Abstract
Adenosine 2A receptor (A2AR) exerts anti-inflammatory effects. However, the role for A2AR in obesity-associated adipose tissue inflammation remains to be elucidated. The present study examined the expression of A2AR in adipose tissue of mice with diet-induced obesity, and determined the effect of A2AR disruption on the status of obesity-associated adipose tissue inflammation. Wild-type C57BL/6J mice and A2AR-disrupted mice were fed a high-fat diet (HFD) for 12 weeks to induce obesity and adipose tissue inflammation. In vitro, bone marrow-derived macrophages from A2AR-disrupted mice and wild-type control mice were treated with palmitate and examined for macrophage proinflammatory activation. Compared with that of low-fat diet (LFD)-fed wild-type mice, A2AR expression in adipose tissue of HFD-fed wild-type mice was increased significantly and was present predominantly in adipose tissue macrophages. The increase in adipose tissue A2AR expression in HFD-fed mice was accompanied with increased phosphorylation states of c-Jun N-terminal kinase 1 p46 and nuclear factor kappa B p65 and mRNA levels of interleukin (Il)-1beta, Il6, and tumor necrosis factor alpha. In A2AR-disrupted mice, HFD feeding induced significant increases in adipose tissue inflammation, indicated by enhanced proinflammatory signaling and increased proinflammatory cytokine expression, and adipose tissue insulin resistance, indicated by a decrease in insulin-stimulated Akt phosphorylation relative to those in wild-type mice. Lastly, A2AR disruption enhanced palmitate-induced macrophage proinflammatory activation. Taken together, these results suggest that A2AR plays a protective role in obesity-associated adipose tissue inflammation, which is attributable to, in large part, A2AR suppression of macrophage proinflammatory activation.
Keywords: adenosine 2A receptor, obesity, adipose tissue inflammation, macrophage
INTRODUCTION
Obesity is an ongoing pandemic and critically contributes to the development and progression of a wide variety of metabolic diseases such as type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease. Over the past decade, much evidence has demonstrated the persistence of low-grade inflammation in adipose tissue during obesity (Lumeng, et al. 2007; Weisberg, et al. 2003; Xu, et al. 2003), and validated adipose tissue inflammation as a critical factor that causes insulin resistance and glucose and fat metabolic dysregulation locally in adipose tissue and distally in key metabolic tissues such as the liver and skeletal muscle (Berg, et al. 2001; Boyle, et al. 2011; Cheung, et al. 2000; Hotamisligil, et al. 1996; Huo, et al. 2010; Huo, et al. 2012; Kabir, et al. 2005; Kamei, et al. 2006; Trujillo and Scherer 2006; Ye, et al. 2007). Given this, a better understanding of adipose tissue inflammation is essential for the development of new and effective approaches for treatment and/or prevention of obesity-associated metabolic diseases.
Since the discovery of macrophage infiltration in adipose tissue of mice with diet-induced obesity (DIO) (Weisberg et al. 2003; Xu et al. 2003), a significant number of studies have investigated how adipose tissue cells, mainly adipocytes and macrophages, regulate the development of adipose tissue inflammation. Using genetically modified mouse models, many researchers have shown that genes/proteins varying from signaling molecules to metabolic and regulatory enzymes (Choe, et al. 2014; Han, et al. 2013; Huo et al. 2010; Huo et al. 2012; Jiao, et al. 2012; Kamei et al. 2006; Kumari, et al. 2016; Menghini, et al. 2009; Nomiyama, et al. 2007; Odegaard, et al. 2007; Saberi, et al. 2009; Solinas, et al. 2007), and even the players of circadian core loop (Xu, et al. 2014) function to either promote or inhibit the development and progression of adipose tissue inflammation. Of note, signaling molecules have drawn particular attention. For instance, signaling molecules such as c-Jun N-terminal kinase 1 (JNK1) and interferon regulatory transcription factor 3 (IRF3) have been validated to play detrimental roles in adipose tissue inflammation (Kumari et al. 2016; Solinas et al. 2007). There is also evidence suggesting the importance of cell surface receptors such as Toll-like receptor 4 (TLR4) and G-protein coupled receptor 120 (GPR120) in the regulation of the pathogenesis of adipose tissue inflammation (Oh, et al. 2010; Saberi et al. 2009). However, it remains to be elucidated how nutrition stress regulates cell surface receptors in the context of altering the development and progression of adipose tissue inflammation.
Adenosine receptors (AR) belong to superfamily of G-protein-coupled receptors. To date, A1, A2A, A2B and A3 are the four AR that have been validated to mediate various physiological functions of adenosine (Haskó, et al. 2008). Among the four AR, A2AR exerts powerful anti-inflammatory effects in immune cells such as macrophages and neutrophils (Gessi, et al. 2000; Haskó et al. 2008). Recent studies have also demonstrated that A2AR activation exhibits anti-inflammatory effects in cultured hepatocytes, and in rodent models of inflammatory liver diseases (Alchera, et al. 2017; Imarisio, et al. 2012). In contrast, using A2AR-disrupted mice, Cai et al. provide complementary evidence to support a protective role for A2AR in inflammatory liver disease (Cai, et al. 2018). Considering that A2AR critically regulates the proinflammatory activation of macrophages that determine adipose tissue inflammation, investigators have postulated A2AR as an essential regulator of adipose tissue inflammation. Indeed, a recent study has suggested a role for A2AR activation in decreasing macrophage infiltration in adipose tissue of DIO mice (DeOliveira, et al. 2017). However, it remains unknown about the effect of nutrition stress, i.e., high-fat diet (HFD) feeding, on altering adipose tissue A2AR expression as it relates to the regulation of adipose tissue inflammation. The present study provides primary evidence to support a protective role for A2AR in obesity-associated adipose tissue inflammation. In addition, A2AR regulates how macrophages respond to palmitate, a major macronutrient accounting for obesity and related diseases.
MATERIALS and METHODS
Animal experiments
Wild-type (WT) C57BL/6J were obtained from Jackson Laboratory (Bar Harbor, ME). A2AR-disrupted (A2AR−/− or A2AR+/−) mice and their WT littermates (A2AR+/+ mice) were generated by breeding male A2AR+/− mice with female A2AR+/− mice (all in C57BL/6J background) as described (Chen, et al. 1999). After weaning, offspring were ear-tagged, and subjected to collection of tail samples for genotyping. Genomic DNA was prepared following manufacturer’s instruction (Extract-N-Amp™ Tissue PCR Kit, Sigma-Aldrich, St. Louis, MO). PCR was performed according to a standard procedure. PCR products were loaded in a 2% agarose gel for visualization. Primers are forward: 5’- GGGCTCCTCGGTGTACA, and reverse: 5’- CCCACAGATCTAGCCTTA. All mice were housed on 12:12-h light-dark cycles (light on at 06:00). Study 1: male WT C57BL/6J mice, at 5 - 6 weeks of age, were fed an HFD (60% fat calories, 20% protein calories, and 20% carbohydrate calories) or low-fat diet (LFD, 10% fat calories, 20% protein calories, and 70% carbohydrate calories) for 12 weeks as described previously (Cai et al. 2018; Huo et al. 2010; Xu et al. 2014) to examine A2AR abundance in relation to diet-induced adipose tissue inflammation. Additional age-matched male WT mice were maintained on a standard chow diet (CD) and served as an additional control. All diets (D12492, D12450J, and D10001) were products from Research Diets, Inc. (New Brunswick, NJ, USA). Study 2: male A2AR−/−, A2AR+/−, and A2AR+/+ mice, at 5 - 6 weeks of age, were fed an HFD for 12 weeks to analyze A2AR regulation of adipose tissue inflammation. Some mice in Study 2 were subjected to the Promethion™ system (Sable Systems International, North Las Vegas, NV) to measure energy metabolism. All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Measurement of plasma glucose levels
After the feeding period, the mice of both studies were fasted for 4 hr prior to collection of blood samples. Briefly, the fasted-mice were anesthetized by ketamine/xylazine. Under anesthesia, mice were subjected to heart puncture to collect blood, which was transferred to a heparinized tube. After centrifugation, plasma was collected and assayed for the levels of glucose using a metabolic assay kit (Sigma, St. Louis, MO).
Histological and immunohistochemical analyses
Paraffin-embedded adipose tissue (epididymal fat) blocks were cut into sections of 5 µm thickness. The sections of adipose tissue from Study 1 were stained for A2AR expression using mouse monoclonal antibodies against A2AR (7F6-G5-A2, Cat# sc-32261, Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Additional sections of adipose tissue from study 1, along with those from Study 2, were stained with H&E and for F4/80 expression with rabbit anti-F4/80 antibodies (1:100) (AbD Serotec, Raleigh, NC). Also, the co-staining of F4/80 (rat anti-mouse, MCA497, Bio-Rad, Hercules, CA) and A2AR in adipose tissue sections was evaluated by double immunofluorescent labeling according to the manufacturer’s instructions (Vector Laboratories, Inc. Burlingame, CA). Following staining, images were obtained using Leica TCS SPE Confocal Microscope System (Buffalo Grove, IL).
Adipose tissue stromal vascular cells and flow cytometry analysis
Adipose tissue stromal vascular cells (SVC) were isolated from epididymal fat depots using the collagenase digestion method (Lumeng et al. 2007; Stienstra, et al. 2008). The isolated SVC were subjected to FACS analyses (Wentworth, et al. 2010; Xu et al. 2014). Briefly, adipose tissue SVC were stained with fluorescence-tagged antibodies: anti-F4/80, anti-CD11b for macrophages, and anti-CD11c and anti-CD206 for macrophage activation, and analyzed using BD Accuri™ C6 Plus flow cytometer (BD Biosciences, San Jose, California, USA). Initially, SVC were analyzed based on FSC-A and SSC-A. Living cells were then examined for F4/80 (FITC) and CD11b (APC) expression. Mature macrophages (F4/80+ CD11b+ cells) were then gated for CD11c (PE/Cy7) and CD206 (PE) expression (macrophage polarization). Mature macrophages that were positive for CD11c but negative for CD206 were considered as proinflammatory (M1) macrophages (F4/80+ CD11b+ CD11c+ CD206− cells) whereas CD11c− CD206+ mature macrophages were considered as alternatively activated (M2) macrophages (F4/80+ CD11b+ CD11c− CD206+ cells).
Cell culture and treatment
Bone marrow cells were isolated from male A2AR−/− and A2AR+/+ mice and differentiated into macrophages (BMDM) as described (Xu et al. 2014). To examine the extent to which A2AR disruption alters the effect of palmitate on macrophage proinflammatory activation, both WT and A2AR−/− BMDM were incubated with media in the presence or absence of palmitate (250 µM, conjugated in bovine serum albumin (BSA)) or BSA for 24 hr. Cell lysates were examined for proinflammatory signaling using Western blot analysis.
Western blot analysis
Frozen adipose tissue (epididymal fat) and cultured cells were prepared in a lysis buffer containing 50 mM HEPES (pH 7.4), 10 mM EDTA, 50 mM sodium pyrophosphate, 0.1 M sodium fluoride, 10 mM sodium orthovanadate, 2 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mM benzamidine, and 1% Triton X-100. After protein electrophoresis and transfer, immunoblots were performed using rabbit anti-serum as primary antibody at a 1:1,000 dilution. This dilution was used for each of the primary antibodies used for the present study. After washing, the blot was incubated with a 1:10,000 dilution of goat anti-rabbit horseradish peroxidase-conjugated secondary antibody and followed by a chemiluminescent kit (Immobilon™ Western; EMD Millipore, Billerica, MA, USA) as described (Qi, et al. 2017). GAPDH was used as a loading control. The maximum intensity of each band was quantified using ImageJ software. Ratios of Pp46/p46, Pp65/65, or P-Akt/Akt were normalized to GAPDH and adjusted relative to the average of LFD- or CD-fed mice, HFD-fed A2AR+/+ mice, phosphate-buffered saline (PBS)-treated A2AR+/+ mice, or BSA-treated A2AR+/+ cells, which was arbitrarily set as 1 (AU). Antibodies against Pp46, p46, Pp65, p65, and hormone-sensitive lipase (HSL), P-HSL were products of Cell Signaling (Danvers, MA, USA). Antibodies against P-Akt and Akt and anti-rabbit IgG antisera were products of Santa Cruz Biotechnology, Inc.
RNA isolation, reverse transcription, and real-time PCR
Total RNA was isolated from adipose tissue (epididymal fat) and cultured macrophages. Reverse transcription was performed using the GoScript™ Reverse Transcription System (Promega) and real-time PCR analysis was performed using SYBR Green (LightCycler® 480 system; Roche) (Guo, et al. 2013; Guo, et al. 2012). The mRNA levels were analyzed for tumor necrosis factor alpha (Tnfa), interleukin 1 beta (Ilb), Il6, Il10, arginase 1, A2AR (Adora2a), peroxisome proliferator-activated receptor gamma (Pparg), macrophage chemoattractant protein 1 (Mcp1), adiponectin, and Hsl. A total of 0.1 μg RNA was used for the determination. Results were normalized to 18s ribosomal RNA and plotted as relative expression to the average of LFD-fed mice or HFD-A2AR+/+ mice, which was set as 1. Primer sequences are provided in Supplementary Table 1.
Statistical Methods
Numeric data are presented as means ± SEM (standard error). Statistical significance was assessed by unpaired, two-way ANOVA (for comparisons including three or more groups) and/or two-tailed Student’s t tests (for variables only involving two groups). Differences were considered significant at the two-tailed P < 0.05. Tukey’s range test was used for post-hoc test.
RESULTS
HFD feeding increases adipose tissue A2AR expression and proinflammatory responses
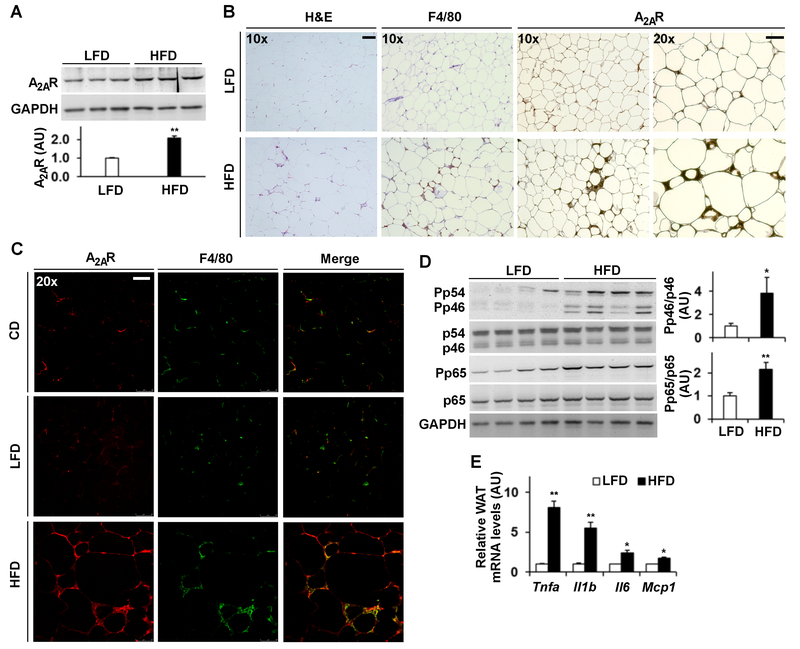
HFD-fed WT mice, a model of DIO, displayed a significant increase in adipose tissue amount of A2AR compared with LFD-fed WT mice (Fig. 1A). When histological and immunohistochemical assays were performed, HFD-fed WT mice revealed significant increases in adipocyte size and in adipose tissue macrophage infiltrations as indicated by immunostaining of F4/80 cells (macrophages) in adipose tissue sections (Fig. 1B). When stained for A2AR expression, adipose tissue sections of HFD-fed WT mice contained markedly more A2AR-positivie cells than those of LFD-fed mice. Also, nearly all A2AR-positive cells belong to stromal cells including macrophages (Fig. 1B). The staining intensity of A2AR in adipose tissue sections of HFD-fed WT mice was much stronger when compared with that of LFD-fed WT mice. Further analysis using immunofluorescent staining not only confirmed that HFD feeding stimulated adipose tissue A2AR expression compared with LFD or CD feeding, but also validated that most A2AR-postive cells were macrophages (Fig. 1C). When adipose tissue inflammatory responses were analyzed, HFD-fed WT mice displayed significant increases in adipose tissue proinflammatory signaling through JNK p46 and NFκB p65 and in adipose tissue mRNA levels of proinflammatory mediators such as Tnfa, Ilb, Il6, and Mcp1 (Fig. 1D,E) compared with LFD- or CD-fed mice. In terms of regulating adipose tissue proinflammatory signaling, LFD did not differ significantly from CD (Supplemental Fig. S1). Taken together, these results suggest that increased adipose tissue A2AR expression is associated with adipose tissue inflammation.
Figure 1. HFD feeding increases adipose tissue adenosine 2A receptor (A2AR) expression and proinflammatory responses.
Male C57BL/6J mice, at 5 – 6 weeks of age, were fed a high-fat diet (HFD) or low-fat diet (LFD) for 12 weeks or maintained on a standard chow diet (CD). (A) Adipose tissue (epididymal fat) lysates were examined for A2AR amount using Western blot analysis. (B) Adipose tissue (epididymal fat) sections were stained with H&E (panels in the first row from top) or stained for F4/80 expression (a macrophage marker) (panels of the second row from top) or A2AR expression (panels of bottom two rows) using immunohistochemistry. Representative images were from HFD-fed mice (right column) and LFD-fed mice (left column). (C) Adipose tissue (epididymal fat) sections were subjected to immunofluorescent staining of A2AR (left column) and/or F4/80 (middle column) expression. (D) Adipose tissue (epididymal fat) lysates were examined for proinflammatory signaling using Western blot analysis. (E) Adipose tissue (epididymal fat) mRNA levels of cytokines were quantified using real-time RT-PCR. For A and D, blots were quantified using densitometry. For A, D, and E, numeric data are means ± SEM. n = 4 - 6. Statistical difference between HFD and LFD: *, P < 0.05 and **, P < 0.01 in bar graphs of A and D or in E for the same gene. For B and C, the scale bar is 75 µm for 10× images or 50 µm for 20× images.
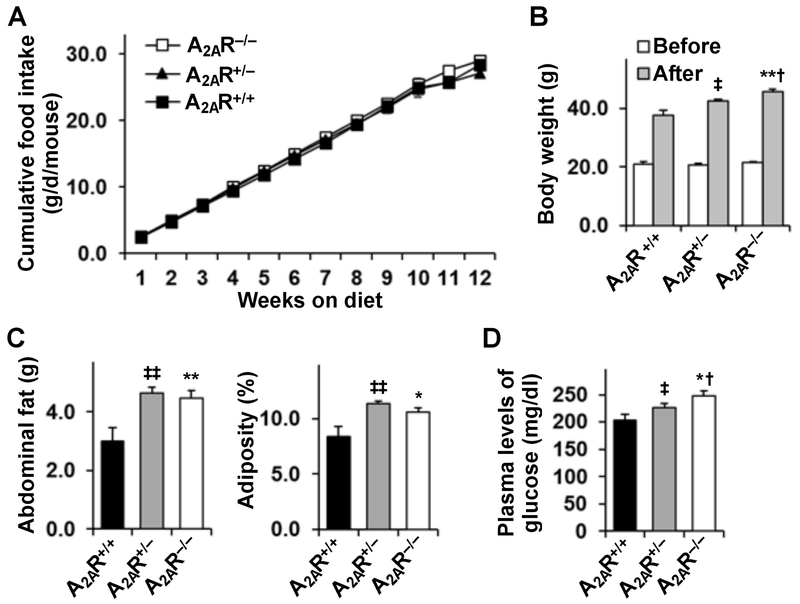
A2AR disruption exacerbates the effects of HFD on inducing weight gain, adiposity, and hyperglycemia
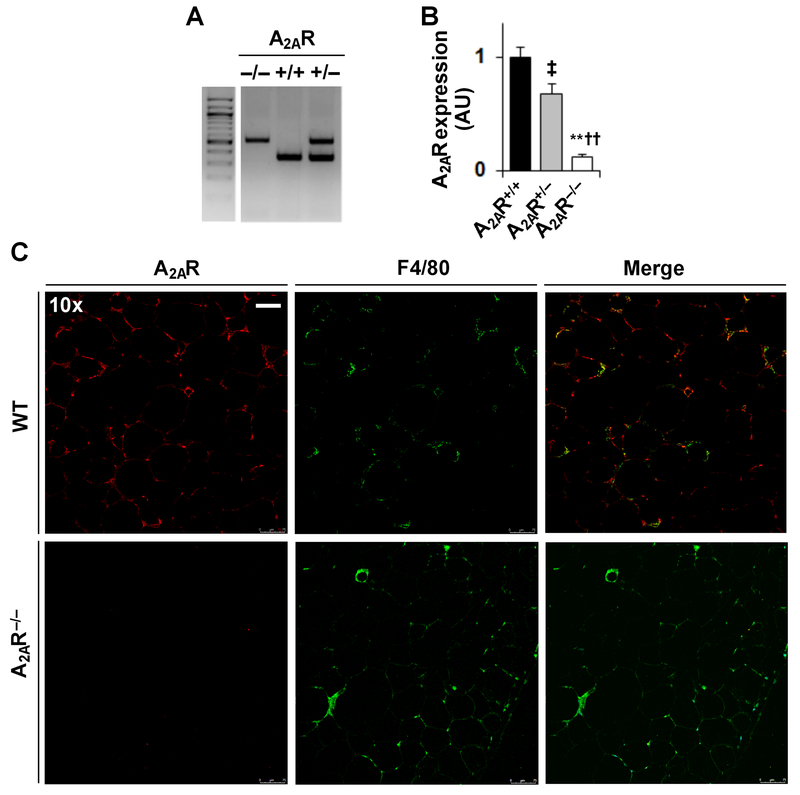
Next, male homozygous A2AR-disrupted (A2AR−/−) mice, heterozygous A2AR-disrupted (A2AR+/−) mice, and their WT (A2AR+/+) littermates (Fig. 2A) were fed an HFD. After the feeding period, adipose tissue A2AR disruption was verified using real-time RT-PCR (Fig. 2B) and immunoflourescent staining (Fig. 2C). Initially, body weight and food intake of the mice were analyzed. Prior to HFD feeding, there was no difference in body weight among the mice (Fig. 3B). After HFD feeding, A2AR−/− or A2AR+/− mice gained much more body weight than A2AR+/+ mice (Fig. 3B); although the mice consumed comparable amount of foods (Fig. 3A). When energy metabolism was analyzed, HFD-fed A2AR−/− mice and HFD-fed A2AR+/+ mice displayed comparable amount of energy expenditure; although HFD-fed A2AR−/− mice, interestingly, revealed a shift in time of peak energy expenditure during night time period (~ 3 hr ahead of HFD-fed A2AR+/+ mice) (Supplemental Fig. S2). Consistently, HFD-fed A2AR−/− or A2AR+/− mice displayed significant increases in abdominal fat mass and adiposity compared with HFD-fed A2AR+/+ mice (Fig. 3C). Also, adipocyte size of HFD-fed A2AR−/− or A2AR+/− mice was significantly increased compared with that of HFD-fed A2AR+/+ mice (see below in Fig. 4A, top panels). At whole animal level, male A2AR-disrupted mice revealed increased severity of HFD-induced systemic insulin resistance and glucose intolerance (supplemental data of the published study by Cai et al. (Cai et al. 2018). Similar analyses were also performed in female mice. However, female A2AR-disrupted mice did not gain more body weight compared with WT mice. Also, female homozygous, but not heterozygous A2AR-disrupted mice revealed an increase in the severity of HFD-induced systemic insulin resistance (Cai et al. 2018). When plasma levels of glucose were examined, A2AR−/− or A2AR+/− mice displayed a greater increase in the severity of HFD-induced hyperglycemia than A2AR+/+ mice (Fig. 3D). Also, HFD-induced hyperglycemia in A2AR−/− mice was severer than that in A2AR+/− mice.
Figure 2. Validation of adipose tissue A2AR disruption.
(A) Genomic DNA was subjected to PCR analysis for genotyping of homozygous A2AR-disrupted (A2AR−/−) mice, heterozygous A2AR-disrupted (A2AR+/−) mice and their wild-type (WT, A2AR+/+) littermates. (B,C) Adipose tissue A2AR disruption. Male A2AR-dirupted (A2AR−/− and A2AR+/−) mice and A2AR+/+ mice, at 5 - 6 weeks of age, were fed an HFD for 12 weeks. For B, adipose tissue (epididymal fat) mRNA levels of A2AR were quantified using real-time RT-PCR. Data are means ± SEM. n = 8 - 10. Statistical difference between A2AR−/− and A2AR+/+: **, P < 0.01; statistical difference between A2AR−/− and A2AR+/−: ††, P < 0.01; statistical difference between A2AR+/‒ and A2AR+/+: ‡, P < 0.05. For C, adipose tissue (epididymal fat) sections were subjected to immunofluorescent staining of A2AR and/or F4/80 expression. The scale bar is 75 µm for 10× images.
Figure 3. A2AR disruption exacerbates the effects of HFD feeding on inducing weight again, adiposity, and hyperglycemia.
Male A2AR−/−, A2AR+/−, and A2AR+/+ mice, at 5 - 6 weeks of age, were fed an HFD for 12 weeks. (A) Cumulative food intake during feeding period. (B) Body weight was measured before and after the feeding period. (C) Abdominal fat mass was calculated as the sum of epididymal fat, mesenteric fat, and perinephric fat. Adiposity was calculated as the ratio of abdominal fat mass to body weight. (D) After the feeding period, mice were fasted for 4 hr prior to collection of blood samples. Plasma levels of glucose were quantified using a metabolic kit. For A - D, data are means ± SEM. n = 4 - 6 (A) or 10 – 12 (C - D). Statistical difference between A2AR−/− and A2AR+/+: *, P < 0.05 and **, P < 0.01 in B after feeding period or in C and D; statistical difference between A2AR−/− and A2AR+/−: †, P < 0.05 in B after feeding period or in D; statistical difference between A2AR+/− and A2AR+/+: ‡, P < 0.05 ‡‡, P < 0.01 in B after feeding period or in C and D.
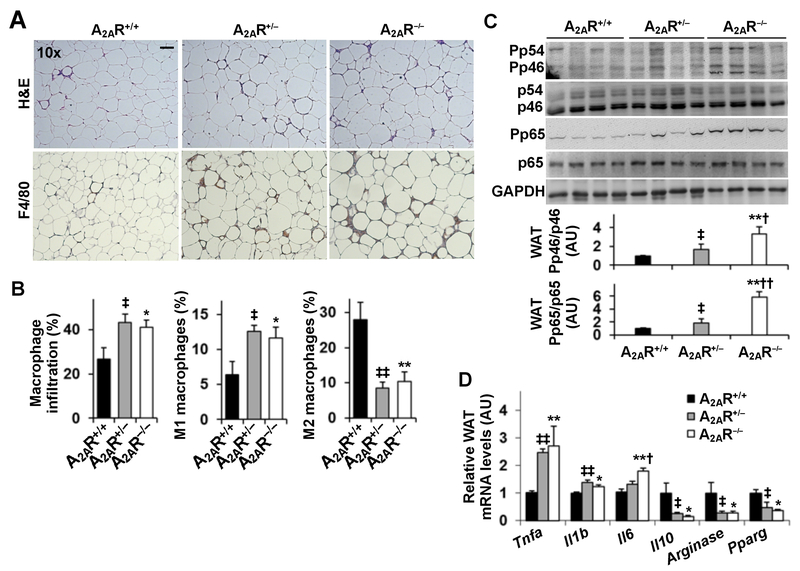
Figure 4. A2AR disruption exacerbates HFD-induced adipose tissue inflammation.
Male A2AR−/−, A2AR+/−, and A2AR+/+ mice, at 5 - 6 weeks of age, were fed an HFD for 12 weeks. (A) Sections of epididymal fat were stained with H&E (top three panels) or stained for F4/80 expression (bottom three panels) using immunohistochemistry. Representative images were presented. (B) Adipose tissue macrophage infiltration and polarization. Stromal vascular cells (SVC) were isolated from epididymal fat depots and analyzed for CD11b and F4/80 expression. Mature macrophages (CD11b+ and F4/80+ cells) were further analyzed for CD11c and CD206 expression. Left panel, percentages of mature macrophages; middle panel, percentages of M1 macrophages (F4/80+ CD11b+ CD11c+ CD206− cells); right panel, percentages of M2 macrophages (F4/80+ CD11b+ CD11c− CD206+ cells). (C) Lysates of epididymal fat were examined for proinflammatory signaling using Western blot analysis. Bar graphs, quantifications of blots. (D) Adipose tissue (epididymal fat) mRNA levels of cytokines and markers related to macrophage polarization were quantified using real-time RT-PCR. For B - D, numeric data are means ± SEM. n = 8 - 10. Statistical difference between A2AR−/− and A2AR+/+: *, P < 0.05 and **, P < 0.01 in B and C or in D for the same gene; statistical difference between A2AR−/− and A2AR+/−: †, P < 0.05 and ††, P < 0.01 in C or in D for the same gene; statistical difference between A2AR+/− and A2AR+/+: ‡, P < 0.05 and ‡‡, P < 0.01 in B and C or in D for the same gene.
A2AR disruption exacerbates HFD-induced adipose tissue inflammation
When adipose tissue inflammation was analyzed, HFD-fed A2AR-disrupted mice accumulated significantly more macrophages in adipose tissue compared with HFD-fed A2AR+/+ mice (Fig. 4A, bottom panels). Among adipose tissue macrophages, there were more proinflammatory (M1) macrophages and fewer alternative or anti-inflammatory (M2) macrophages in HFD-fed A2AR-dirupted mice than in HFD-fed A2AR+/+ mice (Fig. 4B). Consistently, the phosphorylation states of adipose tissue JNK p46 and NFκB p65 and the mRNA levels of proinflammatory cytokines in HFD-fed A2AR-disrupted mice were significantly higher than their respective levels in HFD-fed A2AR+/+ mice (Fig. 4C,D). In contrast, the mRNA levels of Il10, Arginase, and Pparg in HFD-fed A2AR-disrupted mice were significantly lower than their respective levels in HFD-fed A2AR+/+ mice (Fig. 4D). Taken together, these results suggest that A2AR disruption exacerbates diet-induced adipose tissue inflammation.
A2AR disruption exacerbates HFD-induced adipose tissue insulin resistance
We examined A2AR regulation of adipose tissue insulin sensitivity, and observed that insulin-stimulated Akt phosphorylation was significantly decreased in HFD-fed A2AR-disrupted mice in a gene-dose-dependent manner (Fig. 5A). Also, we examined the expression of several genes or enzymes related to adipose tissue metabolic responses. The mRNA levels of adiponectin and Hsl in HFD-fed A2AR−/− mice were significantly decreased compared with their respective levels in HFD-fed A2AR+/+ mice (Fig. 5B). Consistently, the amount and phosphorylation states of HSL in HFD-fed A2AR-disrupted mice were significantly lower than those in HFD-fed A2AR+/+ mice (Fig. 5C). However, Mcp1 mRNAs in HFD-fed A2AR-disupted mice did not differ significantly from those in control mice. These results suggest that A2AR disruption exacerbates diet-induced adipose tissue insulin resistance.
Figure 5. A2AR disruption exacerbates HFD-induced adipose tissue insulin resistance.
Male A2AR−/−, A2AR+/−, and A2AR+/+ mice, at 5 – 6 weeks of age, were fed an HFD for 12 weeks. (A) Adipose tissue insulin signaling. Prior to harvest, mice were given an injection of insulin (1 U/kg body weight) into the portal vein for 5 min. Lysates of epididymal fat were subjected to Western blot analysis. Bar graphs, quantifications of blots. (B) The mRNA levels of genes for adipose tissue metabolic responses were quantified using real-time RT-PCR. (C) Lysates of epididymal fat were examined for HSL amount and phosphorylation states using Western blot analysis. For A and C, numeric data are means ± SEM. n = 6 - 8. Statistical difference between A2AR−/− and A2AR+/+: *, P < 0.05 and **, P < 0.01 in A under the same condition, in B for the same gene, or in C; statistical difference between A2AR−/− and A2AR+/−: †, P < 0.05 and ††, P < 0.01 in A under the same condition, in B for the same gene, or in C; statistical difference between A2AR+/− and A2AR+/+: ‡, P < 0.05 and ‡‡, P < 0.01 in A under the same condition or in C.
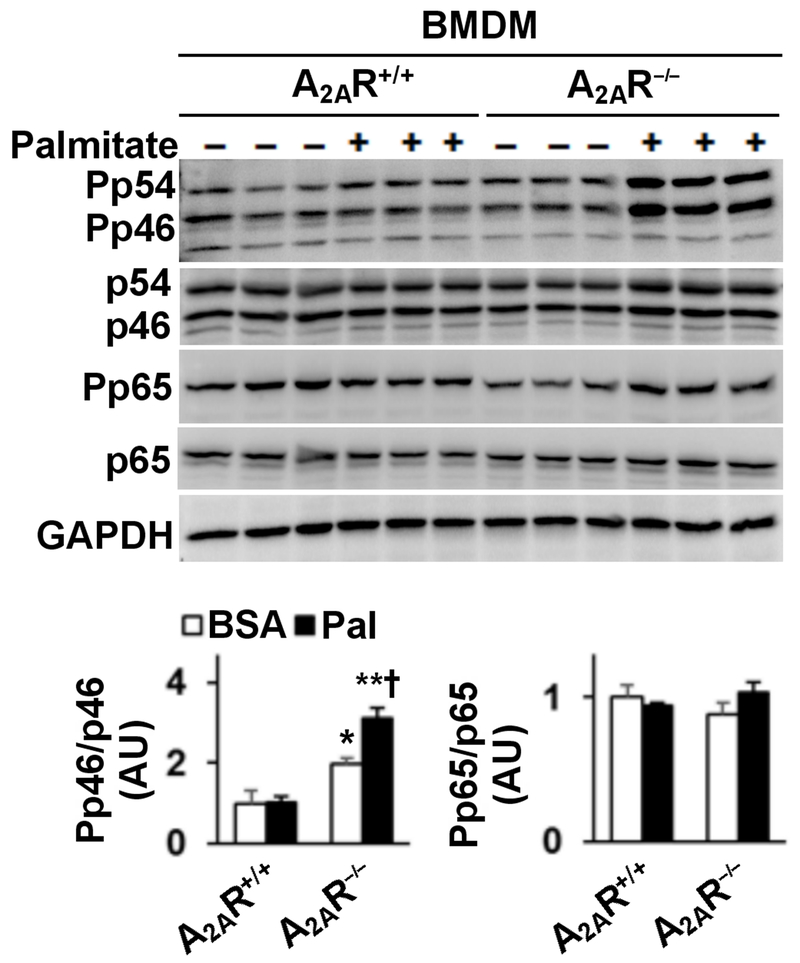
A2AR disruption enhances the effect of palmitate on stimulating macrophage proinflammatory signaling
The present study examined the extent to which A2AR disruption alters the effect of palmitate on regulating macrophage proinflammatory signaling. In WT BMDM, treatment with palmitate caused marginal or insignificant increases in the phosphorylation states of JNK p46 and NFκB p65 (Fig. 6). However, in A2AR−/− BMDM, treatment with palmitate caused a significant increase in the phosphorylation states of JNK p46; although palmitate did not significantly alter the phosphorylation states of NFκB p65 (Fig. 6). These results suggest that A2AR disruption enhances the effect of palmitate on stimulating macrophage proinflammatory signaling.
Figure 6. A2AR disruption enhances macrophage proinflammatory signaling.
Bone marrow cells were isolated from male A2AR−/− and A2AR+/+ mice, and differentiated into macrophages (BMDM). Cells were incubated in growth media in the presence of palmitate (250 µM, conjugated in BSA) or BSA for 24 hr. Cell lysates were subjected to Western blot analysis. Bar graphs, quantifications of blots. Numeric data are means ± SEM. n = 6 - 8. Statistical difference between A2AR−/− and A2AR+/+ with the same treatment: **, P < 0.01.
DISCUSSION
In the present study, we validated an association between increased A2AR expression and adipose tissue inflammation in WT mice upon feeding an HFD. Furthermore, using A2AR-disrupted mice, we demonstrated an increase in the severity of HFD-induced adipose tissue inflammation in relative to that in WT control mice. This finding is complementary to the finding by DeOliveira et al, which indicates a suppressive effect of A2AR activation on adipose tissue inflammation (DeOliveira et al. 2017). At the cellular level, the present study validated that A2AR deficiency exacerbated the stimulatory effect of palmitate on macrophage proinflammatory activation, suggesting that A2AR suppression of adipose tissue inflammation is attributable to, at least in part, the effect of A2AR on inhibiting macrophage activation.
While A2AR is expressed in adipose tissue, the response of A2AR expression to obesity in the context of adipose tissue inflammation is not defined. To address this, the present study examined adipose tissue A2AR amount in WT C57BL/6J mice fed an HFD or LFD, and demonstrated a stimulatory effect of HFD feeding on adipose tissue A2AR expression. Of note, adipose tissue of HFD-fed WT mice contained many more A2AR-positive cells than that of LFD-fed WT mice, and nearly all of the A2AR-positive cells were located in crown structure areas that were mainly composed of macrophages. Upon co-staining A2AR and F4/80 using immunofluorescent assay, it was confirmed that most A2AR-postive cells were macrophages. Furthermore, the staining intensity of A2AR-positive cells in adipose tissue of HFD-fed WT mice was much stronger than that of LFD-fed WT mice. Because of this, it is conceivable that obesity increases adipose tissue A2AR abundance through increasing macrophage infiltration into adipose tissue and stimulating A2AR expression in the infiltrated macrophages. To be noted, increased adipose tissue macrophage A2AR expression in HFD-fed WT mice was accompanied with enhanced adipose tissue inflammatory responses, as indicated by increases in the phosphorylation states of JNK p46 and NFκB p65 and in the mRNA levels of proinflammatory cytokines including Tnfa, Il1b, and Il6. This correlation suggested a defensive response of adipose tissue macrophage A2AR to obesity or HFD feeding rather than a causal role for A2AR in adipose tissue inflammation. This is because the A2AR in macrophage, per se, is anti-inflammatory (Cai et al. 2018; Lukashev, et al. 2004). As substantial evidence, A2AR deficiency exacerbated the effect of HFD feeding on inducing adipose tissue inflammation in vivo and enhanced the effect of palmitate on stimulating macrophage proinflammatory activation in vitro. However, we do not rule out the possibility that prolonged increase in A2AR expression may cause harmful effects, which warrants future investigation.
The regulatory role for A2AR in adipose tissue inflammation is supported by recent evidence obtained from obese mice upon treatment with an A2AR agonist (CGS-21680) (DeOliveira et al. 2017). Specifically, treatment of obese mice with CGS-21680 caused a significant decrease in adipose tissue macrophage infiltration; although treatment with CGS-21680 did not significantly alter the phosphorylation states of adipose tissue JNK p46. As complementary evidence, the present study revealed that the severity of HFD-induced adipose tissue inflammation in A2AR-disrupted mice was significantly greater than that in A2AR+/+ mice. Notably, HFD-fed A2AR-disrupted mice displayed significant increases in adipose tissue macrophage infiltration, as well as percentages of proinflammatory macrophages among adipose tissue macrophages. In addition, HFD-fed A2AR-disrupted mice displayed significant increases in the phosphorylation states of adipose tissue JNK p46 and NFκB p65 and in the mRNA levels of Tnfa, Il1b, and Il6. Taken together, these results argued in favor that A2AR plays a suppressive role in obesity-associated adipose tissue inflammation.
Additional to inflammation, adipose tissue insulin resistance is another feature commonly associated with obesity. In the present study, increased adipose tissue inflammation in HFD-fed A2AR-deficient mice was accompanied with increased severity of HFD-induced adipose tissue insulin resistance. In particular, the states of insulin-induced Akt phosphorylation in HFD-fed A2AR-disrupteted mice were significantly lower than those in HFD-fed control mice. To this point, however, it remains to be explored whether decreased adipose tissue insulin sensitivity in HFD-fed A2AR-deficient mice was due to increased adipose inflammation, increased adipose tissue fat mass, or both. With regard to changes in body weight of HFD-fed mice, our findings are contradictory to those published by Csoka et al.(Csóka, et al. 2017). The exactly reasons for the discrepancy are not clear, but may be attributable to the differences in the responses of WT mice to HFD feeding. In the study by Csoka et al., WT mice displayed a marked increase in body weight upon HFD feeding, and appeared to be hyperphagic compared with the WT mice used by us and others (DeOliveira et al. 2017; Gnad, et al. 2014). Given this, interpretation of the phenotype of A2AR disruption appeared to be influenced by what control was used for comparisons. In the present study, A2AR-deficient mice gained more body weight upon HFD feeding compared with WT mice; although consuming comparable amount of foods and revealing similar energy expenditure. A possible explanation is that HFD-fed A2AR-deficient mice had increased efficiency in energy absorption. This postulation was based on two reasons. First, in rodent models of DIO, intestine inflammation is evident and likely enhances energy absorption. Second, A2AR exerts a suppressive effect on intestine inflammation (Odashima, et al. 2005). However, future study is needed to examine A2AR regulation of intestine inflammation in HFD-fed mice to validate our postulation. In HFD-fed A2AR-disrupted mice, the phosphorylation states of HSL were significantly decreased compared with those in control mice. This appeared to also account for increased adiposity in A2AR-disrupted mice. What should be pointed out is that decreased HSL phosphorylation in HFD-fed A2AR-disrupted mice likely indicates a decrease in adipose tissue release of fatty acids. The latter, when undergoing oxidation, contributes largely to energy expenditure. In other words, decreased adipose tissue HSL phosphorylation was expected to associate with an increase in respiratory quotient in HFD-fed A2AR-disrupted mice relative to control mice. However, this was not the case. We speculate that increased energy absorption could provide more fatty acids to offset a decrease in the availability of fatty acids due to decreased adipose tissue lipolysis. Nonetheless, it is conceivable that A2AR has a role in protecting against HFD-induced adipose tissue inflammation and insulin resistance.
While A2AR exerted anti-inflammatory effects on adipose tissue inflammation, an interesting question raised was to what extent the A2AR in macrophages versus adipocytes contributes to the regulation of adipose tissue inflammation. To address this question may rely on analyzing diet-induced adipose tissue inflammation in mice whose A2AR is disrupted only in adipocytes or myeloid cells (macrophages). However, the results generated by DeOliveira et al. and by the present study appear to indicate a more important role for the A2AR in macrophages in determining overall adipose tissue inflammation; although the data from adipocyte- or macrophage-specific A2AR knockout is not available. This view is supported by three lines of evidence. Firstly, in adipose tissue of HFD-fed WT mice, A2AR was expressed predominantly in macrophages, and barely in adipocytes. Secondly, when adipose tissue macrophage infiltration was increased in HFD-fed A2AR-disrupted mice, adipose tissue amount of Mcp1, whose secretion by adipocytes regulates adipose tissue macrophage infiltration, was not significantly altered. This indicates a role for the A2AR in macrophages in determining adipose tissue macrophage infiltration, which was consistent with the finding that A2AR-disrupted leukocytes (monocytes) likely are easier to infiltrate into inflamamtory tissues when compared with WT cells (Wang, et al. 2010). Thirdly, A2AR activation has been shown to decrease macrophage inflitration, which may be attributable to A2AR actvation in macrophages (Garcia, et al. 2008). Taken together, it appears that the A2AR in macrophages determines obesity-associated adipose tissue inflammation. At the cellular level, A2AR has a direct role in regulating macrophage inflammatory activation, which was validated by the finding that A2AR deficiency enhanced the effect of palmitate, a major component of HFD, on stimulating macrophage proinflammatory activation.
In summary, the present study demonstrated a critical role for A2AR in regulating obesity-associated adipose tissue inflammation. Specifically, A2AR expression was increased in adipose tissue of obese mice, which appeared to be a defensive response. Also, in the absence of A2AR, macrophage proinflammatory status was increased and likely accounted for exacerbation of adipose tissue inflammation, adiposity, and adipose tissue insulin resistance under obese conditions. Accordingly, targeting A2AR to suppress adipose tissue inflammation would be a beneficial approach for management of obesity-associated inflammatory and metabolic diseases. The present study also has several limitations. First, it remains to be determined what signals are responsible for stimulating macrophage A2AR expression in vivo. Second, it is not clear whether increased adiposity in A2AR-disrupted mice was attributable to, in part, increased hepatic production of endogenous fat. Third, it remains to be determined the extent to which A2AR-disruption-related adipose tissue inflammation also acts through causing hepatic and muscle insulin resistance to bring about systemic insulin resistance. We would like to address these questions in our future studies.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part or in whole by grants from the National Institutes of Health (R01DK095828 and R01DK095862 to C.W. and HL095556 to Y.H.), the American Diabetes Association (1-17-IBS-145 to C.W.), and in part by NIH grants DK058411 to GA, SG, FM, and DK115184 to GA. C.W. is supported by the Hatch Program of the National Institutes of Food and Agriculture (NIFA). Y. Cai is supported by China Scholarship Council. In addition, this work was supported in part by the National Key R&D Program of China (2017YFA0105803), the general program of National Natural Science Foundation of China (81770826), the science and technology plan projects of Guangdong Province (2016A050502010), the key special projects of medical and health collaborative innovation of Guangzhou City (201604020016), and the special scientific research project of Guangzhou City (2060404) (all to Y. Chen). This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
DECLARATION of INTEREST
The authors declare that there is no competing financial interest.
REFERENCES
- Alchera E, Rolla S, Imarisio C, Bardina V, Valente G, Novelli F & Carini R 2017. Adenosine A2a receptor stimulation blocks development of nonalcoholic steatohepatitis in mice by multilevel inhibition of signals that cause immunolipotoxicity. Translational Research 182 75–87. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M & Scherer PE 2001. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature Medicine 7 947–953. [DOI] [PubMed] [Google Scholar]
- Boyle JG, Logan PJ, Jones GC, Small M, Sattar N, Connell JMC, Cleland SJ & Salt IP 2011. AMP-activated protein kinase is activated in adipose tissue of individuals with type 2 diabetes treated with metformin: a randomised glycaemia-controlled crossover study. Diabetologia 54 1799–1809. [DOI] [PubMed] [Google Scholar]
- Cai Y, Li H, Liu M, Pei Y, Zheng J, Zhou J, Luo X, Huang W, Ma L, Yang Q, et al. 2018. Disruption of adenosine 2A receptor exacerbates NAFLD through increasing inflammatory responses and SREBP1c activity. Hepatology 68 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-F, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS & Schwarzschild MA 1999. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19 9192–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AT, Wang J, Ree D, Kolls JK & Bryer-Ash M 2000. Tumor necrosis factor-alpha induces hepatic insulin resistance in obese Zucker (fa/fa) rats via interaction of leukocyte antigen-related tyrosine phosphatase with focal adhesion kinase. Diabetes 49 810–819. [DOI] [PubMed] [Google Scholar]
- Choe SS, Shin KC, Ka S, Lee YK, Chun J-S & Kim JB 2014. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes 63 3359–3371. [DOI] [PubMed] [Google Scholar]
- Csóka B, Törő G, Vindeirinho J, Varga ZV, Koscsó B, Németh ZH, Kókai E, Antonioli L, Suleiman M, Marchetti P, et al. 2017. A2A adenosine receptors control pancreatic dysfunction in high-fat-diet-induced obesity. The FASEB Journal 31 4985–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeOliveira CC, Paiva Caria CRe, Ferreira Gotardo EM, Ribeiro ML & Gambero A 2017. Role of A1 and A2A adenosine receptor agonists in adipose tissue inflammation induced by obesity in mice. European Journal of Pharmacology 799 154–159. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Truong LD, Li P, Zhang P, Du J, Chen J-F & Feng L 2008. Adenosine A2A receptor activation and macrophage-mediated experimental glomerulonephritis. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessi S, Varani K, Merighi S, Ongini E & Borea PA 2000. A2A adenosine receptors in human peripheral blood cells. Br J Pharmacol 129 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad T, Scheibler S, von Kugelgen I, Scheele C, Kilic A, Glode A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, et al. 2014. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516 395–399. [DOI] [PubMed] [Google Scholar]
- Guo X, Li H, Xu H, Halim V, Thomas LN, Woo S-L, Huo Y, Chen YE, Sturino JM & Wu C 2013. Disruption of inducible 6-phosphofructo-2-kinase impairs the suppressive effect of PPARγ activation on diet-induced intestine inflammatory response. J Nutr Biochem 24 770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo SL, Walzem RL, Mashek DG, et al. 2012. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE 7 e39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA & Davis RJ 2013. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G, Linden J, Cronstein B & Pacher P 2008. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF & Spiegelman BM 1996. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271 665–668. [DOI] [PubMed] [Google Scholar]
- Huo Y, Guo X, Li H, Wang H, Zhang W, Wang Y, Zhou H, Gao Z, Telang S, Chesney J, et al. 2010. Disruption of inducible 6-phosphofructo-2-kinase ameliorates diet-induced adiposity but exacerbates systemic insulin resistance and adipose tissue inflammatory response. Journal of Biological Chemistry 285 3713–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Fan Y-Y, Ong KT, Woo S-L, et al. 2012. Targeted overexpression of inducible 6-phosphofructo-2-kinase in adipose tissue increases fat deposition but protects against diet-induced insulin resistance and inflammatory responses. Journal of Biological Chemistry 287 21492–21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imarisio C, Alchera E, Sutti S, Valente G, Boccafoschi F, Albano E & Carini R 2012. Adenosine A2a receptor stimulation prevents hepatocyte lipotoxicity and nonalcoholic steatohepatitis (NASH) in rats. Clin Sci 123 323–332. [DOI] [PubMed] [Google Scholar]
- Jiao P, Feng B, Ma J, Nie Y, Paul E, Li Y & Xu H 2012. Constitutive activation of IKKβ in adipose tissue prevents diet-induced obesity in mice. Endocrinology 153 154–165. [DOI] [PubMed] [Google Scholar]
- Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK & Bergman RN 2005. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab 288 E454–461. [DOI] [PubMed] [Google Scholar]
- Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, et al. 2006. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. Journal of Biological Chemistry 281 26602–26614. [DOI] [PubMed] [Google Scholar]
- Kumari M, Wang X, Lantier L, Lyubetskaya A, Eguchi J, Kang S, Tenen D, Roh HC, Kong X, Kazak L, et al. 2016. IRF3 promotes adipose inflammation and insulin resistance and represses browning. Journal of Clinical Investigtion 126 2839–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashev D, Ohta A, Apasov S, Chen J-F & Sitkovsky M 2004. Cutting edge: Physiologic attenuation of proinflammatory transcription by the Gs protein-coupled A2A adenosine receptor in vivo. J Immunol 173 21–24. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, DeYoung SM, Bodzin JL & Saltiel AR 2007. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56 16–23. [DOI] [PubMed] [Google Scholar]
- Menghini R, Menini S, Amoruso R, Fiorentino L, Casagrande V, Marzano V, Tornei F, Bertucci P, Iacobini C, Serino M, et al. 2009. Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology 136 663–672. e664. [DOI] [PubMed] [Google Scholar]
- Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschöp MH, et al. 2007. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. Journal of Clinical Investigtion 117 2877–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, et al. 2005. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129 26–33. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, et al. 2007. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM & Olefsky JM 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T, Chen Y, Li H, Pei Y, Woo S, Guo X, Zhao J, Qian X, Awika J, Huo Y, et al. 2017. A role for PFKFB3/iPFK2 in metformin suppression of adipocyte inflammatory responses. J Mol Endocrinol. 2017 Jul;59(1):49–59. 59 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi M, Woods N-B, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM & Olefsky JM 2009. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab 10 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo J-L, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, et al. 2007. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 6 386–397. [DOI] [PubMed] [Google Scholar]
- Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S & Muller M 2008. Peroxisome proliferator-activated receptor γ activation promotes infiltration of alternatively activated macrophages into adipose tissue. Journal of Biological Chemistry 283 22620–22627. [DOI] [PubMed] [Google Scholar]
- Trujillo ME & Scherer PE 2006. Adipose tissue-derived factors: impact on health and disease. Endocr Rev 27 762–778. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang W, Tang R, Zhu C, Bucher C, Blazar B, Geng J, Zhang C, Linden J, Wu C, et al. 2010. Adenosine A(2A) receptor deficiency in leukocytes increases arterial neointima formation in apolipoprotein E-deficient mice. Arteriosclerosis, Thrombosis, and Vascular Biology 30 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL & Ferrante AWJ 2003. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigtion 112 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, Brien PE & Harrison LC 2010. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 59 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. 2003 Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigtion 112 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li H, Woo S-L, Kim S-M, Shende VR, Neuendorff N, Guo X, Guo T, Qi T, Pei Y, et al. 2014. Myeloid cell-specific disruption of Period1 and Period2 exacerbates diet-induced inflammation and insulin resistance. Journal of Biological Chemistry 289 16374–16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Gao Z, Yin J & He Q 2007. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293 E1118–1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.