Abstract
Cognitive maps are encoded in the hippocampal formation and related regions, and range from the spatial to the purely conceptual. Neural mechanisms that encode information into relational structures, up to an arbitrary level of abstraction, may explain these disparate functions. Research now indicates that social life can also be mapped by these mechanisms: others’ spatial locations, social memory and even a two-dimensional social space framed by social power and affiliation. The systematic mapping of social life onto a relational social space facilitates adaptive social decision-making, akin to social navigation. This emerging line of research has implications for cognitive mapping research, clinical disorders that feature hippocampal dysfunction, and the field of social cognitive neuroscience.
The hippocampal formation performs functions that include spatial representation and episodic memory. These functions may reflect a multi-dimensional “cognitive map” that organizes previous experience to support flexible navigation (Tolman, 1948). The discovery of spatially modulated cells in the hippocampus and entorhinal cortex led to the view that these regions encode cognitive maps (O’Keefe and Nadel, 1978; Eichenbaum and Cohen, 2014). Subsequent research has shown that these regions are also sensitive to a variety of non-spatial and even abstract features, such as sound, time, reward and concepts (Schiller et al., 2015). The hippocampal formation maps and stores this information relationally, enabling inference and decision-making by utilizing stored memory elements (Eichenbaum and Cohen, 2014). Emerging research suggests the hippocampus also represents social stimuli within physical space, information about specific individuals, and abstract social dimensions, such as power and affiliation (Montagrin et al., 2017). The hippocampus and related regions may thus perform social functions and encode “social space” in the form of a cognitive map.
This perspective argues that social cognitive mapping occurs, and is supported by mechanisms that map physical space. The argument presents evidence for spatial mapping, followed by evidence that these same mechanisms also map non-spatial and even abstract information and enable the use of cognitive maps in decision-making. To support the idea that the hippocampus is involved in social cognitive mapping, we highlight research showing that spatially sensitive cells in the hippocampus encode social information, and discuss how this may relate to the role of the hippocampus in social memory. Finally, the “social space” is presented: abstract dimensions of social power and affiliation may be mapped by the same regions that map physical space and thus facilitate social navigation (figure 1). We end with a discussion of how social mapping research advances social cognitive neuroscience.
Figure 1: Increasing abstraction in cognitive mapping.
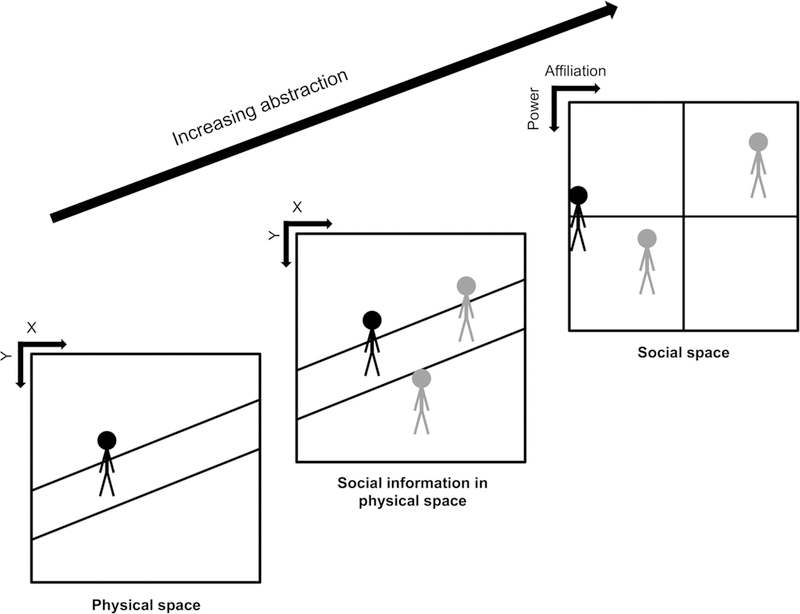
Cognitive mapping occurs at various levels of abstraction. Physical space: An individual’s location (represented by the black stick figure) and the environmental context can be mapped. Social information in physical space: Abstract information within physical environments, such as the locations of conspecifics (represented in grey), can also be cognitively mapped. Other kinds of abstract information, such as reward, are also mapped in this manner. Social space: The social map is fully removed from the sensory details of the environment, and as such is purely abstract. The social space (relative power and affiliation of others, the grey stick figures) is referenced to the individual (black stick figure), in contrast to physical space mapping, which is referenced to the environment (see Box 1 for a brief discussion on reference frames). Evidence shows that other fully abstract spaces, such as reward and concepts are mapped as well.
Mapping spatial dimensions
Tolman formed the cognitive map hypothesis when he observed that animals infer relationships between locations that had never been directly experienced together, a phenomenon that stimulus-response learning could not easily explain (1948). In one illustrative study, rats were trained to travel down a path to a food reward. After training, the rats were placed in a similar environment where the learned path to the food reward was now blocked. Now there were new, alternative routes, one of which led to the reward location. In a single trial test, the rats chose the correct path that angled towards the reward, despite never having been trained to take that specific path. The animals may have retained a representation of the reward’s location and used it to flexibly navigate towards the reward despite obstacles, perhaps by searching an internal “cognitive map” of the relationships within the environment to predict decision outcomes.
The discovery of hippocampus pyramidal cells that encode the animal’s current location (“place cells”) led to the speculation that cognitive maps are encoded in the hippocampal formation (O’Keefe and Nadel, 1978), defined as entorhinal cortex, dentate gyrus, hippocampus CA subfields and subicular complex. Subsequent research has clarified many of the properties of place cells, and they are consistent with the basic idea of a cognitive map. The formation of these neurons’ preferred spatial locations (“place fields”) requires environmental experience but, once stabilized, spiking continues to be spatially specific absent external cues for a period of time (McNaughton et al., 2006), suggesting these representations are learned and at least in part internally maintained. This activity is stable and location specific within spatial contexts, but highly variable across contexts, indicating context dependence (“remapping”; Colgin et al., 2008). Place fields also get continuously larger from the dorsal to ventral hippocampal poles (corresponding to posterior to anterior in humans, respectively), suggesting a functional gradient that may allow for both precise and continuous spatial representations (Strange et al., 2014). This ability to learn and (in part) internally maintain location specific activity within spatial contexts provides some of the machinery necessary to encode the physical environment into a cognitive map.
Regions connected with the hippocampus also contain spatially modulated cell types. In superficial layers of medial entorhinal cortex, the main cortical input to the hippocampus, there are “grid cells” which fire at multiple, regular positions within the environment and are clustered in modules, with distinct scales and orientations (e.g., Hafting et al., 2005; Stensola et al., 2012). Like place cells, grid cells require some experience with the environment and their spiking remaps in new spatial contexts. Other medial entorhinal cortex cells also provide spatial information, including head direction (Sargolini et al., 2006), environmental borders (Solstad, 2008), speed (Kropff et al., 2015), and vectors (distance and direction information) connecting the current location to objects (Høydal et al., 2018). Retrosplenial cortex, an area that is densely interconnected with the hippocampal formation, also contains place and head direction cells (Miller et al., 2014), as well as other spatial cell types. For example, some retrosplenial cells encode sub-routes within a larger environment, at multiple spatial scales, and others map the distance between current location and every other environmental position, giving relational information about complex paths (Alexander and Nitz, 2017). These neuronal subpopulations, among others, may cooperate within a spatially distributed network to support cognitive mapping.
Beyond spatial mapping: Increasing abstraction
Place cells encode more than the animal’s current location: decoded place cell network activity reveals that place cells can represent prospective locations as well as locations the animal just left (Ferbinteanu and Shapiro, 2003). These cells bind recently experienced and to-be-experienced locations together into an orderly trajectory. Place cell sequences can also represent spatial locations that were visited further in the past. During rest or sleep, place cell ensembles often reactivate in patterns that correlate with sequences that were active during environmental experience, in both reverse and forward order (Pfeiffer, 2018). These “replay” sequences may reflect a variety of network level phenomena related to cognitive mapping, such as memory consolidation and memory-based planning (Pfeiffer, 2018). Grid cell replay-like activity has recently been observed as well, suggesting replay might be common across the cognitive map network (O’Neill et al., 2017).
Consistent with the encoding of trajectories that span the past, present and future, the hippocampus can track time (Eichenbaum, 2014). In addition to place cells, the hippocampus contains “time cells” and dual-functions cells that encode both time and place (Eichenbaum, 2014). Independent of spatial information or behavioral factors, time cells fire when an animal is at a particular point in time in a temporally structured experience. For example, when location is held constant, hippocampus CA1 neuronal activity is explained by delay duration, as individual cells fire at different time points to cover the entire delay period (e.g., MacDonald et al., 2011), similar to how place cells fire to cover a linear track. Like place cell remapping, this activity is context dependent: firing patterns change in new contexts such as when a delay period is increased. Therefore this activity does not reflect generic time tracking and instead likely reflects temporal context.
That space and time can be encoded similarly and simultaneously within the same or neighboring cells suggests they may be integrated into a unified spatiotemporal representation. Neural signal at the resolution of functional magnetic resonance imaging (fMRI) supports this interpretation. In a virtual navigation task, hippocampal multivoxel pattern similarity reflected events on both spatial and temporal dimensions, with spatial and temporal similarity having an additive effect on neural similarity (Deuker et al., 2016). Spatial grid cells can also track temporal features (Kraus et al., 2015), bolstering the view that the cognitive map incorporates an abstract temporal dimension.
The representation of space and time likely underpins the role of the hippocampus and related regions in spatial and episodic memory (i.e., memory for events in a specific place and time). Hippocampal neural reinstatement of an item’s spatial or temporal context is key to successful memory retrieval (Flegal et al., 2014), as is differentiating competing spatial or temporal context representations between memories (Copara et al., 2014). Other connected regions have similar memory encoding properties as the hippocampus. Retrosplenial cortex is important to both spatial and episodic memory and contains ensembles that can encode context-specific engrams (Milczarek et al., 2018). It also connects the hippocampus to posterior cingulate cortex (PCC), an area involved in autobiographical memory (Maddock et al., 2001). The interconnections and functional similarities of these regions suggest that the hippocampus, retrosplenial cortex and PCC are nodes within a memory system that builds and stores relational models of the contextual elements of an experience (Ranganath and Ritchey, 2012).
These elements include more than the spatiotemporal context (the “where” and the “when”) of an event, however. The hippocampus can also encode information about specific elements within a spatiotemporal context (the “what” of an episode), such as object identity (Manns and Eichenbaum, 2009). For example, some hippocampus neurons respond optimally to the combination of a specific object and a specific location, and in some cases do not fire at all to the object or location alone (e.g., Komorowski et al., 2009), Conjunctive signaling of this sort may encode what happened where and is a possible consequence of the “what” and “where” visual pathways converging in the hippocampus (Komorowski et al., 2009).
The same mapping mechanisms that encode spatiotemporal contexts and their specific elements might function to organize relationships between environmental elements into a stable, relational framework for visual perception. When individuals visually explored a virtual environment, there was a grid-like BOLD signal in entorhinal cortex, suggesting the presence of visual grid cells with similar properties to spatial grid cells (Julian et al., 2018), a finding supported by single-cell recordings in monkeys (Killian et al., 2012). Additionally, single entorhinal and hippocampal neurons can respond preferentially to the visual perception of spatial layouts (Kreiman et al., 2000). Visual exploration and physical exploration may therefore use some of the same mechanisms to map the physical environment.
Other sensory modalities, such as auditory environments, are similarly encoded by the hippocampal formation, with specific place cells and grid cells responding to specific sound frequencies in a manner akin to spatial mapping (Aronov et al., 2017). Sound frequency exists on a single dimension, like a linear track, and navigation in these two domains yields very similar neural representations, suggesting the same mechanism underlies both spatial and non-spatial dimensional mapping. Additionally, professional piano tuners, who navigate a complex auditory landscape, were found to have larger than normal hippocampal volumes, an effect that was experience-dependent as it was larger in tuners who had more years of practice (Teki et al., 2012). This finding mirrors the larger posterior hippocampal volumes found in longtime taxi drivers (Maguire et al., 2000) and suggests that hippocampal structure changes in response to non-spatial navigation similarly to how it changes in response to spatial navigation.
Hippocampal dimensional encoding extends beyond information encoded by the senses to include abstract information - with abstract defined as latent information that is not directly reducible to immediate sensory perception. This can include abstract information embedded within spatial environments, such as reward location. Indeed, place cell activity is altered by the presence of reward: individual place cells increase their firing around rewards (Poucet and Hok, 2017) and place fields cluster around reward sites (Hok et al., 2007). There is also a dedicated sub-population of hippocampus ventral CA1 and subicular “reward cells” that encode reward location across environments (Gauthier and Tank, 2018). Their activity was not explained by reward related behaviors, suggesting they represent reward location itself.
That the hippocampus encodes behaviorally relevant information like reward location is unsurprising: energy resources are limited and information about the environment is not equally important and thus is not attended to with equal probability. Attentional processes may directly engage the hippocampus to support behaviorally relevant encoding: attention to task goals modulates hippocampal fMRI signal and relates to subsequent task-relevant recall (Aly and Turk-Browne, 2016a, 2016b). Additionally, while task relevant variables, such as spatial context and item identity, can be encoded in the activity of hippocampus CA1 and CA3 ensembles, task irrelevant regularities do not seem to be tracked (McKenzie et al., 2014). Attention guided encoding may thus preferentially map information relevant to the current task, including information outside of the immediate sensory experience of the animal, such as temporal context, object identity and reward - in other words, non-spatial and abstract information.
The relational information between such behaviorally relevant elements is extracted to generate relational models. For example, the hippocampus is important in learning spatial relationships between visual cues (Lavenex et al., 2006), statistical relationships between task items across modalities (Covington et al., 2018), and hierarchical relationships between stimuli via transitive inferences but not discrimination between individual stimuli (Dusek and Eichenbaum, 1997). Similarly, hippocampal damage impairs scene perception that relies upon relationships between features but not perception that relies on individual features (Aly et al., 2013), consistent with a role for the hippocampus in constructing orderly relations between information. The hippocampus therefore helps encode behaviorally relevant statistical patterns into a relational framework, which allows generalizations and inferences about relationships.
This relational mapping likely reflects a general circuit mechanism that may allow for any arbitrary dimension to be encoded, accounting for the wide range of information the hippocampal formation can represent. Purely conceptual information, abstract and not spatially embedded, may be mapped by the hippocampal formation in this same way. Across different images and even the names of the same individual, object or landmark, a subset of hippocampal formation neurons responded to the concept of the item rather than its sensory details (Quiroga et al., 2005). Entorhinal cortex, home of grid cells and an input to the hippocampus, can also map conceptual dimensions. This region contains grid cells that in the spatial domain are conjunctive for location and direction, and fire with a particular spatial periodicity. In fMRI virtual navigation, when participants moved along the orientation of a spatial grid, blood oxygenation level dependent (BOLD) signal in entorhinal cortex oscillated in a manner consistent with grid cell activation (Doeller et al., 2010). Conceptual knowledge can be organized in a similar manner. When participants had to associate birds of varying leg and neck length with specific objects, creating a two-dimensional conceptual space, BOLD activity within entorhinal cortex and other regions (e.g., medial prefrontal cortex) was modulated in a spatial grid-like fashion (Constantinescu et al., 2016). More grid-like representations correlated with better task performance, suggesting representational quality related to memory. These findings were strikingly similar to Doeller and colleagues (2010), again suggesting that abstract space is mapped by the same regions and computations as physical space, with resulting representations being similar when the underlying statistical relationships (e.g., same dimensional space) are similar.
Relational frameworks may also encode the relationships between individual episodic memories. Temporal organization is crucial in recollection and may help explain how the hippocampus organizes memories for specific events across time (Eichenbaum, 2013). The hippocampus is associated with memory for the correct order of sequences (Davachi and DuBrow, 2015), with damage resulting in deficits, even when memory for individual items is spared (Mayes et al., 2001). Furthermore, memories that have overlapping elements, such as in where events occurred and the features within events, may be stored within relational structures, encoded by correlated ensemble firing patterns within CA1 and CA3 (McKenzie et al., 2014). Thus, memory “space” may be mapped by the same mechanisms that map physical space. Within the hippocampus and related structures elements of experience are represented, bound and stored within relational models, including the relationships between events such as their relative order, the locations where they occurred and the features within them.
Using the map: Prediction and navigation
Forming a map and navigating through the environment are two very different operations, however. If the cognitive map is ultimately about navigation there should be mechanisms that use map elements to simulate novel routes and predict decision outcomes, in order to facilitate flexible goal-related navigation across spatial, non-spatial and abstract domains. Several lines of evidence are consistent with this view.
As reviewed above, place and grid cells are sensitive to information about direction, time and speed, and can track progression through space. They may also contribute to path integration, a navigational strategy that does not use landmarks and instead depends upon updating current position and orientation using internal signals (Collett and Graham, 2004). The hippocampus, parahippocampal cortex and retrosplenial cortex contain cells that encode a homing signal that tracks the movement of an animal relative to home (Chrastil et al., 2015). Hippocampal replay sequences can represent all environmental trajectories, whether they have been experienced or not, suggesting active map maintenance that goes beyond simple memory processes (Gupta et al., 2010). These sequences are functionally important for spatial decision-making: disrupting replay sequences during sleep impairs subsequent performance in hippocampus-dependent memory tasks (Jadhav et al., 2012) while pairing reward related stimulation with replay sequences caused rodents to prefer areas represented by those place cells (De Lavilléon et al., 2015). Additionally, grid cells compute vectors that connect locations and therefore represent distances and directions, a key function for goal directed navigation (Høydal et al., 2018; Bush et al., 2015).
Route planning and navigating recruits the hippocampus as well: during virtual spatial navigation in fMRI, hippocampus signal relates to the number of available routes (Javadi et al., 2017), the planning of new routes at choice points (Spiers and Maguire, 2006), the use of novel routes and shortcuts (Marchette et al., 2011), the proximity of the participant to a known goal (Patai et al., 2017), and success in navigation (Suthana et al., 2009). Subpopulations of CA1 place cells signal vectors towards spatial goals, some even when the goals are hidden, indicating a role for memory (Sarel et al., 2017). Different regions within the hippocampal formation may encode complementary information about spatial goals. For example, entorhinal cortex BOLD signal relates to the Euclidean distance to a goal whereas hippocampus BOLD signal relates to distance along a path to the same goal (Howard et al., 2014). Unsurprisingly, individuals with damage to the hippocampal formation have trouble navigating physical locations (Maguire et al., 2006).
To support spatial decision-making the hippocampus may simulate future behavioral possibilities. Rodents often stop at decision points in mazes and perform hesitating behaviors called vicarious trial and error, likely reflecting active planning. When this occurs, place cell trajectories “sweep” ahead of the animal, sequentially sampling the available behavioral options (Redish, 2016). Similarly, place cell trajectories can predict where a rat will travel to next in a decision-making task within an open arena, even when the start and goal locations are novel (Pfeiffer and Foster, 2013). Given that this activity occurs within environments where decision points are cued, it may be stimulus bound with only the most immediate future being simulated.
Other hippocampal ensemble patterns may reflect simulations for future behavior on longer time scales. Prior to an animal’s journey on a given trial and in the absence of an overt stimulus, sequences of place cells can evolve in forward order, consistent with simulating an upcoming journey. These “preplay” events may be constructed from stored place cell sequences (Dragoi and Tonegawa, 2013) and depend upon having some experience with the particular environment (Silva et al., 2015), suggesting that, given knowledge of the environment’s topology, spatial memory can be reconfigured to simulate possible future trajectories. Reward location also influences which trajectories are simulated. Preplay for unexplored regions occurs after seeing reward deposited there, suggesting these ensemble events can be goal-directed (Freyja Ólafsdóttir et al., 2015), while reward specific cells in CA1 and the subiculum fire in prediction of an upcoming reward, and strongly relate to slowing behavior upon reaching the reward location (Gauthier and Tank, 2018). Memory readouts from a spatial cognitive map may simulate paths to future reward, in the service of adaptive behavior.
Consistent with a role for hippocampal memory in decision-making, the hippocampus is important in using relational memory in sequential decision-making tasks (Yee et al., 2014), and is a key region in reconfiguring memory to predict consequences of future scenarios (Barron et al., 2013). Episodic simulation (i.e., imagining future episodes) might depend on these same mechanisms: it shares extensive behavioral and neural similarities with episodic memory, such as temporal and experiential properties, as well as a common neural circuitry that includes the hippocampus (Schacter et al., 2012). Patients with hippocampal damage often cannot describe coherent scenes for imagined future events and will resort to describing each individual component of a scene separately (Hassabis et al., 2007), and they report almost no scene or visual imagery-based mind wandering (McCormick et al., 2018).
An open question is where and how a map’s code is read out to guide action selection. One candidate region is the orbitofrontal cortex (OFC), which is anatomically connected to the hippocampus and has value and decision-making functions, as well as grid-like properties during conceptual mapping (Constantinescu et al., 2016). The OFC may map a “task space,” the set of possible states associated with a given task, by segmenting the world into discrete states and tracking information related to these states, allowing values to be assigned to them (Schuck et al., 2016). This mapping is in some ways analogous to hippocampal cognitive mapping, and these regions may interact to facilitate decision-making. For example, during vicarious trial and error behavior at decision points OFC neurons that are responsive to reward receipt are active around the same time place cell ensembles would be expected to sample the available options (Steiner and Redish, 2012). The close timing suggests that behavioral options simulated by the hippocampus may be subsequently evaluated for reward value in OFC (Wikenheiser and Schoenbaum, 2016). Other work supports this view: suppressing ventral hippocampus output in the ventral subiculum disrupts the formation of OFC representations of task structure and expected outcomes (Wikenheiser et al., 2017), further evincing an interaction between these regions in decision-making related processes.
Nearby medial prefrontal cortex (mPFC) may serve a similar role in cognitive map-based decision-making. Traditionally studied for its role in value representation during decision-making, mPFC may structure a “decision space” via grid-like computations (Constantinescu et al., 2016) that decompose large goals into smaller goals, similar to how grid cells section up physical and conceptual space, and then use hippocampal dependent memory to meet task demands via hippocampal connections (Kaplan et al., 2017). Evidence is consistent with this account: both regions represent goal proximity (Balaguer et al., 2016), and bidirectional covariation between the hippocampus and mPFC during a context memory-guided decision task has been observed (Place et al., 2016). Additionally, increased BOLD covariation between hippocampus and ventromedial PFC accompanies episodic simulation, suggesting ventromedial PFC has a role in reconfiguring memories into a novel episode (Campbell et al., 2018). Hippocampal memories may serve as templates to guide mPFC-based decision-making. Though much work remains to fully elucidate how cognitive maps are used to guide decision-making, this research suggests an intriguing possibility. Relational models, encoded in the hippocampal formation, may allow the simulation of behavioral possibilities, which are subsequently evaluated by OFC and used to guide task relevant behavior by mPFC. This also hints at how greater computational efficiency may be achieved in decision-making even in novel scenarios in which the animal has no experience. The world’s statistical regularity (i.e., reality’s non-randomness) allows memory elements to be generalized and constrain representations of possible behaviors and decision outcomes. This reduces the number of computations needed to generate a decision, greatly increasing the efficiency of decision-making (Kaplan et al., 2017). These processes may support spatial navigation as well as more abstract goal-oriented behavior. This research has slowly been piecing together how Tolman’s map leads to flexible navigation.
Navigating into the social domain
So far, it has been argued that the hippocampal formation and related regions treat spatial and non-spatial information similarly, up to levels of full abstraction. The unique structure of the hippocampus may underlie its functional flexibility: a highly recurrent structure processing all sources of incoming information through its intrinsic circuitry in the same relational manner (Lisman et al., 2017). Internal representations of the statistical structure of information in the world (i.e., ‘cognitive maps’) are generated, which can be generalized to new situations. Thus, non-spatial and abstract information is mapped much like spatial information: this was demonstrated with evidence for non-spatial mapping that requires sensory experience, such as auditory mapping, and evidence for abstract mapping, from time to reward location to purely conceptual spaces.
This same hippocampal centered network likely maps social life. Social information exists in an orderly, dimensional fashion in a manner akin to spatial information. Social dimensions rooted in social memory and relationally bound together frame social interaction, making social space an ideal candidate for cognitive mapping. This kind of social topography may represent individuals as coordinates within social space, from which social vectors can be computed and social inferences and decisions can be generated. Some of the same mechanisms that facilitate adaptive spatial navigation may underlie adaptive social navigation.
Research into social memory supports a role for the hippocampus in organizing social information. For example, faces associated with biographical information or behaviors recruit greater left hippocampus BOLD signal relative to novel faces (Todorov et al., 2007). Other fMRI work corroborates and extends this finding: bilateral hippocampus activity relates to correct recollection for individuals and tracks pre-experiment familiarity of a famous or personally known face, independent of presentation recency (Trinkler et al., 2009). This activity may be specific to social or biological stimuli, as another study showed that landmark familiarity did not relate to hippocampal activity (Viskontas et al., 2009). Social memory also likely underpins social networks: in healthy adults, social network size can be predicted by performance on hippocampal dependent tasks (Stiller and Dunbar, 2007), whereas individuals with hippocampal damage have difficulty retrieving information about individuals (Sanders and Warrington, 1971) and difficulty maintaining relationships, leading to more restricted social networks than healthy individuals (Davidson et al., 2012). These studies, among others, suggest social memory may in part be organizational, systematically managing social information to guide behavior.
Social information in physical spaces
Knowing other animals’ locations is a particularly important piece of social information, as it is key to communication, mating and survival. Given their biological relevance and the role of the hippocampus in representing abstract features of spatial environments, the hippocampus may encode the spatial location of conspecifics. Such “social place cells” were detected in the hippocampus dorsal CA1 when bats had to observe and then perform the same flying behavior as another bat in order to receive a reward (Omer et al., 2018). To investigate the effects of observational learning on place cell activity, separate firing-rate correlation maps were computed for the locations of the observer and the demonstrator. Some cells’ spiking correlated with the observer animal’s current location, in classic place cell fashion. A subset of these cells’ activation patterns also correlated with the location of the demonstrator bat. This conspecific related activity was unlikely to be simple sensory stimulus tracking: inanimate moving objects also elicited place cell activity, but with different representational properties. Rather than sensory details, these cells seem to encode abstract information about the social or behavioral relevance of the conspecific. One possibility was not ruled out in this design, however; this activity could have reflected the simulation of a future self-trajectory.
Another study in rat dorsal CA1 included an experimental condition where future self and current conspecific trajectories could be fully disentangled (Danjo et al., 2018). Rats had to observe a conspecific’s trajectory in a T-maze and, depending on the trial, perform the same or opposite turn as the conspecific. If dorsal CA1 contains social place cells, then their activity during observation should correlate with the conspecific’s location even on trials when the observer had to make the opposite decision. This pattern was observed, which ruled out the hypothesis that this activity was simply future-self- or goal-related and strongly suggested that dorsal CA1 place cells encode the spatial location of other animals.
Dorsal CA2 place cell activity also alters in response to social stimuli. These neurons globally remap in the presence of other rats as well as novel objects, without changes in overall firing rates, perhaps binding social and novelty information to spatial representations (Alexander et al., 2016). Dorsal CA1 neurons did not show this same change in activity, raising a question of what precisely dorsal CA1 and dorsal CA2 are doing in these social processes. Alexander and colleagues did not use an observational learning task as did Danio et al (2018) and Omer et al (2018), thus dorsal CA1 may reflect active spatial tracking of functionally important conspecifics, especially in the service of learning, and dorsal CA2 may reflect passive binding of social information to spatial representation.
Regardless of what explains the differences between these data, these studies show that both CA1 and CA2 have place cells that are sensitive to social context, mirroring previous work showing that place cells are modulated by abstract information. These subfields are also important for social memory. Under normal conditions mice tend to spend more time interacting with unfamiliar mice, a phenomena employed to study social recognition. Optogenetic inhibition of mouse dorsal CA2 neurons prevented the normal decrease in time interacting with a familiar mouse (Hitti and Siegelbaum, 2014), indicating a lack of social recognition for the previously experienced mouse. This effect was not explained by general function loss, such as motor behaviors or spatial or contextual memory. Additionally, optogenetic excitation of mouse dorsal CA2 neurons during memory acquisition increased memory for specific mice, an effect that was dependent on vasopressin receptor function (Smith et al., 2016). In both of these studies, interest in new mice was unaffected, suggesting the effects were specific to social memory rather than social interest. Optogenetic inhibition work showed that ventral CA1 neurons and their projections to nucleus accumbens shell are necessary for social recognition behavior as well. Reactivation of this circuit was sufficient to induce social recognition behavior, suggesting this circuit stores social memory engrams (Okuyama et al., 2016). A subpopulation of neurons within ventral CA1 has been shown to reliably encode reward location across environments. It is possible that a dedicated subpopulation of social cells also exists, or that conspecifics are in part represented by hippocampal reward circuitry.
This research demonstrates that cell populations within the CA1 and CA2 hippocampal subfields are important for processing both the current location of conspecifics and for a specifically social form of recognition. These functions may be related: social place cell activity may underlie social recognition. Although Alexander and colleagues (2016) found that firing rates in dorsal CA2 place cells did not differ between the presence of novel and familiar rats, when the animals were placed back into the original environment without the conspecific, the original place fields did not return, suggesting a memory trace in environments in which social interaction previously occurred. This mirrors evidence that cells in lateral entorhinal cortex continue to code for an object even after the object has been removed from an environment (Tsao et al., 2013).
In seeming contradiction to the evidence of hippocampal involvement in social recognition, hippocampal damage in humans can spare facial recognition while impairing other memory functions (Aly et al., 2010; Bird et al., 2007). This seems to argue against the points just made: if the hippocampus is essential to social recognition, then hippocampal damage should profoundly affect this function. There are several possible explanations for this data. First, there may be other circuits that can support face recognition and thus compensate for hippocampal damage. Another possibility is that different regions subserve facial and social recognition: the hippocampus may not contribute to memory for faces devoid of social relevance. Those studies testing patients’ recognition of previously unknown faces did not pair the faces with any behavioral or social information (Aly et al., 2010; Bird et al., 2007). In other domains, hippocampal damage can impair relational memory while sparing memory for individual items (e.g., Dusek and Eichenbaum, 1997; Mayes et al., 2001), suggesting hippocampal social memory functions may be specific to relational mapping, rather than individual social items like faces. An unusual property of CA2 may also explain this: sources of tissue damage to the hippocampus, from diverse causes such as epilepsy, ischemia, hypoxia and trauma, spare CA2 relative to other subfields (Dudek et al., 2016). Research on patients with hippocampal damage does not always account for subfield damage differences, leaving open the possibility that spared facial recognition reflects a relatively spared CA2.
In addition to resilience to damage, a variety of other factors differentiate CA2 from the other hippocampal subfields and may be relevant to understanding subfield contributions to cognitive mapping. These include gene expression, molecular profile, long-term potentiation, dendritic branching, density of inhibitory interneurons and connectivity (Dudek et al., 2016). CA2 pyramidal neurons have a larger excitatory response to entorhinal input than CA1 neurons, likely because of greater innervation and more efficient signal propagation (Srinivas et al., 2017). Entorhinal projections to the hippocampus are also topographically mapped and CA2 largely receives lateral entorhinal cortex inputs that convey non-spatial information (Hargreaves et al., 2005). Consistent with this, CA2 is less essential to spatial representation than the other subfields, as CA2 place fields change more over time than place fields in CA1 and CA3 (Mankin et al., 2015), and CA2 inactivation does not impair spatial performance (Hitti and Siegelbaum, 2014). As such, projections directly from CA3 to CA1 might adequately support spatial functions, while CA2 to CA1 circuitry might be non-spatial and maybe even have a specifically social function. CA2 pyramidal neurons are rich in vasopressin and oxytocin receptors, neuropeptides important in many social behaviors (Young et al., 2006), and plasticity induced by concurrent stimulation of entorhinal inputs and CA3 affects CA1 and CA2 differently: it improves contextual memory in CA1, and improves social recognition in CA2 (Leroy et al., 2017).
The interconnection of the CA2 and CA1 subfields (the deep layer of CA1 receives inputs from CA2) (Kohara et al., 2014), and the relative specificity of their social functions suggests they may contain circuitry specialized for social cognition. During the encoding of social information vasopressin and oxytocin may regulate CA2 activity, which in turn can modulate CA1 excitation (Piskorowski and Chevaleyre, 2018). Through this pathway, social information could have a modulatory role in hippocampal function generally.
Mapping abstract social dimensions
Mirroring the mapping of abstract dimensions, the hippocampal formation may represent abstract social information dimensionally. One such dimension is dominance hierarchy. Understanding the relative social power of individuals is vitally important to social animals, as status affects resource allocation, mating opportunities and physical safety. Accurately representing hierarchy likely requires repeated interactions with conspecifics where status representations get formed and updated. As the hippocampal formation is critical to representing others in space, social memory, and in learning, storing and inferring hierarchical relationships, it is likely also central in social hierarchy.
Indeed, lesions in the hippocampus disrupt hierarchy formation in rodents (Gray and McNaughton, 1983), while hierarchy disruption decreases hippocampal neurogenesis and induces social behavioral changes, such as a preference for familiar over novel animals (Opendak et al., 2016). Dominance hierarchies affect the hippocampus to the level of gene expression, with subordinate mice showing higher expression of serotonin receptor subtypes (Horii et al., 2017). The hippocampus also represents and updates information about hierarchies: hippocampal BOLD activity relates to reading about status hierarchies relative to reading about objects (Muscatell et al., 2012), tracks the emergence of social hierarchies, correlates with confidence in hierarchical rankings (Kumaran et al., 2012), and increases during rank updating (Kumaran et al., 2016).
Work in social psychology suggests another important social dimension in addition to social power: affiliation (e.g., kinship, bonding) is central in social interaction. These two dimensions are important for social relationships in rodents (Insel and Fernald, 2004), nonhuman primates (Brent et al., 2013) and humans (Fiske, 2012). Although there is evidence for the hippocampus processing social hierarchy, evidence is scant for the role of the hippocampus in processing affiliation information. There is some evidence for hippocampal processing in affiliation broadly, as rhesus monkeys with hippocampal lesions respond less to affiliation signals and behaviors (Machado and Bachevalier, 2006). Social psychology theories suggest that dimensions of power and affiliation are computed jointly (Fiske, 2012; Todorov et al., 2008; Wiggins et al., 1989), creating a two-dimensional cognitive map that locates others relative to ourselves in social space (Tavares et al., 2015). Social coordinates of power by affiliation thus may be mapped in a manner akin to two-dimensional egocentric mapping of physical space, providing the scaffold for navigating social relationships (figure 1).
Social navigation and decision-making
Adaptive social behavior requires many similar processes as adaptive navigation: we have to use memory to draw upon experiences, simulate future social situations and infer the mental states of others. These processes (episodic memory, episodic simulation and mentalizing) all activate a common network circuitry, including the hippocampus, retrosplenial cortex, precuneus, PCC and mPFC, roughly the same network that is active during spatial navigation (Spreng et al., 2009). Social space mapping and navigation might engage some of the same mechanisms as cognitive mapping and navigation generally (figure 2).
Figure 2: Navigating through space.
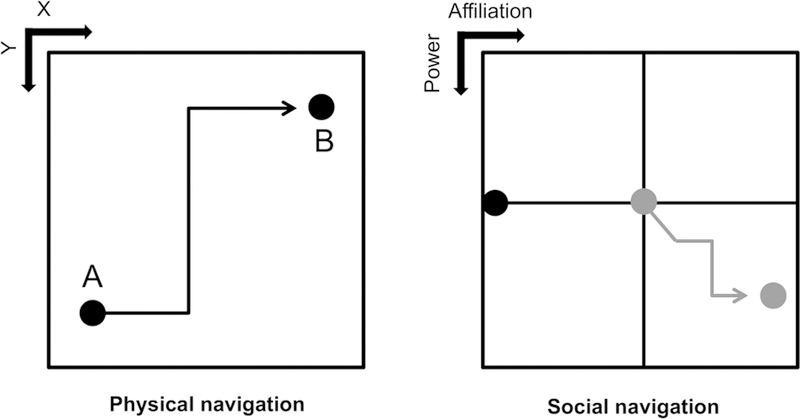
Facilitating navigation through physical space (left panel) is a key function of the spatial cognitive map. Information within the map can be used to simulate trajectories and their consequences, leading to an individual animal (represented by the black dot) navigating adaptively (black trajectory from A to B). Social navigation (right panel) may occur by the same mechanisms, albeit in a more abstract manner. As interactions occur, an individual (grey dot) within the social context will change in power and affiliation (grey trajectory) relative to the observer (black dot). An accurate social map facilitates adaptive social decision making as the interactions accumulate.
Tavares and colleagues (2015) examined this possibility using a role-playing game during fMRI. In the game, participants had just moved to a new town and had to find a job and a place to live. To do this, they interacted with fictional characters and made decisions that altered the relative power and affiliation of the characters. For power interactions, participants could submit to a demand, or refuse or make demands of the characters themselves, increasing or decreasing the relative power of the character. For affiliation interactions, participants could engage in physical touch or personal conversation, or not, increasing or decreasing their affiliation with the character. The choices accumulated into a power by affiliation social coordinate for each character at each decision point throughout the game, from which vector angle and length were calculated using the vector between the participant’s theoretical point of view and the character’s coordinates. Importantly, both of these metrics included information about both the power and affiliation of the character at each decision. The normalized vector angle and length were used as parametric predictors in the fMRI analysis of the decision trials.
As participants made power and affiliation decisions, several different regions had BOLD activity that uniquely correlated with the vector angle to the characters’ locations within the two-dimensional social space. Only one of these regions had activity that correlated with self-reported social skills: the left hippocampus. The relationship between hippocampal activity and the movement of the characters through social space, as measured by the vector angle, was stronger in participants with less self-reported social avoidance and neuroticism. This correlation demonstrates that hippocampus social tracking relates to social skills among healthy individuals, suggesting social cognitive maps are important to real world social behavior.
Hippocampal damage leads to more dramatic and unstable character judgments, perhaps because of an inability to contextualize a person’s current behavior in light of their past behavior, causing an overweighting of current behavioral information (Croft et al., 2010). Taken together with the centrality of the hippocampus in dimensional mapping and the correlation between hippocampus BOLD activity and vector angle in this task, the hippocampus is likely a crucial player in accurately representing the relative social standing of the characters. Under this account, information about an individual, including their identity and previous behavior, facilitates relational mapping of individuals in a power by affiliation social space, reflecting the role of the hippocampus in relational structures more generally.
The well described cell types and circuitry in the hippocampus allows for some speculation on cellular mechanisms. One possibility is that social place cells spiking rate or number of cells recruited is modulated by social information. Research indicates individual hippocampal cells can be sensitive to specific people, responding more to the faces of known people (Kreiman et al., 2000) and invariantly representing the same individual across sensory modalities (Quian Quiroga et al., 2009). It is possible that individual cells, or cell ensembles, can also represent factors such as an individual’s relative power and affiliation. There may even be specialized circuitry (e.g., CA2-CA1) dedicated to representing social dimensions. Another possibility is that reward cells have a more general role that includes social reward: interacting with individuals higher in the social hierarchy might be more rewarding and recruit greater activation.
Two other regions had unique correlations with the vector angle: left inferior parietal lobule and left dorsolateral PFC. The possible roles of these regions are less clear. Inferior parietal lobule, an output for CA1 neurons, was previously implicated in representing distance from oneself in a variety of domains, including spatial, temporal and social familiarity (Parkinson et al., 2014). Additionally, it has been involved in representing spatial locations numerically (Krause et al., 2014) and uncertain decisions (Vickery and Jiang, 2009). One possibility is that the inferior parietal lobule may assist in transforming abstract coordinates into a more concrete form, facilitating distance representation and guiding social decisions under the uncertainty of the social environment.
Dorsolateral PFC can control attention to spatial location (Hagler and Sereno, 2006), influence hippocampal memory expression (Anderson et al., 2004), and may be central in goal-directed and norm-guided behavior, particularly in associating social norms with the value of outcomes (Buckholtz, 2015). What is normative social behavior in any particular situation will depend in large part on social context and might be especially sensitive to the relative power and affiliation of the individuals involved. Dorsolateral PFC, which is connected to the hippocampus, might direct attention to the locations of individuals within the social cognitive map to evaluate social rule-outcome associations given their social standing, ultimately to facilitate social decision-making.
While vector angle is necessary information for locating others in space, the map is incomplete without the distance between the characters and the participant within the space. Only one region related to vector length: posterior cingulate cortex (PCC; extending into precuneus), a region connected with both inferior parietal lobule and the hippocampus (via the retrosplenial cortex). Previous literature has found that PCC contributes to a variety of social cognitive processes: it has been linked to self-referential processes, such as self-judgment (Ochsner et al., 2005) as well as other-referential processes, such as forming and updating first impressions (Schiller et al., 2009; Mende-Siedlecki et al., 2013). Additionally, PCC is anatomically and functionally related to the precuneus, retrosplenial cortex and hippocampal regions: all contribute to the default mode network, a network of areas whose activity is highly correlated with one another and is active when individuals are involved in processes such as remembering and imagining events, thinking about themselves and others, navigating (Spreng et al., 2009)and goal-directed cognition (Spreng et al., 2010).
Mapping properties have been specifically observed in PCC: fMRI research has uncovered a grid-like signal in PCC during the mapping of abstract conceptual dimensions (Constantinescu et al., 2016). Given that grid cells can represent distance (Bush et al., 2015), grid-like representations within PCC might help represent the distance between self and other within social space, with greater PCC signal magnitude reflecting a larger social distance. Calculating social distance might underlie the social functions that have been ascribed to this region, such as mentalizing (i.e., the ability to understand the mental states of others). Consistent with this, an individual’s own social status impacts mentalizing network activity, with lower status individuals showing a greater engagement of this area while encoding social information (Muscatell et al., 2012).
Mentalizing shares similarities with episodic simulation: both require the reconfiguration of mental elements to simulate an experience, and both rely on a similar neural architecture based in the default mode network. Mentalizing operates independently of hippocampus-mediated episodic memory (Rosenbaum et al., 2007), but PCC engagement is seen during the retrieval of autobiographical memories (Maddock et al., 2001) and PCC based memory of one’s own past mental states might inform mental state inferences about others. Social distance might be an important moderating factor in this process. Consistent with this possibility, mentalizing about close others and recalling autobiographical memories recruits many of the same brain areas, including the hippocampus, retrosplenial cortex, PCC and precuneus whereas mentalizing about unfamiliar others does not, suggesting different neural systems subserve these processes (Rabin and Rosenbaum, 2012). While the direction of PCC activation in this study seems to differ from Tavares and colleagues (2015), it may be that mentalizing about known individuals involves social distance representation from one self, whereas mentalizing about unknown individuals may be supported by different mechanisms altogether.
Mentalizing may in part function to improve social decision-making via social predictions. PCC tracked the social vector length at each decision point, when social decisions could affect the mental states and behaviors of others and therefore impact ones own social trajectory. Both passively watching social interactions (Iacoboni et al., 2004) and engaging in social interactions (Rilling et al., 2004) recruit PCC and precuneus, suggesting mentalizing processes may be engaged in support of social decision-making. Speculating from this evidence, PCC grid-like representations might compute social distance, which in turn informs autobiographical memory-mediated mental state inferences.
The correlations between hippocampal social tracking and self-reported social skills suggest that social mapping is important to real world functioning. Social avoidance behaviors, which have been linked to hippocampal function (Lagace et al., 2010), might in part reflect dysfunctional social cognitive mapping, at least among healthy individuals. This opens another possibility: hippocampal dysfunction might cause inaccurate or unstable social maps and lead to psychosocial psychiatric impairments.
Clinical disorders often implicate both hippocampal and social dysfunction, suggesting a link between the two. Schizophrenia patients show dysfunctional social behavior (Fett et al., 2011), as well as reduced hippocampal volume relative to healthy individuals (Adriano et al., 2012). Additionally, they have been found to have reductions in non-pyramidal neurons in CA2 (Benes et al., 1998) including in parvalbumin expressing interneurons (Knable et al., 2004), interesting findings given the significance of CA2 in social memory and behavior. Major depressive disorder is also linked with both hippocampal and social dysfunction. Smaller hippocampal volume in depressed versus healthy individuals is a consistent finding (MacQueen and Frodl, 2011) and ventral hippocampus connections with the nucleus accumbens might be especially important in depressive-like behaviors in rodents (Bagot et al., 2015). Individuals with major depressive disorder also show impaired social functioning (Segrin, 2000). Autism spectrum disorder (ASD) features profound social dysfunction, characterized by an inability to regulate social interactions (Leekam, 2016) as well as hippocampal abnormalities (Amaral et al., 2008).
This review underscores this point: if the social map is instantiated in these circuits social dysfunction should accompany circuit dysfunction. Impaired tracking of dimensions such as power and affiliation might explain some of the social behavior problems observed in these disorders. There are three broad ways in which this could occur. First, map inputs could be dysfunctional. This may include information from structures like the amygdala, which shares bilateral connections with the hippocampus, is an important input region to the hippocampus in social behaviors (Felix-Ortiz and Tye, 2014) and may help drive map properties, such as saliency representation. Second, mapping processes themselves could be dysfunctional, as a result of dysfunction within the hippocampus and related structures. Social stress could induce plasticity within the CA2 to CA1 circuit and via CA1 modulation, for example, affecting both social and non-social hippocampal functions. Third, the map could be misread. Even in cases of normal mapping, downstream regions (e.g., PFC) could be impaired, leading to aberrant social decision-making. For instance, vmPFC volume predicts mentalizing capabilities and social network size (Lewis et al., 2011). Given other work showing spatial overlap between cognitive mapping and mentalizing related regions (Spreng et al., 2009), and that hippocampal measures predict social network size (Stiller and Dunbar, 2007), the interaction between these regions may be important in both healthy and unhealthy social cognition. This framework offers a basic path forward but much more work is needed to precisely delineate the ways in which neural social mapping may relate to social behavior deficits.
Conclusions
We have argued that a common set of computations underlies different types of cognitive maps and their respective forms of “navigation.” Additionally, we have claimed there is a common neurobiology that implements these computations, in regions including the hippocampal formation, retrosplenial cortex, PCC, precuneus and PFC (figure 3). These functions may reflect a multi-dimensional map of the statistical relations between environmental elements, the so-called “cognitive map”. According to this view, contextual information, from spatial to conceptual, is represented, learned and integrated into a relational memory framework, which can be searched to simulate and generate predictions about trajectories. In particular, we explored the social variant of this idea, the social cognitive map. Evidence shows that the hippocampus and related regions encode social information, such as the physical location of conspecifics, information about specific individuals, and power and affiliation in an abstract two-dimensional social space. We argued that social information is relationally mapped to generate an internal model of the social environment - the social space. This mapping may engage the same mechanisms as spatial mapping but there may be additional social computations recruited as well, within specific hippocampal cell populations (e.g., social place cells), subfields (e.g., CA2) and circuitry (e.g., CA2 to CA1). Different pieces of information (e.g., episodic memories, behavioral information) about an individual may relate to specific social coordinates within this social space. Therefore, cue-independent (i.e., across cues and cue types) activation related to an individual may reflect the concept of that individual and their mapping within the social space. Adaptively navigating social interactions might depend on this map.
Figure 3: Visual summary of the current evidence on the neural systems mediating navigational computations in spatial, non-spatial and abstract domains.
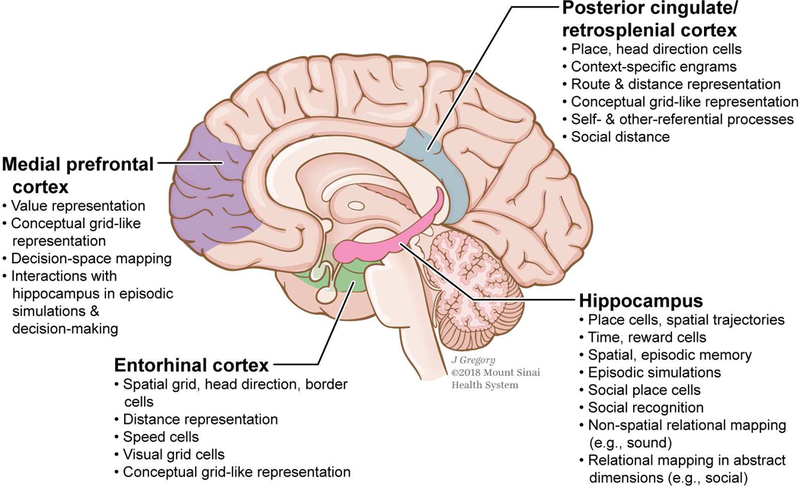
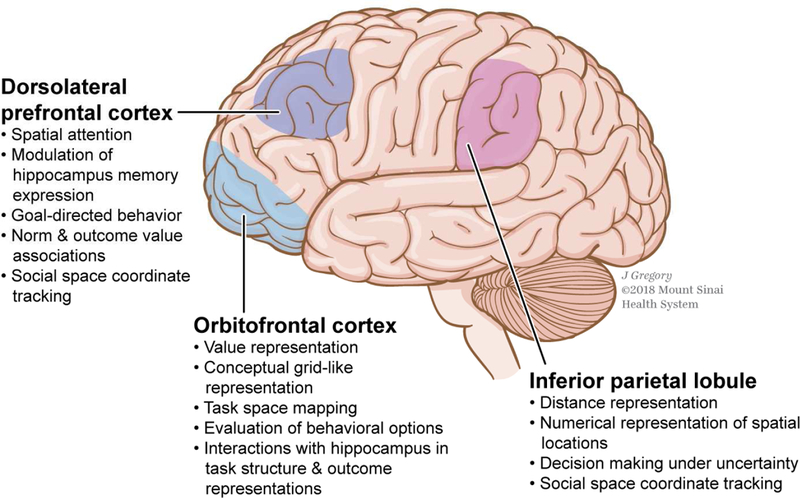
Key regions in cognitive mapping and some of their relevant functions are highlighted. Colors indicate approximate functional distinctions.
Thinking about social interaction in this way allows the field to advances in several ways. For one, applying the cognitive mapping framework to social interaction gives researchers a large literature (place cells, episodic memory, etc.) from which to generate hypotheses and gather methods. The merger of social cognition with cognitive mapping is likely to generate many new testable predictions (Box 1). Relatedly, this point of view offers specific candidate neural mechanisms which may underlie social processes (e.g., social place cells), which can be explored in depth in model organisms. For human work, Tavares and colleagues (2015) generated a naturalistic, role-playing task that more closely models real-world behavior than traditional social cognitive experiments. This task captures movement through social space as social trajectories, allowing a rich exploration of dynamic social relationships. Ecologically valid experiments will be crucial to exploring complex social behaviors. The social cognitive map also provides a novel route forward for examining social deficits in clinical disorders: links may exist between dysfunctional social cognitive mapping and social behavior. Many other questions remain to be explored and while social cognitive mapping research is in its early stages, its growth is sure to provide a valuable new perspective for social cognitive neuroscience.
Box 1: Outstanding Issues.
How do maps in different domains, such as place, time, reward and social, interact to form a “life space” and guide episodic memory encoding and behavior? On a cellular level, one possible mechanism is conjunctive encoding - cells that fire optimally to the combination of different factors and may integrate these factors into a single representation.
What are the properties of hippocampal social cells and social space mapping? Clarifying these properties can probe mechanisms of representation, integration and the domain specificity of the network’s computations. Questions include whether cell populations overlap between spatial, social and other types of cells; whether they exhibit replay and preplay; and whether they remap (an individual’s coordinates might depend upon social context, e.g., work versus an after work happy hour).
How do reference frames (i.e., mapping from the observer’s point of view, or egocentric; object to object mapping, or allocentric) play out across domains? In spatial mapping these frames can be hard to separate: for example, place and grid fields are often not simply allocentric, as they can be modulated by variables like head direction and running speed - egocentric information. It is also possible that there are separate systems for reference frames, or that the brain does not respect this reference frame distinction, and we need new ways to conceptualize reference framing in cognitive mapping.
Does system consolidation (i.e., hippocampal memories become independent of the hippocampus over time) apply to other domains? For example, it is possible that long-term information regarding an individual’s power and affiliation eventually becomes hippocampus independent.
How is within-individual social information mapped and stored? A single social coordinate could index a wide variety of information related to that specific individual. A related question concerns the neural representation of individuals within the space – how are individuals with adjacent social space coordinates discriminated despite similar locations?
How does the structure and shape of cognitive maps affect behavior and decision-making? As Tolman predicted, could “narrow” or distorted maps impair behavioral functioning and lead to aberrant decision-making? The study of psychosocial and cognitive symptoms may benefit from testing within such an explicit computational and relational framework.
This review highlights emerging work that suggests the hippocampal formation encodes relational maps in spatial, non-spatial and abstract domains. Additionally, this review argues that these functions extend to the social domain – and may include specialized social computations.
Acknowledgments
Funding was provided by Klingenstein-Simons Fellowship Award in the Neurosciences to D.S.; and by T32AG049688 to M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriano F, Caltagirone C, and Spalletta G (2012). Hippocampal volume reduction in first-episode and chronic schizophrenia: A review and meta-analysis. Neuroscientist 18, 180–200. [DOI] [PubMed] [Google Scholar]
- Alexander AS, and Nitz DA (2017). Spatially Periodic Activation Patterns of Retrosplenial Cortex Encode Route Sub-spaces and Distance Traveled. Curr. Biol 27, 1551–1560. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Farris S, Pirone JR, Zheng C, Colgin LL, and Dudek SM (2016). Social and novel contexts modify hippocampal CA2 representations of space. Nat. Commun 7, 10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, and Turk-Browne NB (2016a). Attention promotes episodic encoding by stabilizing hippocampal representations. Proc. Natl. Acad. Sci 113, E420–E429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, and Turk-Browne NB (2016b). Attention Stabilizes Representations in the Human Hippocampus. Cereb. Cortex 26, 783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, Knight RT, and Yonelinas AP (2010). Faces are special but not too special: Spared face recognition in amnesia is based on familiarity. Neuropsychologia 48, 3941–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, Ranganath C, and Yonelinas AP (2013). Detecting Changes in Scenes: The Hippocampus Is Critical for Strength-Based Perception. Neuron 78, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, and Nordahl CW (2008). Neuroanatomy of autism. Trends Neurosci 31, 137–145. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Cooper J, Roberston E, Gabrieli SW, Glover GH, and Gabrieli JD (2004). Neural systems underlying the suppression of unwanted memories. Science 303, 232–235. [DOI] [PubMed] [Google Scholar]
- Aronov Dmitry, Nevers Rhin, Tank DW (2017). Mapping of a non-spatial dimension by the hippocampal/entorhinal circuit. Nature 543, 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Parise EM, Peña CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolaños-Guzmán CA, Cheer J, et al. (2015). Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun 6, 7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaguer J, Spiers H, Hassabis D, and Summerfield C (2016). Neural Mechanisms of Hierarchical Planning in a Virtual Subway Network. Neuron 90, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron HC, Dolan RJ, and Behrens TEJ (2013). Online evaluation of novel choices by simultaneous representation of multiple memories. Nat. Neurosci 16, 1492–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, and Todtenkopf MS (1998). A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol. Psychiatry 44, 88–97. [DOI] [PubMed] [Google Scholar]
- Bird CM, Shallice T, and Cipolotti L (2007). Fractionation of memory in medial temporal lobe amnesia. Neuropsychologia 45, 1160–1171. [DOI] [PubMed] [Google Scholar]
- Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, Pate Skene J.H., and Platt ML (2013). Genetic origins of social networks in rhesus macaques. Sci. Rep 3, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW (2015). Social norms, self-control, and the value of antisocial behavior. Curr. Opin. Behav. Sci 3, 122–129. [Google Scholar]
- Bush D, Barry C, Manson D, and Burgess N (2015). Using Grid Cells for Navigation. Neuron 87, 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Madore KP, Benoit RG, Thakral PP, and Schacter DL (2018). Increased hippocampus to ventromedial prefrontal connectivity during the construction of episodic future events. Hippocampus 28, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrastil ER, Sherrill KR, Hasselmo ME, and Stern CE (2015). There and Back Again: Hippocampus and Retrosplenial Cortex Track Homing Distance during Human Path Integration. J. Neurosci 35, 15442–15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, and Moser MB (2008). Understanding memory through hippocampal remapping. Trends Neurosci 31, 469–477. [DOI] [PubMed] [Google Scholar]
- Collett TS, and Graham P (2004). Animal navigation: Path integration, visual landmarks and cognitive maps. Curr. Biol 14, 475–477. [DOI] [PubMed] [Google Scholar]
- Constantinescu AO, O’Reilly JX, and Behrens TEJ (2016). Organizing conceptual knowledge in humans with a gridlike code. Science 352, 1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copara MS, Hassan AS, Kyle CT, Libby LA, Ranganath C, and Ekstrom AD (2014). Complementary Roles of Human Hippocampal Subregions during Retrieval of Spatiotemporal Context. J. Neurosci 34, 6834–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington NV, Brown-Schmidt S, and Duff MC (2018). The Necessity of the Hippocampus for Statistical Learning. J. Cogn. Neurosci 30, 680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft KE, Duff MC, Kovach CK, Anderson SW, Adolphs R, and Tranel D (2010). Detestable or marvelous? Neuroanatomical correlates of character judgments. Neuropsychologia 48, 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo T, Toyoizumi T, and Fujisawa S (2018). Spatial representations of self and other in the hippocampus. Science 359, 213–218. [DOI] [PubMed] [Google Scholar]
- Davachi L, and DuBrow S (2015). How the hippocampus preserves order: the role of prediction and context. Trends Cogn. Sci 19, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PSR, Drouin H, Kwan D, Moscovitch M, and Rosenbaum RS (2012). Memory as social glue: Close interpersonal relationships in amnesic patients. Front. Psychol 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuker L, Bellmund JL, Navarro Schröder T, and Doeller CF (2016). An event map of memory space in the hippocampus. Elife 5, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CCF, Barry C, and Burgess N (2010). Evidence for grid cells in a human memory network. Nature 463, 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, and Tonegawa S (2013). Distinct preplay of multiple novel spatial experiences in the rat. Proc. Natl. Acad. Sci 110, 9100–9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Alexander GM, and Farris S (2016). Rediscovering area CA2: unique properties and functions. Nat. Rev. Neurosci 17, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, and Eichenbaum H (1997). The hippocampus and memory for orderly stimulus relations. Proc. Natl. Acad. Sci 94, 7109–7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2013). Memory on time. Trends Cogn. Sci 17, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2014). Time cells in the hippocampus: a new dimension for mapping memories. Nat. Rev. Neurosci 15, 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, and Cohen NJ (2014). Can We Reconcile the Declarative Memory and Spatial Navigation Views on Hippocampal Function? Neuron 83, 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, and Tye KM (2014). Amygdala Inputs to the Ventral Hippocampus Bidirectionally Modulate Social Behavior. J. Neurosci 34, 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, and Shapiro ML (2003). Prospective and retrospective memory coding in the hippocampus. Neuron 40, 1227–1239. [DOI] [PubMed] [Google Scholar]
- Fett AKJ, Viechtbauer W, Dominguez M de G, Penn DL, van Os J, and Krabbendam L (2011). The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neurosci. Biobehav. Rev 35, 573–588. [DOI] [PubMed] [Google Scholar]
- Fiske ST (2012). Journey to the edges: Social structures and neural maps of inter-group processes. Br. J. Soc. Psychol 51, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KE, Marín-Gutiérrez A, Ragland JD, and Ranganath C (2014). Brain Mechanisms of Successful Recognition through Retrieval of Semantic Context. J. Cogn. Neurosci 26, 1694–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyja Ólafsdóttir H, Barry C, Saleem AB, Ha ssabis D, and Spiers HJ (2015). Hippocampal place cells construct reward related sequences through unexplored space. Elife 4, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier JL, and Tank DW (2018). A Dedicated Population for Reward Coding in the Hippocampus. Neuron 99, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, and McNaughton N (1983). Comparison between the behavioural effects of septal and hippocampal lesions: A review. Neurosci. Biobehav. Rev 7, 119–188. [DOI] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MAA, Touretzky DS, and Redish AD (2010). Hippocampal Replay Is Not a Simple Function of Experience. Neuron 65, 695– 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M-B, and Moser EI (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, and Sereno MI (2006). Spatial maps in frontal and prefrontal cortex. Neuroimage 29, 567–577. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, and Knierim JJ (2005). Major Dissociation Between Medial and Lateral Entorhinal Input to Dorsal Hippocampus. Science (80-. ) 308, 1792–1794. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, and Maguire EA (2007). Patients with hippocampal amnesia cannot imagine new experiences. Proc. Natl. Acad. Sci 104, 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, and Siegelbaum SA (2014). The hippocampal CA2 region is essential for social memory. Nature 508, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V, Lenck-Santini P-P, Roux S, Save E, Muller RU, and Poucet B (2007). Goal-Related Activity in Hippocampal Place Cells. J. Neurosci 27, 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Y, Nagasawa T, Sakakibara H, Takahashi A, Tanave A, Matsumoto Y, Nagayama H, Yoshimi K, Yasuda MT, Shimoi K, et al. (2017). Hierarchy in the home cage affects behaviour and gene expression in group-housed C57BL/6 male mice. Sci. Rep 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LR, Javadi AH, Yu Y, Mill RD, Morrison LC, Knight R, Loftus MM, Staskute L, and Spiers HJ (2014). The Hippocampus and Entorhinal Cortex Encode the Path and Euclidean Distances to Goals during Navigation. Curr. Biol 24, 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høydal ØA, Skytøen ER, Moser M, and Moser EI (2018). Object-vector coding in the medial entorhinal cortex. BioRxiv [DOI] [PubMed]
- Iacoboni M, Lieberman MD, Knowlton BJ, Molnar-Szakacs I, Moritz M, Throop CJ, and Fiske AP (2004). Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage 21, 1167–1173. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser M-B, and Moser EI (2014). Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature 1, 1–20. [DOI] [PubMed] [Google Scholar]
- Insel TR, and Fernald RD (2004). HOW THE BRAIN PROCESSES SOCIAL INFORMATION: Searching for the Social Brain. Annu. Rev. Neurosci 27, 697– 722. [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, and Frank LM (2012). Awake Hippocampal Sharp-Wave Ripples Support Spatial Memory. Science 1454, 1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi AH, Emo B, Howard LR, Zisch FE, Yu Y, Knight R, Pinelo Silva J., and Spiers HJ (2017). Hippocampal and prefrontal processing of network topology to simulate the future. Nat. Commun 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian JB, Keinath AT, Frazzetta G, and Epstein RA (2018). Human entorhinal cortex represents visual space using a boundary-anchored grid. Nat. Neurosci 21, 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R, Schuck NW, and Doeller CF (2017). The Role of Mental Maps in Decision-Making. Trends Neurosci 40, 256–259. [DOI] [PubMed] [Google Scholar]
- Killian NJ, Jutras MJ, and Buffalo EA (2012). A map of visual space in the primate entorhinal cortex. Nature 491, 761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, and Torrey EF (2004). Molecular abnormalities of the hippocampus in severe psychiatric illness: Postmortem findings from the Stanley Neuropathology Consortium. Mol. Psychiatry 9, 609–620. [DOI] [PubMed] [Google Scholar]
- Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, et al. (2014). Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat. Neurosci 17, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, and Eichenbaum H (2009). Robust Conjunctive Item-Place Coding by Hippocampal Neurons Parallels Learning What Happens Where. J. Neurosci 29, 9918–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Brandon MP, Robinson RJ, Connerney MA, Hasselmo ME, and Eichenbaum H (2015). During Running in Place, Grid Cells Integrate Elapsed Time and Distance Run. Neuron 88, 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause F, Lindemann O, Toni I, and Bekkering H (2014). Different Brains Process Numbers Differently: Structural Bases of Individual Differences in Spatial and Nonspatial Number Representations. J. Cogn. Neurosci 26, 768–776. [DOI] [PubMed] [Google Scholar]
- Kreiman G, Koch C, and Fried I (2000). Category-specific visual responses of single neurons in the human. Nat. Neurosci 3, 946–953. [DOI] [PubMed] [Google Scholar]
- Kropff E, Carmichael JE, Moser MB, and Moser EI (2015). Speed cells in the medial entorhinal cortex. Nature 523, 419–424. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Melo HL, and Duzel E (2012). The Emergence and Representation of Knowledge about Social and Nonsocial Hierarchies. Neuron 76, 653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Banino A, Blundell C, Hassabis D, and Dayan P (2016). Computations Underlying Social Hierarchy Learning: Distinct Neural Mechanisms for Updating and Representing Self-Relevant Information. Neuron 92, 1135– 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, and Eisch AJ (2010). Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci 107, 4436–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, and Lavenex P (2006). Hippocampal Lesion Prevents Spatial Relational Learning in Adult Macaque Monkeys. J. Neurosci 26, 4546– 4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lavilléon G, Lacroix MM, Rondi-Reig L, and Benchenane K (2015). Explicit memory creation during sleep demonstrates a causal role of place cells in navigation. Nat. Neurosci 18, 493–495. [DOI] [PubMed] [Google Scholar]
- Leekam S (2016). Social cognitive impairment and autism: What are we trying to explain? Philos. Trans. R. Soc. B Biol. Sci 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, Brann DH, Meira T, and Siegelbaum SA (2017). Input-Timing-Dependent Plasticity in the Hippocampal CA2 Region and Its Potential Role in Social Memory. Neuron 95, 1089–1102. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Rezaie R, Brown R, Roberts N, and Dunbar RIM (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage 57, 1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, and Redish AD (2017). Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci 20, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, and Eichenbaum H (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 71, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, and Bachevalier J (2006). The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav. Neurosci 120, 761–786. [DOI] [PubMed] [Google Scholar]
- MacQueen G, and Frodl T (2011). The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research. Mol. Psychiatry 16, 252–264. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, and Buonocore MH (2001). Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104, 667–676. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, and Frith CD (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci 97, 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, and Spiers HJ (2006). Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain 129, 2894–2907. [DOI] [PubMed] [Google Scholar]
- Mankin EA, Diehl GW, Sparks FT, Leutgeb S, and Leutgeb JK (2015). Hippocampal CA2 Activity Patterns Change over Time to a Larger Extent than between Spatial Contexts. Neuron 85, 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, and Eichenbaum H (2009). A cognitive map for object memory in the hippocampus A cognitive map for object memory in the hippocampus 616–624. [DOI] [PMC free article] [PubMed]
- Marchette SA, Bakker A, and Shelton AL (2011). Cognitive Mappers to Creatures of Habit: Differential Engagement of Place and Response Learning Mechanisms Predicts Human Navigational Behavior. J. Neurosci 31, 15264–15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes AR, Isaac CL, Holdstock JS, Hunkin NM, Montaldi D, Downes JJ, MacDonald C, Cezayirli E, and Roberts JN (2001). Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cogn. Neuropsychol 18, 97–123. [DOI] [PubMed] [Google Scholar]
- McCormick C, Rosenthal CR, Miller TD, and Maguire EA (2018). Mind-wandering in people with hippocampal damage. J. Neurosci 1812–1817. [DOI] [PMC free article] [PubMed]
- McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, and Eichenbaum H (2014). Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron 83, 202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, and Moser MB (2006). Path integration and the neural basis of the “cognitive map.” Nat. Rev. Neurosci 7, 663–678. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Baron SG, and Todorov A (2013). Diagnostic Value Underlies Asymmetric Updating of Impressions in the Morality and Ability Domains. J. Neurosci 33, 19406–19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milczarek MM, Vann SD, and Sengpiel F (2018). Spatial Memory Engram in the Mouse Retrosplenial Cortex. Curr. Biol 28, 1975–1980. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AMP, Vedder LC, Law LM, and Smith DM (2014). Cues, context, and long-term memory: the role of the retrosplenial cortex in spatial cognition. Front. Hum. Neurosci 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagrin A, Saiote C, and Schiller D (2017). The social hippocampus. Hippocampus 1–8. [DOI] [PubMed]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, Lieberman MD, Dapretto M, and Eisenberger NI (2012). Social status modulates neural activity in the mentalizing network. Neuroimage 60, 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, and Nadel L (1978). The hippocampus as a cognitive map (Oxford: Clarendon press; ). [Google Scholar]
- O’Neill J, Boccara CN, Stella F, Schoenenberger P, and Csicsvari J (2017). Superficial Layers of the Medial Entorhinal Cortex Replay Independent of the Hippocampus. Science 188, 184–188. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JDE, Kihsltrom JF, and D’Esposito M (2005). The neural correlates of direct and reflected self-knowledge. Neuroimage 28, 797–814. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, and Tonegawa S (2016). Ventral CA1 neurons store social memory. Science 353, 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer DB, Maimon SR, Las L, and Ulanovsky N (2018). Social place-cells in the bat hippocampus. Science 359, 218–224. [DOI] [PubMed] [Google Scholar]
- Opendak M, Offit L, Monari P, Schoenfeld TJ, Sonti AN, Cameron HA, and Gould E (2016). Lasting Adaptations in Social Behavior Produced by Social Disruption and Inhibition of Adult Neurogenesis. J. Neurosci 36, 7027–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson C, Liu S, and Wheatley T (2014). A Common Cortical Metric for Spatial, Temporal, and Social Distance. J. Neurosci 34, 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patai EZ, Javadi A-H, Ozubko JD, O’Callaghan A, Ji S, Robin J, Grady C, Winocur G, Rosenbaum SR, Moscovitch M, et al. (2017). Long-term consolidation switches goal proximity coding from hippocampus to retrosplenial cortex. BioRxiv 167882. [DOI] [PMC free article] [PubMed]
- Pfeiffer BE (2018). The content of hippocampal “replay.” Hippocampus 1–13. [DOI] [PMC free article] [PubMed]
- Pfeiffer BE, and Foster DJ (2013). Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorowski RA, and Chevaleyre V (2018). Memory circuits: CA2. Curr. Opin. Neurobiol 52, 54–59. [DOI] [PubMed] [Google Scholar]
- Place R, Farovik A, Brockmann M, and Eichenbaum H (2016). Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat. Neurosci 19, 992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucet B, and Hok V (2017). Remembering goal locations. Curr. Opin. Behav. Sci 17, 51–56. [Google Scholar]
- Quian Quiroga R., Kraskov A, Koch C, and Fried I (2009). Explicit Encoding of Multimodal Percepts by Single Neurons in the Human Brain. Curr. Biol 19, 1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, and Fried I (2005). Invariant visual representation by single neurons in the human brain. Nature 435, 1102–1107. [DOI] [PubMed] [Google Scholar]
- Rabin JS, and Rosenbaum RS (2012). Familiarity modulates the functional relationship between theory of mind and autobiographical memory. Neuroimage 62, 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, and Ritchey M (2012). Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci 13, 713–726. [DOI] [PubMed] [Google Scholar]
- Redish AD (2016). Vicarious trial and error. Nat. Rev. Neurosci 17, 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, and Cohen JD (2004). The neural correlates of theory of mind within interpersonal interactions. Neuroimage 22, 1694–1703. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Stuss DT, Levine B, and Tulving E (2007). Theory of mind is independent of episodic memory. Science 318, 1257. [DOI] [PubMed] [Google Scholar]
- Sanders HI, and Warrington EK (1971). Memory for remote events in amnesic patients. Brain 94, 661–668. [DOI] [PubMed] [Google Scholar]
- Sarel A, Finkelstein A, Las L, and Ulanovsky N (2017). Vectorial representation of spatial goals in the hippocampus of bats. Science (80-. ) 355, 176–180. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, and Moser M-B (2006). Conjunctive Representation of Position, Direction, and Velocity in Entorhinal Cortex. Science 312, 758–762. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, and Szpunar KK (2012). The Future of Memory: Remembering, Imagining, and the Brain. Neuron 76, 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, and Phelps EA (2009). A neural mechanism of first impressions. Nat. Neurosci 12, 508–514. [DOI] [PubMed] [Google Scholar]
- Schuck NW, Cai MB, Wilson RC, and Niv Y (2016). Human Orbitofrontal Cortex Represents a Cognitive Map of State Space. Neuron 91, 1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrin C (2000). Social skills deficits associated with depression. Clin. Psychol. Rev 20, 379–403. [DOI] [PubMed] [Google Scholar]
- Silva D, Feng T, and Foster DJ (2015). Trajectory events across hippocampal place cells require previous experience. Nat. Neurosci 18, 1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Williams Avram S.K., Cymerblit-Sabba A, Song J, and Young WS (2016). Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry 21, 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T (2008). Representation of Geometric Bordersin the Entorhinal Cortex. Science 1865, 1–5. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, and Maguire EA (2006). Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage 31, 1826–1840. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, and Kim ASN (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J. Cogn. Neurosci 21, 489–510. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, and Schacter DL (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53, 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas KV, Buss EW, Sun Q, Santoro B, Takahashi H, Nicholson DA, and Siegelbaum SA (2017). The Dendrites of CA2 and CA1 Pyramidal Neurons Differentially Regulate Information Flow in the Cortico-Hippocampal Circuit. J. Neurosci 37, 3276–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AP, and Redish AD (2012). The road not taken: Neural correlates of decision making in orbitofrontal cortex. Front. Neurosci 6, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensola H, Stensola T, Solstad T, FrØland K, Moser MB, and Moser EI (2012). The entorhinal grid map is discretized. Nature 492, 72–78. [DOI] [PubMed] [Google Scholar]
- Stiller J, and Dunbar RIM (2007). Perspective-taking and memory capacity predict social network size. Soc. Networks 29, 93–104. [Google Scholar]
- Strange BA, Witter MP, Lein ES, and Moser EI (2014). Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci 15, 655–669. [DOI] [PubMed] [Google Scholar]
- Suthana NA, Ekstrom AD, Moshirvaziri S, Knowlton B, and Bookheimer SY (2009). Human Hippocampal CA1 Involvement during Allocentric Encoding of Spatial Information. J. Neurosci 29, 10512–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, and Schiller D (2015). A Map for Social Navigation in the Human Brain. Neuron 87, 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki S, Kumar S, von Kriegstein K, Stewart L, Lyness CR, Moore BCJ, Capleton B, and Griffiths TD (2012). Navigating the Auditory Scene: An Expert Role for the Hippocampus. J. Neurosci 32, 12251–12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Gobbini MI, Evans KK, and Haxby JV (2007). Spontaneous retrieval of affective person knowledge in face perception. Neuropsychologia 45, 163–173. [DOI] [PubMed] [Google Scholar]
- Todorov A, Said CP, Engell AD, and Oosterhof NN (2008). Understanding evaluation of faces on social dimensions. Trends Cogn. Sci 12, 455–460. [DOI] [PubMed] [Google Scholar]
- Tolman EC (1948). Cognitive maps in rats and men. Psychol. Rev 55, 189–208. [DOI] [PubMed] [Google Scholar]
- Trinkler I, King JA, Doeller CF, Rugg MD, and Burgess N (2009). Neural bases of autobiographical support for episodic recollection of faces. Hippocampus 19, 718–730. [DOI] [PubMed] [Google Scholar]
- Tsao A, Moser M-B, and Moser EI (2013). Traces of Experience in the Lateral Entorhinal Cortex. Curr. Biol 23, 399–405. [DOI] [PubMed] [Google Scholar]
- Vickery TJ, and Jiang YV (2009). Inferior parietal lobule supports decision making under uncertainty in humans. Cereb. Cortex 19, 916–925. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, Quiroga RQ, and Fried I (2009). Human medial temporal lobe neurons respond preferentially to personally relevant images. Proc. Natl. Acad. Sci 106, 21329–21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JS, Phillips N, and Trapnell P (1989). Circular reasoning about interpersonal behavior: Evidence concerning some untested assumptions underlying diagnostic classification. J. Pers. Soc. Psychol 56, 296–305. [Google Scholar]
- Wikenheiser AM, and Schoenbaum G (2016). Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat. Rev. Neurosci 17, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Marrero-Garcia Y, and Schoenbaum G (2017). Suppression of Ventral Hippocampal Output Impairs Integrated Orbitofrontal Encoding of Task Structure. Neuron 95, 1197–1207. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee LTS, Hannula DE, Tranel D, and Cohen NJ (2014). Short-term retention of relational memory in amnesia revisited: accurate performance depends on hippocampal integrity. Front. Hum. Neurosci 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, and Palkovits M (2006). The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143, 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]


