic blacks and those living in poverty being more likely to be exposed.[7–9] Underrepresented populations are also more burdened by asthma in the US, with higher prevalence of asthma morbidity and mortality in African Americans and Puerto Ricans compared to European Americans.[10,11] SHS exposure is linked with asthma exacerbations, poor asthma control and increased asthma symptoms among children with asthma.[12–17]
Objective measures of SHS exposures in epidemiological studies rely on biochemical testing for assessment of tobacco smoke, which usually focus on metabolites of nicotine, one of the major constituents of tobacco smoke and the primary determinant of addiction to tobacco products.[18] Quantitative dose-response relationships between SHS exposure and asthma outcomes using biomarkers of exposure, however, are not widely reported in the literature. Furthermore, quantification of exposure using nicotine metabolites may be further complicated by race/ethnicity. Nicotine is metabolized at different rates by different racial/ethnic groups, with African Americans on average metabolizing nicotine at a slower rate compared to European Americans, while smaller or not significant differences between Latinos and European Americans are reported.[19–22] Higher measured levels of cotinine, the nicotine metabolite most commonly used to assess nicotine exposure, observed in African Americans[23] may not only be the result of higher tobacco smoke exposures but also slower metabolism and clearance.[24] It is therefore possible that race/ethnicity specific differences in nicotine metabolism may also lead to perceived differences of dose-response by race/ethnicity that are not necessarily due to differential susceptibility to exposure effects.
In this study we examined the relationship of SHS exposure as measured by plasma cotinine, the primary proximate metabolite of nicotine, with exacerbations and disease control in African American and Latino children with asthma. Our analyses included a quantitative assessment of dose-response between levels of plasma cotinine and odds of asthma outcomes.
METHODS
Study population
The study is based on subsamples of participants from the Gene-environments and Admixture in Latino Americans (GALA II) study and the Study of African-Americans, Asthma, Genes, and Environments (SAGE II) from whom plasma cotinine data were available. GALA II and SAGE II are described in detail elsewhere.[25] Briefly, GALA II and SAGE II are parallel case-control studies of asthma conducted among Latino and African American children, respectively. GALA II recruited Latinos from five regions (Chicago, IL; Bronx, NY; Houston, TX; San Francisco Bay Area, CA; and Puerto Rico) and SAGE II recruited African Americans from the San Francisco Bay Area only. Participants whose parents identified as Latino or African American (or self-identified as Latino or African American if 18 or older), and had four Latino or African American grandparents, were recruited for the two studies, respectively. Additionally, participants were 8–21 years old, aiming to ensure a homogenous case population among those who were old enough to perform spirometry, as lung function was one of the outcomes considered in the parent studies. Pregnant women in their 3rd trimester were excluded, because the progression of their pregnancy would have interfered with lung function measurements. Additional exclusion criteria included a history of lung or chronic diseases other than asthma, and current smokers, or those with at least 10 pack-years smoking history. Asthma cases were defined as subjects with a physician diagnosis of asthma, plus two or more symptoms of coughing, wheezing, or shortness of breath in the past 2 years. The current study was restricted to a subsample of 1208 asthma cases, selected for cotinine analysis as part of a separately funded study. Supplemental Table S1 summarizes demographic characteristics in the selected 1208 cases and the total number cases from the two parent studies.
Outcomes and covariates
Asthma exacerbations and asthma control were the primary outcomes of interest and were assessed based on information collected through questionnaires administered in-person with the children’s parents/caretakers by trained bilingual (English–Spanish) interviewers, or the participants themselves if they were 18 or older. Asthma exacerbations in the previous year were defined as any asthma-related hospitalization or emergency room (ER) visit, or prescription of oral steroids for asthma during the 12 months prior to recruitment. Each of (1) asthma-related hospitalizations, (2) asthma-related ER visits and (3) prescription of oral steroids, as well as a combined category of all three types of exacerbations were assessed as separate measures of the outcome. Asthma control was classified according to National Heart, Lung, and Blood Institute measures[26] into three categories: controlled, not well controlled, and very poorly controlled asthma. Relationships between “controlled” asthma as a referent category compared to “not well controlled” and “very poorly controlled” as a combined category, as well as each of the “not well controlled” and “very poorly controlled” categories to “controlled” asthma were assessed separately.
Measures of demographic information (including maternal education and whether the family had health insurance), medical histories, and environmental exposures, were collected through the questionnaires. Information was also collected on maternal smoking during pregnancy, as well as current smoking habits of both parents in the house, other smokers in the house, and total number of current smokers in the household.
Cotinine and nicotine metabolite ratio
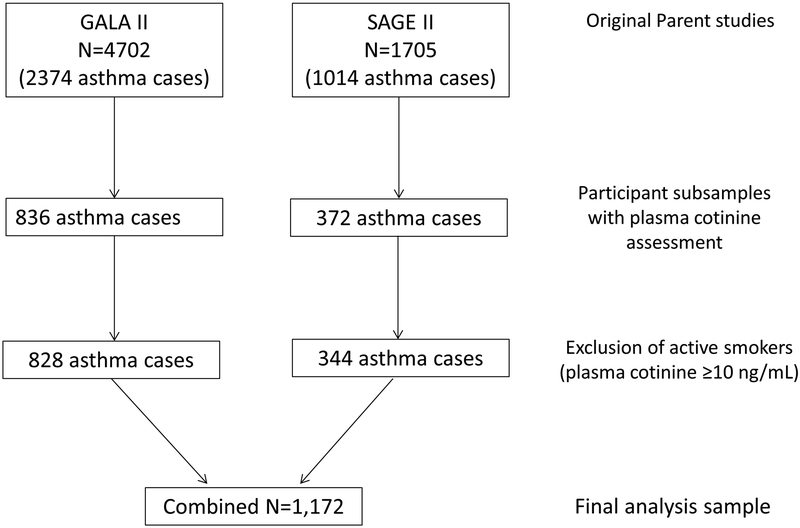
Blood samples from participating children were collected at baseline at the same time as questionnaire administration. Samples from participants in the current study were analyzed for the nicotine metabolites cotinine and 3-hydroxycotinine (3HC) by liquid chromatography–tandem mass spectrometry.[27] The lower limit of quantitation (LOQ) for this assay depends on the volume of plasma available. The volumes available in our study ranged from 0.1 to 1.0 mL, with the LOQ ranging from 0.2 to 0.02 ng/mL, respectively. The LOQ was 0.05 ng/mL or less for 1,112 (92.1%) of the samples. Participants with plasma cotinine levels >10 ng/mL (n=36) were considered to be active smokers[28] and excluded from the analyses for the relationships between SHS exposure and asthma outcomes resulting in a final sample of 1,172 participants. Figure 1 illustrates participant inclusion from each of the parent studies, GALA II and SAGE II, resulting in an analytical sample of 1,172.
Figure 1:
Flow chart illustrating participant inclusion from the two parent studies (GALA II & SAGE II) in the current study.
A binary exposure variable was created with subjects deemed exposed to SHS if plasma cotinine levels were above 0.05 ng/mL, or above the LOQ if that was higher than 0.05 ng/mL, and otherwise unexposed. In a previous study using NHANES data, cotinine concentrations less than 0.05 ng/mL were deemed to result from not being recently been exposed to SHS, or to have been exposed at low levels that the exposure could not be detected.[28] A continuous exposure variable (log-transformed) was used in analyses for dose-response, with subjects without quantitative measures assigned the sample LOQ. The nicotine metabolite ratio (NMR), the ratio of 3HC and cotinine, is an indicator of enzymatic activity of the liver enzyme CYP2A6, which is primarily responsible for nicotine metabolism.[23] Higher NMR is correlated with higher rate of nicotine metabolism. NMR values were available only in participants with both cotinine and 3HC levels measured above the LOQ (n=421, of which 372 had plasma cotinine <10 ng/mL).
Statistical analyses
We assessed associations of self-reported SHS exposure (any current smokers reported in the household versus none) and cotinine-assessed SHS exposure with asthma outcomes. Associations between binary cotinine exposure and asthma exacerbations in the previous year were examined using logistic regression models (separate models for asthma-related hospitalizations, asthma-related ER visits, oral steroid prescription, and any of the three exacerbations combined). Logistic regression was also used to assess associations between binary cotinine and asthma control using “controlled” asthma as reference compared to those with “not well controlled” and “very poorly controlled” in a combined category. We also applied multinomial logistic regression comparing each of the “not well controlled” and “very poorly controlled” categories to “controlled” asthma separately. All of the models included age, sex, race/ethnicity, recruitment region, an indicator variable for self-reported maternal smoking during pregnancy, a categorical variable for maternal education (less than high school, high school or equivalent, at least some college) and an indicator variable for whether the family had health insurance. We also considered NMR as a covariate using 50 imputed datasets to account for missing NMR values in models for the entire study sample as well as stratified by parent study, as a proxy for racial/ethnic group. Multiple imputation was implemented using Multivariate Imputation by Chained Equations. [29]
Quantitative dose-response relationships between continuous cotinine levels and each of the above outcomes were assessed in generalized additive models, extensions of generalized linear models in which the linear predictors depends on smoothing functions of predictors such as spline functions.[30] We used penalized spline terms for the effect of cotinine levels. We also fitted generalized additive models including an interaction term between exposure and an indicator variable for parent study (GALA II vs. SAGE II, as a proxy for racial/ethnic group).[31] All analyses were carried out using R software (version 3.3.3, R Development Core Team, Vienna Austria).
RESULTS
Demographic characteristics for the study sample in question are summarized in Table 1. Briefly, mean± standard deviation (sd) age was 13.1±3.4, and 53% of participants were male with similar distribution of sex between participants from GALA II and SAGE II.
Table 1:
Demographic characteristics in a sample of 1,208 Latino and African American children with asthma, from the GALA II (N=836) and SAGE II (N=372) parent studies, recruited between 2006–2011.
| Characteristic | GALA II n (%) |
SAGE II n (%) |
|---|---|---|
| Age (mean (sd)) | 13.1 (3.4) | 13.1 (3.4) |
| Male | 442 (52.9) | 201 (54.0) |
| Race/ethnicity | ||
| Mexican | 157 (18.8) | |
| Other Latino | 15 (1.8) | |
| Puerto Rican | 664 (79.4) | |
| African American | 372 (100) | |
| Maternal education | ||
| 5th grade or less | 25 (3.0) | 0 (0) |
| Grades 6th–11th | 183 (22.0) | 18 (4.9) |
| High school grad. or equivalent | 223 (26.8) | 103 (28.1) |
| At least some college | 401 (48.2) | 245 (66.9) |
| Health insurance | 806 (97.2) | 369 (99.2) |
| Any current smokers reported in household* | 159 (23.4) | 99 (26.6) |
| Recruitment region | ||
| Chicago | 74 (8.9) | |
| Houston | 34 (4.1) | |
| New York | 81 (9.7) | |
| Puerto Rico | 598 (71.5) | |
| SF Bay Area | 49 (8.9) | 372 (100) |
| Asthma control† | ||
| Controlled | 132 (19.4) | 123 (33.1) |
| Not well controlled | 265 (39.1) | 106 (28.5) |
| Very poorly controlled | 281 (41.4) | 143 (38.4) |
| Asthma exacerbations in the past 12 months† | ||
| Hospitalizations | 92 (11.1) | 8 (2.2) |
| ER visits | 478 (58.3) | 112 (31.2) |
| Oral steroid prescription | 370 (44.7) | 62 (17.0) |
Self-reported number of current smokers in the household is limited to 679 of the GALA II subjects.
Asthma control was available for 678 of the GALA II subjects due to missingness in the information required for the variable definition. Information on asthma exacerbations was available in 1182, 1179 and 1192 subjects respectively for hospitalizations, ER visits and oral steroid prescription.
Percentages for variables with missingness are based on the totals with complete data.
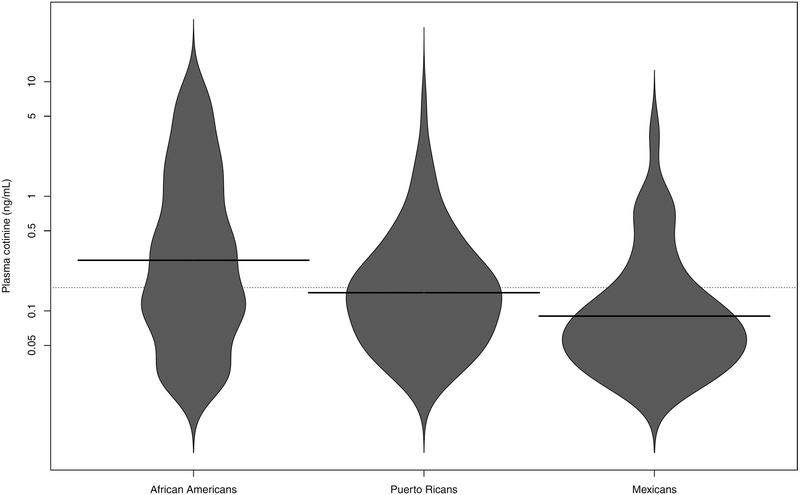
Figure 2 illustrates plasma cotinine distributions by race/ethnicity among those with plasma cotinine measured above the sample LOQ. African Americans had higher cotinine levels compared to Latinos, (respective mean ± sd of 0.51±1.41 and 0.20±0.58, two sample t-test p-value=0.00013, with sample LOQ assigned to participants without levels measured above the LOQ). There was an increasing trend of plasma cotinine levels with reported number of current smokers in the household, though 315 of 777 (41%) participants with no current smokers reported in the household had measured levels of cotinine above 0.05 ng/mL (or the LOQ if higher than 0.05 ng/mL). Summary statistics on plasma cotinine levels, overall and by self-reported number of smokers in household are presented in supplemental Table 2.
Figure 2:
Beanplots for plasma cotinine levels by race/ethnicity. The shapes of the beans represent density traces for the respective distributions in each group, mirrored on the vertical axis. Solid lines represent the group specific median values, while the dotted line represents the overall median value.
Table 2:
Summary statistics for plasma cotinine levels, overall and by self-reported number of smokers in the household (participants without measured cotinine levels above the LOQ were assigned the sample LOQ).
| Participants by self-reported SHS | Median plasma cotinine and IQR (ng/mL) |
|---|---|
| Overall (n=1,172) | 0.05 (0.02, 0.17) |
| Current smokers in the household* | |
| 0 (n=777) | 0.03 (0.02, 0.12) |
| 1 (n=181) | 0.14 (0.04, 0.42) |
| >=2 (n=58) | 0.28 (0.08, 0.99) |
Data restricted to 1016 participants with self-reported SHS exposure data
Table 3 summarizes results for the associations between binary variables of self-reported (presence of any current smokers in the household) and biomarker based (binary cutoff of 0.05 ng/mL or LOQ if higher than 0.05 ng/mL) measures of SHS exposure and asthma outcomes. Associations between self-report of any current smoker in the household and asthma exacerbation variables were not strong, but self-reported exposures were associated with increased odds of uncontrolled asthma with the OR comparing “very poorly controlled” to “controlled” asthma reaching statistical significance (OR: 1.79 (95% confidence interval (CI): 1.18 – 2.70)). Exposure to any SHS determined by plasma cotinine levels above 0.05 ng/mL (or LOQ if higher than 0.05 ng/mL) was positively associated with all asthma outcomes. Although not always statistical significant, associations for asthma exacerbations were above the null for all definitions, but stronger and statistical significant for oral steroid prescriptions which seem to be driving the overall association. Analyses adjusting for NMR using multiple imputed datasets yielded similar estimates, with an OR for experiencing asthma exacerbations in the previous year for cotinine levels ≥0.05 ng/mL, compared to <0.05 ng/mL of 1.37 (1.01 – 1.87). ORs for asthma control were 1.41 (0.99 – 2.00) for “not well controlled” vs “controlled” asthma and 1.82 (1.28 – 2.59) for “very poorly controlled” vs “controlled” asthma.
Table 3:
Associations between SHS exposure (defined using self-report and measured plasma cotinine (>0.05 ng/mL or above the limit of quantification if higher than 0.05 ng/mL)) and asthma outcomes.
| Asthma outcomes | OR (95% CI) | |
|---|---|---|
| Self-reported exposure* | Plasma cotinine determined exposure | |
| Asthma exacerbations in the previous 12 months† | 1.08 (0.75 – 1.57) | 1.40 (1.03 – 1.89) |
| Hospitalizations | 1.77 (0.79 – 3.95) | 1.26 (0.60 – 2.67) |
| ER visits | 1.21 (0.70 – 2.09) | 1.07 (0.65 – 1.75) |
| Oral steroid prescription | 1.00 (0.71 – 1.41) | 1.54 (1.18 – 2.04) |
| Asthma control (binary outcome)‡ | 1.62 (1.09 – 2.38) | 1.53 (1.12 – 2.13) |
| Not well controlled vs controlled | 1.43 (0.93 – 2.21) | 1.39 (0.98 – 1.98) |
| Very poorly controlled vs controlled | 1.79 (1.18 – 2.70) | 1.67 (1.18 – 2.36) |
Self-reported exposure to SHS was determined as any vs no reported current smokers in the household.
Asthma exacerbations assessed as a combined variable for any exacerbations (hospitalizations, ER visits, prescription of oral steroids) vs none
Asthma control assessed as binary outcome with a combined “not well controlled” and “very poorly controlled” category compared to controlled asthma
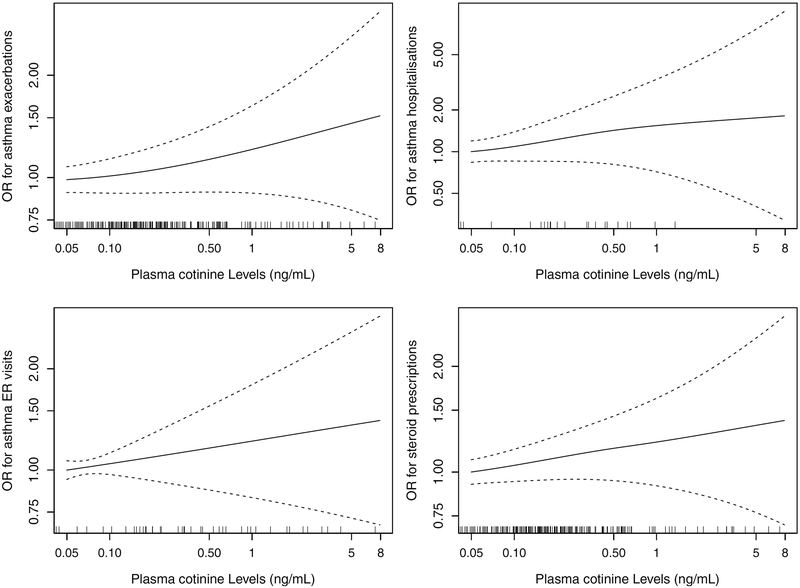
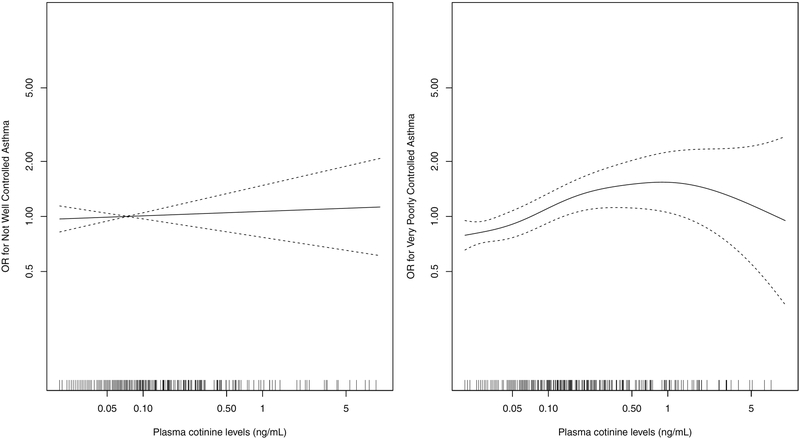
Quantitative dose-response relationships between continuous plasma cotinine levels and asthma exacerbations are presented in Figure 3, and relationships for asthma control are presented in Figure 4. An increasing dose-response was observed for asthma exacerbations over the range of cotinine levels though p-values for the overall null hypothesis for the smoothing terms were not statistically significant. Increasing dose-response was observed for asthma control when comparing “very poorly controlled” to “controlled”, with a steeper increase at lower levels of exposure and a plateauing effect at higher levels (p-value=0.024), but no apparent dose-response was seen when comparing “not well controlled” to “controlled”. The OR comparing ‘very poorly controlled’ to ‘controlled’ asthma was 1.68 (95% CI: 1.16, 2.43) for an increase in exposure from 0.05 ng/ml (used as cutoff for unexposed) to 1 ng/ml (considered indicative of minor to moderate SHS exposures[28]).
Figure 3:
Dose-response of plasma cotinine and asthma exacerbations in the past 12 months, from generalized additive models using penalized splines. Results presented for total asthma exacerbations and separately for asthma-related hospitalizations, asthma-related ER visits and prescription of oral steroids for asthma.
Figure 4:
Dose-response of plasma cotinine and asthma control, from generalized additive models using penalized splines. Results presented comparing each of “not well controlled” and “very poorly controlled” to “controlled” asthma separately.
Effect estimates by study for a binary exposure by study from multiply imputed datasets for NMR are depicted in Table 4. Using a binary exposure cutoff, effect estimates for asthma control appeared stronger in African American children compared to Latino children, but the same was not true for asthma exacerbations. Quantitative dose-response relationships by study from models using penalized splines are illustrated in Supplemental Figure S1.
Table 4:
Associations between SHS exposure (defined as measured plasma cotinine >0.05 ng/mL or above the limit of quantification if higher than 0.05 ng/mL) and asthma outcomes by study (as proxy for race/ethnicity) using multiple imputation data to account for missing NMR values.
| Asthma outcomes | OR (95% CI) | |
|---|---|---|
| Study | ||
| GALA II | SAGE II | |
| Asthma exacerbations in the previous 12 months* | 1.38 (0.95 – 2.01) | 1.27 (0.74 – 2.19) |
| Asthma control (binary outcome)† | 1.27 (0.84 – 1.91) | 2.16 (1.27 – 3.67) |
| Not well controlled vs controlled | 1.21 (0.66 – 2.22) | 1.58 (0.87 – 2.91) |
| Very poorly controlled vs controlled | 1.33 (0.80 – 2.22) | 2.77 (1.51 – 5.00) |
Asthma exacerbations assessed as a combined variable for any exacerbations (hospitalizations, ER visits, prescription of oral steroids) vs none
Asthma control assessed as binary outcome with a combined “not well controlled” and “very poorly controlled” category compared to controlled asthma
DISCUSSION
We observed increased odds of asthma exacerbations as well as increased odds of having poorly controlled asthma among African American and Latino children associated with SHS exposures determined by plasma cotinine levels. We also observed suggestive dose-response relationships even at lower exposure concentrations. To our knowledge, such quantitative dose-response relationships between SHS exposure measured using plasma cotinine levels and asthma outcomes have not been previously reported in the literature. The relative increase in odds of adverse asthma outcomes with increasing exposure, especially concerning poor asthma control, was steeper at the lower range of exposures, with large increases in odds of adverse outcomes reached even at levels of cotinine associated with only minor SHS exposures (0–1 ng/mL).[28] These findings suggest that even low levels of SHS exposure can result in adverse asthma-related effects, further supporting the guidelines stating that there are no safe levels of SHS exposure.[4]
Associations between asthma outcomes in children and SHS exposure have been reported in the literature with exposures associated with increased risk of asthma, asthma exacerbations, wheezing, and reduced lung function.[4,14,16,17] Tobacco smoke is a mixture of compounds, including carbon and nitrogen oxides, particulate matter, nitrosamines, polycyclic aromatic hydrocarbons, carbonyls, and other chemicals, many of which are known toxicants that can induce inflammation and altered immune responses.[32] We observed associations between SHS exposure and increased odds of poor asthma control, which appeared stronger in African American children compared to Latino children, but associations with asthma exacerbations were more similar by race/ethnicity. Findings of differences in the strengths of the cotinine-asthma outcomes relationships by race/ethnicity may indicate a difference in susceptibility, but should be interpreted with caution as metabolic rates of cotinine differ by race/ethnicity.[28,33] Given the observed metabolic differences, cotinine levels in African Americans may result from lower external exposure to tobacco smoke than the same levels in populations that metabolize nicotine at faster rates.[24] Measured cotinine in African Americans may therefore not necessarily be as representative of higher exposure to other tobacco smoke constituents. Additionally, asthma control is a composite outcome based on asthma symptoms, pulmonary function, history of exacerbations (including ER visits), and medication use,[26] all of which may carry racial/ethnic differences as well. For example, while African Americans and Mexican-Americans have established reference equations for pulmonary function based on a representative sample of the US population,[34] the same is not true for Puerto Ricans.[35–38] Misclassification of the outcome is more likely to occur in populations without dedicated prediction equations.
Self-reported measures of SHS exposure did not show good specificity when compared to measures of plasma cotinine. Approximately 41% of children whose parents reported no active smokers in the house had detectable levels of plasma cotinine, though mean levels of plasma cotinine did increase with increasing numbers of smokers reported in the household. This is consistent with previous findings and with SHS exposure being under-reported when relying on self-reported data, particularly in the case of exposures happening outside the home, which would not be captured by the self-reported assessment in the current study.[16,39–41] Third-hand smoke exposures are also likely to be unaccounted by self-reported measures, but may contribute to measured cotinine concentrations and lead to adverse effects.[42]
Biochemical assessment of SHS exposure allows for more objective characterization of tobacco smoke exposure and epidemiologic assessment of exposure-outcome relationships unburdened by exposure misclassification bias seen in self-reported measures of exposure. Plasma cotinine, the biomarker used to determine exposure in the current study, is the major proximate metabolite of nicotine and has been considered a reliable, objective measure of SHS exposure for some time.[43] Nicotine and its metabolites, however, are not necessarily causative agents of the adverse effects of tobacco smoke examined in this study, with effects most likely driven by other compounds as described above.
The data in the current study are based on a subsample of cases from two parent case-control studies. Outcomes in the parent studies were assessed based on retrospective self-reported data (potentially leading to over or under estimation of some of the outcomes), while the exposure was only quantified in one point in time. The plateauing effect observed at analyses of dose-response at higher exposures may represent a true exposure-response function, but also could be the result of less stable functions due to the scarce data at higher ranges of exposure (also evidenced by the less stable confidence intervals at higher exposures), or due to reverse-causation as parents of children with worse asthma may be more likely to reduce their children’s exposures. The binary definition of exposure using a cut-off may have also led to misclassification, especially in samples with LOQ greater than the cut-off. However, this misclassification is expected to be entirely non-differential with respect to the outcomes, and would likely produce an underestimate of the true effect size.
The cross-sectional nature of the data did not allow us to address possible limitations such as these potential issues of reverse causation. Although point prevalence measures of cotinine are useful for assessing recent and ongoing SHS exposure,[44] a longitudinal study with prospective outcome assessment as well as biomarker exposure assessed at multiple time points would be better equipped to address these issues. Furthermore, analyses with interactions were underpowered and we did not examine for effects within separate categories for the different Latino groups.
Strengths of the study include information obtained through use of a detailed questionnaire on potential confounding factors including indicators of socioeconomic status, and the relatively large sample size for an understudied study population, composed of Latino and African American children. There is a lack of minority representation in asthma-related research,[45] despite these populations being disproportionately affected by asthma morbidity and SHS exposure.
In summary, we demonstrated increasing odds of asthma exacerbations and poorer asthma control with increasing levels of SHS exposure measured with plasma cotinine levels in children from underrepresented populations. This pattern of dose-response was seen within the range of cotinine levels indicative of light SHS exposure, suggesting no safe levels of SHS exposure. Differences in exposure levels were also observed by race/ethnicity.
Supplementary Material
-
- What is the key question?
This study aims to assess the shape of the dose-response relationship between secondhand smoke exposures as measured by plasma cotinine and asthma outcomes, in minority children with asthma.
-
- What is the bottom line?
Our results suggest an increase in odds of asthma exacerbations and poor asthma control with increasing exposure, even at levels associated with light secondhand smoke exposures.
-
- Why read on?
We provide a quantitative dose-response assessment of the relationship between secondhand smoke exposure and asthma outcomes in a vulnerable population that is understudied in the respiratory health literature.
Acknowledgments:
We thank Polly Cheung for performing cotinine analyses.
Funding: This study was supported in part by the Sandler Family Foundation, the American Asthma Foundation, the RWJF Amos Medical Faculty Development Program, Harry Wm and Diana V Hind Distinguished Professor in Pharmaceutical Sciences II, the Tobacco-Related Disease Research Program under Award No 24RT-0025, a grant by the Flight Attendants Medical Research Institute (FAMRI), and the following institutes of the National Institutes of Health: National Heart Lung and Blood Institute (NHLBI) 1R01HL117004, R01Hl128439, R01HL135156, 1X01HL134589, R01HL118267; National Institute of Environmental Health Sciences (NIEHS) R01ES015794, R21ES24844, K99ES027511; the National Institute on Minority Health and Health Disparities (NIMHD) 1P60MD006902, U54MD009523, 1R01MD010443; and National Institute of Allergy and Infectious Diseases (NIAID) R01AI079139.
Footnotes
Competing interests: None declared.
REFERENCES
- 1.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ 1997;315:980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation 2005;111:2684–98. [DOI] [PubMed] [Google Scholar]
- 3.Eisner MD. Secondhand smoke and obstructive lung disease: A causal effect? Am J Respir Crit Care Med 2009;179:973–4. [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: 2014. [Google Scholar]
- 5.Öberg M, Jaakkola MS, Prüss-Üstün A, et al. Second-hand smoke. Assessing the burden of disease at national and local levels. (WHO; Environmental Burden of Disease Series, No. 18). 2010. [Google Scholar]
- 6.Öberg M, Jaakkola MS, Woodward A, et al. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011;377:139–46. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Vital signs: nonsmokers’ exposure to secondhand smoke --- United States, 1999–2008. MMWR Surveill Summ 2010;59:1141–6. [PubMed] [Google Scholar]
- 8.Kit BK, Simon a E, Brody DJ, et al. US Prevalence and Trends in Tobacco Smoke Exposure Among Children and Adolescents With Asthma. Pediatrics 2013;131:407–14. [DOI] [PubMed] [Google Scholar]
- 9.Homa DM, Neff LJ, King BA, et al. Vital signs: disparities in nonsmokers’ exposure to secondhand smoke--United States, 1999–2012. MMWR Surveill Summ 2015;64:103–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in Asthm a Prevalence, Health Care Use, and Mortality in the United States, 2001–2010. 2012;:1–8. [PubMed] [Google Scholar]
- 11.Barr RG, Avil L, Davis SM, et al. Pulmonary Disease and Age at Immigration among Hispanics. Results from the Hispanic Community Health Study / Study of Latinos. Am J Respir Crit Care Med 2016;193:386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaakkola JJK, Jaakkola MS. Effects of environmental tobacco smoke on the respiratory health of children. Scand J Work Environ Heal 2002;28:71–83. [PubMed] [Google Scholar]
- 13.Oh SS, Tcheurekdjian H, Roth LA, et al. Effect of secondhand smoke on asthma control among black and Latino children. J Allergy Clin Immunol 2012;129:1478–1483.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2001;163:429–36. [DOI] [PubMed] [Google Scholar]
- 15.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax 1998;53:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between Exposure to Environmental Tobacco Smoke and Exacerbations of Asthma in Children. N Engl J Med 1993;328:1665–9. [DOI] [PubMed] [Google Scholar]
- 17.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and Passive Smoke Exposure and Incidence of Asthma and Wheeze: Systematic Review and Meta-analysis. Pediatrics 2012;129:735–44. [DOI] [PubMed] [Google Scholar]
- 18.Benowitz NL. Nicotine addiction. N Engl J Med 2010;362:2295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benowitz NL, Perez-Stable EJ, Fong I, et al. Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther 1999;291:1196–203. [PubMed] [Google Scholar]
- 20.Pérez-Stable EJ, Herrera B, Jacob P III, et al. Nicotine Metabolism and Intake in Black and White Smokers. JAMA 1998;280:152. [DOI] [PubMed] [Google Scholar]
- 21.Benowitz NL, Pérez-Stable EJ, Herrera B, et al. Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst 2002;94:108–15. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Park SL, Stram DO, et al. Associations between genetic ancestries and nicotine metabolism biomarkers in the multiethnic cohort study. Am J Epidemiol 2015;182:945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dempsey DA, Meyers MA, Oh SS, et al. Determination of Tobacco Smoke Exposure by Plasma Cotinine Levels in Infants and Children Attending Urban Public Hospital Clinics. Arch Pediatr Adolesc Med 2012;166:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu AZX, Renner CC, Hatsukami DK, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in cyp2a6: The influence of genetics, race, and sex. Cancer Epidemiol Biomarkers Prev 2013;22:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura KK, Galanter JM, Roth LA, et al. Early-Life air pollution and asthma risk in minority children the GALA II and SAGE II studies. Am J Respir Crit Care Med 2013;188:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Asthma Education and Prevention Program (National Heart Lung and Blood Institute). Expert Panel Report 3: Guidelines for the diagnosis and management of asthma: full report 2007. Bethesda, MD: 2007. [Google Scholar]
- 27.Jacob P, Yu L, Duan M, et al. Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2. J Chromatogr B Anal Technol Biomed Life Sci 2011;879:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 2009;169:236–48. [DOI] [PubMed] [Google Scholar]
- 29.Buuren S van, Groothuis-Oudshoorn K. mice : Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45. [Google Scholar]
- 30.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: : Chapman & Hall/CRC; 2006. [Google Scholar]
- 31.Coull BA, Ruppert D, Wand MP. Simple incorporation of interactions into additive models. Biometrics 2001;57:539–45. [DOI] [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: 2006. [Google Scholar]
- 33.Benowitz NL, Dains KM, Dempsey D, et al. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res 2011;13:772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hankinson JL, Odencrantz JR, Fedan KB. Spirometrie reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 35.Hankinson JL, Kawut SM, Shahar E, et al. Performance of american thoracic society-recommended spirometry reference values in a multiethnic sample of adults the multi-ethnic study of atherosclerosis (MESA) lung study. Chest 2010;137:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pino-Yanes M, Thakur N, Gignoux CR, et al. Genetic ancestry influences asthma susceptibility and lung function among Latinos. J Allergy Clin Immunol 2015;135:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brehm JM, Acosta-Pérez E, Klei L, et al. African ancestry and lung function in Puerto Rican children. J Allergy Clin Immunol 2012;129:1484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar R, Seibold MA, Aldrich MC, et al. Genetic ancestry in lung-function predictions. N Engl J Med 2010;363:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisner MD, Klein J, Hammond SK, et al. Directly measured second hand smoke exposure and asthma health outcomes. Thorax 2005;60:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arheart KL, Lee DJ, Fleming LE, et al. Accuracy of self-reported smoking and secondhand smoke exposure in the US workforce: The national health and nutrition examination surveys. J Occup Environ Med 2008;50:1414–20. [DOI] [PubMed] [Google Scholar]
- 41.Max W, Sung HY, Shi Y. Who is exposed to secondhand smoke? Self-reported and serum cotinine measured exposure in the U.S., 1999–2006. Int J Environ Res Public Health 2009;6:1633–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matt GE, Quintana PJE, Destaillats H, et al. Thirdhand tobacco smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect 2011;119:1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect 1999;107:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US Department of Health and Human Services. Children and Secondhand Smoke Exposure. Excerpts from The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 2007. [Google Scholar]
- 45.Burchard EG, Oh SS, Foreman MG, et al. Moving toward True Inclusion of Racial/Ethnic Minorities in Federally Funded Studies. A Key Step for Achieving Respiratory Health Equality in the United States. Am J Respir Crit Care Med 2015;191:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.