Abstract
Childhood absence epilepsy (CAE) is the most common pediatric epilepsy syndrome and is characterized by typical absence seizures (AS). AS are non-convulsive epileptic seizures characterized by a sudden loss of awareness and bilaterally generalized synchronous 2.5–4 Hz spike and slow-wave discharges (SWD). Gamma butyrolactone (GBL) is an acute pharmacological model of AS and induces bilaterally synchronous SWDs and behavioral arrest. Despite the long use of this model, little is known about its strain and sex-dependent features. We compared the dose-response profile of GBL-evoked SWDs in three rat strains (Long Evans, Sprague-Dawley, and Wistar), and examined the modulatory effects of estrous cycle on SWDs in female Wistar rats. We evaluated the number of seizures, the cumulative time seizing, and the average seizure duration as a function of dose, strain, and sex/estrous phase. Long Evans rats displayed the greatest sensitivity to GBL, followed by Wistar rats, and then by Sprague-Dawley rats. GBL-evoked SWDs were modulated by estrous cycle in female rats, with the lowest sensitivity to GBL occurring during metestrus. Wistar rats showed the greatest variability as a function of dose, and the least variability within dose; these features make this strain desirable for interventional studies. Moreover, our finding that the SWD response to GBL differs as a function of estrous cycle underscores the importance of cycle monitoring in studies examining female animals using this model. Together, these strain and sex-dependent findings provide guidance for future studies.
Keywords: GHB, GBL, Sex differences, estrous cycle, Epilepsy, Generalized Spike-and-Wave discharge, Typical Absence Seizures, Reticular Thalamic Nucleus
1. Introduction
Childhood absence epilepsy (CAE) is the most common pediatric epilepsy syndrome (Jallon et al., 2001) and has a typical onset between 3 and 8 years of age. The prevalence of CAE is ~15–20% among children with epilepsy; of these, 20–70% of cases resolve, often around adolescence (Panayiotopoulos, 2001). CAE, in combination with Juvenile absence epilepsy, which has a later onset (i.e., 10–17 years of age), have a combined incidence of ~100 per 100,000 person-years (Olsson, 1988).
Typical absence seizures (AS) are non-convulsive epileptic seizures characterized by a sudden loss of awareness; multiple daily seizures are commonly reported (Crunelli and Leresche, 2002). The electrographic hallmark of AS is bilaterally generalized synchronous 2.5–4 Hz spike and slow-wave discharges (SWD) (Berg et al., 2009). These oscillations originate in a corticothalamocortical network (Crunelli and Leresche, 2002; Sitnikova, 2010; Sitnikova and van Luijtelaar, 2009), critically engaging intrathalamic connections in the reticular thalamic nucleus and thalamocortical cells in the ventrobasal thalamus (Sorokin et al., 2017; Tancredi et al., 2000). First-line pharmacotherapies for absence seizures include ethosuximide and valproate, both of which disrupt absence seizures through blockade of T-Type calcium channels (Glauser et al., 2010).
Both genetic (e.g., Genetic Absence Epilepsy Rats from Strasbourg [GAERS], Wistar Albino Glaxo/Rij [WAG/Rij]) and pharmacological models of AS have proven fruitful in understanding the fundamental mechanisms underlying AS (Akman et al., 2010a; D’Antuono et al., 2005). Gamma butyrolactone (GBL), in particular, has been widely used as a pharmacological model of AS (Snead, 1992a; Venzi et al., 2015). GBL induces AS across species, including mouse, rat, non-human primate, and humans (although see (Venzi et al., 2015) for caveats regarding this model in mice). GBL is biologically inactive (Snead, 1991) but is rapidly converted into gamma-hydroxybutyric acid (GHB) by active lactonases present in serum and liver (Roth et al., 1967). Systemic administration of GBL reliably meets all criteria of an absence seizure model in rats (Snead, 1992a, 1988, 1984) and produces electrographic and behavioral events similar to human absence seizures (Cortez et al., 2016; Crunelli and Leresche, 2002).
GBL-evoked ASs are sensitive to canonical anti-absence drugs and are exacerbated by drugs that worsen ASs in humans, underscoring the predictive validity of this model (Snead, 1978; Venzi et al., 2015). The neural mechanisms of GBL action include activation of both the γ-aminobutyric acid type B (GABAB) receptor and a GHB-specific receptor (Emri et al., 1996). Despite the extensive use of this model, sex-specific features remain uncharacterized. This is of interest because absence epilepsies have a 2–5 times greater incidence in females as compared to males (Christensen et al., 2005; Waaler et al., 2000). Moreover, ovarian hormones regulate both the severity of atypical absence seizures and GABAB receptor binding throughout estrous cycle (Persad et al., 2004). However, there is a comparable incidence of SWDs among males and females from inbreed GAERS and WAG/Rij strains (Coenen and Van Luijtelaar, 1987; van Luijtelaar et al., 2014). Ideally, a model mirroring the clinical population should show a divergence in response between female and male animals.
In addition to poverty of information regarding sex differences in the GBL model, little is known regarding strain differences. The model was originally characterized in Sprague-Dawley rats, and has been used to a lesser extent in Wistar and Long Evans rats. These strains differ in the expression of spontaneous absence-like discharges (Cortez et al., 2001; Pinault et al., 2001), as well as other behavioral (Turner and Burne, 2014), pharmacological (Woolfolk and Holtzman, 1995) and epilepsy-related phenotypes (Löscher et al., 2017; Twele et al., 2016). With the increasing availability of transgenic rat strains, background strain influences on phenotypes have become a more pressing consideration. Transgenic strains on each of these backgrounds have been developed, and the feasibility of the multi-generation backcrosses needed to homogenize their backgrounds remains out of reach for most laboratories. Thus, determining strain-dependent effects in this model may enhance future study design using diverse background strains.
To address these gaps, we compared the dose-response profile of GBL evoked SWDs in three strains of rats (Long Evans, Sprague-Dawley and Wistar), and examined the modulatory effects of estrous cycle on SWDs in female Wistar rats.
2. Materials and Methods
2.1. Animals
Experiments were performed on male Long Evans (LE, Charles River, n=6), Sprague-Dawley (SD, Envigo, n=8), and Wistar rats (WIS, Envigo, n=9). After completing the dose-response studies in male rats, we selected a single strain (Wistar) to investigate estrous cycle effects in female rats (Envigo, n=10), as this strain displayed the greatest variability as a function of dose in males. All rats were 6–8 weeks of age at the time of surgery. The animals were housed in the Georgetown University Division of Comparative Medicine under environmentally controlled conditions (12 hr light/dark cycle, lights on between 6:00 A.M. and 6:00 P.M.; ambient temperature 23°C ± 1°C) with food (Lab Diet, #5001) and water provided ab libitum. All experiments were performed during the light phase. Experimental procedures were performed in compliance with Association for Assessment and Accreditation of Laboratory Animal Care standards, the Guide for the Care and Use of Laboratory Animals (National Research Council (U.S.) et al., 2011), and were approved by the Georgetown University Animal Care and Use Committee.
2.2. Estrous Cycle Monitoring
For female rats, estrous cycle stages were assessed by taking vaginal smears from each animal in the morning of each experimental day (between 8 and 9 a.m.). The smears were transferred to a clean slide and then visualized by light microscopy. The phase of the estrous cycle was estimated on the basis of the predominant cell type: proestrus - large round, usually nucleated cells; estrus - masses of the anucleated cornified (keratinized) irregularly squamous epithelial cells; metestrus - round nucleated epithelial cells with infiltration of leukocytes; and diestrus - predomination of leukocytes. All the experiments were conducted before 1 p.m., to avoid possible changes of the cycle phase or hormonal interference. Each animal was tested at each phase of the estrous cycle. We cannot rule out the possibility that stress associated with repeated handling or vaginal smears altered the cycle rhythmicity. However, suggesting that our staging was accurate, and that stressors did not alter the cycle in an obvious manner, the stage of the cycle varied by the expected phase when animals were checked intermittently over the course of several weeks.
2.3. Surgery and Electrode Implantation
Surgeries were performed as previously described (Dunn et al., 2018). Briefly, rats were anesthetized with equithesin (2.8 mg/kg, i.p.) and placed into a stereotaxic frame (Kopf, Tujunga, CA). Five epidural EEG screw electrodes were implanted through holes in the skull. Electrodes were placed over the frontal and parietal cortices; frontal electrodes were placed just posterior to coronal suture and just lateral to the sagittal suture, parietal electrodes were placed ~2mm anterior to the lambdoidal suture at the approximate midpoint of the parietal bone. A reference/ground electrode was placed over the cerebellum. EEG wires were routed into a plastic pedestal (PlasticsOne, Roanoke, VA) and held in place with dental acrylic. During the recordings, the animals were awake and tethered to an EEG preamplifier and amplifier (Pinnacle Technologies, Lawrence, KS). Data were recorded using LabChart 8 (AD instruments, Colorado Spring, CO) with a 60 Hz low pass filter. Electrographic traces are derived from frontal and parietal leads referenced to the contralateral cortex. Recordings were 30 min in duration for all animals (both sexes and all strains), and all recordings fell within the first half of the light-dark period.
Four male rats of each strain were also implanted with a pair of stainless steel electrodes (PlasticsOne) in the left thalamic reticular nucleus (AP −1.8; ML 2.6; DV 6.0). At the conclusion of experiments, these animals were overdosed with pentobarbital based euthanasia solution, and perfused transcardially with saline followed by 4% paraformaldehyde. Brains were post-fixed for 24–72 hours, cryoprotected, frozen, and sectioned. Tissue were stained using thionin, as previously described (West et al., 2012). Only rats with histologically confirmed placement of depth electrodes in the reticular nucleus were used for depth EEG analysis.
2.4. Drugs
Gamma-Butyrolactone (GBL, Sigma) was diluted in 0.9% saline at a concentration of 100 mg/ml and administered via intraperitoneal (i.p.) injection. Animals were monitored for the occurrence of spike-and-wave discharge (SWDs) activity for 30 min after the time of injection. Four doses were tested, 50, 75, 100 and 200 mg/kg with the interval of at least 1 day (but typically 2–4 days) between tests. The dose range was selected on the basis of effective doses in prior studies using this compound (Snead, 1991, 1988; Soper et al., 2016). The dosing order was randomized across animals. In both blood and brain, GHB (the active metabolite of GBL) levels rise within minutes after drug administration, followed by a rapid redistribution phase. Moreover, GBL displays non-linear pharmacokinetics with saturation (zero-order) kinetics evident at high concentrations (Giarman and Roth, 1964; Lettieri and Fung, 1979; R. H. Roth and Giarman, 1965; Robert H. Roth and Giarman, 1965). While estimating the precise half-time in our studies is not possible from published data, it is worth noting that the duration of time in which SWDs are observed even after the highest doses of GBL we used does not exceed 1 hour. Moreover, even at doses 2–10 times those we employed, behavioral effects only persist ~2 hr after administration (c.f., (McCabe et al., 1971). Thus, even with an intra-dose interval of 24h, drug accumulation is not expected to be a concern.
The use of a repeated measure design minimized the number of animals used for this study, consistent with ARRIVE guidelines. We cannot rule out the possibility that repeated administration of GBL resulted in neuroplastic changes. Indeed, after continuous administration of GHB prodrugs can produce dependence in baboons ((Goodwin et al., 2013), and reduced sleep-time after high dose GBL in rodents has been reported after twice daily injections ((Van Sassenbroeck et al., 2003)). Alterations in GABAA receptor subunit levels in the thalamus have been reported in the hours following GHB-evoked seizures, but these return to baseline levels within 24h; moreover, the total duration of GHB-evoked seizures is decreased when a second dose is given 6, but not 24 h after an initial dose (Banerjee et al., 1998). Thus, while tolerance has been reported after repeated high-dose GBL, the doses and intervals we selected likely avoid this effect.
2.5. EEG analysis
SWDs were assessed offline using LabChart 8 by a dose- and strain- blind observer; for analysis of data from females, the observer was also blinded to estrous cycle phase. The signal was filtered (band pass 2–50Hz) and SWDs were differentiated from normal activity, including sleep spindles (6–12Hz) based on amplitude: SWDs showed peak-to-peak amplitude that was at least double the background activity with a typical crescendo-decrescendo pattern. SWDs were only counted as ASs if they were visible on all EEG leads. While the morphology of the SWDs differed slightly across strains, these criterion were effective in each strain. We quantified the GBL effect in terms of cumulative duration of SWDs per 30 min epoch after GBL administration. EEG recordings was analyzed for every dose tested. Mean EEG power frequency during SWDs was calculated using LabChart 8. We quantified the mean power for each SWD observed. This was averaged within-subject to produce a single value for each rat at each dose tested. Cross-correlation and event-triggered spectral analyses were performed using NeuroExplorer (Version 5, Plexon), and were derived from a minimum of 20 events in 2–3 subjects per strain. Only animals with electrodes correctly positioned in the nRT were used for the cross correlation and event-triggered spectral analyses.
2.6. Hypnotic State
High dose GBL has been reported to induce a hypnotic state characterized by the emergence of continuous slow-wave EEG activity (3 to 5Hz); this deep hypnotic state is characterized behaviorally by loss of posture and righting reflex (Godschalk et al., 1977; Snead, 1984; Venzi et al., 2015). We calculated the duration of predominate slow-wave activity as a metric of hypnosis following high-dose GBL.
2.7. Statistical and Data analysis
Data were analyzed using GraphPad Prism version 6 (GraphPad Inc., La Jolla, CA) and SPSS version 25 (IBM). For males, data were analyzed by two-way analysis of variance with strain as a between subjects factor and dose as a within subjects factor. For females, both dose and estrous phase was analyzed as a within subjects factor. For analysis of duration of hypnotic state, data were not normally distributed and thus analyzed by Kruskal-Wallis test. Pairwise comparisons were conducted following the finding of a significant main effect or interaction and were subject to Holm-Sidak correction for familywise error rate. Linear trend analysis was conducted as a post-test following repeated measures ANOVA in GraphPad Prism following the method of Sheskin (Sheskin, 2011). P values less than 0.05 were considered to be statistically significant.
3. Results
3.1. Strain- and Dose- Dependent Effects
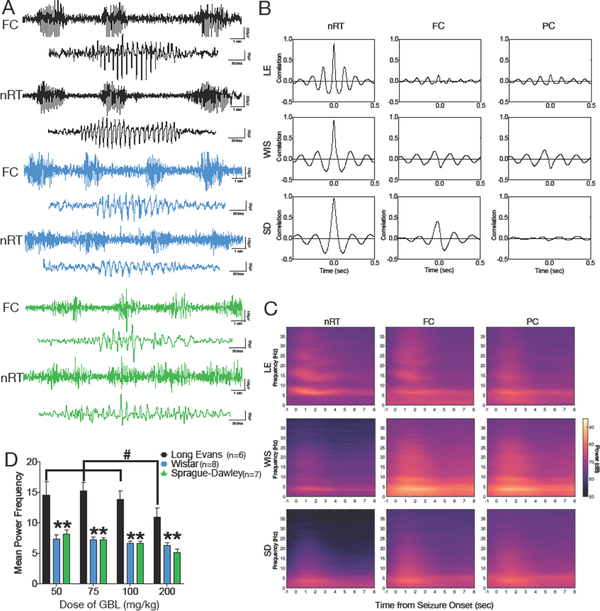
Here we examined the dose dependent effect of systemic administration of GBL on evoked SWDs in adult rats of the Long Evans, Wistar, and Sprague-Dawley strains. Following the GBL injection, bursts of bilaterally synchronous spike-and-wave discharges (SWDs) were evident in EEG recorded from the frontal and parietal cortices, as well as in depth electrodes located in the thalamic reticular nucleus. The localization of electrodes is shown in Supplemental Figure 1. The first episodes of SWD occurred within 3–5 min of GBL administration and lasted for the duration of the observation window (30 min). Representative EEGs for each strain are shown in Fig 1A. Sudden behavioral arrest (cessation of ongoing activity) and vibrissa twitching were evident for the duration of the EEG paroxysm. At the conclusion of the electrographic discharge, normal motor behavior resumed. Cross-correlation analyses revealed synchronized activity across thalamic and cortical leads in all strains (Figure 1B). Peri-event spectrograms, derived from a minimum of 20 discharges per subject, with 2–3 rats averaged per strain, indicated an elevation in thalamic EEG power and an elevation in cortical EEG power (Figure 1C). Spectral characteristics of the discharges surprisingly differed across strains (Figure 1D): Wistar and Sprague-Dawley rats displayed peak power in the 5–7 Hz range, whereas Long Evans displayed peak power in the 10–15 Hz range. ANOVA revealed a main effect of strain (F (2, 13) = 25.9, P=0.00003), a significant main effect of dose (F (3, 39) = 7.547, P=0.0004), but no dose-by-strain interaction (F (6, 39) = 1.33, P=0.27). The strain effect was due to significantly higher mean power frequency in Long Evans as compared to Wistar (P=0.000005) and Sprague-Dawley (P=0.0001) strains. The dose effect was due to significant reduction in mean power frequency in Long Evans rats treated with the highest dose of GBL (200 mg/kg); mean power frequency for each of the lower doses differed significantly from that following the 200 mg/kg dose (50 vs 200: p=0.007; 75 vs 200: p=0.001; 100 vs. 200: p=0.03, Holm-Sidak corrected post-tests). The analyses in Figure 1D represent the average of all events recorded from the frontal cortical lead of each rat; mean power was averaged within subject and the single subject values were then averaged to produce group means.
Figure 1. Electrographic characterization of Spike-and-Wave Discharges across strain.
(A) Representative electrographic recordings from Long Evans (LE, black, top), Wistar (WIS, blue, middle) and Sprague-Dawley (SD, green, bottom) strains. Recordings show both cortical and thalamic discharge during SWDs. (B) Cross-correlation between EEG channels shows, indicating a high correlation of activity between thalamic and cortical sites in each strain. (C) Event-triggered power spectra for each strain. (D) Mean power frequency as a function of GBL dose across strains. # indicates significantly greater than the 200 mg/kg dose within the Long Evans strain; * indicates significant difference from the Long Evans strain within each dose (P<0.05). FC = frontal cortex, nRT = thalamic reticular nucleus, PC = parietal cortex
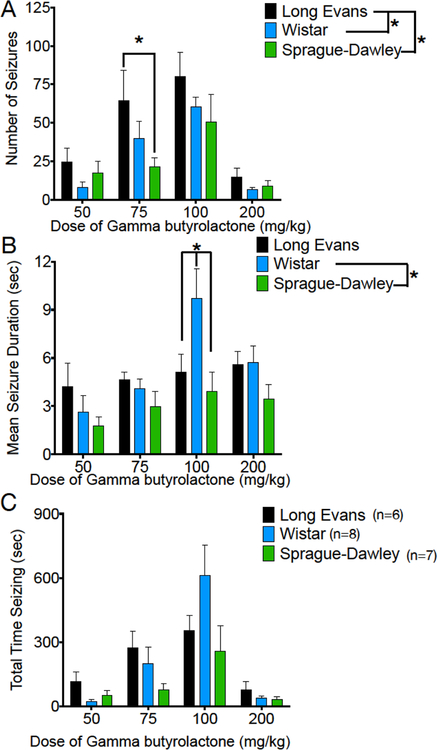
All the doses tested led to the emergence of SWDs and behavior arrest in all three strains (Figure 2). To quantify this response, we measured the number of SWDs, the cumulative time spent displaying SWDs, and the average SWD duration as a function of dose (Figure 2A–C, respectively). With respect to the number of SWDs (Figure 2A), we found a main effect of dose (F (3, 54) = 18.17, P=0.00000003), a main effect of strain (F (2, 18) = 4.992, P=0.019) but no strain-by-dose interaction (F (6, 54) = 0.8579, P=0.5). The strain effect was driven by the significantly greater number of SWDs in Long Evans strain compared to the other two strains (simple effect of strain, Long Evans vs Sprague-Dawley: P=0.02; Long Evans vs Wistar: P=0.045; Holm-Sidak corrected). In all strains, we noted a decrease in SWD number at the highest dose tested, likely due to the emergence of a hypnotic state (see Figure 5). Strain-dependent (F (2, 18) = 3.99, P=0.04) and dose-dependent (F (3, 54) = 6.45, P=0.0008) effects, as well as a strain-by-dose interaction F (6, 54) = 2.35, P=0.04) were also apparent for average SWD duration (Figure 2B). This was driven by significantly longer SWD durations in the Wistar strain as compared to the Sprague-Dawley strain (simple effect of strain, P=0.03; Holm-Sidak corrected), which was particularly evident at the 100 mg/kg dose of GBL (P=0.006).
Figure 2. GBL induces dose- and strain-dependent Spike-and-Wave discharges.
(A) Number of SWDs detected during the observation period as a function of strain and dose, (B) mean duration of individual SWDs as a function of strain and dose, (C) total time displaying SWDs (number × mean duration) as a function of strain and dose. * = significantly different, P<0.05. Figures show means and standard errors of the mean.
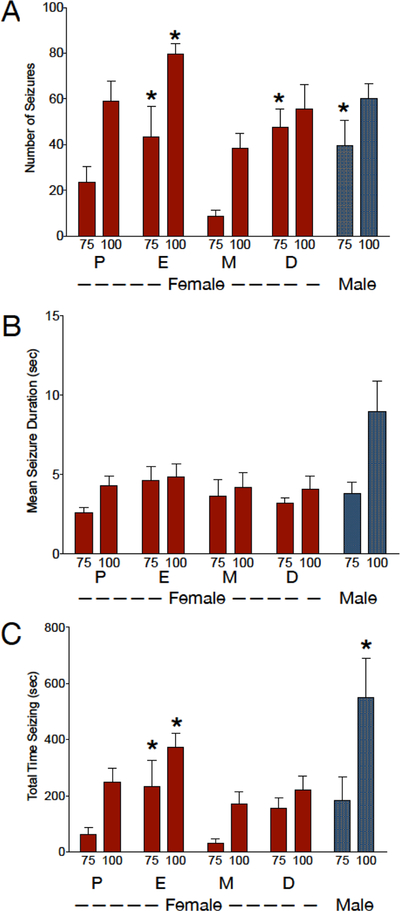
Figure 5. Sex- and Estrous Cycle Dependent effects of GBL in Wistar rats.
(A) Number of SWDs detected during the observation period as a function of estrous cycle phase and dose, (B) mean duration of individual SWDs as a function of estrous cycle phase and dose, (C) total time displaying SWDs (number × mean duration) as a function of estrous cycle phase and dose. * = significantly different from metestrus, P<0.05. Figures show means and standard errors of the mean. P = Proestrus, E = Estrus, M = Metestrus, D = Diestrus
Despite these differences in characteristics of individual SWDs, there was only a marginal main effect of strain on the cumulative parameter, total time displaying SWDs (F (2, 18) = 3.406; P=0.06; Fig 2C). As with the other parameters, there was a main effect of dose (F (3, 54) = 15.7, P=0.0000002) on total time displaying SWDs. In all three strains, total duration increased as a function of dose until decreasing at the highest dose; as with number of SWDs, the decrease following 200 mg/kg of GBL may be due to the emergence of a hypnotic state (Figure 4). When we analyzed the lowest three doses collapsed across strain (repeated measures ANOVA, effect of dose F(1.3,26)=13.1, P=0.0006), a significant linear trend of increasing cumulative SWD duration as a function of increasing dose was evident (R2=0.300, P=0.00001). The general profile observed was a right shifted dose-response for Long Evans as compared to the other strains (number of SWDs), and a striking increase in length of SWDs in Wistar rats. This trend was also evident within Long Evans (R2=0.303, P=0.048) and Wistar (R2=0.481, P=0.0005) rats, but only approached significance in Sprague-Dawley rats (R2=0.175, P=0.076).
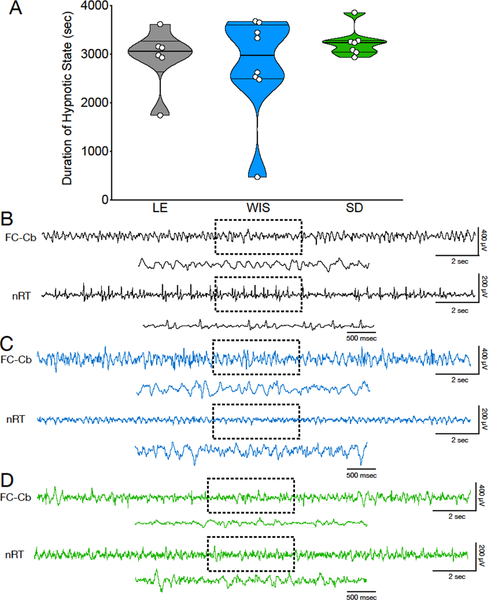
Figure 4. Hypnotic state induced by 200 mg/kg GBL as a function of strain.
(A) Violin plots showing duration of hypnotic state across strains. (B-D) electrographic pattern of hypnotic state in Long Evans (LE; Panel B), Wistar (WIS; Panel C) and Sprague-Dawley (SD; Panel C) strains.
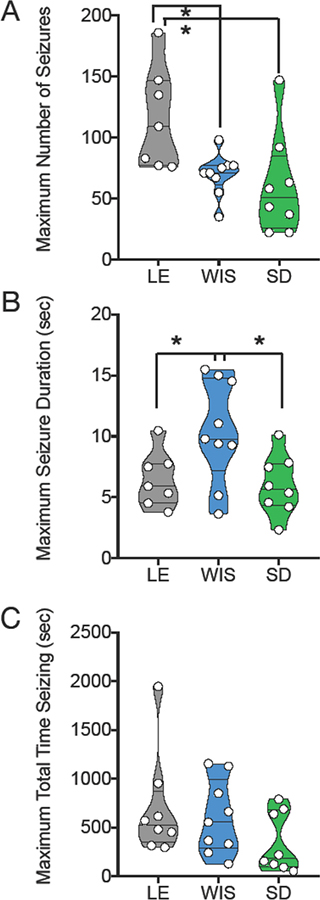
Strain differences were further underscored by analysis of the within-subject maximum response (Figure 3). For this analysis, we calculated the maximum number of SWDs, maximum mean SWD duration, and maximum total time displaying SWDs across doses on a within-subject basis. The maximum number of SWDs differed significantly across strain (F(2,21)=5.5, P=0.01), an effect driven by the significantly greater number of SWDs in the Long Evans, as compared to Wistar and Sprague-Dawley strains (Holm-Sidak corrected Ps = 0.028 and 0.016, respectively). Consistent with our observation in Fig 2 that Wistar animals display longer SWDs, maximum SWD duration likewise differed across strains (F (2,21) = 4.8, P=0.02), an effect driven by the increased duration in Wistar as compared to Long Evans and Sprague-Dawley strains (Holm-Sidak corrected Ps = 0.048 and 0.030, respectively).
Figure 3. Strain effects with maximum response collapsed across dose.
Violin plots showing (A) Number of SWDs detected during the observation period, (B) mean duration of individual SWDs, (C) total time displaying SWDs (number × mean duration). Circles indicate individual subjects. Data for this figure represents the maximum within subject response for each parameter. * = significantly different, P<0.05. LE = Long Evans, WIS = Wistar, SD = Sprague-Dawley
We evaluated the duration of hypnotic state generated by high dose GBL (200 mg/kg; Figure 4A). High dose GBL (200 mg/kg) has been previously reported to induce SWD that gradually evolved into a continuous hypersynchronous state at with a lower mean power frequency (3–5 Hz) (Godschalk et al., 1977; Snead, 1992a, 1992b). During these states, animals remain frozen with their eyes open, displaying decreased muscle tone. Electrographically, this state is characterized by the emergence of high amplitude slow-wave activity resembling that seen during slow wave sleep. This electrographic pattern is quite different than the “spindle-like” pattern seen during spike-and-wave discharges; i.e., there is no spike component (Venzi et al., 2015). This state often lasts for several minutes with absence of movement and the presence of continuous low irregular EEG frequency (Figure 4B). Duration of hypnotic state did not differ as a function of strain (Kruskal-Wallis test, H=0.91, P=0.7).
3.2. Sex differences and impact of the estrous cycle
To determine if the estrous cycle affect GBL-evoked SWDs, we evaluated GBL responses (75 and 100 mg/kg) in female Wistar rats across estrous phases (Figure 5). With respect to SWD count, we found a significant main effect of both dose (F (1, 4) = 19.7, P=0.01) and estrous cycle phase (F (3, 12) = 7.4, P=0.005), but no dose-by-estrous phase interaction (F (3, 12) = 2.1, P=0.2). This was driven by the significantly lower number of SWD counts during metestrus compared to estrus and diestrus (P=0.01 and P=0.007, respectively; Holm-Sidak corrected). We compared the response for each phase of the estrous cycle to the response in males. We only detected a main effect of sex when comparing males to females during metestrus (F (1, 11) = 11.8, P=0.006), this was driven by a lower number of SWDs in females as compared to males, with the 75 mg/kg dose of GBL (P<0.05, Holm-Sidak corrected). There was no effect of sex when comparing males to females during proestrus [F(1,11)=1.03, P=0.33], diestrus [F(1,11)=0.027, P=0.9], or estrus [F(1,11)=1.44, P=0.25].
When we evaluated mean SWD duration, we found a main effect of dose (F (1, 4) = 14.2, P=0.02), but neither an effect of estrous phase (F (3, 12) = 1.7, P=0.2) nor a dose-by-estrous phase interaction (F (3, 12) = 0.63, P=0.6). Females did not differ from males at any point in the estrus cycle (proestrus [F(1,11)=4.8, P=0.052], diestrus [F(1,11)=3.99, P=0.07], metestrus [F(1,11)=2.9, P=0.12], or estrus [F(1,11)=1.39, P=0.26].
Finally, we evaluated total time spent displaying SWDs. We found a main effect of dose (F (1, 4) = 59.2, P=0.002), a main effect of estrous phase (F (3, 12) = 5.3, P=0.02), but no dose-by-estrous phase interaction (F (3, 12) = 0.69, P=0.6). The main effect of estrous phase was driven by a significantly shorter time spent displaying SWDs in metestrus compared to estrus (P=0.03; Holm-Sidak corrected). Males differed from females only during the metestrus phase (F (1, 11) = 5.8, P=0.03), an effect evident at the 100 mg/kg dose (P=0.03; Holm-Sidak corrected).
4. Discussion
Our data demonstrate that the response to GBL differs as a function of rat strain, estrous cycle, and sex. Long Evans rats displayed enhanced sensitivity to GBL, with a greater number of induced SWDs (absence seizures) than Sprague-Dawley and Wistar rats at similar doses. In addition, SWDs in Long Evans rats consisted of higher-frequency spectral components than in the other strains. Wistar rats displayed a surprising increase in duration of individual SWDs as compared to the other strains. In addition, we found that the response to GBL differed as a function of phase of the estrous cycle with higher sensitivity to GBL found during estrus and diestrus compared to metestrus. Moreover, responses in females differed from males only during metestrus. These findings will enable more efficient study design using this common model across strains and sexes.
4.1. Strain Differences
Differences across rat strains, substrains, and suppliers, have been well-documented in several models of epilepsy; likewise differences across mouse strains have been reported in a variety of epilepsy models (for review see: (Löscher et al., 2017)). Many of these studies, however, have focused on models of temporal lobe epilepsy (Brandt et al., 2003; Honndorf et al., 2011; Hort et al., 2000; Langer et al., 2011, 2011; Löscher et al., 1998; Portelli et al., 2009; Xu et al., 2004). While the brain networks generating absence seizures differ substantially from those that generate temporal lobe-like seizures, strain differences have also been reported in models of AS (Willoughby and Mackenzie, 1992).
Consistent with our findings with GBL, Long Evans rats displayed a baseline occurrence of SWD in naïve animals (Cortez et al., 2001; Huang et al., 2012; Pinault et al., 2001); up to 90% of Long Evans rats displayed this phenotype (Shaw, 2004). Sprague-Dawley rats have also been reported to display spontaneous SWDs, although the penetrance (~14%) of this phenotype is less than that reported in Long Evans rats. Interestingly, this phenotype was sex-dependent: while 20% of female Sprague-Dawley rats displayed SWDs, they were uncommon or absent in males (Pearce et al., 2014). Finally, Wistar rats have likewise been reported to display spontaneous SWDs with a penetrance of ~40%; as with Sprague-Dawley rats, these discharges were more common in females as compared to males (80% vs 34%, respectively) (Vergnes et al., 1982). While the functional significance of these SWDs remains a topic of debate (Ewell, 2017; Kaplan, 1985; Rodgers et al., 2015; Taylor et al., n.d.; Wiest and Nicolelis, 2003), the greater sensitivity to GBL in Long Evans animals is seemingly consistent with a predisposition to initiate spontaneous thalamocortical oscillations in this strain. Anecdotally, we have regularly observed spontaneous SWDs in Long Evans rats obtained from Charles River; and less frequently in Wistar and Sprague-Dawley rats obtained from Harlan/Envigo. Further characterization of the baseline SWD phenotype of these strains/sub-strains would enable an assessment of the degree to which spontaneous SWD occurrence predicts susceptibility to GBL-evoked discharges.
Following GBL administration, we noted a divergence in mean power frequency of SWDs across strains. Consistent with prior reports in Sprague-Dawley rats (Pearce et al., 2014) and Wistar rats (Vergnes et al., 1982), the mean power frequency was ~6 Hz. By contrast, Long Evans rats displayed a higher mean power frequency for SWDs, consistent with reports (Shaw, 2007; Wiest and Nicolelis, 2003) in which oscillations displayed 5–14 (or 7–12 Hz) peak power. The mechanisms resulting in these differences remain unexplored.
Interestingly, the common inbred rat models of absence epilepsy (e.g., GAERS, WAG/Rij) were both derived from colonies of Wistar rats (Akman et al., 2010b). In our analysis, Wistar rats displayed the least variability in SWD number (see Fig 3A), and the longest average SWDs (Fig 2B, 3B). Depending on the experimental purpose, this tighter clustering across Wistar rats may offer an advantage or disadvantage. These data also raise the possibility that given the higher variability in both LE and SD strains, they may be suited to develop further absence-prone or absence resistant strains.
4.2. Sex differences and impact of estrous cycle
As with strain differences, sex differences in epilepsy phenotypes have been well-reported but primarily in the context of temporal lobe or generalized tonic-clonic epilepsy models (Bujas et al., 1997; Mejías-Aponte et al., 2002; Nicoletti et al., 1985; Pericić and Bujas, 1997; Scharfman and MacLusky, 2014). Of particular relevance for the present study, progestins, including both progesterone and its metabolite allopregnanolone exerted anticonvulsant effects in models of temporal lobe epilepsy (Lonsdale et al., 2006; Lonsdale and Burnham, 2007, 2003), presumably through action on GABAA receptors (Wu et al., 1990). However, this profile may differ for absence-like seizures, which are typically exacerbated by drugs that enhance GABAergic neurotransmission. Along these lines, progesterone administration significantly increased SWDs in WAG/Rij, while progesterone antagonists, estradiol, and estrogen receptor antagonists failed to impact SWDs (van Luijtelaar et al., 2001). Consistent with this finding, progesterone has also anecdotally been reported to increase typical absence seizures in humans (Grünewald et al., 1992).
Levels of estrogens, androgens, and progestins change dynamically over the course of the estrous cycle. Estrogen levels climb during metestrus and diestrus, peak during proestrus, and drop precipitously during estrus. Progesterone levels likewise peak during proestrus, drop during estrus, and are low during metestrus and diestrus (Lebron-Milad and Milad, 2012; Smith et al., 1975). Accordingly, it is not surprising that cycle stage is associated with differential effects on seizures. For example, proestrus is associated with increased vulnerability whereas estrus is associated with decreased vulnerability to status evoked by pilocarpine (Scharfman et al., 2005). Moreover, progesterone levels, but not estradiol levels modulated SWDs in WAG/Rij rats: the number of SWDs increased during the peak in serum levels of progesterone during the estrous cycle (i.e., during proestrus) (van Luijtelaar et al., 2001). This differs from the peak sensitivity to GBL we observed during estrus, which may result from a difference in mechanism between evoked SWDs (present study) and spontaneous SWDs (van Luijtelaar et al., 2001).
GABAB receptors are a primary target of GBL/GHB and display estrous-cycle dependent changes in expression (al-Dahan et al., 1994). This effect occurs in a progesterone-dependent manner, with a surge in GABAB receptor levels observed 4h after progesterone priming (al-Dahan and Thalmann, 1996). Thus, although progesterone levels are low during estrus, GABAB receptor levels are thus expected to be high following the progesterone surge during proestrus. This may explain the enhanced sensitivity to GBL we observed during estrus. Consistent with a more general role for GABAB receptors in the emergence of SWDs, GABAB antagonists reduce spontaneous SWDs in aged Wistar rats (Puigcerver et al., 1996) and these receptors are downregulated in models of absence seizures (Inaba et al., 2009; Merlo et al., 2007).
In addition to fluctuations in GABAB receptor levels during the estrus cycle, glutamic acid decarboxylase and vesicular GABA transporter levels are increased in the thalamus during the estrous phase, including in the nRT (Umorin et al., 2016). Increased GABA signaling may also contribute to the exacerbation of SWDs during estrus. Finally, estrous cycle differences in sensitivity of GBL and SWDs may also be related to neurosteroid levels, which are modulated as a function of their precursor hormones. For example, allopregnanolone, which is synthesized from progesterone, significantly increases SWDs in WAG/Rij rats (Budziszewska et al., 1999). As discussed above, progesterone and its metabolites potentiate GABAA receptor signaling, which may in turn exacerbate SWDs (Biagini et al., 2010).
In the present study, 30 min EEGs were collected during the first half of the light period of the light-dark cycle, however the peak levels of estradiol and progesterone typically occur in close proximity to shifts in light-dark cycle (Smith et al., 1975). Thus, a shift of only a few hours may result in quite different levels of circulating ovarian hormones, even within a given phase of the cycle. Because we did not monitor hormone levels, we cannot directly associate either estradiol or progesterone levels with the change in sensitivity we observed across the estrous cycle.
4.3. Conclusions
Here we have described the dose-response characteristics of GBL-evoked SWDs across three strains of rats: Long Evans rats displayed the greatest sensitivity, followed by Wistar rats, followed by Sprague-Dawley rats. Moreover, we report that GBL-evoked SWDs are modulated by estrous cycle in female rats, with the peak sensitivity occurring during estrus. Given that Wistar rats displayed the greatest variability as a function of dose, and the least variability within dose; this strain may desirable for future interventional studies. Moreover, our finding that the SWD response to GBL differs as a function of estrous cycle underscores the importance of cycle monitoring in studies examining female animals using this model. Together, these strain and sex-dependent findings provide guidance for future studies enabling optimization of dose selection by strain and sex.
Supplementary Material
Highlights.
Laboratory rat strains differ in response to gamma butyrolactone (GBL) evoked spike-and-wave discharges
Long Evans rats display greater sensitivity to GBL than do Sprague-Dawley or Wistar rats
Responses to GBL differ as a function of sex and estrus cycle
Acknowledgements
This work was supported by R01NS097762 (PAF), KL2TR001432 (PAF, IK), UL1TR0010409 (IK), K01MH110647 (IK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akman O, Demiralp T, Ates N, Onat FY, 2010a. Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy. Epilepsy Res. 89, 185–193. 10.1016/j.eplepsyres.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Akman O, Demiralp T, Ates N, Onat FY, 2010b. Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy. Epilepsy Res. 89, 185–193. 10.1016/j.eplepsyres.2009.12.005 [DOI] [PubMed] [Google Scholar]
- al-Dahan MI, Jalilian Tehrani MH, Thalmann RH, 1994. Regulation of gamma-aminobutyric acidB (GABAB) receptors in cerebral cortex during the estrous cycle. Brain Res. 640, 33–9. [DOI] [PubMed] [Google Scholar]
- al-Dahan MI, Thalmann RH, 1996. Progesterone regulates gamma-aminobutyric acid B (GABAB) receptors in the neocortex of female rats. Brain Res. 727, 40–8. [PubMed] [Google Scholar]
- Banerjee PK, Tillakaratne NJ, Brailowsky S, Olsen RW, Tobin AJ, Snead OC, 1998. Alterations in GABAA receptor alpha 1 and alpha 4 subunit mRNA levels in thalamic relay nuclei following absence-like seizures in rats. Exp. Neurol 154, 213–223. 10.1006/exnr.1998.6928 [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, French J, Glauser TA, 2009. Revised terminology and concepts for organization of the epilepsies : Report of the Commission on Classification and 1–19. [DOI] [PubMed]
- Biagini G, Panuccio G, Avoli M, 2010. Neurosteroids and epilepsy. Curr. Opin. Neurol 23, 170–176. 10.1097/WCO.0b013e32833735cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Glien M, Potschka H, Volk H, Löscher W, 2003. Epileptogenesis and neuropathology after different types of status epilepticus induced by prolonged electrical stimulation of the basolateral amygdala in rats. Epilepsy Res. 55, 83–103. 10.1016/S0920-1211(03)00114-1 [DOI] [PubMed] [Google Scholar]
- Budziszewska B, Van Luijtelaar G, Coenen AM, Leśkiewicz M, Lasoń W, 1999. Effects of neurosteroids on spike-wave discharges in the genetic epileptic WAG/Rij rat. Epilepsy Res. 33, 23–9. [DOI] [PubMed] [Google Scholar]
- Bujas M, Pericić D, Jazvinsćak M, 1997. Influence of gender and gonadectomy on bicuculline-induced convulsions and on GABAA receptors. Brain Res. Bull 43, 411–6. [DOI] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P, 2005. Gender differences in epilepsy. Epilepsia 46, 956–60. 10.1111/j.1528-1167.2005.51204.x [DOI] [PubMed] [Google Scholar]
- Coenen AM, Van Luijtelaar EL, 1987. The WAG/Rij rat model for absence epilepsy: age and sex factors. Epilepsy Res. 1, 297–301. [DOI] [PubMed] [Google Scholar]
- Cortez MA, Kostopoulos GK, Snead OC, 2016. Acute and chronic pharmacological models of generalized absence seizures. J. Neurosci. Methods 260, 175–84. 10.1016/j.jneumeth.2015.08.034 [DOI] [PubMed] [Google Scholar]
- Cortez MA, McKerlie C, Snead OC, 2001. A model of atypical absence seizures: EEG, pharmacology, and developmental characterization. Neurology 56, 341–9. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Leresche N, 2002. Childhood Absence Epilepsy: Genes, Channels, Neurons and Networks. Nat. Rev. Neurosci 3, 371–382. 10.1038/nrn811 [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Inaba Y, Biagini G, D’Arcangelo G, Tancredi V, Avoli M, 2005. Synaptic hyperexcitability of deep layer neocortical cells in a genetic model of absence seizures: Neocortical excitability and absence epilepsy. Genes Brain Behav. 5, 73–84. 10.1111/j.1601-183X.2005.00146.x [DOI] [PubMed] [Google Scholar]
- Dunn R, Queenan BN, Pak DTS, Forcelli PA, 2018. Divergent effects of levetiracetam and tiagabine against spontaneous seizures in adult rats following neonatal hypoxia. Epilepsy Res. 140, 1–7. 10.1016/j.eplepsyres.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Emri Z, Antal K, Crunelli V, 1996. Gamma-hydroxybutyric acid decreases thalamic sensory excitatory postsynaptic potentials by an action on presynaptic GABAB receptors. Neurosci. Lett 216, 121–4. [DOI] [PubMed] [Google Scholar]
- Ewell LA, 2017. Assessing Levels of Awareness During Seizures in Animal Models. Epilepsy Curr. 17, 372–373. 10.5698/1535-7597.17.6.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarman NJ, Roth RH, 1964. Differential Estimation of Gamma-butyrolactone and Gamma-hydroxybutyric Acid in Rat Blood and Brain. Science 145, 583–584. 10.1126/science.145.3632.583 [DOI] [PubMed] [Google Scholar]
- Glauser TA, Cnaan A, Shinnar S, Hirtz DG, Dlugos D, Masur D, Clark PO, Capparelli EV, Adamson PC, Childhood Absence Epilepsy Study Group, 2010. Ethosuximide, Valproic Acid, and Lamotrigine in Childhood Absence Epilepsy. N. Engl. J. Med 362, 790–799. 10.1056/NEJMoa0902014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godschalk M, Dzoljic MR, Bonta IL, 1977. Slow wave sleep and a state resembling absence epilepsy induced in the rat by gamma-hydroxybutyrate. Eur. J. Pharmacol 44, 105–11. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Gibson KM, Weerts EM, 2013. Physical dependence on gamma-hydroxybutrate (GHB) prodrug 1,4-butanediol (1,4-BD): Time course and severity of withdrawal in baboons. Drug Alcohol Depend. 132, 427–433. 10.1016/j.drugalcdep.2013.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald RA, Aliberti V, Panayiotopoulos CP, 1992. Exacerbation of typical absence seizures by progesterone. Seizure 1, 137–8. [DOI] [PubMed] [Google Scholar]
- Honndorf S, Lindemann C, Töllner K, Gernert M, 2011. Female Wistar rats obtained from different breeders vary in anxiety-like behavior and epileptogenesis. Epilepsy Res. 94, 26–38. 10.1016/j.eplepsyres.2010.12.012 [DOI] [PubMed] [Google Scholar]
- Hort J, Brozek G, Komárek V, Langmeier M, Mares P, 2000. Interstrain differences in cognitive functions in rats in relation to status epilepticus. Behav. Brain Res. 112, 77–83. [DOI] [PubMed] [Google Scholar]
- Huang H-Y, Lee H-W, Chen S-D, Shaw F-Z, 2012. Lamotrigine ameliorates seizures and psychiatric comorbidity in a rat model of spontaneous absence epilepsy. Epilepsia 53, 2005–2014. 10.1111/j.1528-1167.2012.03664.x [DOI] [PubMed] [Google Scholar]
- Inaba Y, D’Antuono M, Bertazzoni G, Biagini G, Avoli M, 2009. Diminished Presynaptic GABAB Receptor Function in the Neocortex of a Genetic Model of Absence Epilepsy. Neurosignals 17, 121–131. 10.1159/000197864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallon P, Loiseau P, Loiseau J, 2001. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Coordination Active du Réseau Observatoire Longitudinal de l’ Epilepsie. Epilepsia 42, 464–75. [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, 1985. The epileptic nature of rodent electrocortical polyspiking is still unproven. Exp. Neurol 88, 425–36. [DOI] [PubMed] [Google Scholar]
- Langer M, Brandt C, Löscher W, 2011. Marked strain and substrain differences in induction of status epilepticus and subsequent development of neurodegeneration, epilepsy, and behavioral alterations in rats. [corrected]. Epilepsy Res. 96, 207–24. 10.1016/j.eplepsyres.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Lebron-Milad K, Milad MR, 2012. Sex differences, gonadal hormones and the fear extinction network: Implications for anxiety disorders. Biol. Mood Anxiety Disord. 2, 3 10.1186/2045-5380-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettieri JT, Fung HL, 1979. Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J. Pharmacol. Exp. Ther 208, 7–11. [PubMed] [Google Scholar]
- Lonsdale D, Burnham WM, 2007. The anticonvulsant effects of allopregnanolone against amygdala-kindled seizures in female rats. Neurosci. Lett 411, 147–51. 10.1016/j.neulet.2006.10.023 [DOI] [PubMed] [Google Scholar]
- Lonsdale D, Burnham WM, 2003. The anticonvulsant effects of progesterone and 5alpha-dihydroprogesterone on amygdala-kindled seizures in rats. Epilepsia 44, 1494–9. [DOI] [PubMed] [Google Scholar]
- Lonsdale D, Nylen K, McIntyre Burnham W, 2006. The anticonvulsant effects of progesterone and its metabolites on amygdala-kindled seizures in male rats. Brain Res. 1101, 110–6. 10.1016/j.brainres.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Löscher W, Cramer S, Ebert U, 1998. Differences in kindling development in seven outbred and inbred rat strains. Exp. Neurol 154, 551–9. 10.1006/exnr.1998.6948 [DOI] [PubMed] [Google Scholar]
- Löscher W, Ferland RJ, Ferraro TN, 2017. The relevance of inter- and intrastrain differences in mice and rats and their implications for models of seizures and epilepsy. Epilepsy Behav. 73, 214–235. 10.1016/j.yebeh.2017.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe ER, Layne EC, Sayler DF, Slusher N, Bessman SP, 1971. Synergy of Ethanol and a Natural Soporific--Gamma Hydroxybutyrate. Science 171, 404–406. 10.1126/science.171.3969.404 [DOI] [PubMed] [Google Scholar]
- Mejías-Aponte CA, Jiménez-Rivera CA, Segarra AC, 2002. Sex differences in models of temporal lobe epilepsy: role of testosterone. Brain Res. 944, 210–8. [DOI] [PubMed] [Google Scholar]
- Merlo D, Mollinari C, Inaba Y, Cardinale A, Rinaldi AM, D’Antuono M, D’Arcangelo G, Tancredi V, Ragsdale D, Avoli M, 2007. Reduced GABAB receptor subunit expression and paired-pulse depression in a genetic model of absence seizures. Neurobiol. Dis 25, 631–641. 10.1016/j.nbd.2006.11.005 [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.), Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.), 2011. Guide for the care and use of laboratory animals, 8th ed ed. National Academies Press, Washington, D.C. [Google Scholar]
- Nicoletti F, Speciale C, Sortino MA, Summa G, Caruso G, Patti F, Canonico PL, 1985. Comparative effects of estradiol benzoate, the antiestrogen clomiphene citrate, and the progestin medroxyprogesterone acetate on kainic acid-induced seizures in male and female rats. Epilepsia 26, 252–7. [DOI] [PubMed] [Google Scholar]
- Olsson I, 1988. Epidemiology of absence epilepsy. I. Concept and incidence. Acta Paediatr. Scand 77, 860–6. [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos CP, 2001. Treatment of typical absence seizures and related epileptic syndromes. Paediatr. Drugs 3, 379–403. [DOI] [PubMed] [Google Scholar]
- Pearce PS, Friedman D, LaFrancois JJ, Iyengar SS, Fenton AA, MacLusky NJ, Scharfman HE, 2014. Spike–wave discharges in adult Sprague–Dawley rats and their implications for animal models of temporal lobe epilepsy. Epilepsy Behav. 32, 121–131. 10.1016/j.yebeh.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pericić D, Bujas M, 1997. Sex differences in the response to GABA antagonists depend on the route of drug administration. Exp. Brain Res. 115, 187–90. [DOI] [PubMed] [Google Scholar]
- Persad V, Ting Wong CG, Cortez MA, Wang YT, Snead OC, 2004. Hormonal regulation of atypical absence seizures. Ann. Neurol 55, 353–361. 10.1002/ana.10831 [DOI] [PubMed] [Google Scholar]
- Pinault D, Vergnes M, Marescaux C, 2001. Medium-voltage 5–9-Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons. Neuroscience 105, 181–201. [DOI] [PubMed] [Google Scholar]
- Portelli J, Aourz N, De Bundel D, Meurs A, Smolders I, Michotte Y, Clinckers R, 2009. Intrastrain differences in seizure susceptibility, pharmacological response and basal neurochemistry of Wistar rats. Epilepsy Res. 87, 234–46. 10.1016/j.eplepsyres.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Puigcerver A, van Luijtelaar EL, Drinkenburg WH, Coenen AL, 1996. Effects of the GABAB antagonist CGP 35348 on sleep-wake states, behaviour, and spike-wave discharges in old rats. Brain Res. Bull 40, 157–162. [DOI] [PubMed] [Google Scholar]
- Rodgers KM, Dudek FE, Barth DS, 2015. Progressive, Seizure-Like, Spike-Wave Discharges Are Common in Both Injured and Uninjured Sprague-Dawley Rats: Implications for the Fluid Percussion Injury Model of Post-Traumatic Epilepsy. J. Neurosci. Off. J. Soc. Neurosci 35, 9194–204. 10.1523/JNEUROSCI.0919-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RH, Giarman NJ, 1965. PRELIMINARY REPORT ON THE METABOLISM OF GAMMA-BUTYROLACTONE AND GAMMA-HYDROXYBUTYRIC ACID. Biochem. Pharmacol 14, 177–178. [DOI] [PubMed] [Google Scholar]
- Roth Robert H., Giarman NJ, 1965. Preliminary report on the metabolism of γ-butyrolactone and γ-hydroxybutyric acid. Biochem. Pharmacol 14, 177–178. 10.1016/0006-2952(65)90073-0 [DOI] [PubMed] [Google Scholar]
- Roth RH, Levy R, Giarman NJ, 1967. Dependence of rat serum lactonase upon calcium. Biochem. Pharmacol 16, 596–8. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Rigoulot M-A, Berger RE, Walling SG, Mercurio TC, Stormes K, Maclusky NJ, 2005. Seizure susceptibility in intact and ovariectomized female rats treated with the convulsant pilocarpine. Exp. Neurol 196, 73–86. 10.1016/j.expneurol.2005.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ, 2014. Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol. Dis 72 Pt B, 180–192. 10.1016/j.nbd.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw F-Z, 2007. 7–12 Hz high-voltage rhythmic spike discharges in rats evaluated by antiepileptic drugs and flicker stimulation. J. Neurophysiol 97, 238–47. 10.1152/jn.00340.2006 [DOI] [PubMed] [Google Scholar]
- Shaw F-Z, 2004. Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J. Neurophysiol 91, 63–77. 10.1152/jn.00487.2003 [DOI] [PubMed] [Google Scholar]
- Sheskin D, 2011. Handbook of parametric and nonparametric statistical procedures, 5th ed ed. CRC Press, Boca Raton. [Google Scholar]
- Sitnikova E, 2010. Thalamo-cortical mechanisms of sleep spindles and spike-wave discharges in rat model of absence epilepsy (a review). Epilepsy Res. 89, 17–26. 10.1016/j.eplepsyres.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Sitnikova E, van Luijtelaar G, 2009. Electroencephalographic precursors of spike-wave discharges in a genetic rat model of absence epilepsy: Power spectrum and coherence EEG analyses. Epilepsy Res. 84, 159–71. 10.1016/j.eplepsyres.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD, 1975. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96, 219–26. 10.1210/endo-96-1-219 [DOI] [PubMed] [Google Scholar]
- Snead OC, 1992a. Pharmacological models of generalized absence seizures in rodents. J. Neural Transm. Suppl. 35, 7–19. [DOI] [PubMed] [Google Scholar]
- Snead OC, 1992b. Evidence for GABAB-mediated mechanisms in experimental generalized absence seizures. Eur. J. Pharmacol 213, 343–9. [DOI] [PubMed] [Google Scholar]
- Snead OC, 1991. The gamma-hydroxybutyrate model of absence seizures: correlation of regional brain levels of gamma-hydroxybutyric acid and gamma-butyrolactone with spike wave discharges. Neuropharmacology 30, 161–7. [DOI] [PubMed] [Google Scholar]
- Snead OC, 1988. gamma-Hydroxybutyrate model of generalized absence seizures: further characterization and comparison with other absence models. Epilepsia 29, 361–8. [DOI] [PubMed] [Google Scholar]
- Snead OC, 1984. Ontogeny of gamma-hydroxybutyric acid. II. Electroencephalographic effects. Brain Res. 317, 89–96. [DOI] [PubMed] [Google Scholar]
- Snead OC, 1978. Gamma hydroxybutyrate in the monkey. II. Effect of chronic oral anticonvulsant drugs. Neurology 28, 643–8. [DOI] [PubMed] [Google Scholar]
- Soper C, Wicker E, Kulick CV, N’Gouemo P, Forcelli PA, 2016. Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol. Dis 87, 102–115. 10.1016/j.nbd.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin JM, Davidson TJ, Frechette E, Abramian AM, Deisseroth K, Huguenard JR, Paz JT, 2017. Bidirectional Control of Generalized Epilepsy Networks via Rapid Real-Time Switching of Firing Mode. Neuron 93, 194–210. 10.1016/j.neuron.2016.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, 2018. Brain maps 4.0-Structure of the rat brain: An open access atlas with global nervous system nomenclature ontology and flatmaps. J. Comp. Neurol 526, 935–943. 10.1002/cne.24381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi V, Biagini G, D’Antuono M, Louvel J, Pumain R, Avoli M, 2000. Spindle-Like Thalamocortical Synchronization in a Rat Brain Slice Preparation. J. Neurophysiol 84, 1093–1097. 10.1152/jn.2000.84.2.1093 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Rodgers KM, Bercum FM, Carmen X, Booth J, Dudek FE, Daniel X, Barth S, n.d. Voluntary Control of Epileptiform Spike–Wave Discharges in Awake Rats. 10.1523/JNEUROSCI.3235-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KM, Burne THJ, 2014. Comprehensive behavioural analysis of long Evans and Sprague-Dawley rats reveals differential effects of housing conditions on tests relevant to neuropsychiatric disorders. PLoS ONE 9 10.1371/journal.pone.0093411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twele F, Töllner K, Brandt C, Löscher W, 2016. Significant effects of sex, strain, and anesthesia in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. Epilepsy Behav. 55, 47–56. 10.1016/j.yebeh.2015.11.027 [DOI] [PubMed] [Google Scholar]
- Umorin M, Stinson C, Bellinger LL, Kramer PR, 2016. Genes in the GABA Pathway Increase in the Lateral Thalamus of Sprague-Dawley Rats During the Proestrus/Estrus Phase. J. Cell. Physiol 231, 1057–64. 10.1002/jcp.25198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luijtelaar G, Budziszewska B, Jaworska-Feil L, Ellis J, Coenen A, Lasoń W, 2001. The ovarian hormones and absence epilepsy: a long-term EEG study and pharmacological effects in a genetic absence epilepsy model. Epilepsy Res. 46, 225–39. [DOI] [PubMed] [Google Scholar]
- van Luijtelaar G, Onat FY, Gallagher MJ, 2014. Animal models of absence epilepsies: what do they model and do sex and sex hormones matter? Neurobiol. Dis 72 Pt B, 167–79. 10.1016/j.nbd.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sassenbroeck DK, De Paepe P, Belpaire FM, Boon PA, Buylaert WA, 2003. Tolerance to the hypnotic and electroencephalographic effect of gamma-hydroxybutyrate in the rat: pharmacokinetic and pharmacodynamic aspects. J. Pharm. Pharmacol 55, 609–615. 10.1211/002235703765344513 [DOI] [PubMed] [Google Scholar]
- Venzi M, Di Giovanni G, Crunelli V, 2015. A Critical Evaluation of the Gamma-Hydroxybutyrate (GHB) Model of Absence Seizures. CNS Neurosci. Ther 21, 123–140. 10.1111/cns.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Micheletti G, Reis J, Depaulis A, Rumbach L, Warter JM, 1982. Spontaneous paroxysmal electroclinical patterns in rat: a model of generalized non-convulsive epilepsy. Neurosci. Lett 33, 97–101. [DOI] [PubMed] [Google Scholar]
- Waaler PE, Blom BH, Skeidsvoll H, Mykletun A, 2000. Prevalence, classification, and severity of epilepsy in children in western Norway. Epilepsia 41, 802–10. [DOI] [PubMed] [Google Scholar]
- West EA, Forcelli PA, Murnen AT, McCue DL, Gale K, Malkova L, 2012. Transient inactivation of basolateral amygdala during selective satiation disrupts reinforcer devaluation in rats. Behav. Neurosci 126, 563–574. 10.1037/a0029080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest MC, Nicolelis MAL, 2003. Behavioral detection of tactile stimuli during 7–12 Hz cortical oscillations in awake rats. Nat. Neurosci 6, 913–914. 10.1038/nn1107 [DOI] [PubMed] [Google Scholar]
- Willoughby JO, Mackenzie L, 1992. Nonconvulsive electrocorticographic paroxysms (absence epilepsy) in rat strains. Lab. Anim. Sci 42, 551–554. [PubMed] [Google Scholar]
- Woolfolk DR, Holtzman SG, 1995. Rat strain differences in the potentiation of morphine-induced analgesia by stress. Pharmacol. Biochem. Behav 51, 699–703. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH, 1990. Inverse modulation of gamma-aminobutyric acid- and glycine-induced currents by progesterone. Mol. Pharmacol 37, 597–602. [PubMed] [Google Scholar]
- Xu B, McIntyre DC, Fahnestock M, Racine RJ, 2004. Strain differences affect the induction of status epilepticus and seizure-induced morphological changes. Eur. J. Neurosci 20, 403–18. 10.1111/j.1460-9568.2004.03489.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.