Summary
The branched-chain amino acids (BCAAs) are vital to both growth and virulence of the human pathogen Staphylococcus aureus. In addition to supporting protein synthesis, the BCAAs serve as precursors for branched-chain fatty acids (BCFAs), which are predominant membrane fatty acids, and, in association with the global regulatory protein CodY, the BCAAs are key co-regulators of virulence factors. Despite these critical functions, S. aureus represses Leu and Val synthesis, instead preferring to acquire them from the extracellular milieu. We previously identified BrnQ1 as a BCAA transporter, yet a brnQ1 mutant remained capable of BCAA acquisition. Here, we describe BcaP as an additional BCAA transporter, and determine that it plays a secondary role to BrnQ1 during S. aureus growth in a chemically defined medium. Furthermore, membrane fatty acid composition analysis revealed that BrnQ1, and not BcaP, is required for transporting Leu and Val to be used for iso-BCFA synthesis. Despite a predominant role for BrnQ1 in vitro, both BrnQ1 and BcaP are required for S. aureus fitness in vivo in a hematogenous spread infection model and a nasal colonisation model. These data demonstrate the importance of BrnQ1 and BcaP for growth, environmental adaptation and virulence of S. aureus.
Introduction
Staphylococcus aureus, and its methicillin-resistant variant, is associated with significant human infectious morbidity and mortality. It is capable of infecting nearly everybody site to cause infections that range from mild skin and soft tissue infections to life-threatening pneumonia, necrotizing fasciitis and sepsis (Gillet et al., 2002; Gonzalez et al., 2005; Miller et al., 2005; King et al., 2006). It is also an asymptomatic colonizer of the nares in approximately one third of the population (Gorwitz et al., 2008). The synthesis and acquisition of nutrients are critical to support S. aureus metabolism and virulence factor production and are prerequisites for infection and colonisation (Mei et al., 1997; Coulter et al., 1998; Benton et al., 2004; Krismer et al., 2014). Such nutrients include amino acids, which S. aureus is presumably starved for in the host given that genes for amino acid synthesis and transport are upregulated in vivo (Malachowa et al., 2011; Krismer et al., 2014). Yet the specific amino acid requirements of S. aureus in diverse host niches and the mechanisms to maintain these nutrients are not fully understood.
The branched-chain amino acids (BCAAs; Ile, Leu, Val) are vital nutrients for bacterial physiology, as their importance extends beyond supporting high levels of protein synthesis. In most Gram-positive bacteria, the BCAAs are precursors for the synthesis of branched-chain fatty acids (BCFAs), which are a major constituent of membrane fatty acids and include Leu/Val-derived iso-fatty acids and Ile-derived anteiso-fatty acids. In S. aureus, BCFAs comprise 65% of membrane fatty acids and straight-chain fatty acids comprise 35% (Singh et al., 2008). BCFA composition is adjusted to regulate membrane fluidity, an alternative strategy to the incorporation of unsaturated fatty acids, which are toxic to S. aureus (Butcher et al., 1976). Of the BCFAs, anteiso-fatty acids are most important for promoting a more fluid membrane and as such, their abundance in the membrane is increased at low temperatures to support growth (Annous et al., 1997; Klein et al., 1999; Edgcomb et al., 2000; Zhu et al., 2005a; Giotis et al., 2007; Singh et al., 2008). The iso:anteiso ratio is also important for pH stress tolerance, resistance to membrane stressors including antimicrobial peptides and virulence (Giotis et al., 2007; Singh et al., 2008; Sun and O’Riordan, 2010; Sun et al., 2012); thus, the BCFAs serve an important role in adaptation to various environmental conditions.
Intracellular levels of BCAAs are monitored by the global regulatory protein CodY (Shivers and Sonenshein, 2004; Handke et al., 2008; Villapakkam et al., 2009). CodY is a highly conserved transcriptional repressor in low %mol G + C Gram-positive bacteria that regulates >100 genes involved in amino acid and peptide metabolism and transport in response to BCAAs and GTP (Guédon et al., 2001; Sonenshein, 2005; Pohl et al., 2009). CodY also regulates virulence factors in S. aureus (Majerczyk et al., 2008; Montgomery et al., 2012; Rivera et al., 2012; Waters et al., 2016), implicating CodY as an important link between the metabolic status of the cell and virulence. Identifying the mechanisms that maintain intracellular levels of BCAAs is therefore a key step towards understanding how CodY activity is regulated.
S. aureus was previously reported to be auxotrophic for Leu and Val, despite encoding the ilv-leu operon required for BCAA synthesis (Onoue and Mori, 1997; Pohl et al., 2009). In replicating this, we found that in fact, S. aureus is capable of synthesizing Leu and Val, but it represses their synthesis during early stage growth (Kaiser et al., 2015). This indicated that S. aureus prefers to acquire these nutrients and must therefore be able to scavenge BCAAs from the host. Indeed, free BCAAs are available to S. aureus at sites of infection or colonisation in the micromolar range (50–300 μM except for Ile which is undetectable in nasal secretions) (Psychogios et al., 2011; Krismer et al., 2014). We have, therefore, focused our studies on investigating mechanisms of BCAA transport. BCAA transporters in other Gram-positive bacteria include the secondary transporters BrnQ, BraB and BcaP (Stucky et al., 1995; den Hengst et al., 2006; Belitsky, 2015). We previously characterized three BrnQ homologs (BrnQ1, BrnQ2 and BrnQ3) in the community-associated methicillin-resistant S. aureus strain USA300. We determined that BrnQ1 transports all three BCAAs, BrnQ2 is a dedicated Ile transporter and BrnQ3 did not appear to function as a BCAA transporter. Yet a strain lacking all three brnQ genes remained capable of BCAA transport (Kaiser et al., 2015). BLAST searches revealed that USA300 also possesses a BcaP homolog. In this study, we describe the role of BcaP in BCAA acquisition and demonstrate the importance of BCAA transport to S. aureus growth, membrane BCFA content and fitness in vivo.
Results
Identification of CodY-regulated bcaP as a BCAA transporter in S. aureus
We hypothesized that one or more BCAA transporter(s) exist in S. aureus, in addition to the three brnQ genes, since we previously demonstrated that uptake of Ile, Leu and Val is not completely abolished in a brnQ1-2-3 mutant (Kaiser et al., 2015). We prioritized the gene SAUSA300_2538 to investigate since it exhibited 34% and 63% total similarity to BcaP, a BCAA transporter in Bacillus subtilis and Lactococcus lactis respectively (den Hengst et al., 2006; Belitsky, 2015). The SAUSA300_2538 gene, hereafter referred to as bcaP, possesses a CodY-binding site (CodY box) 102 base pairs upstream of the start codon (Fig. 1A). Consistent with this, we observed that expression of bcaP in S. aureus strain USA300 increased in the absence of CodY activity (Fig. 1B). This is in agreement with previous findings in S. aureus strains Newman and UAMS-1, although the magnitude of bcaP upregulation observed in USA300 is lower than what was previously reported, possibly a consequence of strain variation (Pohl et al., 2009; Majerczyk et al., 2010).
Fig. 1.

BcaP is a CodY-regulated gene in USA300.
A. Genomic context of bcaP in the USA300 FPR3757 genome.
B. Expression of bcaP was evaluated by RT-qPCR in WT USA300 and a codY mutant grown to an OD600 of 0.6 in TSB. Data were normalized relative to the expression of rpoB, and expression of bcaP in WT cells was set to 1 as the comparator. Data are the mean of three biological replicates +/− SD. Data were analysed using a Student’s unpaired t test. *** P≤0.001.
To determine whether BcaP functions as a BCAA transporter, we mutated bcaP alone and in combination with the brnQ genes and measured uptake of 14C-labeled Ile, Leu or Val in the various mutant strains. The bcaP mutation alone had a minor effect on transport of all three BCAAs compared to the wild type (WT) strain (Fig. 2A). However, when bcaP was mutated in the brnQ1-2-3 mutant background, uptake of all three BCAAs was completely abolished (Fig. 2A), indicating a clear role for BcaP in BCAA transport. Leu transport was completely abolished in the brnQ1-bcaP mutant, suggesting that these are the only genes responsible for Leu transport. Ile transport was reduced in the brnQ1-bcaP mutant compared to the bcaP mutant alone, and introduction of the brnQ2 mutation into the brnQ1-bcaP mutant resulted no Ile transport, indicating that the brnQ1, brnQ2 and bcaP genes all participate in Ile transport. Unexpectedly, the brnQ1-bcaP mutant retained some Val transport (Fig. 2A). The addition of the brnQ3 mutation in the brnQ1-bcaP mutant background resulted in no Val transport (Fig. 2A), uncovering an involvement of BrnQ3 in Val transport that we previously had overlooked (Kaiser et al., 2015), likely due to the transport activity of BcaP.
Fig. 2.
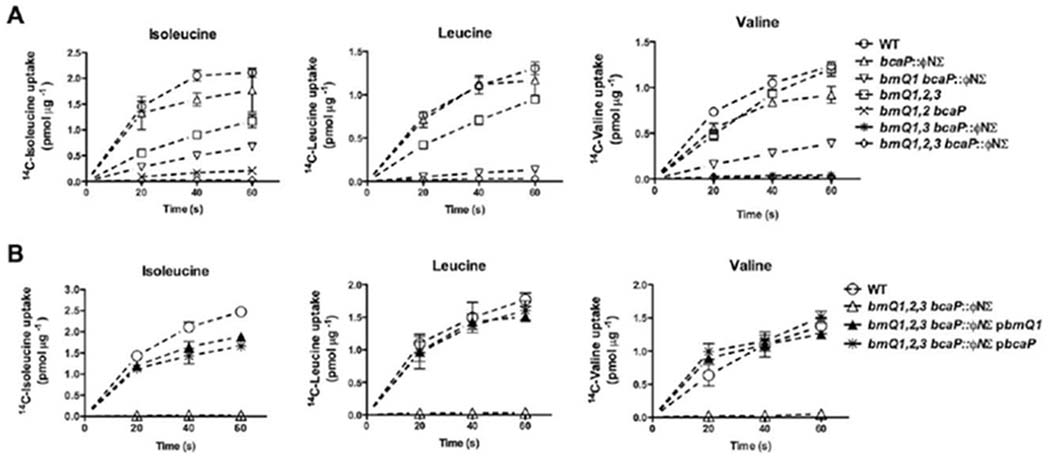
BcaP is a BCAA transporter.
A and B. Cells were harvested from cultures grown to mid-late exponential phase in complete CDM, or CDM supplemented with 10 mg mL−1 tryptone in the case of the brnQ1,brnQ2,brnQ3,bcaP mutant. Transport of 14C-labeled BCAA, as indicated, was measured at 20, 40 and 60 seconds and expressed relative to total protein. Data points shown are the mean of three biological replicates +/− SD.
To confirm a direct role for BcaP in BCAA transport, we tested whether expression of bcaP in trans could restore transport in the brnQ1-2-3-bcaP mutant. BcaP restored transport of all three BCAAs in the quadruple mutant to levels comparable to WT or the quadruple mutant complemented with BrnQ1 (Fig. 2B), thus confirming the role of BcaP as a BCAA transporter. Despite our observation that BrnQ3 contributes to Val transport (Fig. 2A), expression of brnQ3 from a complementation vector (confirmed using RT-qPCR; data not shown) was unable to restore Val transport in the quadruple mutant (data not shown). At this point, we have no conclusive evidence that implicates BrnQ3 as a bona fide BCAA transporter.
BCAA transporters in L. lactis and B. subtilis are capable of transporting amino acids structurally similar to BCAAs (den Hengst et al., 2006; Belitsky, 2015). To test the transport specificity of the BrnQ and BcaP transporters in S. aureus, we investigated 14C-Leu uptake with 10-fold, 100-fold and 1000-fold excess of each unlabeled amino acid. Thousand-fold excess was required to reduce transport to less than 50% (data not shown). Alanine, cysteine, methionine and threonine were able to reduce BrnQ1- or BcaP-mediated Leu transport whereas only aspartic acid was able to compete for Ile transport mediated by BrnQ2 (Supporting Information Fig. S1). The transport kinetics for these amino acids is beyond the scope of this study; however, we investigated whether BrnQ1 and BcaP are required for growth in chemically defined media (CDM) with limiting concentrations of Ala, Cys, Thr or Met. The double mutant did not exhibit a growth delay in these media any more so than in complete CDM (Supporting Information Fig. S2), suggesting that either (i) they bind to, but do not transport these amino acids, (ii) there are other transporters for these amino acids or (iii) at least in the case of Ala, Thr and Met (for which S. aureus is not auxotrophic) they are synthesized inside the cell, masking a transport-dependent growth defect.
BrnQ1 is the predominant BCAA transporter required for growth in media containing free amino acids
We previously demonstrated that despite encoding the BCAA biosynthetic operon, USA300 prefers Leu and Val acquisition over their biosynthesis during growth in CDM (Kaiser et al., 2015). We, therefore, determined the biological relevance of each of BcaP and BrnQ1 in S. aureus for growth in complete CDM, which contains excess concentrations of Leu and Val (684 μM). A mutation in bcaP did not impede growth of USA300 in complete CDM, whereas a brnQ1 mutation resulted in a reduced growth rate (Fig. 3A). Combining both mutations further reduced the growth rate (Fig. 3A). The growth of the brnQ1-bcaP mutant did not require a compensatory mutation, as when these cells were plated for isolated colonies, picked and re-inoculated into the same medium, an identical growth phenotype was observed. Thus, either another transporter(s) or induction of BCAA synthesis is able to supply Leu and Val for growth. To minimize the effects of the existence of a potentially low-affinity transporter, we reduced the concentration of Leu or Val in the media by tenfold. In Leu-limited CDM, the bcaP mutant exhibited a growth rate comparable to the WT strain, whereas the brnQ1 mutant exhibited a growth lag of approximately 12 h (Fig. 3B). Addition of the bcaP mutation into the brnQ1 mutant background extended the growth lag to ~14 h (Fig. 3B). The bcaP mutant also grew comparably to the WT strain in Val-limited CDM, whereas the brnQ1 and brnQ1-bcaP mutants both exhibited a growth lag of ~ 18 h. Growth of the brnQ1-bcaP mutant in all media could be restored to WT levels with the addition of multi-copy complementation vectors expressing either brnQ1 or bcaP (Fig. 3A–C). Together these data indicate that BrnQ1 is the predominant transporter required for Leu and Val acquisition during growth in media containing free amino acids, and BcaP contributes to Leu acquisition, but only noticeably in the absence of BrnQ1.
Fig. 3.

BrnQ1 is the predominant transporter required for growth in free amino acid containing medium. Strains were grown in complete CDM to mid-late exponential phase and sub-cultured to a starting OD600 of 0.0025 in either A) complete CDM (684 μM each Leu and Val) or CDM where all amino acids were present at the same concentration as in complete CDM, except for alterations to the concentration of Leu (panel B) or Val (panel C). Data are the mean readable ODs of three biological replicates +/− SD. pRMC2 is the empty vector.
To test whether the growth of the mutants in Leu- and Val-limited conditions following an extended growth lag was the result of compensatory mutations, we plated the cultures that eventually grew in these conditions and, using these cells, repeated the growth experiment in the Leu- and Val-limited conditions. The brnQ1 and brnQ1-bcaP mutants from the Leu-limited CDM exhibited the same growth lag upon sub-culture into Leu-limited CDM indicating that the eventual growth in this condition does not result in a genetically heritable compensatory mutation (data not shown). Interestingly, the brnQ1 and brnQ1-bcaP mutant cultures from Val-limited CDM were able to grow without a growth lag upon subculture into the same condition (data not shown) and, in fact, both strains exhibited unimpeded growth in CDM with Val omitted (data not shown). These data suggest that the growth of the brnQ1 and brnQ1-bcaP mutants in the Val-limited media resulted in a genetically heritable compensatory mutation that likely results in Val biosynthesis. The identity of the mutation, or mutations, is currently under investigation.
Predominance of BrnQ1 is dictated by promoter strength
We next investigated the underlying basis for the predominant role of BrnQ1 over BcaP for growth in CDM. We determined the apparent KM and Vmax of each transporter using the quadruple mutant complemented with either brnQ1 or bcaP. Both transporters had an apparent KM for each BCAA in the low micromolar range (1–4 μM) (Table 1). Vmax values further revealed that under the conditions where growth is assessed, both transporters are functioning at maximum velocity, and in fact, the Vmax of BrnQ1 is lower than the Vmax of BcaP (Table 1). These data indicate that, when over-expressed, both transporters function comparably, and therefore, transporter kinetics are not likely sufficient to explain their different roles in growth.
Table 1.
Transporter kinetics.
| Isoleucine |
Leucine |
Valine |
||||
|---|---|---|---|---|---|---|
| Transporter | KMa | Vmaxa | KM | Vmax | KM | Vmax |
| BcaP | 3.4 ±0.8 | 110 ±12 | 2.9 ±0.8 | 28 ±3 | 3.7 ±0.6 | 155 ± 12 |
| BrnQ1 | 1.4 ±0.4 | 55 ±7 | 1.1 ±0.7 | 10 ±2 | 2.5 ±1.7 | 92 ± 39 |
KM units μM, Vmax units pmol μg−1 min−1; units of activity are expressed relative to total protein.
Values are the average of three biological replicates ± SD.
We next turned to a lux promoter-reporter assay to determine the promoter activity of each of brnQ1 and bcaP during growth, as we were not able to directly determine protein abundance of each transporter due to lack of antibodies. Promoter activities of both brnQ1 and bcaP were higher in a codY mutant compared to the WT strain (Fig. 4A and B), confirming they are both repressed by CodY. Notably, the promoter of brnQ1 drove higher expression of the lux genes from the reporter plasmid than the bcaP promoter, which exhibited little/no lux expression (Fig. 4A and B), suggesting that brnQ1 expression is higher than bcaP Since CodY promoters do not necessarily respond equally to changes in the level of CodY activity (Brinsmade et al., 2014; Waters et al., 2016), we also assessed promoter activity in the low Leu condition, and observed the same trends in promoter activity as in complete CDM (data not shown). Together, with the observation that over-expression of bcaP from a multi-copy plasmid restores growth of the brnQ1-bcaP mutant, these data suggest that the predominant role of BrnQ1 is due to protein abundance and not differences in transport kinetics between the two transporters.
Fig. 4.

Promoter activity of brnQ1::lux is higher than bcaP::lux. Growth (OD600; closed symbols) and relative luminescence units (RLU; open symbols) of WT USA300 and the codY mutant harboring either A) the pGYbrnQ1::lux reporter vector or B) the pGYbcaP::lux reporter vector. Strains were grown to mid-late exponential phase in complete CDM and sub-cultured to an OD600 of 0.01 in complete CDM. Data are the mean +/− SD of three biological replicates.
BCFA content in S. aureus membranes is dependent on BrnQ1
BCFAs are synthesized from the BCAAs and comprise 50–65% of total membrane fatty acids of S. aureus grown in rich media (Singh et al., 2008). Ile, Leu and Val are used for the synthesis of anteiso-fatty acids, odd-chained iso-fatty acids and even-chained iso-fatty acids respectively, with anteiso-fatty acids being the most abundant (Singh et al., 2008). Since S. aureus relies on transporters to acquire the BCAAs, we hypothesized that transporter mutants would lack the corresponding BCFAs in their membranes. When grown in complex media (TSB), BCFAs made up 43% of total fatty acids in the WT strain and 48% of total fatty acids in a brnQ1-bcaP mutant, with no difference in the proportion of anteiso- and iso-fatty acids between strains (Table 2). These data suggest that, independent of the free amino acid transporters BrnQ1 and BcaP, S. aureus is able to acquire BCAAs from peptide sources present in a rich medium. In contrast, when grown in CDM, the proportion of BCFAs increased to 76% of total fatty acids in the WT strain and 88% in the brnQ1-bcaP mutant, with drastic differences in the proportions of anteiso- and iso-fatty acids between the WT and mutant. Whereas BCFAs in the WT strain membrane included both anteiso- and iso-fatty acids, BCFAs in the brnQ1-bcaP mutant membrane were derived solely from anteiso-fatty acids, with no detection of Leu-derived odd-chain iso-fatty acids or Val-derived even-chain iso-fatty acids. The absence of Leu- and Val-derived BCFAs in this strain was the consequence of the brnQ1 mutation since the brnQ1 mutant alone lacked Leu- and Val-derived BCFAs, whereas the fatty acid profile of the bcaP mutant resembled that of the WT strain (Table 2). Notably, the anteiso-fatty acid content was not affected in a strain lacking all Ile transporters (e.g. brnQ1-brnQ2-bcaP) (Table 2). Altogether, these data indicate that, in a defined, free-amino acid-containing medium, BrnQ1 is required for isofatty acid synthesis, and Ile synthesis is sufficient to maintain anteiso-fatty acid content in the absence of BCAA transport.
Table 2.
Fatty acid profiles of S. aureus BCAA transporter mutants.
| % (wt/wt) of total fatty acida |
|||||||
|---|---|---|---|---|---|---|---|
| WT |
brnQ1brnQ2bcaP |
brnQ1bcaP CDM | brnQ1 CDM | bcaP CDM | |||
| Fatty acid | TSB | CDM | TSB | CDM | |||
| SCFAs | 54 ± 2 | 23 ± 3 | 52 ± 2 | 11 ± 4 | 11 ± 3 | 11 ± 1 | 23 ± 1 |
| BCFAs | 44 ± 4 | 77 ± 5 | 48 ± 2 | 88 ± 5 | 88 ± 4 | 86 ± 3 | 77 ± 2 |
| Anteiso | 27 ± 5 | 47 ± 3 | 33 ± 4 | 88 ± 5 | 88 ± 3 | 86 ± 3 | 47 ± 1 |
| Iso (Odd) | 8 ± 2 | 24 ± 2 | 6 ± 2 | <1.0 | <1.0 | <1.0 | 25 ± 1 |
| Iso (Even) | 9 ± 2 | 5 ± 1 | 10 ± 1 | <1.0 | <1.0 | <1.0 | 5 ± 1 |
Values are the average percentage of fatty acids from three biological replicates ± SD. Fatty acids less than 1% are not reported.
BrnQ1 and BcaP are required for growth in CDM at temperatures below 37 °C
The adaptation of Gram-positive bacteria to growth at low temperature requires an increase in anteiso-fatty acid content to increase membrane fluidity (Klein et al., 1999; Edgcomb et al., 2000; Zhu et al., 2005b). We, therefore, hypothesized that the brnQ1 mutant would be better adapted than the WT strain to growth at lower temperatures since anteiso-fatty acids comprise 88% of membrane fatty acids in this strain. In contrast to this hypothesis, the brnQ1 mutant exhibited growth attenuation relative to the WT strain that was even more evident when grown at 25°C compared to 37°C (Fig. 5A). This temperature-dependent growth phenotype was even more so evident with the brnQ1-bcaP mutant (Fig. 5A). Growth of the brnQ1-bcaP mutant could be restored with expression of either brnQ1 or bcaP from a complementation vector (Fig. 5B), or addition of peptide source to the growth medium (Fig. 5C). Since BcaP contributes to this growth phenotype, but is not required for BCFA synthesis, we suspect this growth-attenuated phenotype is independent of membrane content, yet nonetheless noteworthy.
Fig. 5.

BCAA acquisition is required for growth in CDM at 25°C.
A and B. Strains were grown in complete CDM to mid-late exponential phase, sub-cultured to a starting OD600 of 0.0025 in complete CDM and incubated at either 25°C or 37°C.
C. Strains were grown in CDM supplemented with 10 mg mL−1 of tryptone to mid-late exponential phase and sub-cultured to a starting OD600 of 0.0025 in CDM 10 mg mL−1 of tryptone. Data are the mean readable ODs of three biological replicates +/− SD.
Staphyloxanthin abundance is increased in the membrane of the brnQ1 mutant
In the course of our experiments as described above, we noticed that the cell pellet of the brnQ1 mutant was brighter yellow than that of the WT strain when grown in CDM (Fig. 6A). We therefore quantified the abundance of the pigmented carotenoid staphyloxanthin in the membrane of the WT strain and brnQ1 and bcaP mutants. Indeed, the membrane of the brnQ1 mutant contained more staphyloxanthin than either the WT strain or the bcaP mutant, and this increase could be restored to WT levels upon complementation (Fig. 6B and C). Staphyloxanthin is a polar carotenoid that increases membrane rigidity (Wisniewska et al., 2006), thus its presence likely helps to counterbalance the high proportion of membrane fluidity-increasing anteiso-fatty acids in the brnQ1 mutant membrane (Edgcomb et al., 2000). To determine if the presence of staphyloxanthin masked the growth benefit of the brnQ1 mutant at low temperatures, we obtained a mutant incapable of producing staphyloxanthin (crtM) and mobilized this mutation into the brnQ1 mutant background to assess the ability of this strain to grow at low temperatures. Staphyloxanthin did not alter growth of the brnQ1 mutant at 25°C (Fig. 6D), supporting our conclusion that this phenotype is not related to membrane BCFAs. Staphyloxanthin is considered a virulence factor in S. aureus due to its antioxidant properties which protect cells against oxidative stress, and its proposed role in resisting antimicrobial peptides (Liu et al., 2005; Clauditz et al., 2006; Mishra et al., 2011a). We, therefore, investigated whether the brnQ1 mutant was more resistant to these stressors. We found that the brnQ1 mutant was equally susceptible, compared to the WT strain, to H2O2 (data not shown). The brnQ1 mutant and WT strain were also equally susceptible to the membrane targeting antibiotics daptomycin (DAP) and polymyxin B (PMB), with minimum inhibitory concentrations (MIC) values in the range of those typically reported for S. aureus (Fig. 6E and F) (Mishra et al., 2011b). Altogether, these data indicate that although staphyloxanthin is more abundant in the brnQ1 mutant membrane, it is not sufficiently high to impart enhanced resistance to membrane stressors.
Fig. 6.

Staphyloxanthin abundance is increased in the brnQ1 mutant membrane.
A. Cell pellets of cultures grown overnight in complete CDM.
B and C. Staphyloxanthin was extracted using warm methanol from the cell pellets of strains grown to log-phase in complete CDM. Staphyloxanthin was quantified at an absorbance of 465 and expressed relative to the OD600 of log-phase cultures. Data are the mean of three biological replicates +/− SD and were analysed using one-way ANOVA with the Tukey post test. * P≤ 0.05, ** P≤ 0.01, *** P≤ 0.001.
D. Cells were grown in complete CDM to mid-late exponential phase, sub-cultured to a starting OD600 of 0.0025 in complete CDM and incubated at 25°C. Data are the mean of readable ODs of three biological replicates +/− SD.
E and F. Strains were grown in complete CDM to mid-late exponential phase and sub-cultured to a starting OD600 of 0.0025 in complete CDM containing serial dilutions of each antibiotics. The MIC was determined by examining visible growth of two biological replicates after 24 h.
BrnQ1 and BcaP are required for S. aureus infection and colonisation
High throughput studies have identified amino acid synthesis and transport genes as a major category of genes required for S. aureus virulence (Coulter et al., 1998; Benton et al., 2004). These genes are also some of the most upregulated during growth in human nasal secretions (Krismer et al., 2014). We, therefore, assessed the contribution of Leu/Val transport to USA300 fitness in vivo using a murine systemic infection model and nasal colonisation model. Mice infected systemically with either the WT strain or the single mutants lost approximately 20% of their body weight by day 4 of infection and exhibited reduced activity and grooming. In contrast, mice infected with the brnQ1-bcaP mutant lost on average approximately 5% of their body weight and appeared active and well groomed (Fig. 7A). Despite these apparent differences in disease severity, we did not observe statistically significant differences in kidney bacterial burden between mice in any of these groups (data not shown). We did, however, observe reduced bacterial burden of the brnQ1 mutant and the brnQ1-bcaP mutant in the hearts (Fig. 7B) and there appeared to be a trend towards decreased CFUs in the liver for the brnQ1, bcaP and brnQ1-bcaP mutants (Fig. 7C). These data suggest that the requirement of Leu/Val transport for growth in vivo is niche-specific.
Fig. 7.
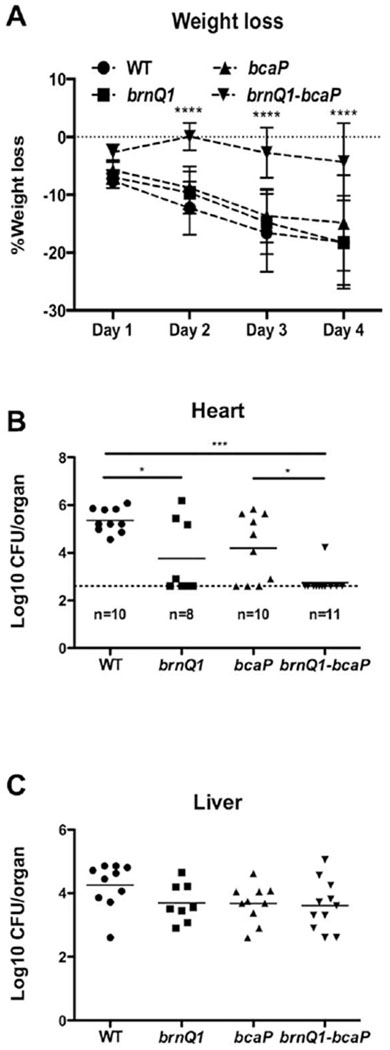
BCAA acquisition is required for full virulence of S. aureus in a murine systemic infection model. Balb/c mice were infected with 6–7 × 106 CFU of the WT strain (n = 10), brnQ1 mutant (n = 8), bcaP mutant (n = 10) or brnQ1-bcaP mutant (n = 11) via tail vein injection.
A. Weight loss was monitored every 24 h post-infection. Data were analysed for significance relative to the WT strain using two-way ANOVA with the Dunnett’s post test. Mice were sacrificed on day four of infection and B) hearts and C) livers were harvested and homogenized, and dilutions were plated for CFU. The dotted line in panel B indicates the limit of detection. Log transformed data were analysed using one-way ANOVA with the Tukey post test. Data in panel C are not significant. * P≤0.05, *** P≤0.001, **** P≤0.0001.
Using a nasal colonisation model, we found that all mice inoculated with either the WT strain or the brnQ1 or bcaP mutants remained colonized on day 7, with comparable CFUs recovered. In contrast, the brnQ1-bcaP mutant could not be recovered on day 7 from 27% (8/30) of mice (Fig. 8). These data suggest that both BrnQ1 and BcaP are required for fitness of S. aureus during nasal colonisation.
Fig. 8.

BCAA acquisition is required for S. aureus murine nasal colonisation. C57BL/6 mice were treated with 2 mg mL−1 streptomycin sulfate in the drinking water 24 h prior to inoculation to remove the microbiota.
Mice were intranasally inoculated with 1 × 108 CFU of the streptomycin resistant WT strain (n=25), brnQ1 mutant (n=12), bcaP mutant (n=9) or brnQ1-bcaP mutant (n=30) under anesthesia. On day 7 post-inoculation, mice were sacrificed and snouts were excised, homogenized and plated for CFU. The dotted line indicates the limit of detection. Log transformed data were analysed using one-way ANOVA with the Tukey post test. ** P≤0.01.
Discussion
S. aureus requires BCAAs for several physiological processes, yet it does not readily synthesize Leu or Val and rather relies on the acquisition of these nutrients to support its metabolism (Kaiser et al., 2015). We previously identified BrnQ1 as a Leu/Val transporter, however, a brnQ1 mutation was not sufficient to abolish Leu/Val transport (Kaiser et al., 2015). Here, we identify BcaP as an additional Leu/Val transporter in S. aureus, which, when mutated in combination with a brnQ1 mutation, results in abrogation of Leu transport and almost a complete loss of Val transport. Identification of this additional, BcaP-dependent mechanism of Leu/Val transport enabled us to characterize the consequence of Leu/Val limitation on S. aureus physiology, and discern which transporters are required for growth, membrane BCFA synthesis and virulence. Our data indicate that although BrnQ1 is the dominant transporter for supplying Leu and Val for growth and BCFA synthesis in vitro, both BrnQ1 and BcaP contribute to S. aureus infection and colonisation.
We attribute the minimal contribution of BcaP to S. aureus growth and BCFA synthesis in vitro to its lower expression relative to BrnQ1. Although this was measured indirectly, this is corroborated by the observation that over-expression of bcaP in the brnQ1-bcaP mutant is sufficient to restore growth to WT levels. This finding contrasts BCAA transport in L. lactis and B. subtilis, where BcaP serves as the more efficient transporter (den Hengst et al., 2006; Belitsky, 2015). It should be cautioned that the relative importance of BrnQ1 and BcaP in USA300 should not be generalized across all S. aureus strains, since when expressed to similar levels off of a multi-copy vector, BrnQ1 and BcaP in S. aureus exhibit comparable KM and Vmax values. Thus, any differences in transporter gene expression across strains would likely affect the relative importance of BrnQ1 and BcaP
Despite a contribution of BcaP to growth in CDM in the absence of BrnQ1, BcaP played no role in supplying Leu and Val for BCFA biosynthesis. Instead, the cells replaced iso-fatty acids with anteiso-fatty acids, presumably supported by Ile synthesis. This compensatory mechanism is unique to S. aureus, as other Grampositive bacteria, such as B. subtilis and L. monocytogenes, are able to synthesize all three BCAAs to maintain BCFA content in the absence of exogenous BCAAs (Klein et al., 1999; Zhu et al., 2005b; Beranová et al., 2008). Although the mechanism that regulates this is currently unknown, it can be speculated that this ensures Leu and Val are conserved for protein synthesis, and might be one explanation as to why S. aureus has retained Ile synthesis.
This study highlights a paradox in S. aureus, in which even upon Leu and Val starvation, endogenous synthesis from the intact BCAA biosynthetic operon does not readily compensate. Our data suggest that BCAA biosynthesis is eventually de-repressed following an extended growth lag of the transporter mutants (14–18 h) when either Leu or Val are limited, a mechanism that is likely to involve known regulators of BCAA biosynthesis, including CodY, CcpA or YeaZ (Ludwig et al., 2002; Pohl et al., 2009; Majerczyk et al., 2010; Lei et al., 2014; Waters et al., 2016). Intriguingly, BCAA biosynthesis in the transporter mutants grown in Val-limited conditions requires a compensatory mutation, whereas a Leu-limited growth medium did not result in genetically heritable suppressor mutations. We had previously made a similar observation with WT USA300 grown in media with either Val or Leu omitted; that is growth in media with Val omitted required a compensatory mutation, whereas growth in media with Leu omitted did not (Kaiser et al., 2015). We are currently investigating the regulation of BCAA synthesis and why a compensatory mutation is required for Val but not Leu synthesis.
Although BcaP plays a secondary role in growth in vitro, it has an additive function with BrnQ1 in vivo. BcaP function might have been underappreciated in vitro due to differences in growth conditions and/or gene expression. For example, the activation state of CodY in vivo might differ from that in vitro, and it is possible that brnQ1 and bcaP expression respond differently to the activation state of CodY (Brinsmade et al., 2014; Waters et al., 2016). Although both are CodY regulated, fine-tuning of expression could occur, and as such the requirement of each transporter would depend on the availability of CodY effectors; BCAAs and GTP Mechanisms of fine-tuning BCAA transporter expression in B. subtilis include multiple upstream CodY binding sites (Belitsky and Sonenshein, 2011) and direct and indirect CodY regulation (Belitsky et al., 2015). Additionally, the affinity of CodY for each promoter could determine the timing of expression in relation to nutrient availability (Belitsky and Sonenshein, 2013). A more detailed characterisation of the regulation of these genes might provide insight as to how these genes are regulated in vivo.
The systemic infection model revealed that mutations in both brnQ1 and bcaP not only resulted in reduced bacterial burden in the heart but also in significantly less weight loss and disease severity. The ability of this strain to reach such a high bacterial burden in the kidneys (e.g. 108 CFU, data not shown) but not cause signs of infection could be the result of altered toxin secretion in vivo and will need to be explored in future studies. The ability of the brnQ1 and brnQ1-bcaP mutants to replicate efficiently in some organs (e.g. livers and kidneys) and not others (e.g. heart) could reflect differences in nutrient availability (e.g., peptides sources and/or host-derived fatty acids). Indeed, S. aureus encodes a di/tri-peptide permease (dtpT) and several oligopeptide permeases that might supply BCAAs in vivo (Hiron et al., 2007), although the requirement for oligopeptide transport for S. aureus virulence is not known. Moreover, S. aureus is able to incorporate host-derived fatty acids in its membrane (Altenbern, 1977) and, when grown in serum, BCFAs comprise less than 40% of membrane fatty acids in USA300 (Sen et al., 2016). Finally, although both brnQ1 and bcaP are upregulated in human serum and blood, so are the genes for BCAA biosynthesis (Malachowa et al., 2011). Thus it is possible that Leu and Val are synthesized in vivo.
Our data suggest a dichotomy in the ability of the brnQ1-bcaP mutant to colonize murine nares, as the brnQ1-bcaP mutant was not recovered from several mice on day 7, yet in some mice was recovered at CFUs comparable to the WT strain. At this point, it is unclear whether this is a result of the inability of the brnQ1-bcaP mutant to establish colonisation in some circumstances, or perhaps it is cleared at a more rapid rate, albeit asynchronously, than the WT strain. We hypothesize the former; cells must maintain a threshold level of BCAAs to support growth in this environment, and BCAA transport is essential to do so. Free amino acid transport might be critical in this environment due to limited availability of peptide sources of BCAAs. The finding that an S. aureus strain incapable of synthesizing Met has a reduced capacity for nasal colonisation suggests that peptides are not sufficient to complement amino acid deficiency in this environment (Krismer et al., 2014). With limited availability of peptides, the transporter mutant must rely on BCAA biosynthesis for growth. Although the BCAA biosynthesis genes are upregulated when S. aureus is grown in media that mimics human nasal secretions (Krismer et al., 2014), the biosynthetic enzymes require iron (Rosario-Cruz et al., 2015), which is likely limited in this environment (Burian et al., 2010). Thus, in the absence of transport, cells will only be viable if there is sufficient iron and/or carbon to support BCAA synthesis. The variability in nutrient levels across mice might be sufficient to create this threshold for growth. More studies will be required to test this hypothesis.
Altogether, this study provides a detailed characterisation of the role of BCAA transport in S. aureus general physiology, and lays a foundation for understanding the link between BCAA metabolism and virulence in S. aureus.
Experimental procedures
Strains and growth conditions
Bacterial strains and plasmids used in this study are described in Table 3. The WT strain in all experiments is the MRSA isolate pulsed-field gel electrophoresis type USA300 LAC that has been cured of the erythromycin resistance plasmid. For experiments involving S. aureus grown in tryptic soy broth (TSB) (EMD Millipore, Billerica, MA), two to three colonies were inoculated from TSB agar into TSB and grown to mid-exponential phase at 37°C, then sub-cultured into fresh TSB to a starting OD600 equivalent of 0.0025. For growth curves performed in CDM, described previously (Kaiser et al., 2015), two to three colonies were inoculated from TSB agar into CDM and were grown to mid-late-exponential phase at 37°C, then sub-cultured to a starting OD600 equivalent of 0.0025 in either complete CDM or CDM with altered concentrations of amino acids. The brnQ1, brnQ2, brnQ3, bcaP mutant was treated the same; however, media was supplemented with 10 mg mL−1 of Bacto™ tryptone (BD Biosciences; Sparks, MD) to support growth. Strains were grown at 37°C or 25°C where indicated. Growth curves were performed either in 100-well plates and read using the Bioscreen C visible spectrophotometer (Growth Curves USA; Piscataway, NJ), or in flasks using a flask:volume ratio of at least 5:1. Where required, ampicillin (100 μg mL−1), chloramphenicol (10 μg mL−1), erythromycin (3 μg mL−1), streptomycin (500 μg mL−1) and tetracycline (4 μg mL−1) were added to the growth medium.
Table 3.
Bacterial strains and plasmids used in this study.
| Strain/Plasmid | Descriptiona | Source or reference |
|---|---|---|
| S. aureus | ||
| USA300 | USA300 LAC cured of antibiotic resistance plasmid | (Arsic et al., 2012) |
| RN4220 | ; capable of accepting foreign DNA | (Kreiswirth et al., 1983) |
| bcaP | USA300 bcaP∷ΦNΣ; EmR (SAUSA300_2538) | (Fey et al., 2013) |
| brnQ1 | USA300 ΔbrnQ1 | (Kaiser et al., 2015) |
| brnQ1-bcaP | USA300 ΔbrnQ1 bcaP∷ΦNΣ; EmR | This study |
| brnQ1-2-3 | USA300 ΔbrnQ1 ΔbrnQ2 brnQ3∷Tc; TcR | (Kaiser et al., 2015) |
| brnQ1-2-3-bcaP | USA300 ΔbrnQ1 ΔbrnQ2 brnQ3∷Tc bcaP∷ΦNΣ; EmR, TcR | This study |
| brnQ1-2-bcaP | USA300 ΔbrnQ1 ΔbrnQ2 bcaP∷ΦNΣ; EmR | This study |
| brnQ1-3-bcaP | USA300 ΔbrnQ1 brnQ3∷Tc bcaP∷ΦNΣ; EmR, TcR | This study |
| codY | USA300 codY∷ ΦNΣ; EmR | (Fey et al., 2013) |
| crtM | USA300 crtM∷ ΦNΣ; EmR | (Fey et al., 2013) |
| brnQ1-crtM | USA300 ΔbrnQ1 crtM∷ ΦNΣ; EmR | This study |
| E. coli | ||
| DH5α | F− ΅80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 () supE44 relA1 deoR Δ(lacZYA-argF)U169phoA | Promega |
| Plasmids | ||
| pRMC2 | Anhydrotetracycline-inducible expression vector; ApR in E. coli; CmR in S. aureus | (Corrigan and Foster, 2009) |
| pBrnQ1 | pRMC2 containing brnQ1; CmRpRMC2 containing bcaP; CmR | (Kaiser et al., 2015) |
| pBcaP | Vector harboring promoterless luxABCDE operon; CmR | This study |
| pGYlux | lux reporter vector with brnQ1 promoter | (Mesak et al., 2009) |
| pGYbrnQ1∷lux | lux reporter vector with bcaP promoter | This study |
| pGYbcaP∷lux | This study |
Abbreviations: TcR, CmR, EmR and ApR designate resistance to tetracycline, chloramphenicol, erythromycin and ampicillin respectively.
Mutagenesis and construction of plasmids
Strains containing a transposon insertion in either the bcaP gene (bcaP∷ΦNΣ) or crtM (crtM∷ΦNΣ) gene were identified in the Nebraska Transposon Mutant Library (Fey et al., 2013). The bcaP transposon was transduced into our laboratory strain of USA300 and into the brnQ1, brnQ1-2-3, brnQ1-2 and brnQ1-3 backgrounds using phage 80α. The same method was used to transduce the crtM transposon into WT USA300 and the brnQ1 mutant. Transposon insertions were confirmed by PCR. All plasmids were first constructed as shuttle vectors in Escherichia coli DH5α. Once confirmed, plasmids were subsequently electroporated into S. aureus RN4220 as an intermediate host, and then into the desired S. aureus USA300 strains. Primers used for plasmid construction are listed in Supporting Information Table S1.
RT-qPCR
Cultures were grown to mid-exponential phase (OD600 of 0.6 in TSB) and RNA was extracted using an Aurum Total RNA Mini Kit as described by the manufacturer (Bio-Rad; Hercules, CA). RNA (500 ng) was reverse transcribed using Superscript II (Invitrogen; Carlsbad, CA) according to manufacturers’ instructions and using 500 μg mL−1 of random hexamers. cDNA was PCR-amplified using SensiFast SYBR (Bioline; Taunton, MA). The primers used and their reaction efficiencies are listed in Supporting Information Table S1. Data were normalized relative to expression of the reference gene rpoB.
Radioactive transport assays
Cultures were grown overnight at 37°C in complete CDM and sub-cultured into complete CDM at a starting OD600 of 0.1. Bacteria were grown to mid/late-exponential phase (OD600 of 1.0), harvested by filtration on 0.45 μm pore-size membrane filters and washed with PBS before being re-suspended in CDM lacking amino acids. Cells were warmed to 37°C for 10 min prior to the assay. The 14C-labeled amino acid of interest (Perkin Elmer, MA) was added to cells at a final concentration of 1 μM. An aliquot of cells was removed at 20, 40 and 60 s and rapidly filtered through 0.45 μm membrane filters. The filters were immediately washed with 10 mL of 0.1 M LiCl2 at room temperature. Filters were dried and placed in scintillation vials containing 4 mL of Cytoscint™ scintillation cocktail (Fisher Scientific). 14C radioactivity was measured using the LS 6500 scintillation system (Beckman). Radioactivity was normalized to total protein; 1 mL of culture with an OD of 1.0 corresponded to approximately 115 μg of protein. For competition assays, 1 mM of non-labeled amino acid was added in the presence of 1 μM of either 14C-Leu or 14C-Ile and cells were filtered at 60 s and processed as above.
To determine transport kinetics, cells, prepared as above, were incubated with 500 nM, 1 μM, 2 μM or 4 μM of a 14C-labeled amino acid. An aliquot of cells was filtered as described above after 20 s. The velocity of uptake for each concentration was normalized to total protein and plotted to determine the Michaelis-Menten equation and the apparent KM and Vmax.
Lux reporter gene assays
The WT USA300 strain and codY mutant containing either pGYbrnQ1∷lux or pGYbcaP∷lux were grown to mid-late exponential phase in complete CDM and sub-cultured to a starting OD600 of 0.01 (200 uL well−1) of complete CDM in a clear bottom 96 well Microfluor 2 White Plate (Thermo). Plates were incubated at 37°C with constant shaking. Luminescence and OD600 were ready at hourly intervals on the BioTek Synergy H4 Hybrid Reader (BioTek, Winooski, VT) with 1 s of integration and a gain of 200.
Membrane fatty acid analysis and staphyloxanthin quantification
Strains were grown overnight in either TSB or CDM and sub-cultured to an OD600 of 0.05 in the same media. Cells were harvested at mid-exponential phase (OD600 0.4–0.6) by centrifugation at 3000 × g at 4°C for 15 min, and the pellet was washed three times with cold sterile distilled water. The fatty acids in the bacterial cells (30 to 40 mg [wet weight]) were saponified, methylated and extracted for fatty acid methyl ester (FAME) analysis. The resulting methyl ester mixtures were separated using an Agilent 5890 dual-tower gas chromatograph and the fatty acyl groups were identified using the MIDI microbial identification system (Sherlock 4.5 microbial identification system) at Microbial ID, (Newark, DE) (Julotok et al., 2010; Sen et al., 2015). Minor fatty acids (<1% of the total) are not reported in the tables. Quantification of the carotenoid pigment was performed as per the warm methanol extraction protocol described previously (Davis et al., 2005; Liu et al., 2005). Cells were harvested from strains grown overnight in CDM, and carotenoid was extracted with warm (55°C) methanol for 5 min. The absorbance at 465 nm of the supernatant after centrifugation was measured and expressed relative to the OD600 of overnight cultures.
Antibiotic susceptibility assays
The MIC for DAP and PMB were determined based on growth of S. aureus strains in tubes containing 2 mL of complete CDM with a range of twofold dilutions of each antibiotic. PMB (Sigma-Aldrich) was resuspended in H2O to a stock concentration of 50 mg mL−1 and DAP (Cayman Chemicals) was resuspended in 100% DMSO to a stock concentration of 5 mg mL−1. The concentration of DMSO did not exceed 5% of the culture volume in any experiment. Cells were grown to mid-late-exponential phase in complete CDM and sub-cultured into CDM containing antibiotics at a starting CFU of approximately 1 × 105. Cultures were incubated at 37°C and were monitored for visible growth after 24 h.
Murine model of systemic infection
All protocols were reviewed and approved by the University of Western’s Ontario Animal Use Subcommittee, a subcommittee of the University Council on Animal Care. The systemic infection was performed as previously described (Kaiser et al., 2015). Briefly, 7 week old BALB/c mice purchased from Charles River Laboratories Canada were housed in microisolator cages. Strains were grown in TSB to mid-exponential phase, washed twice in PBS and resuspended in PBS. Mice were infected with approximately 6 × 106 – 7 × 106 CFU of S. aureus via tail-vein injection. Mice were monitored for activity and weight changes every 24 h for the duration of the experiment. Mice were sacrificed 96 h post challenge and kidneys, livers and hearts were removed, homogenized in 0.01% Triton X-100 and plated on TSB agar to determine CFUs.
Murine nasal colonisation model
All protocols were reviewed and approved by the University of Western’s Ontario Animal Use Subcommittee, a subcommittee of the University Council on Animal Care. To evaluate S. aureus nasal colonisation, the endogenous murine microbiota, which are non-permissive to S. aureus colonisation, were depleted with use of streptomycin sulfate, as previously described (Kiser et al., 1999). Since S. aureus is not naturally resistant to Sm, Sm resistant colonies were selected for independently in the WT and mutant backgrounds by plating overnight cultures on TSB agar containing 500 μg mL−1 of streptomycin sulfate. Sm-resistant clones were confirmed to be free of any obvious growth defects, compared to the Sm-sensitive colonies, during growth in TSB or CDM. Seven week old C57BL/6 mice purchased from Charles River Laboratories Canada, were housed in microisolator cages. Mice received 2 mg mL−1 streptomycin sulfate in the drinking water 24 h prior to inoculation with S. aureus, which was changed every 3–4 days for the duration of the experiment. Strains were grown in TSB to mid-exponential phase, washed twice in PBS and resuspended in PBS. Isoflurane-anesthetized mice were inoculated with 5 uL bacterial suspensions into each naris for a total of 108 CFU of S. aureus and monitored over 7 days. On day 7, mice were sacrificed and the snout was excised, homogenized in PBS with 0.01% Triton X-100 and plated on TSB agar for CFUs.
Statistics
All data were analysed using the statistical tests indicated in figure legends using GraphPad Prism version 6.0.
Supplementary Material
Acknowledgements
This research was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council to DEH. BJW is supported by grant R15 AI099977 from the National Institutes of Health. JCK was supported by a Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institutes of Health Research, and an RGE Murray Graduate Scholarship from the Department of Microbiology & Immunology. We thank Sameha Omer for constructing the BrnQ mutants. The authors have no conflict of interest to declare. Author contributions: JCK and DEH conceived and designed the study. JCK, SS and AS performed, analysed and interpreted experiments. BJW assisted in the design and interpretation of experiments. JCK and DEH wrote the manuscript.
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Altenbern RA (1977) Cerulenin inhibited cells of Staphylococcus aureus resume growth when supplemented with either a saturated or an unsaturated fatty acid. Antimicrob Agents Chemother 11: 574–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annous B, Becker L, Bayles D, Labeda D, and Wilkinson B (1997) Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol 63: 3887–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsic B, Zhu Y, Heinrichs DE, and McGavin MJ (2012) Induction of the staphylococcal proteolytic cascade by antimicrobial fatty acids in community acquired methicillin resistant Staphylococcus aureus. PLoS One 7: e45952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitsky BR (2015) Role of branched-chain amino acid transport in Bacillus subtilis CodY activity. J Bacteriol 197: 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitsky BR, Brinsmade SR, and Sonenshein AL (2015) Intermediate levels of Bacillus subtilis CodY activity are required for derepression of the branched-chain amino acid permease, BraB. PLoS Genet 11: e1005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitsky BR, and Sonenshein AL (2011) Contributions of multiple binding sites and effector-independent binding to CodY-mediated regulation in Bacillus subtilis. J Bacteriol 193: 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belitsky BR, and Sonenshein AL (2013) Genome-wide identification of Bacillus subtilis CodY-binding sites at single-nucleotide resolution. Proc Natl Acad Sci USA 110: 7026–7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton BM, Zhang JP, Bond S, Pope C, Christian T, Lee L, et al. (2004) Large-scale identification of genes required for full virulence of Staphylococcus aureus. J Bacteriol 186: 8478–8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranová J, Jemioła-Rzemińska M, Elhottová D, Strzałka K, and Konopásek I (2008) Metabolic control of the membrane fluidity in Bacillus subtilis during cold adaptation. Biochim Biophys Acta 1778: 445–453. [DOI] [PubMed] [Google Scholar]
- Brinsmade SR, Alexander EL, Livny J, Stettner AI, Segrè D, Rhee KY, et al. (2014) Hierarchical expression of genes controlled by the Bacillus subtilis global regulatory protein CodY. Proc Natl Acad Sci U S A 111: 8227–8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burian M, Rautenberg M, Kohler T, Fritz M, Krismer B, Unger C, et al. (2010) Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis 201: 1414–1421. [DOI] [PubMed] [Google Scholar]
- Butcher GW, King G, and Dyke KG (1976) Sensitivity of Staphylococcus aureus to unsaturated fatty acids. J Gen Microbiol 94: 290–296. [DOI] [PubMed] [Google Scholar]
- Clauditz A, Resch A, Wieland KP, Peschel A, and Götz F (2006) Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun 74: 4950–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan RM, and Foster TJ (2009) An improved tetracycline-inducible expression vector for Staphylococcus aureus. Plasmid 61: 126–129. [DOI] [PubMed] [Google Scholar]
- Coulter SN, Schwan WR, Ng EY, Langhorne MH, Ritchie HD,Westbrock-Wadman S, et al. (1998) Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol Microbiol 30: 393–404. [DOI] [PubMed] [Google Scholar]
- Davis AO, O’leary JO, Muthaiyan A, Langevin MJ, Delgado A, Abalos AT, et al. (2005) Characterization of Staphylococcus aureus mutants expressing reduced susceptibility to common house-cleaners. J Appl Microbiol 98: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hengst CD, Groeneveld M, Kuipers OP, and Kok J (2006) Identification and functional characterization of the Lactococcus lactis CodY-regulated branched-chain amino acid permease BcaP (CtrA). J Bacteriol 188: 3280–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgcomb MR, Sirimanne S, Wilkinson BJ, Drouin P, and Morse RD (2000) Electron paramagnetic resonance studies of the membrane fluidity of the foodborne pathogenic psychrotroph Listeria monocytogenes. Biochim Biophys Acta 1463: 31–42. [DOI] [PubMed] [Google Scholar]
- Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, et al. (2013) A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio 4: 12–e00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, et al. (2002) Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359: 753–759. [DOI] [PubMed] [Google Scholar]
- Giotis ES, McDowell DA, Blair IS, and Wilkinson BJ (2007) Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes. Appl Environ Microbiol 73: 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez BE, Martinez-Aguilar G, Hulten KG, Hammerman WA, Coss-Bu J, Avalos-Mishaan A, et al. (2005) Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics 115: 642–648. [DOI] [PubMed] [Google Scholar]
- Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, et al. (2008) Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J Infect Dis 197: 1226–1234. [DOI] [PubMed] [Google Scholar]
- Guédon E, Serror P, Ehrlich SD, Renault P, and Delorme C (2001) Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol 40: 1227–1239. [DOI] [PubMed] [Google Scholar]
- Handke LD, Shivers RP, and Sonenshein AL (2008) Interaction of Bacillus subtilis CodY with GTP. J Bacteriol 190: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiron A, Borezée-Durant E, Piard JC, and Juillard V (2007) Only one of four oligopeptide transport systems mediates nitrogen nutrition in Staphylococcus aureus. J Bacteriol 189: 5119–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julotok M, Singh AK, Gatto C, and Wilkinson BJ (2010) Influence of fatty acid precursors, including food preservatives, on the growth and fatty acid composition of Listeria monocytogenes at 37 and 10°C. Appl Environ Microbiol 76: 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser JC, Omer S, Sheldon JR, Welch I, and Heinrichs DE (2015) Role of BrnQ1 and BrnQ2 in branched-chain amino acid transport and virulence in Staphylococcus aureus. Infect Immun 83: 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MD, Humphrey BJ, Wang YF, Kourbatova EV, and Ray SM (2006) Emergence of Community-Acquired Methicillin-Resistant Staphylococcus aureus USA300 Clone as the Predominant Cause of Skin and Soft-Tissue Infections. Ann Intern Med 144: 309–318. [DOI] [PubMed] [Google Scholar]
- Kiser KB, Cantey-Kiser JM, and Lee JC (1999) Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun 67: 5001–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W, Weber MHW, and Mohamed A (1999) Cold shock response of Bacillus subtilis: Isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J Bacteriol 181: 5341–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth BN, Löfdahl S, Betley MJ, O’reilly M, Schlievert PM, Bergdoll MS, et al. (1983) The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305: 709–712. [DOI] [PubMed] [Google Scholar]
- Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, et al. (2014) Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10: e1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei T, Yang J, and Ji Y (2014) Determination of essentiality and regulatory function of staphylococcal YeaZ in branched-chain amino acid biosynthesis. Virulence 6: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, et al. (2005) Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H, Meinken C, Matin A, and Stulke J (2002) Insufficient Expression of the ilv-leu Operon Encoding Enzymes of Branched-Chain Amino Acid Biosynthesis Limits Growth of a Bacillus subtilis ccpA Mutant. J Bacteriol 184: 5174–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, et al. (2010) Direct Targets of CodY in Staphylococcus aureus. J Bacteriol 192: 2861–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerczyk CD, Sadykov MR, Luong TT, Lee C, Somerville GA, and Sonenshein AL (2008) Staphylococcus aureus CodY negatively regulates virulence gene expression. J Bacteriol 190: 2257–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malachowa N, Whitney AR, Kobayashi SD, Sturdevant DE, Kennedy AD, Braughton KR, et al. (2011) Global changes in Staphylococcus aureus gene expression in human blood. PLoS One 6: e18617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei JM, Nourbakhsh F, Ford CW, and Holden DW (1997) Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol 26: 399–407. [DOI] [PubMed] [Google Scholar]
- Mesak LR, Yim G, and Davies J (2009) Improved lux reporters for use in Staphylococcus aureus. Plasmid 61: 182–187. [DOI] [PubMed] [Google Scholar]
- Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, et al. (2005) Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 352: 1445–1453. [DOI] [PubMed] [Google Scholar]
- Mishra NN, Liu GY, Yeaman MR, Nast CC, Proctor RA, McKinnell J, et al. (2011a) Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob Agents Chemother 55: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra NN, McKinnell J, Yeaman MR, Rubio A, Nast CC, Chen L, et al. (2011b) In vitro cross-resistance to daptomycin and host defense cationic antimicrobial peptides in clinical methicillin-resistant Staphylococcus aureus Isolates. Antimicrob. Agents Chemother 55: 4012–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery CP, Boyle-Vavra S, Roux A, Ebine K, Sonenshein AL, and Daum RS (2012) CodY deletion enhances in vivo virulence of community-associated methicillin-resistant Staphylococcus aureus clone USA300. Infect Immun 80: 2382–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue Y, and Mori M (1997) Amino acid requirements for the growth and enterotoxin production by Staphylococcus aureus in chemically defined media. Int J Food Microbiol 36: 77–82. [DOI] [PubMed] [Google Scholar]
- Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, et al. (2009) CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J Bacteriol 191: 2953–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, et al. (2011) The human serum metabolome. PLoS One 6: e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera FE, Miller HK, Kolar SL, Stevens SMJ, and Shaw LN (2012) The impact of CodY on virulence determinant production in community-associated methicillin-resistant Staphylococcus aureus. Proteomics 12: 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario-Cruz Z, Chahal HK, Mike LA, Skaar EP, and Boyd JM (2015) Bacillithiol has a role in Fe-S cluster biogenesis in Staphylococcus aureus. Mol Microbiol 98: 218–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Johnson SR, Song Y, Sirobhushanam S, Tefft R, Gatto C, et al. (2016) Growth-environment dependent modulation of Staphylococcus aureus branched-chain to straight-chain fatty acid ratio and incorporation of unsaturated fatty acids. biorXiv, in press . Available at: 10.1101/047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Sirobhushanam S, Hantak MP, Lawrence P, Brenna JT, Gatto C, et al. (2015) Short branched-chain C6 carboxylic acids result in increased growth, novel “unnatural” fatty acids and increased membrane fluidity in a Listeria monocytogenes branched-chain fatty acid-deficient mutant. Biochim Biophys Acta 1851: 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers RP, and Sonenshein AL (2004) Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol Microbiol 53: 599–611. [DOI] [PubMed] [Google Scholar]
- Singh VK, Hattangady DS, Giotis ES, Singh AK, Chamberlain NR, Stuart MK, et al. (2008) Insertional inactivation of branched-chain alpha-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl Environ Microbiol 74: 5882–5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein AL (2005) CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol 8: 203–207. [DOI] [PubMed] [Google Scholar]
- Stucky K, Hagting A, Klein JR, Matern H, Henrich B, Konings WN, et al. (1995) Cloning and characterization of brnQ, a gene encoding a low-affinity, branched-chain amino acid carrier in Lactobacillus delbrückii subsp lactis DSM7290. Mol Gen Genet 249: 682–690. [DOI] [PubMed] [Google Scholar]
- Sun Y, and O’riordan MXD (2010) Branched-chain fatty acids promote Listeria monocytogenes intracellular infection and virulence. Infect Immun 78: 4667–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wilkinson BJ, Standiford TJ, Akinbi HT, and O’riordan MXD (2012) Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. J Bacteriol 194: 5274–5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapakkam AC, Handke LD, Belitsky BR, Levdikov VM, Wilkinson AJ, and Sonenshein AL (2009) Genetic and biochemical analysis of the interaction of Bacillus subtilis CodY with branched-chain amino acids. J Bacteriol 191: 6865–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters NR, Samuels DJ, Behera RK, Livny J, Rhee KY, Sadykov MR, et al. (2016) A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Mol Microbiol 101: 495–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska A, Widomska J, and Subczynski WK (2006) Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim Pol 53: 475–484. [PubMed] [Google Scholar]
- Zhu K, Bayles DO, Xiong A, Jayaswal RK, and Wilkinson BJ (2005a) Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon-interrupted branched-chain alpha-keto acid dehydrogenase. Microbiology 151: 615–623. [DOI] [PubMed] [Google Scholar]
- Zhu K, Ding X, Julotok M, and Wilkinson BJ (2005b) Exogenous isoleucine and fatty acid shortening ensure the high content of anteiso-C15:0 fatty acid required for low-temperature growth of Listeria monocytogenes. Appl Environ Microbiol 71: 8002–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


