Abstract
Blum’s laboratory first showed the benefits of naloxone or narcotic antagonists in the treatment of alcohol dependence. This seminal work published in Nature in the early 70’s, in conjunction with many other studies, later served as the basis for the development of the narcotic antagonist (NTX) now used to treat both alcohol and opioid dependence. In 2006 an extended-release injectable of Naltrexone (XR-NTX) was approved by the FDA. Naltrexone is a relatively weak antagonist of κ- and δ-receptors and is also a potent μ-receptor antagonist. Dosages of naltrexone that effectively reduce opioid and alcohol consumption also actively block μ-receptors, but chronically down-regulate mesolimbic dopamine release. While studies show benefit especially in the short term, there is ongoing evidence that the retention and compliance with NTX are not sufficient to characterize adherence as high. However, extended-release NTX opioid treatment is associated with superior outcomes including less likely relapse (defined as daily use), and much longer time to relapse despite higher rates of concurrent non-opioid substance use like cocaine. Regarding long-term extended-release injectable (XR-NTX) for opioid dependence; there was higher compliance with Opioid Use Disorder (OUD) than for Alcohol Use Disorder (AUD.). Consideration of modalities in combination with XR-NTX is imperative. Research by Blum., et al. showed that a combination of Naltrexone and a pro-dopamine regulator neuro-nutrient (KB220) significantly prevented opioid relapse. Thus, early identification of addiction vulnerability with the Genetic Addiction Risk Score (GARS™) a panel of polymorphic risk alleles from ten reward circuitry genes will provide valuable information especially as it relates to genetically guided therapy with the KB220 neuro nutrient termed ‘Precision Addiction Management”.
Keywords: Extended-Release Injectable Naltrexone (XR-NTX), Reward Deficiency, Neuronutrient, Combination Therapy, Opioid Treatment
Introduction
In the early 70’s Blum’s laboratory was the first to show the benefits of Naltrexone (NTX) or narcotic antagonists in the treatment of alcohol dependence published in Nature [1]. This seminal work, in conjunction with many other studies, later served as the basis for the development of the narcotic antagonist Naltrexone now used to treat both opioid and alcohol dependence. In fact, naltrexone is a relatively weak antagonist of κ- and δ-receptors and a potent μ-receptor antagonist, dosages of NTX that effectively reduce opioid and alcohol consumption also actively block μ-receptors, but chronically down-regulate, mesolimbic dopamine release [2]. In 2006 extended-release injectable Naltrexone (XR-NTX) was approved by the FDA.
While many studies show the benefit of NTX, especially in the short term, there is ongoing evidence that the retention and compliance are not sufficient to characterize adherence to treatment as high [3]. However, opioid treatment with extended-release NTX associated with superior outcomes and less likely relapse (defined as daily use), with a longer time to relapse, despite higher rates of concurrent non-opioid substance use like cocaine. Specifically, a meta-analysis, of 22 randomized, controlled trials, found only 3 (14%) met criteria for high levels of adherence assurance, 5 (23%) met medium adherence assurance criteria, and 14 (64%) met low adherence criteria. Moreover, Spearman correlation between risk ratios (for naltrexone vs. placebo) return to heavy drinking and the level of adherence assurance (low vs. medium vs. high) was significant (r = −.62, p = .025). Regarding long-term XR-NTX for opioid dependence, there was higher compliance with Opioid Use Disorder (OUD) than for Alcohol Use Disorder (AUD). After completion of the study, most participants discontinued treatment with XR-NTX primarily due to “feeling cured” and “wanting to do it on my own” rather than external barriers such as cost or side effects [4]. We suggest that to improve adherence to treatment, consideration of NTX in combination with other modalities is imperative. An exploratory trial by Blum., et al. showed that a combination of NTX and a pro-dopamine regulator neuro-nutrient (KB220) significantly prevented opioid relapse [5,6].
The hypothesis is that prevention of opioid dependence relapse is a function of coupling a pro-dopamine regulator KB220PAM and XR-NTX, especially, in patients with identified genetic addiction risk for Reward Deficiency Syndrome (RDS). Neuro nutrient therapy termed “Precision Addiction Management” based on early identification with the Genetic Addiction Risk Score (GARS) can be used to up-regulate required dopaminergic activity [7] while balancing dopamine in caudate-nucleus and cerebellum [8].
Relapse prevention combining pro-dopamine regulator (KB220) and naltrexone (NTX) in rapid detoxification of opioid dependent (methadone) patients
Over two decades ago, a rapid method to detoxify either methadone or heroin-dependent subjects utilizing NTX was of interest in chemical dependency treatment centers throughout the United States, and worldwide. In 2004 Blum., et al. tested the hypothesis that combining narcotic antagonists, and amino-acid therapy now KB220PAM might promote neuronal dopamine release and enhance compliance in methadone patients rapidly detoxified with the narcotic antagonist NTX (Trexan; Dupont, Delaware). The amino-acid therapy used in the experiment consisted of an enkephalinase inhibitor (D-phenylalanine) and neurotransmitter precursors (L-amino-acids). In the pilot study Blum’s group [5,6] found that the combination of NTX and amino-acids resulted in significantly enhanced compliance; the patients prolonged their treatment with NTX and consequently, reduced relapse (see Figure 1).
Figure 1:
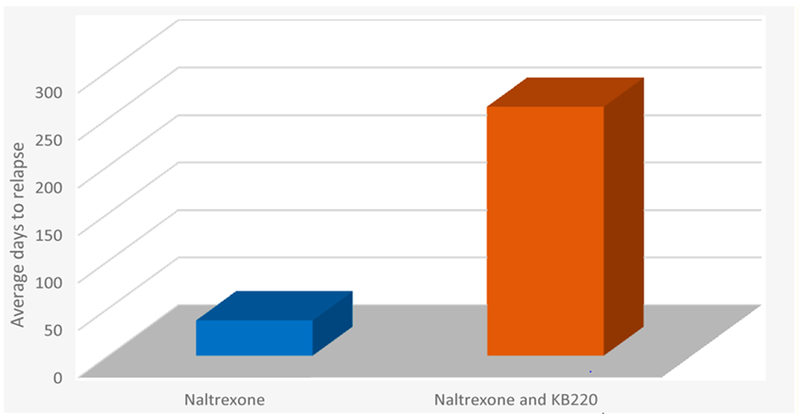
Average days to relapse Naltrexone vs. Naltrexone and KB220. The bar graph shows days to relapse following rapid detoxification of Methadone patients with Naltrexone (n = 1000) vs. Naltrexone and KB220 (n = 13). Days to relapse were 37 and 262 days respectively [5,6].
Compliance calculated on 1000 patients using this rapid detoxification method averaged 37 days without amino-acid therapy. In contrast, the 13 subjects, receiving both the NTX and amino-acid therapy reported an average of continuing the combination treatment for 262 days (p < 0.0001F) [5,6]. Enkephalinase inhibition, while blocking the delta-receptors, with a narcotic antagonist even if weak, may be a promising method to induce rapid detox in chronic methadone patients. The combination of enkephlanase inhibition with XR-NTX may similarly be applicable for treatment and relapse prevention for both opiate and alcohol-dependent individuals. These results also suggest testing this combination both in a larger cohort and with the sublingual combination of the partial opiate mu receptor agonist buprenorphine. Regarding buprenorphine and dopaminergic function, acute doses increase dopamine release, whereas, chronic administration leads to reduced dopamine release [9,10]. The most logical goal of treatment for Substance Use Disorder (SUD) is to induce dopamine homeostasis, and many addiction professionals believe that buprenorphine due to neuroadaptation causes putative dopamine homeostasis. We argue to the contrary and show the differential effects of Buprenorphine for both acute and chronic administration reveal DA dysregulation not balance (Figure 2).
Figure 2:

Acute and chronic effects of buprenorphine on dopamine (DA) Release. Representation of the acute and chronic effects of buprenorphine on mesolimbic dopamine release [10].
Regarding NTX for the treatment of opioid dependence, despite the beneficial impact on the reduction of craving behaviors, opioid maintenance therapy has been associated with adverse effects on cognitive and psychomotor functioning. These adverse effects may limit the outcome of behavioral strategies, rehabilitation, and reintegration into society. To help answer this conundrum Bach., et al. used functional magnetic imaging (fMRI), to investigate the effect of buprenorphine and methadone maintenance therapy on visuospatial working memory performance in a case-controlled study. They found altered neuronal activation in the patients, including brain areas associated with working memory performance and addiction, although, the visuospatial working memory task behavioral performance was similar across groups [11]. It is noteworthy that Jayaram-Lindström., et al. reported in a rat microdialysis study of the modulatory effects of NTX on dopamine levels after acute and chronic amphetamine exposure. They found that chronic, not acute NTX modulates Amphetamine-induced dopamine release. Specifically, they found NTX significantly attenuated dopamine release caused by the reinstatement of amphetamine. The authors concluded that opioid-dopamine interactions reinforce and heighten the addictive effects of amphetamine [12]. These findings may help to facilitate medication development in the field of drug dependence especially as they also relate to buprenorphine/naloxone combinations.
However, in rat models, dopamine release caused by narcotic antagonists is dose-dependent [13]. A short-term stimulatory effect may be due to activation of the glutaminergic VTA drive regulating DA release at the nucleus accumbens (NAc). In this regard, Chartoff and Connery suggested that crosstalk between glutamatergic neurotransmission and MOR-associated G protein signaling leads to immediate and long-term effects on emotional states (like euphoria, depression) and motivated behavior (like drug-seeking, relapse) [14]. It is noteworthy that Cano-Cebrián., et al. found that Acamprosate a drug approved by the FDA to treat alcoholism blocks increases in extracellular dopamine levels in NAc evoked by chemical stimulation of the ventral hippocampus. The effect is that blocked NMDA receptors attenuate the glutaminergic drive to release DA at the NAc [15].
One interesting question evaluated by Dijkstra., et al. involved the effect of the administration of naltrexone on craving level after naltrexone induced rapid opioid detoxification. Does naltrexone effect craving, in abstinent opioid-dependent patients? In contrast to the general opinion, the results suggest that the use of opioids associated with increased craving and that abstinence from opioids associates with less craving, independent of the use of naltrexone [16].
What is precision addiction management (PAM)?
The suggestion here is that the efficiency of XR-NTX treatment for opioid relapse may be improved by a regimen that includes co-therapy with a pro-dopamine regulator KB220PAM, especially in patients with identified genetic addiction risk and RDS. The following sections explain RDS, the cascade of neurotransmission that results in the release of dopamine the pleasure neurotransmitter, the basis of the development of the neuronutrient formulation of KB220 and the results of clinical trials and neuroimaging studies. Finally, Precision Addiction Management; is the use of an individual’s risk alleles identified by the results of the GARS test to select an optimal neuronutrient formulation for that person.
Reward Deficiency Syndrome (RDS)
To understand the concept of patented “Precision Behavioral Management (PBM)”, we are compelled to provide a brief synopsis of RDS. Reward Deficiency Syndrome involves dopamine resistance a form of sensory deprivation of the brain’s reward or pleasure mechanisms. The syndrome occurs because of an individual’s inability to derive reward from ordinary, everyday activities. RDS can be relatively mild or severe, and addiction is one manifestation of RDS. We now know that RDS is a disorder of the neurochemistry of the brain and effects over one-third of the US population. Extensive scientific peer-reviewed research articles back this bold statement, RDS is defined in SAGE Encyclopedia of Abnormal Psychology 2017 [17].
Dopamine is foremost, a component of brain function, and RDS [18] and the key to feelings of well-being and happiness, that depend on excellent brain dopaminergic function. The healthy function of molecular neuroanatomy ultimately results in the release of the neurotransmitter dopamine. Dopamine induces “pleasure” and reduces “stress.” This phenomenon, the neuronal release of dopamine at the reward site of the brain NAc, involves a complicated cascade of neurotransmission called the “Brain Reward Cascade” (BRC). Dopamine released into the synapse results in feelings of well-being and reduced stress. Many other brain chemicals interact to facilitate activation of the dopamine post-receptor site in the brain reward center. The quantity of dopamine released relies on the upstream neurotransmitter serotonin, to stimulate endorphins and enkephalin. Subsequently, endorphins regulate the activity of GABA then GABA regulates the actual release of dopamine in the reward site of the brain. Blum and Kozlowski identified this process in 1989. Figure 3 represents the Brain Reward Cascade [19].
Figure 3:
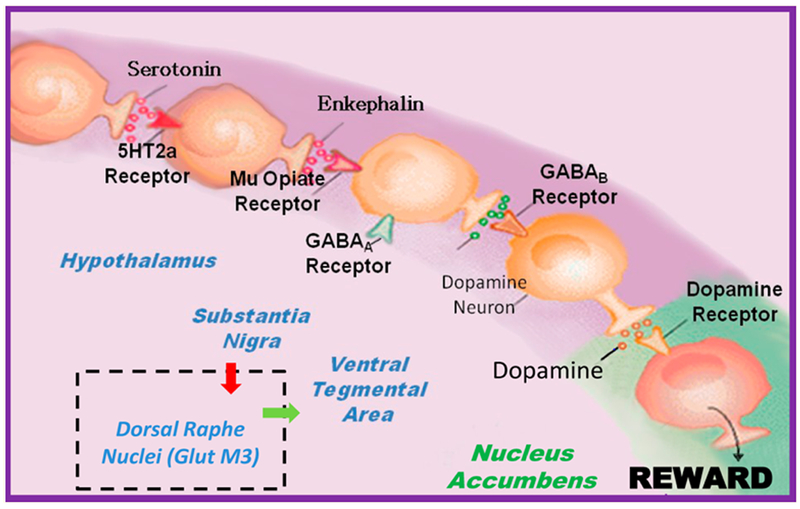
The interaction of the main neurotransmitters of the Brain Reward Cascade (BRC). Schematic of the interaction of neurotransmitters within the mesolimbic reward system [20]. Modified from [21].
The BRC of the mesocorticolimbic dopaminergic pathway plays an especially important role in mediating natural rewards like sexual drive and hunger, as well as unnatural rewards like substance seeking. Natural rewards include satisfaction of physiological drives while unnatural rewards are learned and involve satisfaction of acquired pleasures such as hedonic sensations [22]. Alcohol and other drugs, as well as most positive-reinforces like sex food gambling; aggressive thrills cause activation and neuronal release of brain dopamine into the synapses. The dopamine release can decrease negative feelings and satisfy abnormal cravings for alcohol, cocaine, heroin, nicotine, and with chronic use, exacerbate low dopamine function [23].
Following extensive research, a new understanding of how these substances influence the neurology of dopamine release and addictive behaviors arose. The research determined that alcoholism is like opiates, cocaine, nicotine, food, and some repetitive behaviors, (like gaming, sex addiction), are also similar. Both psychoactive drugs and certain behaviors produce a surge of dopamine in the midbrain (mesolimbic reward center) the biological substrate for addictive behavior. Individuals, genetically predisposed to crave (“want”) dopamine release [24], are at higher risk for addiction due to environmental and genetic factors that can, especially in combination, reduce dopamine release and cause a reward deficiency.
Reward deficiency is a type of flawed dopamine metabolism and function, linked to gene variants that cause hypodopaminergia. These polymorphisms effect the function of the genes involved in the Brain Reward Cascade, for example, the dopamine D2 receptor gene makes D2 receptors, and the polymorphism (Variation) A1 causes a reduction in receptor numbers (30 - 40% fewer receptors at birth) [25].
The established concept of RDS helps to identify a complex array of behaviors, associated molecular dysfunctions in the mesolimbic system of the brain [26]. Essentially, high-risk individuals seek behaviors and substances including alcohol, opiates, cocaine, nicotine, and glucose known to cause the preferential release of dopamine at the Nucleus Accumbens. Activation of the dopaminergic pathways offset low dopaminergic function, caused by gene variants in the BRC. The behaviors include personality disorders and are addictive, impulsive, and compulsive [27] (see table 1).
Table 1:
Lists the reward deficiency syndrome (RDS)behaviors based on shared hypodopaminergia.
| Addictive behaviors | Impulsive behaviors | Obsessive compulsive behaviors | Personality disorders | ||
|---|---|---|---|---|---|
| Substance Related | Non-Substance Related | Spectrum Disorders | Disruptive Impulsive | ||
| Alcohol | Thrill seeking (novelty) | Attention-deficit Hyperactivity | Anti-social | Body Dysmorphic | Paranoid |
| Cannabis | Sexual Sadism | Tourette and Tic Syndrome | Conduct | Hoarding | Schizoid |
| Opioids | Sexual Masochism | Autism | Intermittent Explosive | Trichotillomania (hair pulling) | Borderline |
| Sedatives/Hypnotics | Hypersexual | Oppositional Defiant | Excoriation (skin picking) | Schizotypal | |
| Stimulants | Gambling | Exhibitionistic | Non-suicidal Self-Injury | Histrionic | |
| Tobacco | Internet Gaming | Narcissistic | |||
| Glucose | Avoidant | ||||
| Food | Dependent | ||||
The RDS concept involves shared genes and behavioral tendencies [28]. The RDS behaviors include substance use disorders. Dependence on Alcohol [29], psycho-stimulants [30], marijuana [31], nicotine (smoking) [32] and Opioid misuse [33] with altered opiate receptor function [34], carbohydrates; sugar-binging [35] and obesity [36] are substance-related RDS. Pathological gambling [37], sex addiction [38], reactive aggression [39], pathological aggression [40–42] and certain personality disorders [43] are non-substance RDS behaviors. The RDS personality disorders include novelty seeking [44] and non-suicide self-mutilation [45].
Poly-genes are involved, and these substances misuse and non-substance behaviors induce pre-synaptic dopamine release in the NAc [46]. Spectrum disorders; such as Attention Deficit Hyperactivity Disorder (ADHD), Tourette’s syndrome, and Autism involve dopamine deficiency due to genetic dopamine dysregulation [47]. These are some of the study results that support the theory that polymorphisms, of the reward genes identified in the brain reward system, are significantly associated with the reward-dependent traits.
Genetic Addiction Risk Score (GARS)
Following an extensive literature review on alleles that contributed most to the hypodopaminergic trait, RDS, eleven polymorphisms in ten genes were selected for the GARS test. The selection involved thousands of studies associating genetic risk with drug and non-drug addictive behavior.
The GARS test for RDS behaviors, identified genetic variation in the BRC that involves at least the Dopamine D1 to D4 Receptor genes, the Dopamine and Serotonin Transporter genes, the Mu-opiate and GABA-B3 receptor genes, the Mono-Amine-Oxidase A gene and the Catecholamine-Methyl-Transferase gene. The test uses DNA from a non-invasive cheek swab. The report describes the function of each genetic risk variation and the behaviors that individuals may have a genetic risk or predisposition for, identified by the individuals’ GARS panel.
The use of genetic testing for RDS risk diagnosis, unlike tests at birth for certain rare diseases, (like Huntington’s disease. Phenylketonuria, Congenital hypothyroidism. Galactosemia, and Sickle cell anemia), RDS is polygenetic and about a third of the US population have some RDS polymorphisms [48]. However, early GARS testing for early risk stratification and non-pharmacologic interventions in for example ADHD is parsonomiuos. Pro-dopaminergic therapies may be used to ameliorate the hypodopaminergia and prevent the emergence of RDS behaviors. Some pro-dopamine therapies that can be used to reduce the impact of RDS are exercise, diet, parent-training, computer-assisted learning, music and safe non-stimulant nutraceutical dopaminergic agonist therapy [49]. Overall, early risk diagnosis is beneficial for both prevention and positive treatment outcomes.
In clinical practice, the GARS test can be used to reduce denial and guilt, corroborate family genograms, predict relapse probability and identify therapeutic targets based on known gene polymorphisms [50,51]. GARS results can impact decisions about appropriate therapies including pain medications, level of care placement, for example, in-patient, out-patient, intensive outpatient, and residential, and “length of stay” in treatment. Genetic severity-based relapse and recovery liability and vulnerability for addiction can be identified. Pharmacogenetic medical monitoring for better clinical outcomes; knowing that, for example, a person with the A1 allele of the DRD2 gene has reduced binding to delta (endorphin) receptors in the brain will provide information directed toward MAT treatment. Finally, customization of the KB220 formulation to match the individual’s risk-reward gene polymorphisms makes possible the amelioration of reward deficiency.
The KB220PAM Formulations
The oral neuronutrient formulation of KB220PAM is a glutaminergic-dopaminergic optimization complex [52]. The natural non-drug active components of KB220PAM were selected to restore the neurological neurotransmitter balance disrupted by hypodopaminergic variations of the genes that regulate the BRC. The ingredients include amino-acid precursors to neurotransmitters like Serotonin, Glutamine, and Dopamine, and inhibitors of Endorphin breakdown enzymes known to clear Dopamine from the synapse.
As now defined by the American Society of Addiction Medicine (ASAM 2011), addiction is a primary, chronic disease of the brain that involves; reward, motivation, memory and related circuitry (Smith). Dysfunction in these neurological circuits leads to characteristic biological, psychological, social and spiritual manifestations. These are reflected in persons compulsively pursuing reward and relief by misuse of substances and other behaviors. Although addiction cannot be cured, remission can be accomplished through a program of treatment, abstinence from all psychoactive substances, and supported-recovery. In 2012 ASAM recognized that alcohol and other drugs and non-substance addictive, impulsive and obsessive behaviors are a chronic brain disorder that involves relapse, progressive development, and the potential for fatality if not treated.
Impairment of the reward circuitry must be addressed to prevent relapse, support recovery and an enhanced quality of life. In fact, carriers of the DRD2 A1 variant are known to have higher relapse and mortality rates than noncarriers [53]. At least 110 million in the United States of America are genetically prone to RDS behaviors. Many genes are involved in our ability to perceive life positively, and normal gene function results in a “Happy Brain” [54]. Genes or the environment (especially stress) are causes of impairment within the brain reward cascade. Neuro-physiologically an “Unhappy Brain” is in an impaired state. The chemical messenger that regulates neuronal dopamine release is a powerful neurotransmitter called GABA. Excessive GABA leads to a reduction of Dopamine and reduced ability to cope with stress (see figure 5).
Figure 5: Brain Reward Cascade (normal and hypodopaminergic state).
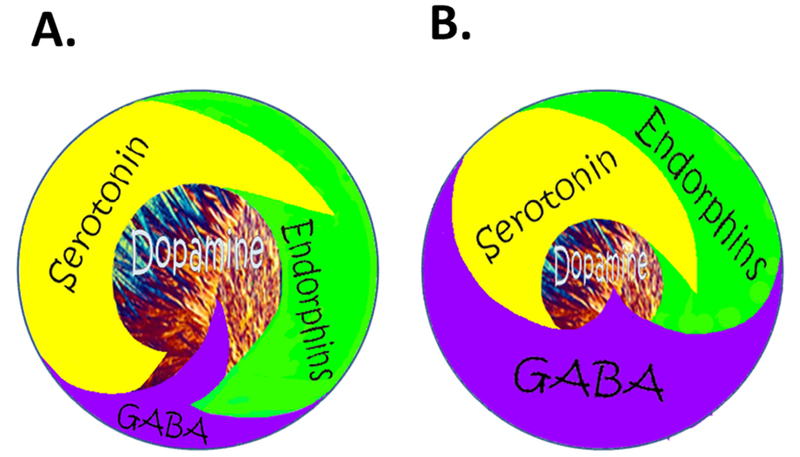
A. Happy Brain: Represents the normal physiologic state of the neurotransmitter interaction at the mesolimbic region of the brain. Briefly, serotonin in the hypothalamus stimulates neuronal projections of methionine enkephalin in the hypothalamus that, in turn, inhibits the release of GABA in the substantia nigra, thereby allowing for the normal amount of Dopamine to be released at the Nucleus Accumbens (NAc); reward site of the brain. B Unhappy Brain: Represents hypodopaminergic function of the mesolimbic region of the brain. The hypodopaminergic state is due to gene polymorphisms as well as environmental elements, including both stress and neurotoxicity from misuse of psychoactive drugs like. alcohol, heroin, cocaine and genetic variables [54].
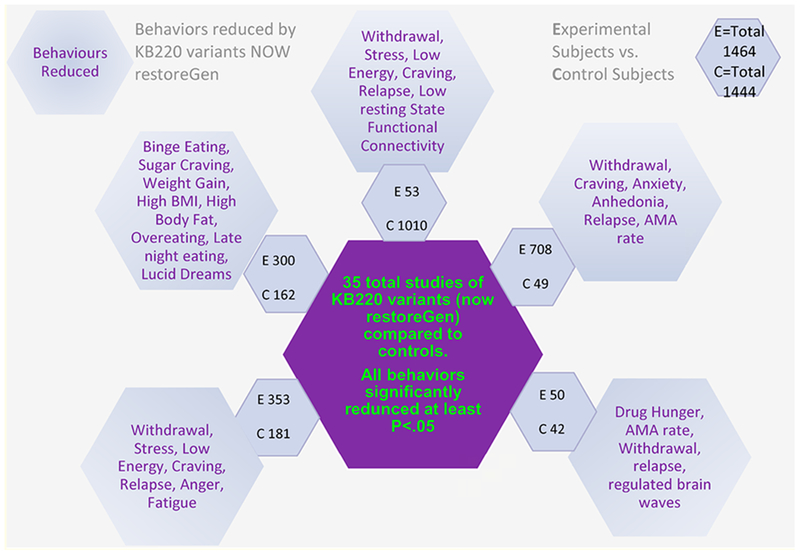
The hypothesis is that chronic pain opiate use, once initiated, continues as substance seeking, due primarily to a hypodopaminergic trait (genetic) or state (epigenetic). Any major solution must address the low Dopamine brain function early in the recovery process especially when an individual seeks help and clinicians try to promote long-term balancing of Dopamine function with the laudable goal of inducing “Dopamine Homeostasis” (regulation). There is continuing excitement concerning the consistent positive effects of KB220 with thirty-five published human studies showing benefits to society, anti-craving effects, enhanced well-being (stress reduction); reduced discharge against Medical Advice (AMA) rates; increased focus; reduced relapse and overall redemption of joy (see figure 6).
Figure 6:
Schematic of Precision Behavioral Management (PBM).
Using neuroimaging tools, KB220PAM has been shown to activate neurobiological targets, restore “Dopamine Homeostasis” and most importantly enhanced feelings of well-being and happiness. New neuroimaging studies have unraveled the Mechanism of Action (MOA) of this complex [55] (see figure 7).
Figure 7:
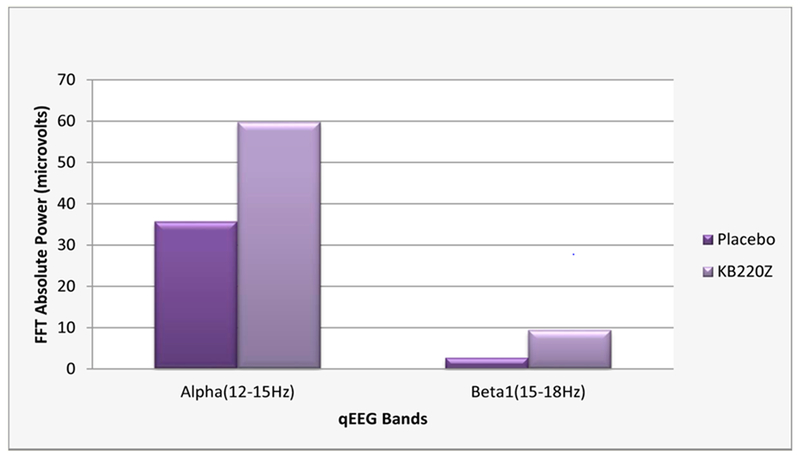
The results of qEEG one hour after oral administration of the KB220Z to abstinent psychostimulant addicts. One hour after administration of KB220Z, calming by increasing alpha waves (~40%) along with increasing low beta waves (~68%) were shown [55].
Figure 7 represents the results of qEEG one hour after oral administration of the KB220Z to abstinent psychostimulant addicts (cocaine, methamphetamine). This triple-blinded placebo comparison shows an increase in alpha and low beta waves showing regulation of an area known to control relapse (the orbital-frontal Cortex-Cingulate Gyrus). It is well-known that increased in alpha waves and increased in low beta waves cause a feeling of well-being and calm [55].
In another published study [8] at Beijing University Imaging Center, using fMRI to evaluate abstinent heroin use disorder patients for the effects of KB220Z, one-hour after administration. This triple-blinded placebo comparison demonstrated significantly activated reward site dopamine. Seeing reward site dopamine activity provides a mechanism (MOA) whereby this natural substance acting as a Dopamine D2 stimulant (agonist) increases dopamine release in Caudate Nucleus while also reducing the hyperemotional state observed in an associated brain region (Cerebellum), thus inducing dopamine balance (Figure 8).
Figure 8:
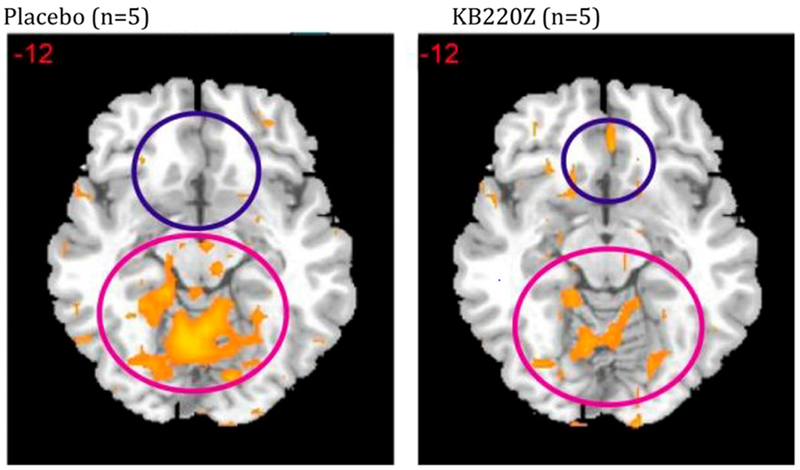
Shows resting state fMRI (rsfMRI) in abstinent heroin users KB220Z vs. placebo. In figure 8 rsfMRI data analysis in abstinent heroin users (n = 5) before and one hour after oral KB220Z and placebo showing significant increases in rsfMRI response with KB220Z [8] and putative DA homeostasis
NIDA proposed that lack of connectivity may be key to all addictions [56]. Findings in both human and animal studies consistently show a remarkable increase in resting state functional connectivity and increased neuronal recruitment within 15 (animal) to 60 (human) minutes post administration. Additional dopamine neuronal firing was noted in brain areas involved in reward processing with possible neuroplasticity even in the long-term, see figure 9.
Figure 9:
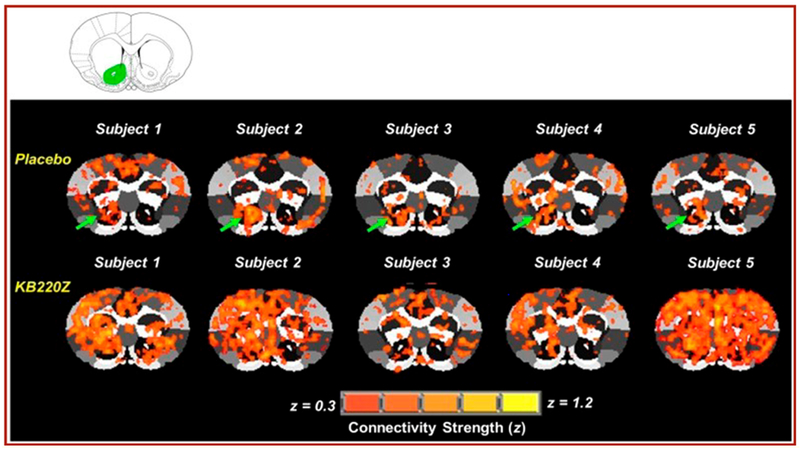
Represents cross-correlation fMRI maps of five rodents that show: Placebo compared to KB220Z treated rats. The maps correspond to resting state connectivity for the NAc (highlighted in green in the atlas map above the figure; only left seed is shown). Note the distributed but significant connectivity between various brain regions and the NAc in the placebo subjects. KB220Z increased connectivity, especially between left-right accumbens, dorsal striatum, and limbic cortical areassuch as the anterior cingulate, prelimbic and infralimbic regions. Correlation maps for representative subjects presented at a threshold between 0.3 ≤ z ≥ 1.2 [56].
The KB220Z, Opioid treatment model, has been shown to help restore brain function during opioid withdrawal and attenuate relapse potentially by increasing resting state functional connectivity, connectivity volume due to enhanced neuronal recruitment [56].
Relief from chronic pain (by finding ways to induce long-term amelioration of the symptoms of chronic pain) can succeed due to induction of Dopamine Homeostasis [42]. The pain-suppression system is activated by acute stress, via the release of endogenous opioids (endorphins) and substance P within the ventral tegmental area. However, prolonged exposure to unavoidable stress produces both reductions of dopamine output in the nucleus accumbens and development of persistent hyperalgesia (abnormally heightened sensitivity to pain) [57]. The proposal is that a stress-related reduction of dopaminergic tone within the nucleus accumbens contributes to the development of hyperalgesia and thus plays a role in the pathogenesis of chronic pain conditions. The hypothesis is that normalized dopamine release, in the nucleus accumbens, will feedback to the BRC, whereby the Mu and Delta (endorphin type) opioid receptors will be activated to control pain sensitivity. In the condition, low dopamine release impairs this mechanism and higher sensitivity to pain develops [58,59].
Summary
Dopamine homeostasis can be achieved by supplying the patient with just the right amount of dopamine to induce balance between the pro-dopamine releaser (glutaminergic) and actual neuronal dopamine synthesis and subsequent release. This balance can be accomplished with glutaminergic-dopaminergic optimization; moderating the need for additional opiate antagonists (like naltrexone) and other more potent agonists (methadone or buprenorphine). Based on the studies described above, a patient, with chronic pain at risk of substance misuse, will have a brain balancing technique that can yield healthy experience free of addictive agents like opiates, methadone, and buprenorphine. It has been observed in humans that KB220 variations are active anti-withdrawal agents in opioid dependence and can improve retention and compliance on NTX. Many published studies, have found that continued use of KB220, leads to feelings of well being, relief from stress and promotes recovery from Substance Use Disorder and a life free of the pain and addiction.
To reiterate, in the early 70’s Blum’s laboratory was the first to show the benefits of naloxone or narcotic antagonists in the treatment of alcohol dependence published in Nature. This seminal work, in conjunction with many other studies, later served as the basis to develop the narcotic antagonist Naltrexone for treatment of both opioid and alcohol dependence. In 2006 the extended-release injectable of Naltrexone (XR-NTX) approved by the FDA.
While these studies show benefit naltrexone chronically down-regulates mesolimbic dopamine release, while in the short term, there is ongoing evidence that the retention and compliance on NTX are not sufficient to characterize adherence as high [3]. However, opioid treatment with the XR-NTX associated with superior outcomes; higher compliance with OUD than for AUD, longer time to relapse and less likely relapse, despite higher rates of concurrent non-opioid substance use like cocaine [4]. Before the release of long-term extended-release injectable (XR-NTX) for opioid dependence, further, research by Blum., et al. showed that a combination of a pro-dopamine regulator neuro-nutrient (KB220) and Naltrexone and significantly prevented relapse to OUD [5,6]. Early identification with the GARS test will provide a basis for co-therapy with individualized genetically guided pro-dopaminergic neuronutrient and XR-NXT. Larger trials of the combination of ‘PBM” and long-term XR-NXT are prudent and imperative in the search for a treatment that can prevent relapse to OUD.
Figure 4:
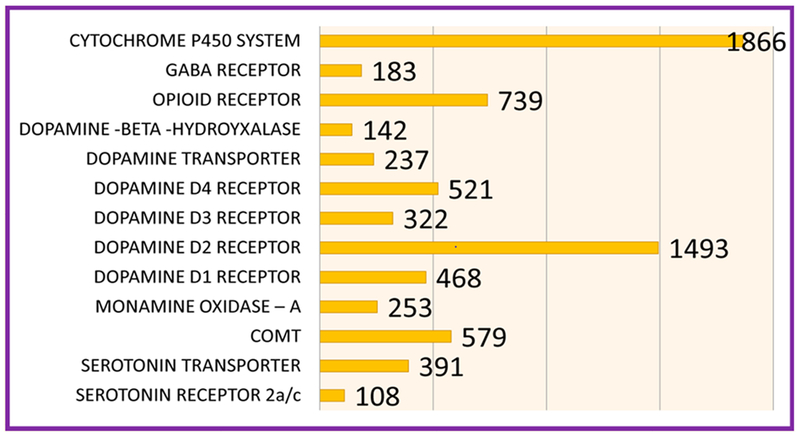
Lists the number of association studies per genetic risk allele as of November 12th 2017.
Acknowledgement
We the authors appreciate the expert edits by Margaret A. Madigan. We are indebted to the expert support of Justin Jones and Erin Gallagher and Geneus Health staff. We also acknowledge the clinical support of Sherief Moustafa, RN and Rami Sleiman. The authors want to express their appreciation to Mary Hauser and staff of Dominion Diagnostics.
Funding Source
Dr. Elman is supported by grant# 1 I01 CX001118-01A2. Dr. Thanos is funded by the NY Research Foundation (RIAQ0940) and the NIH (DA035923 and DA035949). Dr. Badgaiyan is supported by the National Institutes of Health grants 1R01NS073884 and 1R21MH073624, and VA Merit Review Awards CX000479 and CX000780. Dr. Blum is funded by NIH/NIMHD grant R41MD12318 along with Marjorie-Gondre-Lewis of Howard University. Marcelo Febo is the recipient of NIH DA019946 and is funded by the McKnight Brain Institute Foundation.
Abbreviations
- XR
Extended-release
- NTX
Naltrexone
- RDS
Reward Deficiency Syndrome
- BRC
Brain Reward Cascade
- GARS
Genetic Addiction Risk Score
- OUD
Opioid Use Disorder
- AUD
Alcohol Use Disorders
- PAM
Precision Addiction Management
Footnotes
Conflict of Interest
Drs. Blum and Siwicki are member of the Board of Directors and own stock in both Geneus Health. LLC. and RestoreGen LLC., exclusive distributors of the Genetic Addiction Risk Score(GARS)™ and KB220PAM of Blum’s patents issued and pending. Dr. Blum is the Chief Scientific Advisor of Dominion Diagnostics and Dr. Siwicki is a member of the Board of Directors. Dr. Blum is Chief Neurogenetics and Addiction Therapy advisor of Florida House. Dr. Blum is Chairman of the Board and CSO of Geneus Health and Drs. Modestino, Badgaiyan, Baron, Thanos, Elman, Siwicki, Febo, and Gold. Are on Geenus Health Scientific Advisory Board.
Bibliography
- 1.Blum K, et al. “Naloxone-Induced Inhibition of Ethanol Dependence in Mice”. Nature 2655589 (1977): 49–51. [DOI] [PubMed] [Google Scholar]
- 2.Fois GR and Diana M. “Opioid Antagonists Block Acetaldehyde-Induced Increments in Dopamine Neurons Activity”. Drug and Alcohol Dependence 158 (2016): 172–176. [DOI] [PubMed] [Google Scholar]
- 3.Swift R, et al. “Adherence Monitoring in Naltrexone Pharmacotherapy Trials: A Systematic Review”. Journal of Studies on Alcohol and Drugs 726 (2011): 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams AR., et al. “Long-Term Follow-up Study of Community-Based Patients Receiving Xr-Ntx for Opioid Use Disorders”. American Journal on Addictions 264 (2017): 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen TJ., et al. “Are Dopaminergic Genes Involved in a Predisposition to Pathological Aggression? Hypothesizing the Importance of “Super Normal Controls” in Psychiatric genetic Research of Complex Behavioral Disorders”. Medical Hypotheses 654 (2005): 703–707. [DOI] [PubMed] [Google Scholar]
- 6.Chen TJ., et al. “Narcotic Antagonists in Drug Dependence: Pilot Study Showing Enhancement of Compliance with Syn-10, Amino-Acid Precursors and Enkephalinase Inhibition Therapy”. Medical Hypotheses 633 (2004): 538–548. [DOI] [PubMed] [Google Scholar]
- 7.Willuhn I, et al. “Excessive Cocaine Use Results from Decreased Phasic Dopamine Signaling in the Striatum”. Nature Neuroscience 175 (2014): 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum K, et al. “Rsfmri Effects of Kb220z on Neural Pathways in Reward Circuitry of Abstinent Genotyped Heroin Addicts”. Postgraduate Medical Journal 1272 (2015): 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira FC., et al. “Buprenorphine Modulates Methamphetamine-Induced Dopamine Dynamics in the Rat Caudate Nucleus”. Neurotoxicity Research 191 (2011): 94–101. [DOI] [PubMed] [Google Scholar]
- 10.Blum K, et al. “Buprenorphine and Naloxone Combinations and Dopamine”. Current Psychopharmacology 62 (2017). [Google Scholar]
- 11.Bach P, et al. “Diminished Brain Functional Magnetic Resonance Imaging Activation in Patients on Opiate Maintenance Despite Normal Spatial Working Memory Task Performance”. Clinical Neuropharmacology 354 (2012): 153–160. [DOI] [PubMed] [Google Scholar]
- 12.Jayaram-Lindstrom N, et al. “Naltrexone Modulates Dopamine Release Following Chronic, but Not Acute Amphetamine Administration: A Translational Study”. Translational Psychiatry 74 (2017): e1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Chiara G and Imperato A. “Opposite Effects of Mu and Kappa Opiate Agonists on Dopamine Release in the Nucleus Accumbens and in the Dorsal Caudate of Freely Moving Rats”. Journal of Pharmacology and Experimental Therapeutics 2443 (1988): 1067–1080. [PubMed] [Google Scholar]
- 14.Chartoff EH and Connery HS. “It’s More Exciting Than Mu: Crosstalk between Mu Opioid Receptors and Glutamatergic Transmission in the Mesolimbic Dopamine System”. Frontiers in Pharmacology 5 (2014): 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cano-Cebrian MJ., et al. “Acamprosate Blocks the Increase in Dopamine Extracellular Levels in Nucleus Accumbens Evoked by Chemical Stimulation of the Ventral Hippocampus”. Naunyn-Schmiedeberg’s Archives of Pharmacology 3684 (2003): 324–327. [DOI] [PubMed] [Google Scholar]
- 16.Dijkstra BA., et al. “Does Naltrexone Affect Craving in Abstinent Opioid-Dependent Patients?” Addiction Biology 122 (2007): 176–182. [DOI] [PubMed] [Google Scholar]
- 17.Blum K “Reward Deficiency Syndrome”. The SAGE Encyclopedia of Abnormal and Clinical Psychology Editors. Wenzel Amy. University of Pennsylvania School of Medicine, USA: Sage Publications, Inc; (2017). [Google Scholar]
- 18.Saddoris MP. “Terminal Dopamine Release Kinetics in the Accumbens Core and Shell Are Distinctly Altered after Withdrawal from Cocaine Self-Administration”. eNeuro 35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum K and Kozlowski GP. “Ethanol and Neuromodulators Interaction: A Cascade Model of Reward”. Alcohol and Behavior. Editors. Ollat H, Parvez S and Parvez H. The Netherlands: VSP Press Utrecht; (1990): 131–150. [Google Scholar]
- 20.Blum K, et al. “Neurogenetics and Nutrigenomics of Neuro-Nutrient Therapy for Reward Deficiency Syndrome (RDS): Clinical Ramifications as a Function of Molecular Neurobiological Mechanisms”. Journal of Addiction Research and Therapy 35 (2012): 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson C “The Science of Addiction”. New York, New York: W.W. Norton and Co; (2007). [Google Scholar]
- 22.Gold MS., et al. “A Shared Molecular and Genetic Basis for Food and Drug Addiction: Overcoming Hypodopaminergic Trait/State by Incorporating Dopamine Agonistic Therapy in Psychiatry”. Psychiatric Clinics of North America 383 (2015): 419–462. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF. “The Dark Side of Emotion: The Addiction Perspective”. European Journal of Pharmacology 753 (2015): 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson MJ., et al. “Roles of “Wanting” and “Liking” in Motivating Behavior: Gambling, Food and Drug Addictions”. Current Topics in Behavioral Neurosciences 27 (2016): 105–136. [DOI] [PubMed] [Google Scholar]
- 25.Noble EP., et al. “Allelic Association of the D2 Dopamine Receptor Gene with Receptor-Binding Characteristics in Alcoholism”. Archives of General Psychiatry 487 (1991): 648–654. [DOI] [PubMed] [Google Scholar]
- 26.Baik JH. “Dopamine Signaling in Reward-Related Behaviors”. Frontiers in Neural Circuits 7 (2013): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum K, et al. “The D2 Dopamine Receptor Gene as a Determinant of Reward Deficiency Syndrome”. Journal of the Royal Society of Medicine 897 (1996): 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koob GF and Volkow ND. “Neurobiology of Addiction: A Neurocircuitry Analysis”. Lancet Psychiatry 38 (2016): 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberlin BG., et al. “Limbic Responses to Reward Cues Correlate with Antisocial Trait Density in Heavy Drinkers”. Neuroimage 601 (2012): 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez-Cuesta J, et al. “Effects of Genetic Deletion of Endogenous Opioid System Components on the Reinstatement of Cocaine-Seeking Behavior in Mice”. Neuropsychopharmacology 3913 (2014): 2974–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blum K, et al. “Enhancing Brain Pregnenolone May Protect Cannabis Intoxication but Should Not Be Considered as an Anti-Addiction Therapeutic: Hypothesizing Dopaminergic Blockade and Promoting Anti-Reward”. Journal of Reward Deficiency Syndrome 11 (2015): 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fowler JS., et al. “Monoamine Oxidase and Cigarette Smoking”. Neurotoxicology 241 (2003): 75–82. [DOI] [PubMed] [Google Scholar]
- 33.Hwang IC., et al. “Oprm1 A118g Gene Variant and Postoperative Opioid Requirement: A Systematic Review and Meta-Analysis”. Anesthesiology 1214 (2014): 825–834. [DOI] [PubMed] [Google Scholar]
- 34.Cui Y, et al. “Targeted Expression of Mu-Opioid Receptors in a Subset of Striatal Direct-Pathway Neurons Restores Opiate Reward”. Nature Neuroscience 172 (2014): 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blum K, et al. “Dopamine and Glucose, Obesity, and Reward Deficiency Syndrome”. Frontiers in Psychology 5 (2014): 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thanos PK., et al. “Food Restriction Markedly Increases Dopamine D2 Receptor (D2r) in a Rat Model of Obesity as Assessed with in-Vivo Mupet Imaging ([11c] Raclopride) and in-Vitro ([3h] Spiperone) Autoradiography”. Synapse 621 (2008): 50–61. [DOI] [PubMed] [Google Scholar]
- 37.Gyollai A, et al. “The Genetics of Problem and Pathological Gambling: A Systematic Review”. Current Pharmaceutical Design 2025 (2014): 3993–3999. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho J, et al. “Hypersexuality and High Sexual Desire: Exploring the Structure of Problematic Sexuality.” Journal of Sexual Medicine 126 (2015): 1356–1367. [DOI] [PubMed] [Google Scholar]
- 39.Haller J “The Neurobiology of Abnormal Manifestations of Aggression--a Review of Hypothalamic Mechanisms in Cats, Rodents and Humans”. Brain Research Bulletin 93 (2013): 97–109. [DOI] [PubMed] [Google Scholar]
- 40.Godar SC., et al. “The Role of Monoamine Oxidase a in Aggression: Current Translational Developments and Future Challenges”. Progress in Neuro-Psychopharmacology and Biological Psychiatry 69 (2016): 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chester DS and DeWall CN. “The Pleasure of Revenge: Retaliatory Aggression Arises from a Neural Imbalance toward Reward”. Social Cognitive and Affective Neuroscience 117 (2016): 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen AL., et al. “Hypothesizing That Brain Reward Circuitry Genes Are Genetic Antecedents of Pain Sensitivity and Critical Diagnostic and Pharmacogenomic Treatment Targets for Chronic Pain Conditions”. Medical Hypotheses 721 (2009): 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conner BT., et al. “Genetic, Personality, and Environmental Predictors of Drug Use in Adolescents”. Journal of Substance Abuse Treatment 382 (2010): 178–190. [DOI] [PubMed] [Google Scholar]
- 44.Noble EP., et al. “D2 and D4 Dopamine Receptor Polymorphisms and Personality”. American Journal of Medical Genetics 813 (1998): 257–267. [PubMed] [Google Scholar]
- 45.Esposito CL and Clum GA. “The Relative Contribution of Diagnostic and Psychosocial Factors in the Prediction of Adolescent Suicidal Ideation”. Journal of Clinical Child and Adolescent Psychology 323 (2003): 386–395. [DOI] [PubMed] [Google Scholar]
- 46.McCutcheon JE., et al. “Optical Suppression of Drug-Evoked Phasic Dopamine Release”. Frontiers in Neural Circuits 8 (2014): 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding K, et al. “Dat1 Methylation Is Associated with Methylphenidate Response on Oppositional and Hyperactive-Impulsive Symptoms in Children and Adolescents with ADHD”. World Journal of Biological Psychiatry 184 (2017): 291–299. [DOI] [PubMed] [Google Scholar]
- 48.Blum K, et al. “Can Genetic Testing Provide Information to Develop Customized Nutrigenomic Solutions for Reward Deficiency Syndrome?” Clinical Medical Reviews and Case Reports 21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Downs B, et al. “Have We Hatched the Addiction Egg: Reward Deficiency Syndrome Solution System™”. Journal of Genetic Syndromes and Gene Therapy 4136 (2013): 14318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blum K, et al. “Global Opioid Epidemic: Doomed to Fail without Genetically Based Precision Addiction Medicine (Pam™): Lessons Learned from America”. Precision Medicine 21 (2017): 17–22. [PMC free article] [PubMed] [Google Scholar]
- 51.Blum K, et al. “The Benefits of Customized DNA Directed Nutrition to Balance the Brain Reward Circuitry and Reduce Addictive Behaviors”. Precision Medicine 11 (2016): 18–33. [PMC free article] [PubMed] [Google Scholar]
- 52.Duquette Lucien L, et al. “Neurobiologyof Kb220z-Glutaminergic-Dopaminergic Optimization Complex [Gdoc] as a Liquid Nano: Clinical Activation of Brain in a Highly Functional Clinician Improving Focus, Motivation and Overall Sensory Input Following Chronic Intake”. Clinical Medical Reviews and Case Reports 35 (2016): 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahlgren A, et al. “Do Alcohol-Dependent Individuals with DRD2 A1 Allele Have an Increased Risk of Relapse? A Pilot Study”. Alcohol and Alcoholism 465 (2011): 509–513. [DOI] [PubMed] [Google Scholar]
- 54.Blum K, et al. “Neuropsychiatric Genetics of Happiness, Friendships, and Politics: Hypothesizing Homophily (“Birds of a Feather Flock Together”) as a Function of Reward Gene Polymorphisms”. Journal of Genetic Syndromes and Gene Therapy 3112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blum K, et al. “Overcoming Qeeg Abnormalities and Reward Gene Deficits During Protracted Abstinence in Male Psychostimulant and Polydrug Abusers Utilizing Putative Dopamine D (2) Agonist Therapy: Part 2”. Postgraduate Medical Journal 1226 (2010): 214–226. [DOI] [PubMed] [Google Scholar]
- 56.Febo M, et al. “Enhanced Functional Connectivity and Volume between Cognitive and Reward Centers of Naïve Rodent Brain Produced by Pro-Dopaminergic Agent Kb220z”. PLOS One 124 (2017): e0174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wood PB. “Stress and Dopamine: Implications for the Pathophysiology of Chronic Widespread Pain”. Medical Hypotheses 623 (2004): 420–424. [DOI] [PubMed] [Google Scholar]
- 58.Yi P and Pryzbylkowski P. “Opioid Induced Hyperalgesia”. Pain Medicine 161 (2015): S32–S36. [DOI] [PubMed] [Google Scholar]
- 59.Ogata M, et al. “Characterization of Nociceptive Response to Chemical, Mechanical, and Thermal Stimuli in Adolescent Rats with Neonatal Dopamine Depletion”. Neuroscience 289 (2015): 43–55. [DOI] [PubMed] [Google Scholar]


