Abstract
Growth hormone (GH) has long been known to stimulate lipolysis and insulin resistance; however, the molecular mechanisms underlying these effects are unknown. In the present study, we demonstrate that GH acutely induces lipolysis in cultured adipocytes. This effect is secondary to the reduced expression of a negative regulator of lipolysis, Fat Specific Protein 27 (FSP27) at both the mRNA and protein level. These effects are mimicked in vivo as transgenic over-expression of GH leads to a reduction of FSP27 expression. Mechanistically, we show GH modulation of FSP27 expression is mediated through activation of both MEK/ERK and STAT5 dependent intracellular signaling. These two molecular pathways interact to differentially manipulate peroxisome proliferator-activated receptor gamma activity (PPARγ) on the FSP27 promoter. Furthermore, over-expression of FSP27 is sufficient to fully suppress GH-induced lipolysis and insulin resistance in cultured adipocytes. Taken together, these data decipher a molecular mechanism by which GH acutely regulates lipolysis and insulin resistance in adipocytes.
Keywords: PPPAR-gamma, metabolism, acromegaly, adipose tissue, obesity, diabetes
Introduction
Although growth hormone (GH) has been primarily studied for its effects on linear growth, pronounced stimulation of lipolysis was among the first metabolic effects reported in human subjects following the introduction of pituitary derived human GH (Raben and Hollenberg 1959). Studies in mice have indicated that ablation of the GH receptor or Jak2 in adipose tissue reduces GH induced lipolysis (List, et al. 2013; Nordstrom, et al. 2013; Shi, et al. 2014). In addition, pharmacological blockade of the hormone sensitive lipase (HSL) abrogates the lipolytic effects of GH in human subjects (Nielsen, et al. 2001). However, the direct molecular mechanisms by which GH induces lipolysis have not been fully elucidated.
The importance of deciphering this mechanism is clear, as several lines of clinical evidence demonstrate that GH regulates insulin sensitivity in humans as a direct result of its lipolytic action. In both healthy volunteers and patients with GH deficiency, the insulin resistance caused by acute GH treatment is reversed by a pharmacological blockade of lipolysis (Cornford, et al. 2012; Moller, et al. 2009; Salgin, et al. 2009; Segerlantz, et al. 2003). Additionally, the “dawn phenomenon”, which describes an early morning increase in insulin resistance in diabetic patients, has been directly attributed to the diurnal peak of GH levels (Bolli and Gerich 1984; Bouchonville, et al. 2014; Monnier, et al. 2013; Monnier, et al. 2012; Schmidt, et al. 1981). Strikingly, the dawn phenomenon can be almost entirely corrected by reducing GH levels (Campbell, et al. 1985; Davidson, et al. 1988) or by blocking the lipolytic action of GH (Salgin et al. 2009). Taken together, these studies demonstrate that GH-mediated lipolysis is a critical regulator of insulin sensitivity in both healthy and diabetic patients.
Lipolysis in adipose tissue requires the sequential activation of several enzymes including the rate limiting enzyme Adipose triglyceride lipase (ATGL) and Hormone sensitive lipase (HSL) (Zimmermann, et al. 2004). The Cell Death-Inducing DNA Fragmentation Factor Alpha-like Effector (CIDE)-family proteins associate with lipid droplets and regulate fatty acid (FA) homeostasis in adipocytes (Puri, et al. 2008a; Puri, et al. 2008b). CIDEC, also known as FSP27, regulates lipid droplet dynamics and lipolysis in adipocytes through suppression of the catalytic capacity as well as transcription of ATGL (Grahn, et al. 2014; Singh, et al. 2014). Consistent with these studies, mutation of FSP27 in humans leads to increased lipolysis (Rubio-Cabezas, et al. 2009). In addition, adipose-specific disruption of FSP27 causes insulin resistance in high fat fed mice (Tanaka, et al. 2015).
In the present study, we demonstrate that GH-induced lipolysis is associated with an acute reduction in FSP27 mRNA and protein expression. Mechanistically, we show GH induced reduction of FSP27 is mediated through GH activation of both MEK/ERK and STAT5 dependent signaling which coordinately regulate peroxisome proliferator-activated receptor gamma (PPARγ) transcriptional activity. Finally, we demonstrate that over-expression of FSP27 is alone sufficient to fully repress GH induced lipolysis and insulin resistance in adipocytes. Taken together, these data clearly demonstrate a transcriptional mechanism by which GH acutely regulates lipolysis and insulin action in adipocytes.
Material and Methods
Mice
bGH and STAT5ΔN/ ΔN mice were housed at 22°C under a 14-hour light, 10-hour dark cycle, 3–4 mice per cage, and ad libitum access to water and standard laboratory chow (ProLab RMH 3000). All experiments were approved by the Ohio University Institutional Animal Care and Use Committee.
Cell Culture
3T3L1 (from ATCC: CL-173; passages 4–12) were grown in DMEM, high glucose (4.5 g/L) supplemented with Glutamax, Pen-Strep, and 10% FBS. For differentiation into adipocytes, cells were seeded at 200,000 cells/well in 6-well plates. After cells reached confluence, the medium was replaced by differentiation medium (growth medium with 1 μM dexamethasone, 0.5 μM isobutylmethylxanthine, 100 nM insulin, and 1 μM rosiglitazone. After 2 days, the medium was replaced with growth medium containing 100 nM insulin and 1 μM rosiglitazone for 2 more days, then for 2–3 days with growth medium alone.
Lipolysis
Differentiated adipocytes were serum deprived in DMEM, high glucose for two hours then treated in KRH buffer supplemented with 2 mM sodium pyruvate in the presence or absence of U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene), rosiglitazone, or STAT5 inhibitor, and GH for 2 h. The cells were washed carefully and incubated in Krebs Hepes-bicarbonate (KRH) buffer supplemented with 2 mM sodium pyruvate in the presence or absence of U0126, rosiglitazone, or STAT5 inhibitor, and GH for an additional hour. Glycerol release was measured by colorimetric reaction as previously described (Lee, et al. 2013).
Real-time RT-PCR
Analysis of gene expression was conducted using real-time reverse transcriptase quantitative PCR (qPCR). TriZol Reagent (Life Technologies) was used to extract total RNA from adipose tissue samples, and RNA was quantified by measuring absorbance at 260 and 280 nm with a ratio ≥ 1.8. For a list of real time primers and sequences see Supplementary Table 1.
Western Blot Analysis and Nuclear Fractionation
Proteins were extracted from adipose tissue and cells, subjected to SDS-PAGE, transferred to polyvinylidine fluoride membranes, and blots were blocked and probed with antibodies as specified in Supplementary Table 2. Protein were quantified as a ratio to the content of β-actin except for Pparγ in the nuclear fractionation studies in which proliferating cell nuclear antigen (PCNA) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) were utilized as loading controls. Nuclear Fractionation was performed utilizing the Rapid, Efficient And Practical (REAP) method as previously described (Suzuki, et al. 2010).
Reporter assays
293T cells (from ATCC: CRL-3216; passages 4–8) were transfected with polyethylenimine (PEI) transfection reagent while still in suspension in 96-well plates. Each well was transfected with 50ng of a previously described FSP27 luciferase construct (Kim, et al. 2008), along with a total of 50ng of expression plasmids containing STAT5A, STAT5B, or empty vector controls and 10ng of Renilla-TK plasmid. The cells were harvested 24 h later and luciferase activity was measured using a Dual Renilla Luciferase II Assay Kit, and normalized to Renilla luciferase measurements (Promega). Site directed mutatagenesis of the FSP27 constructs by PCR was performed using the primers listed in Supplementary Table 1.
FSP27 overexpression
Fully differentiated 3T3-L1 adipocytes (day 6) (from ATCC: CRL-173; passages 2–6) were either infected with control adenovirus or adenovirus expressing FSP27 (1–2 moi/cell). Cells were treated 24 hours later.
Statistics
Normality was tested with the Shapiro-Wilk test and by using the normal probability plot on raw data. Non-normal distributed data were ln-transformed. Differences between groupswere tested using a Student’s t-test or a two-way repeated measurements analysis of variance (ANOVA) as appropriate. Statistical significance was assumed for p < 0.05. Data are presented as arithmetic means ± SE unless otherwise stated.
Results
GH Acutely Induces Lipolysis and Reduces FSP27 Expression
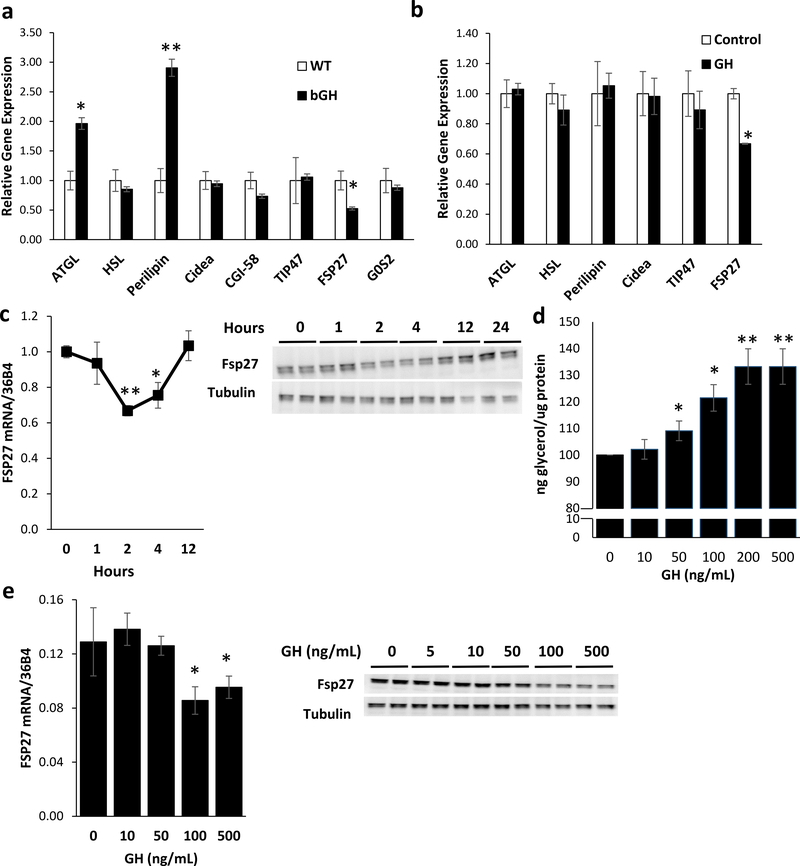
In order to determine the effects of GH on lipolysis, we examined the expression of known regulators of lipolysis in the perigonadal adipose tissue of bovine (b)GH mice transgenic mice. bGH mice have serum GH levels of approximately 2 μg/mL, leading to a 2-fold increase in serum IGF-1 levels (Chen, et al. 1991; Chen, et al. 1990). In the perigondal adipose tissue, mRNA levels of ATGL and perilipin were increased 2 and 3-fold, respectively, while levels of FSP27 were reduced by 40% compared to levels in wild-type controls (Figure 1a). In order to determine which of these regulators was directly and acutely regulated by GH action, differentiated 3T3-L1 adipocytes were treated with 500 ng/μL of recombinant bGH for 2 hrs. qPCR analysis indicates expression of FSP27 was acutely reduced by ~35% by GH treatment, with no changes in mRNA levels of other regulators of lipolysis (Figure 1b). This GH-mediated decrease in FSP27 mRNA and protein expression was transitory, as qPCR and Western blot analysis indicates that FSP27 mRNA levels and protein levels were reduced by 35–40% at 2–4 hours after GH treatment with levels returning to baseline by 12 hours (Figure 1c; Supplemental Figure 1a). On the other hand, the known target gene of GH, insulin-like growth factor I (Igf1) was significantly increased at 4, 12 and 24 hours after GH treatment (Chia, et al. 2010) (Supplemental Figure 1b). Furthermore, treatment of differentiated 3T3-L1 adipocytes with GH for 2 hours reveals that lipolysis, as measured by glycerol release, is increased in a dose dependent manner (Figure 1d). This increased lipolytic rate is accompanied by a rate dependent decrease in FSP27 mRNA and protein levels at 2 hours (Figure 1e; Supplemental Figure 1c). Thus, in cultured adipocytes, GH treatment directly increases lipolysis that is associated with a rapid and transient decrease in FSP27 expression.
Figure 1. GH Acutely Induces Lipolysis and Reduces FSP27 Expression in a Time and Dose Dependent Manner.
a) qPCR analysis of mRNA levels of regulators of lipolysis: ATGL, HSL, Perilipin, Cidea, Comparative gene identification-58 (CGI-58), Tail-interacting protein of 47 kD (TIP47), G0/G1 switch gene 2 (G0S2), and FSP27 mRNA was compared in RNA isolated from perigonadal fat of 4 month old male bGH mice. Data are shown as mean ± SEM of 8–10 Samples. * p<0.05; **p<0.01; ***p<0.01.
b) qPCR analysis of ATGL, HSL, Perilipin, Cidea, CGI-58, TIP47, and FSP27 mRNA in RNA isolated from 3T3-L1 adipocytes treated with 500ng/mL recombinant bovine GH (bGH) for 2 hours. Data are shown as mean ± SEM of 3 independent experiments.
c) qPCR and Western Blot analysis of FSP27 mRNA and protein isolated from 3T3-L1 adipocytes treated with 500ng/mL recombinant bovine GH (bGH). Data are shown as mean ± SEM of 3 independent experiments.
d) Lipolysis as measured by glycerol release from 3T3-L1 adipocytes treated with bGH for two hours. Data are shown as mean ± SEM of 3 independent experiments.
e) qPCR and Western Blot analysis of FSP27 mRNA and protein isolated from 3T3-L1 adipocytes treated with bGH for 2 hours. Data are shown as mean ± SEM of 3 independent experiments.
GH Regulates Lipolysis in a MEK and PPARγ Dependent Manner
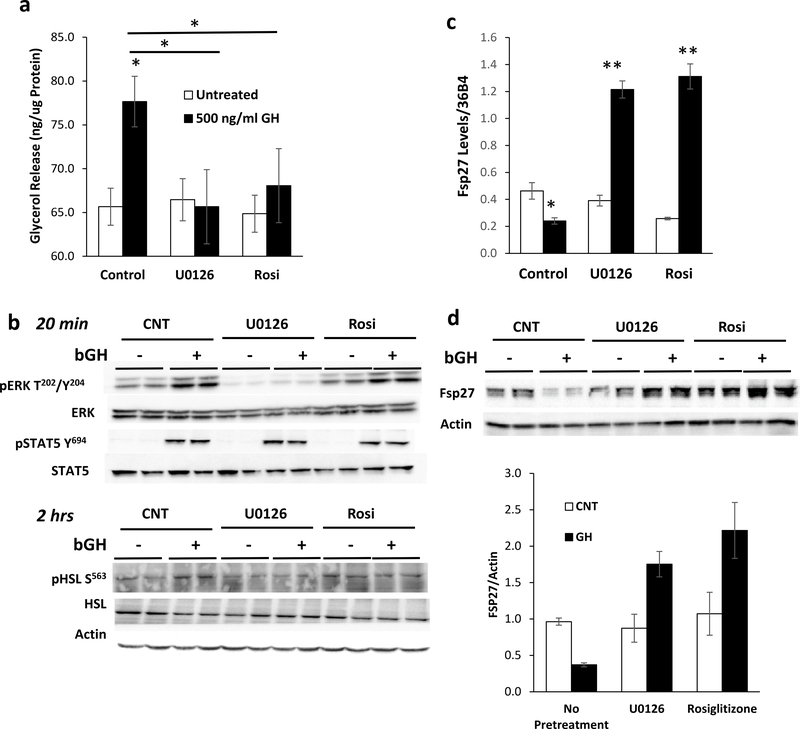
Since FSP27 is transcriptionally regulated by PPARγ (Kim et al. 2008; Puri et al. 2008a) and its expression is affected by PPARγ phosphorylation (Banks, et al. 2015; Choi, et al. 2011; Tan, et al. 2016) mediated through MEK/ERK, we investigated if GH modulates PPARγ levels and/or activity through these pathways. Although no changes in total PPARγ protein levels were noted, we did find that the effects on lipolysis are mediated through both MEK/ERK and PPARγ. A 2 hour pre-treatment with 10 μM U0126, to block the phosphorylation of MEK, or 1 μM rosiglitazone, to fully activate PPARγ, was sufficient to completely inhibit GH-induced lipolysis (Figure 2a). Western blot analysis indicates that 20 min of GH treatment induced phosphorylation of ERK on T202/Y204 and STAT5 on Y694 without changes in total protein levels. Pretreatment with U0126 abolished ERK phosphorylation without affecting STAT5 phosphorylation, while rosiglitazone pretreatment had no effect on the phosphorylation of either ERK1 or STAT5. Phosphorylation of HSL at S563 was induced by GH treatment at the 2 hour time point, and this induction was blunted by U0126, but not significantly altered by rosiglitazone pretreatment (Figure 2b; Supplemental Figure 2a).
Figure 2. GH Regulates Lipolysis in a MEK and Pparγ Dependent Manner.
a) Lipolysis as measured by glycerol release from 3T3-L1 adipocytes treated with 500ng/mL bGH for 2 hours after 2 hours of pre-treatment with 10 μM U0126 or 1 μM Rosiglitazone. Data are shown as mean ± SEM of 3 independent experiments. Asterisks indicate a significant differences in all panels * p<0.05; **p<0.01.
b) Representative Western blot analysis of pERK T202/Y204 , total ERK, pSTAT5 Y694, total STAT5, pHSL S563 , and total HSL in 3T3-L1 adipocytes treated with 500ng/mL bGH for either 20 minutes or 2 hours after 2 hours of pre-treatment with 10 μM U0126 or 1 μM Rosiglitazone. Actin is used as a loading control.
c-d) qPCR and Western blot analysis of FSP27 mRNA and protein isolated from cells treated with 500ng/mL bGH for 2 hours after 2 hours of pre-treatment with 10 μM U0126 or 1 μM Rosiglitazone. Actin is used as a loading control. Quantitation of Western blot data are shown as mean ± SEM of 4 independent experiments.
Interestingly, both U0126 and rosiglitazone pretreatment not only prevented GH-induced reduction of FSP27, but led to a GH-dependent three-fold increase in the level of FSP27 mRNA and protein (Fig. 2c-d). Levels of other regulators of lipolysis including ATGL, HSL, and CIDEA were not significantly altered either by U0126 and rosiglitazone pretreatment or GH treatment. Furthermore, no changes in total PPARγ levels were noted (Supplemental Figures 2b-d, Figure 3a). Taken together, these data indicate that GH suppresses FSP27 expression through MEK/ERK and PPARγ dependent mechanisms.
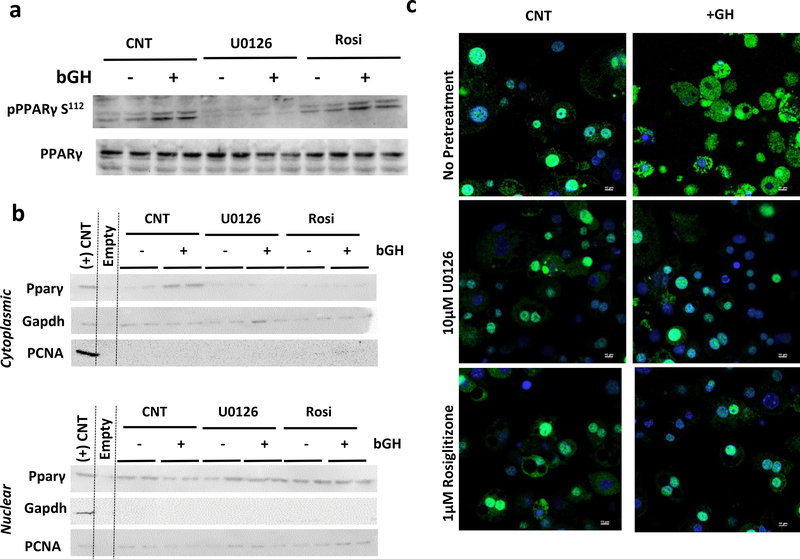
GH Treatment Results in PPARγ Translocation to the Cytoplasm
MEK has been shown to directly interact with and phosphorylate PPARγ leading to its nuclear export after stimulation with tetradecanoyl phorbol acetate or TNF-α stimulation (Burgermeister, et al. 2007; Burgermeister and Seger 2007; Tan et al. 2016). GH also acts through these mechanisms as 3T3-L1 adipocytes treated with 500ng/mL bGH for 20 minutes increased phosphorylation of pPPARγ on S112 without effecting total pPPARγ levels. Pre-treatment with 10 μM U0126 abolished this effect, while pre-treatment with rosiglitazone had no effect (Figure 3a). Nuclear fractionation experiments one hour after GH treatment, demonstrated that GH leads to a rapid increase in cytoplasmic PPARγ and a reduction in nuclear PPARγ. This translocation was inhibited by both U0126 and rosiglitazone (Figure 3b). These effects were confirmed by immunofluorescence for PPARγ (green) with nuclei counterstained with DAPI (blue) in 3T3-L1 adipocytes treated with 500ng/mL bGH for one hour GH treatment resulted in a rapid translocation of PPARγ from the nucleus to the cytoplasm in 3T3-L1 adipocytes, and pretreatment with 10 μM U0126 or 1 μM rosiglitazone blocked this effect (Figure 3c).
Figure 3. GH Treatment Results in Rapid PPARγ Translocation.
a) Representative Western blot analysis of pPPARγ S112 and total pPPARγ in 3T3-L1 adipocytes treated with 500ng/mL bGH for 20minutes after 2 hours of pre-treatment with 10 μM U0126 or 1 μM Rosiglitazone.
b) Representative Western blot analysis for PPARγ in 3T3-L1 adipocytes treated with 500ng/mL bGH for 1 hour after 2 hours of pre-treatment with MEK1 inhibitor, 10 μM U0126, or 1 μM Rosiglitazone following nuclear fractionation. The positive control is whole cell lysate of 3T3-L1 adipocytes. Gapdh and PCNA are loading controls for the cytoplasmic and nuclear fractions, respectively.
c) Immunofluorescence for PPARγ (green) with nuclei counterstained with DAPI (blue) of in 3T3-L1 adipocytes treated with 500ng/mL bGH for 1 hour after 2 hours pre-treatment with 10 μM U0126 or 1 μM Rosiglitazone.
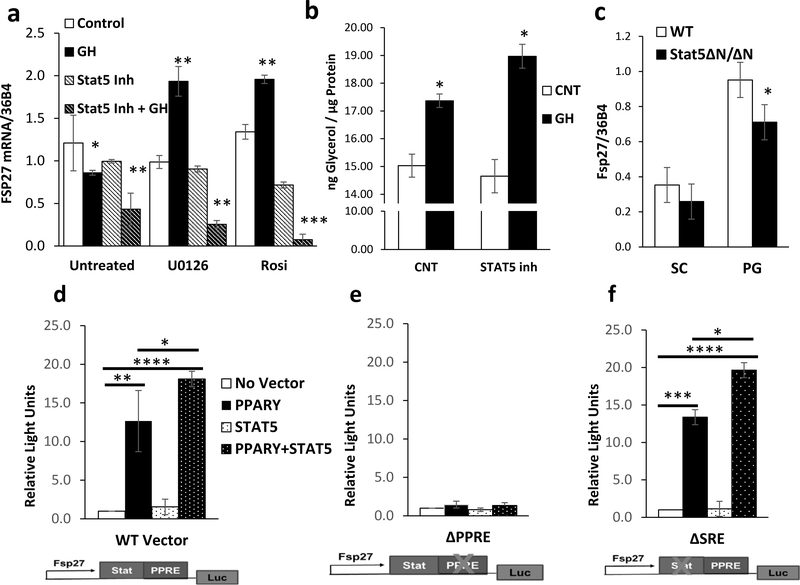
STAT5 increases PPARγ Activity on the FSP27 Promoter
The 3-fold increase of FSP27 mRNA in 3T3-L1 adipocytes that we observed upon U0126 or rosiglitazone pretreatment and subsequent GH treatment (Figure 2c) lead us to hypothesize that an additional regulatory mechanism is responsible for FSP27 regulation. Nuclear fractionation indicates that STAT5 translocates to the nucleus in a MEK and PPARγ independent manner (Supplemental Figure 3a). Since STAT5 mediates many GH-dependent effects, we pretreated 3T3-L1 adipocytes with the specific STAT5 inhibitor, CAS 285986–31-4 (Muller, et al. 2008). The STAT5 inhibitor reduced GH-induced phosphorylation of STAT5 on Y694 by ~70% without changes in total protein levels (Supplemental Figure 3b-c). Although pretreatment with the STAT5 inhibitor alone had limited effect on mRNA levels of FSP27, pretreatment with STAT5 inhibitor in combination with GH treatment leads to 60% down-regulation of FSP27 mRNA. In addition, the STAT5 inhibition completely suppressed the upregulation of FSP27 mRNA that was observed with U0126 and rosiglitazone pretreatment and subsequent GH treatment, and lead to an 80–90% downregulation of FSP27 mRNA (Figure 4a). Inhibition of STAT5 increased GH-mediated lipolysis by ~15%, however these results did not quite reach statistical significance (Figure 4b). Furthermore, qPCR analysis of RNA isolated from subcutaneous and perigonadal fat of mice which express hypomorphic forms of both STAT5a and STAT5b, STAT5ΔN/ΔN mutant mice (Cui, et al. 2004; Teglund, et al. 1998), demonstrates that FSP27 is significantly reduced in the perigonadal fat and tends to be reduced in subcutaneous fat (Figure 4c).
Figure 4. STAT5 and PPARγ Regulate FSP27 Expression.
a) qPCR analysis of FSP27 in RNA isolated from 3T3-L1 adipocytes treated with vehicle or 500ng/mL bGH for 2 hours after 2 hours of pre-treatment with 10 μM U0126, 1 μM Rosiglitazone, or 200 μM STAT5 inhibitor. Data are shown as mean ± SEM of 3 independent experiments. Asterisks indicate a significant differences in all panels * p<0.05; **p<0.01 ; ***p<0.001.
b) Lipolysis as measured by glycerol release from 3T3-L1 adipocytes treated with vehicle or 500ng/mL bGH for 2 hours after 2 hours of pre-treatment with 200 μM STAT5 inhibitor. Data are shown as mean ± SEM of 2 independent experiments, each with 3 replicates/group.
c) Expression level of FSP27 mRNA was compared using quantitative real-time PCR (qPCR) of RNA isolated from subcutaneous (SC) and perigonadal (PG) fat of 4 month old male Stat5ΔN/ΔN mice. Data are shown as mean ± SEM of six samples.
d-f) Luciferase activity of 293T cells transfected with a 0.9-kb WT FSP27 luciferase reporter, with the PPARγ response element mutated, or with a presumptive STAT5 response element mutated. The reporter vector was co-transfected either a vector control or 25 ng of Pparγ expression vector and 25 ng of its obligate heterodimer RXRα. The cells were also co-transfected with either a vector control or 25 ng of a STAT5 expression vector. Data are shown as mean ± SEM of three independent experiments, each with three replicates.
The dependence of FSP27 expression on both STAT5 and PPARγ prompted us to test whether FSP27 might be directly regulated by the cooperative action of these factors. A reporter construct harboring a 0.9-kb upstream fragment of the human FSP27 gene linked to a luciferase reporter gene (FSP27-luc) was co-transfected with either a vector control or 25 ng of PPARγ expression vector and 25 ng of its obligate heterodimer RXRα. The cells were also co-transfected with either a vector control or 25 ng of a STAT5 expression vector. Luciferase assays demonstrated that basal FSP27 promoter activity in 293T cells was very low, and no change in activity was seen following STAT5 expression. The FSP27-luc activity was potently activated by co-transfection of PPARγ and its obligate heterodimer partner RXRα. Although we could not detect direct interaction between PPARγ and STAT5 (Supplemental Figure 3d), co-transfection of STAT5 with PPARγ resulted in a synergistic 50% increase in FSP27-luc activation compared to PPARγ alone (Figure 4d). Both STAT5A and STAT5B were equally able to transactivate the FSP27 promoter construct when co-transfected with PPARγ, while a constitutively active form of STAT5B (Farrar 2010) lead to further transactivation of the promoter construct (Supplemental Figure 3e).
Examination of the FSP27 promoter sequence revealed the presence of half of a consensus STAT binding site (StatRE) adjacent to the known PPARγ response element (PPRE) (Kim et al. 2008). Site directed mutagenesis of the PPRE (ΔPPRE) in the FSP27 promoter abolishes its response to both PPARγ and STAT5 (Figure 4e). On the other hand, mutation of the StatRE (ΔSRE) does not significantly affect activation either by PPARγ or co-transfection of PPARγ and Stat5 (Figure 4f).
FSP27 Over-expression Prevents GH Induced Lipolysis and Insulin Resistance.
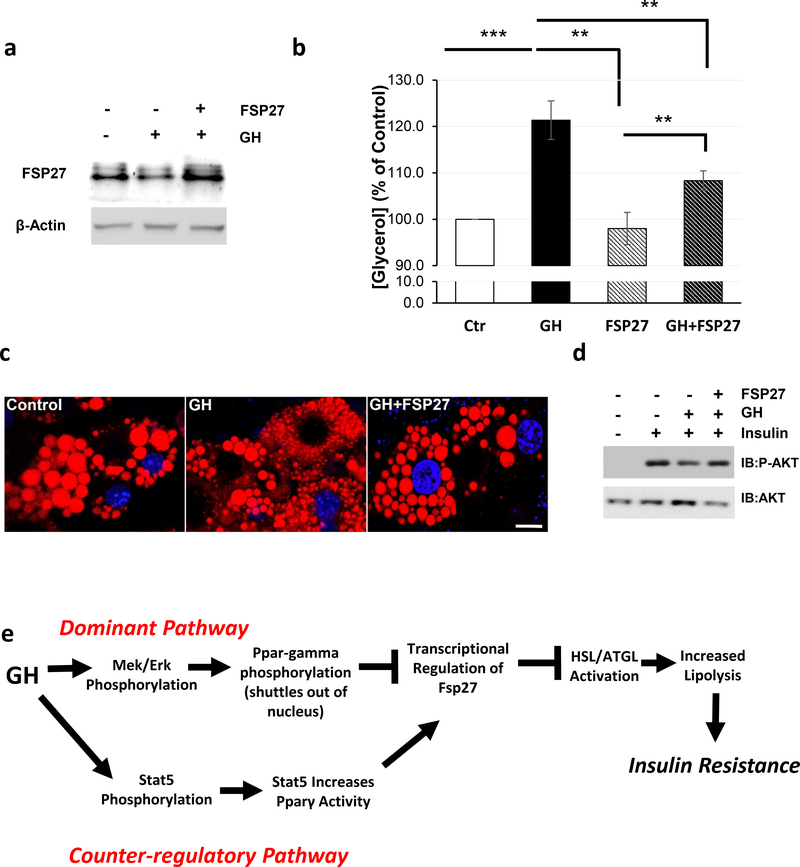
Our data indicate that GH-induced lipolysis is associated with a reduction of FSP27 expression. Thus, we hypothesized that FSP27 over-expression might protect adipocytes from GH-induced lipolysis and insulin resistance. To test our hypothesis we utilized adenovirus to stably over-express human FSP27 in 3T3-L1 adipocytes. Interestingly, FSP27 over-expression in adipocytes almost completely suppressed GH-induced lipolysis (Figure 5a-b). Furthermore, since lipolysis is associated with lipid droplet fragmentation (Walther and Farese 2012), we assessed lipid droplet number in control and FSP27 over-expressing cells. Control 3T3-L1 adipocytes each contained ~20 lipid droplets/cell, and 24 hours of GH treatment lead to over a 5-fold increase in the number of lipid droplets. FSP27 over-expression was sufficient to completely abolish the effect GH on lipid fragmentation (Figure 5c, Supplemental Figure 4a). Since the FFAs liberated by lipolysis have long been known to cause insulin resistance (Boden 1997), we assessed the insulin-stimulated phosphorylation of AKT S473 in these adipocytes. Treatment of control adipocytes with 10nM insulin for 15 minutes strongly induces phosphorylation of AKT on S473, while pretreatment with 250ng/mL GH for two hours reduced this phosphorylation by ~60%. However, the inhibitory effect of GH on AKT S473 phosphorylation was abolished in adipocytes over-expressing FSP27 (Figure 5d, Supplemental Figure 4b). Thus, over-expression of FSP27 is sufficient to prevent both GH-induced lipolysis, lipid fragmentation, and insulin resistance.
Figure 5. FSP27 Over-expression Prevents GH-Induced Lipolysis and Insulin Resistance.
a) Representative Western blot analysis of FSP27 in WT 3T3-L1 adipocytes and FSP27 over-expressing adipocytes treated with 500ng/mL bGH for two hours.
b) Lipolysis as measured by glycerol release from WT 3T3-L1 adipocytes and FSP27 over-expressing adipocytes treated with 250ng/mL bGH for two hours. Data are shown as mean ± SEM of 3 independent experiments. Asterisks indicate a significant differences * p<0.05, ** p<0.01, *** p<0.001
c) Immunofluorescence of Nile Red Stained WT and FSP27 over-expressing 3T3-L1 adipocytes treated with vehicle or 500ng/mL bGH for 24 hours.
d) Representative Western blot analysis of pAKT S473 and total AKT in WT 3T3-L1 adipocytes and FSP27 over-expressing adipocytes treated with 250ng/mL bGH for two hours and 10nM insulin for 15 minutes.
d) Proposed mechanism of GH-induced lipolysis.
Discussion
In the present study, we present a molecular mechanism by which GH regulates lipolysis in adipose tissue. These studies are consistent with human studies in adipose tissue, as GH receptor levels have been shown to be directly correlated with FSP27 levels in human subcutaneous adipose tissue (Karastergiou, et al. 2016) and acute infusion of 30ng/min of GH in human subjects is sufficient to induce lipolysis and leads to reduction in FSP27 expression at the mRNA and protein levels (Sharma V, et al.[submitted manuscript]). Our data demonstrates that GH induced MEK/ERK activation promotes PPARγ phosphorylation and translocation from the nucleus. This serves as the dominant pathway controlled by GH and led to reduced transcription of FSP27. As a counter-regulatory mechanism, GH-induced phosphorylation of STAT5 which enhances PPARγ activity on the FSP27 promoter. Together, these intersecting pathways act together to tightly regulate the lipolytic effects GH (Figure 5e).
The molecular mechanisms described in this study, indicate that GH-induced lipolysis is secondary to its reduction in the expression of FSP27. This mechanism is distinct from previous studies describing pathways by which GH chronically induces lipolysis through HSL activation (Bergan, et al. 2013; Dietz and Schwartz 1991). In agreement with these previous findings, we also observed an increase in the MEK/ERK mediated phosphorylation of HSL (Figure 2b). However, since ATGL hydrolysis of triglycerides is the first and rate-limiting step in lipolysis, it suggests that the changes in the ATGL activity by modulation of FSP27 may be the key regulatory step in control of GH-induced lipolytic rate. Furthermore, except in cases of acromegaly, GH secretion is pulsatile and regulated in diurnal rhythms (Ho, et al. 1987). This pulsatile secretion leads us to espouse a biological model by which GH acutely and transiently regulates lipolysis over the effects observed in cellular models of chronic GH treatment. Interestingly, FSP27 levels are chronically, rather than transiently, reduced in bGH animals with consistently high GH levels. bGH has similar somatotropic effects as hGH, but does not bind the prolactin receptor, and thus does not have the severe lactogenic and reproductive effects observed upon over-expression of hGH effects (Bartke, et al. 1992; Bartke, et al. 1994; Posner 1976). In addition to reduced fat mass, bGH mice also have numerous changes within their adipose tissue, including increased immune cell infiltration within adipose tissue, increased circulating levels of inflammatory cytokines, and dramatically altered adipokine expression (Benencia, et al. 2015; Berryman and List 2017; Wang, et al. 2007). All of these factors have been shown to affect FSP27 expression (Becerril, et al. 2018; Tan et al. 2016), and may contribute to its chronically reduced expression in bGH adipose tissue.
In contrast to the extremely rapid kinetics of catecholamine-induced lipolysis, in which measurable FFA release occurs within minutes, administration of a physiological GH bolus has been shown to stimulate lipolysis after a time lag of 2–3 hours (Moller, et al. 1990; Morimoto, et al. 2001). Consistent with this, the expression of FSP27 is reduced 2 hours after GH treatment (Figures 1b-c). Since FSP27 protein is very unstable and has a short half-life of ~15 minutes (Nian, et al. 2010; Yang, et al. 2011), our data indicate that the reduced PPARγ-dependent transcription caused by GH leads to a rapid reduction in its mRNA and protein expression. Importantly, levels of other regulators of lipolysis including ATGL, HSL, CIDEA, and CGI-58 are not acutely changed by GH treatment. Furthermore, although chronic GH treatment upregulates transcription of PPARγ mRNA in a STAT5 dependent manner (Kawai, et al. 2007), the acute lipolytic effects of GH occur in the absence of increased PPARγ expression (Figure 4a). In addition, these effects are also independent of IGF-1, as levels of IGF-1 remain unchanged after 2 hours of GH treatment in cultured adipocytes (Supplemental Figure 2a). Although we could not detect direct protein-protein interaction of STAT5 and PPARγ (Supplemental Figure 3d), our data clearly indicate that STAT5, in a GH-dependent manner, is critical for maintaining FSP27 expression and may play a role in moderating the magnitude of GH-induced lipolysis (Figure 4a-b). Treatment with the STAT5 inhibitor tended to increase lipolysis, this difference did not quite reach statistical significance. This may be due to the secondary role of STAT5 pathway in this process, as the MEK/ERK is the predominant pathway. In addition, the STAT5 inhibitor did not fully suppress STAT5 phosphorylation, thus limiting its effect on lipolysis. Elucidating the exact molecular mechanism by which STAT5 controls PPARγ activity will be of importance in future studies.
Our data demonstrate that reduction of lipolysis by over-expression of FSP27 blocks the GH-mediated suppression of insulin signaling in adipocytes. These results are in agreement with previous reports that indicate circulating FFAs are the dominant inducer of insulin resistance and non-suppressible hepatic glucose production in patients with T2DM (Titchenell, et al. 2016). Furthermore, both current and early studies have consistently demonstrated that acute administration of GH has a strong diabetogenic effect that is primarily due to its lipolytic action (Cornford et al. 2012; Houssay 1936; Moller et al. 2009; Salgin et al. 2009; Segerlantz et al. 2003). These diabetogenic effects of GH are also manifested in acromegalic patients with high levels of GH that display increased rates of insulin resistance, hyperinsulinemia, and type 2 diabetes (Hansen, et al. 1986; Melmed, et al. 2009; Rizza, et al. 1982). This induction of insulin resistance is, in all likelihood, a critical part of the response of adipocytes to GH, as it serves to inhibit the anti-lipolytic action of insulin (Okada, et al. 1994) and allows lipolysis to proceed. Although previous experiments in mice have shown that the diabetogenic effect of GH can, at least in part, be explained by alterations in the ability of insulin to activate PI-3 kinase in insulin target cells (del Rincon, et al. 2007; Dominici, et al. 2005), studies in humans have questioned the role of PI-3 kinase in GH-induced insulin resistance (Jessen, et al. 2005; Krusenstjerna-Hafstrom, et al. 2011a; Krusenstjerna-Hafstrom, et al. 2011b; Nielsen, et al. 2008). Importantly, the “dawn phenomenon”, which describes an early morning increase in insulin resistance in patients with diabetes, can be almost entirely corrected by reducing GH levels or GH-induced lipolysis (Campbell et al. 1985; Davidson et al. 1988; Salgin et al. 2009). Although the dawn phenomenon was first described in Type 1 Diabetes mellitus (T1DM), recent studies utilizing the advent of continuous glucose monitoring systems has demonstrated the dawn phenomenon occurs in ~50% of patients with both T1DM and T2DM, significantly increases HbA1c levels (~0.4% in T2DM), and leads to frequent hyperglycemic episodes (Bouchonville et al. 2014; Monnier et al. 2013; Monnier et al. 2012). Taken together, these studies demonstrate that GH-mediated lipolysis is a critical regulator of insulin resistance in both healthy subjects and patients with diabetes.
Clinical studies have consistently demonstrated that GH treatment reduces visceral fat mass and improves metabolic parameters in GH deficient patients, as well as those with visceral obesity (Beauregard, et al. 2008; Bredella, et al. 2013). This reduction in visceral fat mass is presumably, at least in part, due to lipolytic action of GH. On the other hand, in diabetic patients, GH-induced lipolysis leads to frequent hyperglycemic episodes in the dawn phenomenon (Bouchonville et al. 2014; Monnier et al. 2013; Monnier et al. 2012). Thus, understanding the molecular mechanisms which underlie GH-induced lipolysis is crucial for the treatment of a wide variety of patients. Our studies identify several molecular targets of intervention to manipulate GH-induced lipolysis. Many of these targets already have pharmacological agents in clinical use, including: MEK inhibitors (Trametinib), PPARγ agonists (Thiazolidinediones), and anti-lipolytic agents (Acipimox). Therefore, we strongly believe that these data have clear bench to bedside ramifications and can have significant and immediate clinical impact.
Supplementary Material
Supplemental Figure 1.
Data are shown as mean ± SEM of 3 independent experiments.
a) Quantitation of Western blot analysis of FSP27 protein isolated from 3T3-L1 adipocytes treated with 500ng/mL bGH depicted in Figure 1c. Data are normalized to tubulin levels and shown as mean ± SEM of 2 independent experiments.
b) qPCR analysis of Igf1 mRNA in RNA isolated from 3T3-L1 adipocytes treated with 500ng/mL recombinant bovine GH (bGH). Data are shown as mean ± SEM of 3 independent experiments.. Asterisks indicate a significant differences in all panels * p<0.05; **p<0.01.
c) Quantitation of Western blot analysis of FSP27 protein isolated from 3T3-L1 adipocytes treated with increasing concentrations of bGH depicted in Figure 1e. Data are normalized to tubulin levels and shown as mean ± SEM of 2 independent experiments.
Supplemental Figure 2.
a) Quantitation of Western blot analysis of pHSL S563 in protein isolated from 3T3-L1 adipocytes depicted in Figure 2b. Data are normalized to HSL levels and shown as mean ± SEM of 2 independent experiments.
b-d) qPCR analysis of ATGL, HSL, and Cidea mRNA in RNA isolated from 3T3-L1 adipocytes treated with 500ng/mL bGH for 2 hours after 2 hours of pre-treatment with 10 μM U0126 or 1 μM Rosiglitazone. Data are shown as mean ± SEM of 4 independent experiments.
Supplemental Figure 3.
Western blot analysis for nuclear STAT5 following bGH treatment and cell fractionation. PCNA is used as a loading control.
b) Representative Western blot analysis of pSTAT5 Y694 and total STAT5 in 3T3-L1 adipocytes treated with 500ng/mL bGH for 20 minutes after 2 hours of pre-treatment with 200 μM STAT5 inhibitor, CAS 285986–31-4. Actin is used as a loading control.
b) Quantitation of Western blot analysis for pSTAT5 Y694 and total STAT5 depicted in Supplementary Figure 3b. Data are shown as mean ± SEM of 2 independent experiments. * p<0.05; **p<0.01; *** p<0.001.
d) Immunoblot for PPARγ or STAT5 following co-immunoprecipitation between in-vitro transcribed and translated PPARγ and STAT5 proteins. Proteins were mixed, immunoprecipitation by either PPARγ or STAT5 antibodies. Input control (5%) shown as a positive control.
e) Luciferase activity of 293T cells transfected with the 0.9-kb WT FSP27 luciferase reporter. The reporter vector was co-transfected with either a vector control or 25 ng of Pparγ expression vector and 25 ng of its obligate heterodimer RXRα. The cells were also co-transfected with either a vector control or 25 ng of a Stat5a, Stat5b, or a Stat5b-constiuatively active (CA) expression vector. Data are shown as mean ± SEM of three independent experiments, each with three replicates.
Supplemental Figure 4.
a) Quantitation of lipid droplet number in cells shown in Figure 6b. Data are shown as mean ± SEM of 14–17 cells/condition. **** p<0.0001.
b) Quantitation of Western blot analysis for pAKT S473 and total AKT in Figure 6d. Data are shown as mean ± SEM of 3 independent experiments. ** p<0.01.
Acknowledgments
The authors would like to thank: Dr. Sang Hoon Kim (Kyung Hee University), Dr. Ann-Hwee Lee (Cornell University), Dr. Sander Kersten (Wageningen University), Dr. Michael Farrar (University of Minnesota), Dr. Bruce Spiegelman (Dana Farber Cancer Institute) and Dr. C. Ronald Kahn (Joslin Diabetes Center) for vector constructs.
Funding
This work was supported by start-up funds from Ohio University College of Osteopathic Medicine (KYL), the American Diabetes Association Junior Faculty Development Award 1–17-JDF-055 (KYL), the NIH/NIDDK grant DK101711 (VP), the Osteopathic Heritage Foundation’s Vision 2020 (VP), the state of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll (JJK).
Footnotes
Declaration of of Interest
The authors declare no conflicts of interest.
References
- Banks AS, McAllister FE, Camporez JP, Zushin PJ, Jurczak MJ, Laznik-Bogoslavski D, Shulman GI, Gygi SP & Spiegelman BM 2015. An ERK/Cdk5 axis controls the diabetogenic actions of PPARgamma. Nature 517 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Naar EM, Johnson L, May MR, Cecim M, Yun JS & Wagner TE 1992. Effects of expression of human or bovine growth hormone genes on sperm production and male reproductive performance in four lines of transgenic mice. J Reprod Fertil 95 109–118. [DOI] [PubMed] [Google Scholar]
- Bartke A, Turyn D, Aguilar CC, Sotelo AI, Steger RW, Chen XZ & Kopchick JJ 1994. Growth hormone (GH) binding and effects of GH analogs in transgenic mice. Proc Soc Exp Biol Med 206 190–194. [DOI] [PubMed] [Google Scholar]
- Beauregard C, Utz AL, Schaub AE, Nachtigall L, Biller BM, Miller KK & Klibanski A 2008. Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab 93 2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerril S, Rodriguez A, Catalan V, Mendez-Gimenez L, Ramirez B, Sainz N, Llorente M, Unamuno X, Gomez-Ambrosi J & Fruhbeck G 2018. Targeted disruption of the iNOS gene improves adipose tissue inflammation and fibrosis in leptin-deficient ob/ob mice: role of tenascin C. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]
- Benencia F, Harshman S, Duran-Ortiz S, Lubbers ER, List EO, Householder L, Al-Naeeli M, Liang X, Welch L, Kopchick JJ, et al. 2015. Male bovine GH transgenic mice have decreased adiposity with an adipose depot-specific increase in immune cell populations. Endocrinology 156 1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan HE, Kittilson JD & Sheridan MA 2013. PKC and ERK mediate GH-stimulated lipolysis. J Mol Endocrinol 51 213–224. [DOI] [PubMed] [Google Scholar]
- Berryman DE & List EO 2017. Growth Hormone’s Effect on Adipose Tissue: Quality versus Quantity. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G 1997. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46 3–10. [PubMed] [Google Scholar]
- Bolli GB & Gerich JE 1984. The “dawn phenomenon”--a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med 310 746–750. [DOI] [PubMed] [Google Scholar]
- Bouchonville M, Jaghab J, Duran-Valdez E, Schrader R & Schade D 2014. The Effectiveness and Risks of Programming an Insulin Pump to Counteract the Dawn Phenomenon in Type 1 Diabetes. Endocr Pract 1–25. [DOI] [PubMed] [Google Scholar]
- Bredella MA, Gerweck AV, Lin E, Landa MG, Torriani M, Schoenfeld DA, Hemphill LC & Miller KK 2013. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab 98 3864–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgermeister E, Chuderland D, Hanoch T, Meyer M, Liscovitch M & Seger R 2007. Interaction with MEK causes nuclear export and downregulation of peroxisome proliferator-activated receptor gamma. Mol Cell Biol 27 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgermeister E & Seger R 2007. MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle 6 1539–1548. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Bolli GB, Cryer PE & Gerich JE 1985. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med 312 1473–1479. [DOI] [PubMed] [Google Scholar]
- Chen WY, White ME, Wagner TE & Kopchick JJ 1991. Functional antagonism between endogenous mouse growth hormone (GH) and a GH analog results in dwarf transgenic mice. Endocrinology 129 1402–1408. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wight DC, Wagner TE & Kopchick JJ 1990. Expression of a mutated bovine growth hormone gene suppresses growth of transgenic mice. Proc Natl Acad Sci U S A 87 5061–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DJ, Varco-Merth B & Rotwein P 2010. Dispersed Chromosomal Stat5b-binding elements mediate growth hormone-activated insulin-like growth factor-I gene transcription. J Biol Chem 285 17636–17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, et al. 2011. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature 477 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford AS, Barkan AL, Hinko A & Horowitz JF 2012. Suppression in growth hormone during overeating ameliorates the increase in insulin resistance and cardiovascular disease risk. Am J Physiol Endocrinol Metab 303 E1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW & Hennighausen L 2004. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24 8037–8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MB, Harris MD, Ziel FH & Rosenberg CS 1988. Suppression of sleep-induced growth hormone secretion by anticholinergic agent abolishes dawn phenomenon. Diabetes 37 166–171. [DOI] [PubMed] [Google Scholar]
- del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, Kopchick JJ, Friedman JE, Draznin B & Thorner MO 2007. Growth hormone regulation of p85alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes 56 1638–1646. [DOI] [PubMed] [Google Scholar]
- Dietz J & Schwartz J 1991. Growth hormone alters lipolysis and hormone-sensitive lipase activity in 3T3-F442A adipocytes. Metabolism 40 800–806. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI & Turyn D 2005. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res 15 324–336. [DOI] [PubMed] [Google Scholar]
- Farrar MA 2010. Design and use of constitutively active STAT5 constructs. Methods Enzymol 485 583–596. [DOI] [PubMed] [Google Scholar]
- Grahn TH, Kaur R, Yin J, Schweiger M, Sharma VM, Lee MJ, Ido Y, Smas CM, Zechner R, Lass A, et al. 2014. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. J Biol Chem 289 12029–12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen I, Tsalikian E, Beaufrere B, Gerich J, Haymond M & Rizza R 1986. Insulin resistance in acromegaly: defects in both hepatic and extrahepatic insulin action. Am J Physiol 250 E269–273. [DOI] [PubMed] [Google Scholar]
- Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL & Thorner MO 1987. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64 51–58. [DOI] [PubMed] [Google Scholar]
- Houssay BA 1936. The Hypophysis and Metabolism. N Engl J Med 214 961–985. [Google Scholar]
- Jessen N, Djurhuus CB, Jorgensen JO, Jensen LS, Moller N, Lund S & Schmitz O 2005. Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am J Physiol Endocrinol Metab 288 E194–199. [DOI] [PubMed] [Google Scholar]
- Karastergiou K, Bredella MA, Lee MJ, Smith SR, Fried SK & Miller KK 2016. Growth hormone receptor expression in human gluteal versus abdominal subcutaneous adipose tissue: Association with body shape. Obesity (Silver Spring) 24 1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Namba N, Mushiake S, Etani Y, Nishimura R, Makishima M & Ozono K 2007. Growth hormone stimulates adipogenesis of 3T3-L1 cells through activation of the Stat5A/5B-PPARgamma pathway. J Mol Endocrinol 38 19–34. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Cho SY, Yun CH, Moon YS, Lee TR & Kim SH 2008. Transcriptional activation of Cidec by PPARgamma2 in adipocyte. Biochem Biophys Res Commun 377 297–302. [DOI] [PubMed] [Google Scholar]
- Krusenstjerna-Hafstrom T, Clasen BF, Moller N, Jessen N, Pedersen SB, Christiansen JS & Jorgensen JO 2011a. Growth hormone (GH)-induced insulin resistance is rapidly reversible: an experimental study in GH-deficient adults. J Clin Endocrinol Metab 96 2548–2557. [DOI] [PubMed] [Google Scholar]
- Krusenstjerna-Hafstrom T, Madsen M, Vendelbo MH, Pedersen SB, Christiansen JS, Moller N, Jessen N & Jorgensen JO 2011b. Insulin and GH signaling in human skeletal muscle in vivo following exogenous GH exposure: impact of an oral glucose load. PLoS One 6 e19392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Yamamoto Y, Boucher J, Winnay JN, Gesta S, Cobb J, Bluher M & Kahn CR 2013. Shox2 is a molecular determinant of depot-specific adipocyte function. Proc Natl Acad Sci U S A 110 11409–11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, et al. 2013. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol 27 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, et al. 2009. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab 94 1509–1517. [DOI] [PubMed] [Google Scholar]
- Moller L, Norrelund H, Jessen N, Flyvbjerg A, Pedersen SB, Gaylinn BD, Liu J, Thorner MO, Moller N & Lunde Jorgensen JO 2009. Impact of growth hormone receptor blockade on substrate metabolism during fasting in healthy subjects. J Clin Endocrinol Metab 94 4524–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller N, Jorgensen JO, Schmitz O, Moller J, Christiansen J, Alberti KG & Orskov H 1990. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol 258 E86–91. [DOI] [PubMed] [Google Scholar]
- Monnier L, Colette C, Dejager S & Owens D 2013. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: is this of concern? Diabetes Care 36 4057–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier L, Colette C, Sardinoux M, Baptista G, Regnier-Zerbib A & Owens D 2012. Frequency and severity of the dawn phenomenon in type 2 diabetes: relationship to age. Diabetes Care 35 2597–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C, Kameda K, Tsujita T & Okuda H 2001. Relationships between lipolysis induced by various lipolytic agents and hormone-sensitive lipase in rat fat cells. J Lipid Res 42 120–127. [PubMed] [Google Scholar]
- Muller J, Sperl B, Reindl W, Kiessling A & Berg T 2008. Discovery of chromone-based inhibitors of the transcription factor STAT5. Chembiochem 9 723–727. [DOI] [PubMed] [Google Scholar]
- Nian Z, Sun Z, Yu L, Toh SY, Sang J & Li P 2010. Fat-specific protein 27 undergoes ubiquitin-dependent degradation regulated by triacylglycerol synthesis and lipid droplet formation. J Biol Chem 285 9604–9615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C, Gormsen LC, Jessen N, Pedersen SB, Moller N, Lund S & Jorgensen JO 2008. Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab 93 2842–2850. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Moller N, Christiansen JS & Jorgensen JO 2001. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 50 2301–2308. [DOI] [PubMed] [Google Scholar]
- Nordstrom SM, Tran JL, Sos BC, Wagner KU & Weiss EJ 2013. Disruption of JAK2 in adipocytes impairs lipolysis and improves fatty liver in mice with elevated GH. Mol Endocrinol 27 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Kawano Y, Sakakibara T, Hazeki O & Ui M 1994. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem 269 3568–3573. [PubMed] [Google Scholar]
- Posner BI 1976. Characterization and modulation of growth hormone and prolactin binding in mouse liver. Endocrinology 98 645–654. [DOI] [PubMed] [Google Scholar]
- Puri V, Ranjit S, Konda S, Nicoloro SM, Straubhaar J, Chawla A, Chouinard M, Lin C, Burkart A, Corvera S, et al. 2008a. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc Natl Acad Sci U S A 105 7833–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri V, Virbasius JV, Guilherme A & Czech MP 2008b. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol (Oxf) 192 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben MS & Hollenberg CH 1959. Effect of growth hormone on plasma fatty acids. J Clin Invest 38 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ & Gerich JE 1982. Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes 31 663–669. [DOI] [PubMed] [Google Scholar]
- Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CS, Bottomley W, Vigouroux C, Magre J, et al. 2009. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med 1 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgin B, Marcovecchio ML, Williams RM, Jackson SJ, Bluck LJ, Humphreys SM, Acerini CL & Dunger DB 2009. Effects of growth hormone and free fatty acids on insulin sensitivity in patients with type 1 diabetes. J Clin Endocrinol Metab 94 3297–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MI, Hadji-Georgopoulos A, Rendell M, Margolis S & Kowarski A 1981. The dawn phenomenon, an early morning glucose rise: implications for diabetic intraday blood glucose variation. Diabetes Care 4 579–585. [DOI] [PubMed] [Google Scholar]
- Segerlantz M, Bramnert M, Manhem P, Laurila E & Groop LC 2003. Inhibition of lipolysis during acute GH exposure increases insulin sensitivity in previously untreated GH-deficient adults. Eur J Endocrinol 149 511–519. [DOI] [PubMed] [Google Scholar]
- Shi SY, Luk CT, Brunt JJ, Sivasubramaniyam T, Lu SY, Schroer SA & Woo M 2014. Adipocyte-specific deficiency of Janus kinase (JAK) 2 in mice impairs lipolysis and increases body weight, and leads to insulin resistance with ageing. Diabetologia 57 1016–1026. [DOI] [PubMed] [Google Scholar]
- Singh M, Kaur R, Lee MJ, Pickering RT, Sharma VM, Puri V & Kandror KV 2014. Fat-specific protein 27 inhibits lipolysis by facilitating the inhibitory effect of transcription factor Egr1 on transcription of adipose triglyceride lipase. J Biol Chem 289 14481–14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Bose P, Leong-Quong RY, Fujita DJ & Riabowol K 2010. REAP: A two minute cell fractionation method. BMC Res Notes 3 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Cao Z, Li M, Xu E, Wang J & Xiao Y 2016. TNF-alpha downregulates CIDEC via MEK/ERK pathway in human adipocytes. Obesity (Silver Spring) 24 1070–1080. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Takahashi S, Matsubara T, Jiang C, Sakamoto W, Chanturiya T, Teng R, Gavrilova O & Gonzalez FJ 2015. Adipocyte-specific disruption of fat-specific protein 27 causes hepatosteatosis and insulin resistance in high-fat diet-fed mice. J Biol Chem 290 3092–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G & Ihle JN 1998. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93 841–850. [DOI] [PubMed] [Google Scholar]
- Walther TC & Farese RV Jr. 2012. Lipid droplets and cellular lipid metabolism. Annu Rev Biochem 81 687–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Masternak MM, Al-Regaiey KA & Bartke A 2007. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology 148 2845–2853. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang X, Heckmann BL, Lu X & Liu J 2011. Relative contribution of adipose triglyceride lipase and hormone-sensitive lipase to tumor necrosis factor-alpha (TNF-alpha)-induced lipolysis in adipocytes. J Biol Chem 286 40477–40485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306 1383–1386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1.
Data are shown as mean ± SEM of 3 independent experiments.
a) Quantitation of Western blot analysis of FSP27 protein isolated from 3T3-L1 adipocytes treated with 500ng/mL bGH depicted in Figure 1c. Data are normalized to tubulin levels and shown as mean ± SEM of 2 independent experiments.
b) qPCR analysis of Igf1 mRNA in RNA isolated from 3T3-L1 adipocytes treated with 500ng/mL recombinant bovine GH (bGH). Data are shown as mean ± SEM of 3 independent experiments.. Asterisks indicate a significant differences in all panels * p<0.05; **p<0.01.
c) Quantitation of Western blot analysis of FSP27 protein isolated from 3T3-L1 adipocytes treated with increasing concentrations of bGH depicted in Figure 1e. Data are normalized to tubulin levels and shown as mean ± SEM of 2 independent experiments.
Supplemental Figure 2.
a) Quantitation of Western blot analysis of pHSL S563 in protein isolated from 3T3-L1 adipocytes depicted in Figure 2b. Data are normalized to HSL levels and shown as mean ± SEM of 2 independent experiments.
b-d) qPCR analysis of ATGL, HSL, and Cidea mRNA in RNA isolated from 3T3-L1 adipocytes treated with 500ng/mL bGH for 2 hours after 2 hours of pre-treatment with 10 μM U0126 or 1 μM Rosiglitazone. Data are shown as mean ± SEM of 4 independent experiments.
Supplemental Figure 3.
Western blot analysis for nuclear STAT5 following bGH treatment and cell fractionation. PCNA is used as a loading control.
b) Representative Western blot analysis of pSTAT5 Y694 and total STAT5 in 3T3-L1 adipocytes treated with 500ng/mL bGH for 20 minutes after 2 hours of pre-treatment with 200 μM STAT5 inhibitor, CAS 285986–31-4. Actin is used as a loading control.
b) Quantitation of Western blot analysis for pSTAT5 Y694 and total STAT5 depicted in Supplementary Figure 3b. Data are shown as mean ± SEM of 2 independent experiments. * p<0.05; **p<0.01; *** p<0.001.
d) Immunoblot for PPARγ or STAT5 following co-immunoprecipitation between in-vitro transcribed and translated PPARγ and STAT5 proteins. Proteins were mixed, immunoprecipitation by either PPARγ or STAT5 antibodies. Input control (5%) shown as a positive control.
e) Luciferase activity of 293T cells transfected with the 0.9-kb WT FSP27 luciferase reporter. The reporter vector was co-transfected with either a vector control or 25 ng of Pparγ expression vector and 25 ng of its obligate heterodimer RXRα. The cells were also co-transfected with either a vector control or 25 ng of a Stat5a, Stat5b, or a Stat5b-constiuatively active (CA) expression vector. Data are shown as mean ± SEM of three independent experiments, each with three replicates.
Supplemental Figure 4.
a) Quantitation of lipid droplet number in cells shown in Figure 6b. Data are shown as mean ± SEM of 14–17 cells/condition. **** p<0.0001.
b) Quantitation of Western blot analysis for pAKT S473 and total AKT in Figure 6d. Data are shown as mean ± SEM of 3 independent experiments. ** p<0.01.