Abstract
Smoking is an important risk factor in the development of heart failure with preserved ejection (HFpEF), and prior reports have identified smoking as a significant predictor of death in this population. However, the relationship between smoking and heart failure-specific outcomes has not been examined in patients with HFpEF. This analysis included 1,717 (mean age=71±10 years; 50% male; 78% white) patients with HFpEF enrolled in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial from the Americas. Smoking was ascertained by self-reported history and was categorized as never, former, or current. Multivariable Cox regression was used to examine the risk of hospitalization for heart failure, death, and cardiovascular death across smoking categories. There were 116 (7%) current, 871 (51%) former, and 729 (42%) never smokers in this analysis. Current smoking was associated with an increased risk for hospitalization for heart failure (never: HR=1.0; former: HR=1.25, 95%CI=0.99–1.57; current: HR=1.68, 95%CI=1.08–2.61), death (never: HR=1.0; former: HR=1.02, 95%CI=0.81–1.29; current: HR=1.82, 95%CI=1.19–2.78), and cardiovascular death (never: HR=1.0; former: HR=1.00, 95%CI=0.74–1.35; current: HR=1.85, 95%CI=1.09–3.24) compared with former or never smokers in a multivariable model adjusted for cardiovascular risk factors. A similar increased risk for hospitalization for heart failure (former: HR=1.0; current: HR=1.54, 95%CI=1.01, 2.36), death (former: HR=1.0; current: HR=1.81, 95%CI=1.19, 2.75), and cardiovascular death (former: HR=1.0; current: HR=1.76, 95%CI=1.04, 2.98) was observed for current smokers when we limited the analysis to those with a history of smoking. In conclusion, current smoking is associated an increased risk for adverse outcomes in HFpEF, including hospitalization for heart failure. Smoking cessation strategies possibly have a role to reduce the risk for adverse cardiovascular outcomes in patients with HFpEF.
Keywords: smoking, heart failure, preserved ejection fraction, outcomes
Current smoking has been identified as an independent risk factor for the development of heart failure with preserved ejection fraction (HFpEF).1 Additionally, cigarette smoking has been clearly identified as an important predictor of death in patients with HFpEF.2,3 Although the influence of cigarette smoking on death in HFpEF is clear, the relationship between smoking and heart failure-specific outcomes, such as hospitalization for heart failure, has not been specifically examined in patients with HFpEF. Due to the fact that HFpEF accounts for over 50% of all heart failure cases,4 a better understanding of the relationship between smoking and HFpEF is needed, as smoking possibly represents an important modifiable risk factor to improve outcomes in this subset of heart failure patients. To address this gap in knowledge, we aimed to examine the association between smoking and heart failure-specific outcomes in patients with HFpEF from the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT).
METHODS
The present study used data from TOPCAT obtained from the National Heart, Lung, and Blood Institute, and the data and study materials have been made available to other researchers. TOPCAT was a multi-center, international randomized, double blind, placebo-control study to examine the efficacy of spironolactone in patients with HFpEF. The design, inclusion criteria, and baseline characteristics of the trial have been published previously.5,6 Briefly, 3,445 patients with symptomatic HFpEF from 270 sites in 6 countries were enrolled between August, 2006 and January, 2012. The primary goal of the trial was to determine if spironolactone was associated with a reduction in the composite outcome of cardiovascular mortality, aborted cardiac arrest, or heart failure hospitalization in patients with HFpEF (e.g., documented ejection fraction ≥45%). In this analysis, we examined the relationship between smoking and the risk of hospitalization for heart failure, death, and cardiovascular death. Due to differences in the baseline characteristics and event rates observed between patients recruited in Russia and Georgia versus the Americas,7 we limited our analysis to TOPCAT patients who were enrolled from the Americas. This current analysis was approved by the institutional review board at Emory University School of Medicine, and subjects gave written informed consent prior to participation in TOPCAT.
Patients who participated in TOPCAT underwent a detailed baseline evaluation.6 Age, sex, race, and cigarette smoking were obtained by self-reported history. Smoking was defined as current, former, or never. Medical history for the following diagnoses were obtained by self-report and medical record review: coronary heart disease, stroke, New York Heart Association Class, and prior heart failure hospitalization. Chronic obstructive pulmonary disease was defined by self-reported history followed by medical record review that included pulmonary function testing and the use of relevant medications. Diabetes was ascertained by self-reported history, medical record review, or by the use of diabetes medications (e.g., insulin and oral hypoglycemic agents). Systolic blood pressure and body mass index were obtained by trained staff and laboratory data included serum creatinine. Medication data included aspirin, statins, and antihypertensive medications (beta blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, calcium channel blockers, diuretics, long-acting nitrates, and other antihypertensive medications).
The adjudication of outcomes in TOPCAT, including detailed descriptions for the clinical end-points, have been described.5,8 This analysis included the following outcomes: hospitalization for heart failure, death, and cardiovascular death. Hospitalization for heart failure was defined as the unexpected presentation to an acute care facility requiring overnight stay with symptoms and physical exam findings consistent with heart failure, and treatment with intravenous vasodilators, inotropes, mechanical fluid removal, or hemodynamic support. Cardiovascular death was defined as death due to one of the following: myocardial infarction, worsening heart failure, sudden death, stroke, pulmonary embolism, death occurring during a cardiovascular-related procedure, or other cardiovascular death. Death included the composite of cardiovascular and non-cardiovascular death.
Participants were categorized based on their history of cigarette smoking: current, former, or never. Baseline characteristics were compared across the aforementioned categories. Categorical variables were reported as frequency and percentage, while continuous variables were recorded as mean ± standard deviation. Statistical significance for categorical variables was tested using the chi-square method and for continuous variables the analysis of variance procedure was used. Follow-up time was defined as the time from randomization until one of the following: outcome of interest, death, loss to follow-up, or end of follow-up. Kaplan-Meier estimates were used to examine the unadjusted cumulative incidence of heart failure hospitalization and differences were compared using the log-rank procedure. Survival probabilities also were computed using the Kaplan-Meier method across smoking categories. Cox regression was used to examine the risk of each outcome associated with each smoking category. Multivariable models were constructed with the following clinically relevant variables: Model 1 adjusted for age, sex, and race; Model 2 adjusted for Model 1 covariates plus systolic blood pressure, serum creatinine, diabetes, chronic obstructive pulmonary disease, body mass index, aspirin, antihypertensive medications, statins, randomization group, New York Heart Association Class, coronary heart disease, and stroke. A secondary analysis was performed in which we compared the risk of each outcome associated with current smoking among those with a history of smoking (referent=former smoker). The proportional hazards assumption was not violated in our analyses. Statistical significance was defined as p <0.05. SAS Version 9.4 (Cary, NC) was used for all analyses.
RESULTS
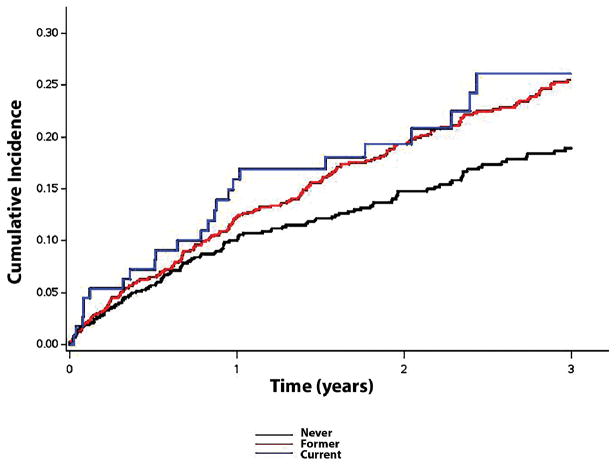
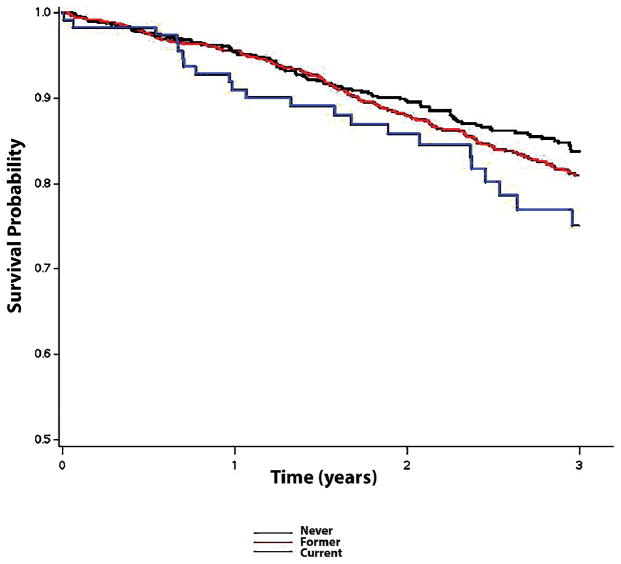
This analysis included 1,717 (mean age=71±10 years; 50% male; 78% white) patients from TOPCAT who were enrolled in the Americas. There were 116 (7%), 871 (51%), and 729 (42%) patients whose smoking status was classified as current, former, or never, respectively. Baseline characteristics across smoker category are shown in Table 1. Over a median follow-up of 2.9 years (25th–75th percentiles=1.9, 4.1 years), a total of 387 hospitalizations for heart failure, 374 deaths, and 218 cardiovascular deaths occurred. The unadjusted cumulative incidence estimates for hospitalization for heart failure (log-rank p=0.0029) and the unadjusted survival probabilities (log-rank p=0.044) across smoking categories are shown in Figures 1 and 2, respectively. After adjustment for several cardiovascular risk factors, current smoking was associated with an increased risk for hospitalization for heart failure, death, and cardiovascular death compared with former or never smokers (Table 2). A similar increased risk for hospitalization for heart failure (former: HR=1.0; current: HR=1.54, 95%CI=1.01, 2.36), death (former: HR=1.0; current: HR=1.81, 95%CI=1.19, 2.75), and cardiovascular death (former: HR=1.0; current: HR=1.76, 95%CI=1.04, 2.98) was observed for current smokers when we limited the analysis to those with a history of smoking.
Table 1.
Baseline Characteristics (N=1,717)
| Characteristics | Smoker | P-value* | ||
|---|---|---|---|---|
|
| ||||
| Never (n=729) | Former (n=872) | Current (n=116) | ||
| Age, mean ± SD (years) | 72±10 | 72±9.2 | 64±8.7 | <0.001 |
| Men | 240 (33%) | 548 (63%) | 69 (59%) | <0.001 |
| White | 549 (75%) | 716 (82%) | 76 (66%) | <0.001 |
| Diabetes mellitus | 310 (43%) | 405 (46%) | 48 (41%) | 0.23 |
| Coronary heart disease | 200 (27%) | 388 (45%) | 45 (39%) | <0.001 |
| Stroke | 58 (8%) | 87 (10%) | 9 (8%) | 0.33 |
| Systolic blood pressure, mean ± SD (mm Hg) | 128±15 | 127±16 | 128±15 | 0.43 |
| Body mass index, mean ± SD (kg/m2) | 34±8.3 | 34±8.1 | 33±8.0 | 0.34 |
| Serum creatinine, mean ± SD (mg/dL) | 1.12±0.32 | 1.20±0.35 | 1.10±0.36 | <0.001 |
| New York Heart Association Class III-IV | 243 (33%) | 333 (38%) | 31 (27%) | 0.017 |
| Prior heart failure hospitalization | 415 (57%) | 518 (59%) | 73 (63%) | 0.37 |
| Chronic obstructive pulmonary disease | 62 (9%) | 178 (20%) | 41 (35%) | <0.001 |
| Aspirin use | 377 (52%) | 555 (64%) | 72 (62%) | <0.001 |
| Statin | 428 (59%) | 618 (71%) | 70 (60%) | <0.001 |
| Spironolactone | 381 (52%) | 427 (49%) | 59 (51%) | 0.42 |
| Beta blockers | 560 (77%) | 706 (81%) | 88 (76%) | 0.092 |
| ACEi/ARB | 579 (79%) | 678 (78%) | 99 (85%) | 0.16 |
| Calcium channel blockers | 272 (37%) | 355 (41%) | 42 (36%) | 0.30 |
| Diuretics | 637 (87%) | 794 (91%) | 97 (84%) | 0.0084 |
| Long-acting nitrate | 114 (16%) | 156 (18%) | 24 (21%) | 0.28 |
| Other antihypertensive medications | 104 (14%) | 158 (18%) | 22 (19%) | 0.089 |
Statistical significance for continuous data was tested using the analysis of variance and categorical data was tested using the chi-square test.
ACEi/ARB= angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; SD=standard deviation.
Figure 1.
Unadjusted Cumulative Incidence of Hospitalization for Heart Failure across Smoking Categories*
*The cumulative incidence curves of hospitalization for heart failure were statistically different across smoking categories (log-rank p=0.0029).
Figure 2.
Unadjusted Kaplan-Meier Survival Probability across Smoking Categories*
*The survival curves were statistically different across smoking categories (log-rank p=0.044).
Table 2.
Risk of Hospitalization for Heart Failure, Death, and Cardiovascular Death across Smoking Categories (N=1,717)
| Outcome | Events/No. at risk | Model 1* HR (95%CI) |
P-value | Model 2† HR (95%CI) |
P-value |
|---|---|---|---|---|---|
|
| |||||
| Hospitalization for Heart Failure | |||||
| Never smoker | 135/729 | Ref | - | Ref | - |
| Former smoker | 225/872 | 1.44 (1.15, 1.80) | 0.0012 | 1.25 (0.99, 1.57) | 0.053 |
| Current smoker | 27/116 | 1.55 (1.01, 2.38) | 0.044 | 1.68 (1.08, 2.61) | 0.020 |
|
| |||||
| Death | |||||
| Never smoker | 142/729 | Ref | - | Ref | - |
| Former smoker | 202/872 | 1.10 (0.88, 1.37) | 0.42 | 1.02 (0.81, 1.29) | 0.85 |
| Current smoker | 30/116 | 1.96 (1.30, 2.97) | 0.0014 | 1.82 (1.19, 2.78) | 0.0057 |
|
| |||||
| Cardiovascular Death | |||||
| Never smoker | 85/729 | Ref | - | Ref | - |
| Former smoker | 113/872 | 1.03 (0.77, 1.38) | 0.85 | 1.00 (0.74, 1.35) | 0.99 |
| Current smoker | 20/116 | 2.06 (1.23, 3.44) | 0.0058 | 1.85 (1.09, 3.14) | 0.022 |
Adjusted for age, sex, and race.
Adjusted for Model 1 covariates plus systolic blood pressure, serum creatinine, diabetes, chronic obstructive pulmonary disease, body mass index, aspirin, antihypertensive medications, statins, randomization group, New York Heart Association Class, coronary heart disease, and stroke.
CI=confidence interval; HR=hazard ratio.
DISCUSSION
In this analysis from TOPCAT, current cigarette use was associated with an increased risk of hospitalization, hospitalization for heart failure, death, and cardiovascular death in patients with HFpEF. Overall, the findings of this analysis have identified current smoking as an important comorbid condition in patients who have HFpEF, and suggest that smoking cessation strategies are beneficial to improve outcomes in this subset of heart failure patients.
Recent reports have demonstrated that smoking is associated with an increased risk of death in patients with HFpEF. An examination of registry data consisting of HFpEF patients who were admitted with heart failure symptoms in Singapore demonstrated that smoking was associated with an increased risk of death after 2 years of follow-up.2 Additionally, data from the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) study found smoking to be significantly associated with mortality in patients with HFpEF.3 Our data confirm that smoking is associated with an increased risk for death, and also extend the negative outcomes associated with smoking to include hospitalization for heart failure and cardiovascular death. Also, we observed that the risk for these outcomes was limited to patients who reported the current use of cigarettes. Therefore, the findings presented in this study suggest that ongoing cigarette smoking is an important modifiable risk factor to reduce adverse cardiovascular outcomes in patients with HFpEF, and we address an important gap in the literature regarding the detrimental influence of cigarette smoking on heart failure-specific outcomes in HFpEF.
There are several pathophysiological mechanisms that link cigarette use with adverse cardiovascular events. Active smoking is associated with structural and functional myocardial changes, such as increased left ventricular mass and diastolic dysfunction.9,10 Increased oxidative stress and inflammation have been proposed as mediators for these structural changes and subsequent development of HFpEF.11,12 Furthermore, current cigarette smoking leads to hemodynamic changes that possibly precipitate adverse events in HFpEF patients, such as increased myocardial oxygen demand and vasoconstriction.13,14 Tobacco exposure also is associated with subclinical myocardial injury that over time possibly leads to decompensation.15 Although we provide several explanations for the observed findings, additional studies are necessary to fully elucidate the underlying mechanisms that explain the increased risk for adverse events associated with current smoking in patients with HFpEF.
Cigarette smoking has important implications for the practicing physician, as it has repeatedly been linked to serious comorbidities and a higher risk of death across multiple medical conditions.11 With the rising prevalence of HFpEF, and a lack of effective disease modifying therapies, smoking cessation possibly represents an important component of HFpEF management to reduce morbidity and mortality in this high-risk group.16 Additionally, smoking cessation may have a role to reduce hospitalization for acute decompensated heart failure. However, current guidelines do not specifically emphasize smoking cessation in the management of HFpEF.17 The data in this report suggest that current smoking represents a serious modifiable risk factor in HFpEF, and further study is needed to determine if smoking cessation strategies are able to reduce heart failure-specific outcomes in this important subset of cardiovascular patients.
Our analysis has several limitations that merit attention. Smoking history was self-reported and subjected our analysis to recall bias. Smoking was assessed at a single point in time and it is possible that our results will vary with repeat assessment for cigarette use. Additionally, we were unable to account for the type of cigarettes smoked, and this data may influence our findings. We also acknowledge that the main aim of TOPCAT was not to investigate the role of cigarette smoking in patients with HFpEF, and our hypothesis regarding smoking cessation measures to improve outcomes in HFpEF is speculative. We further acknowledge that further studies, including clinical trials, are needed to appropriately investigate the role of cigarette smoking, and cessation strategies, in patients who have HFpEF. Lastly, current smokers possibly dropped out of the study sooner and more frequently than non-smokers, resulting in informative censoring bias.
In conclusion, we have demonstrated that current smoking is an important modifiable risk factor for future cardiovascular events in HFpEF. Further investigation is needed to confirm our findings, and to determine if smoking cessation strategies are able to improve heart failure-specific outcomes in this high-risk subgroup of heart failure patients.
Acknowledgments
FUNDING
Pratik B. Sandesara is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA). Wesley T. O’Neal is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under award number F32-HL134290. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This Manuscript was prepared using Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist Trial (TOPCAT) Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT Study or the National Heart, Lung, and Blood Institute.
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yap J, Sim D, Lim CP, Chia SY, Go YY, Jaufeerally FR, Sim LL, Liew R, Ching CK. Predictors of two-year mortality in Asian patients with heart failure and preserved ejection fraction. Int J Cardiol. 2015;183:33–38. doi: 10.1016/j.ijcard.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 3.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN Meta-Analysis Global Group in Chronic Heart F. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 4.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972e910. doi: 10.1016/j.ahj.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Shah SJ, Heitner JF, Sweitzer NK, Anand IS, Kim HY, Harty B, Boineau R, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Lewis EF, Markov V, O’Meara E, Kobulia B, Shaburishvili T, Solomon SD, Pitt B, Pfeffer MA, Li R. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–192. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 8.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 9.Nadruz W, Jr, Claggett B, Goncalves A, Querejeta-Roca G, Fernandes-Silva MM, Shah AM, Cheng S, Tanaka H, Heiss G, Kitzman DW, Solomon SD. Smoking and Cardiac Structure and Function in the Elderly: The ARIC Study (Atherosclerosis Risk in Communities) Circ Cardiovasc Imaging. 2016;9:e004950. doi: 10.1161/CIRCIMAGING.116.004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 12.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, Smith AL, Bauer DC, Newman AB, Kim L, Bibbins-Domingo K, Tindle H, Harris TB, Tang WW, Kritchevsky SB, Butler J. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J. 2012;164:236–242. doi: 10.1016/j.ahj.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinowitz BD, Thorp K, Huber GL, Abelmann WH. Acute hemodynamic effects of cigarette smoking in man assessed by systolic time intervals and echocardiography. Circulation. 1979;60:752–760. doi: 10.1161/01.cir.60.4.752. [DOI] [PubMed] [Google Scholar]
- 14.Suskin N, Sheth T, Negassa A, Yusuf S. Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;37:1677–1682. doi: 10.1016/s0735-1097(01)01195-0. [DOI] [PubMed] [Google Scholar]
- 15.Ali M, Li Y, O’Neal WT, Soliman EZ. Tobacco Exposure as Determined by Serum Cotinine and Subclinical Myocardial Injury in Individuals Free from Cardiovascular Disease. Am J Cardiol. 2017;120:1114–1117. doi: 10.1016/j.amjcard.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Raina A, Kanwar M. New drugs and devices in the pipeline for heart failure with reduced ejection fraction versus heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;11:374–381. doi: 10.1007/s11897-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]