Abstract
We examined the changes in salivary immunoglobulin A (SIgA) and the incidence of upper respiratory syndrome (URS) throughout a college cross-country season as well as the acute effect of a VO 2max test on SIgA. Subjects were 22 cross country athletes (XC) (20.7±0.3 years) and 23 matched controls (C) (20.4±0.2 years). Saliva samples were collected pre and post VO2max and at four training time points (August – November). Weekly logs indicating S&S of URS from which a total symptom score (TSS) was calculated were collected. There was a significant decrease in SIgA F(1,43)=10.742, p<0.001 and the secretion rate of SIgA F(1,43)=15.617, p<0.001 for XC at time points two through four. XC was also significantly lower than controls in those two variables across those time points. The secretion rate of SIgA and URS were negatively correlated at time point four R2=0.443, F(4,22)=26.9, p=0.001. There was a significant acute post exercise decrease in the secretion rate of SIgA, pre (M=21.44, SEM=3.95) and post (M=14.5, SEM=3.0), t(1,21)=2.185, p=0.039. Prolonged training resulted in decreased mucosal SIgA.
Key word: mucosal Immunity, excessive training, illness, immunoglobulins
Introduction
It has been almost 40 years since Tomasi and colleagues reported their groundbreaking work on the link between exercise and upper respiratory tract infections (URTI). This rightly inspired a revival of study on exercise and infection 38 . Mackinnon and colleagues 20 furthered this impetus for more study with their finding connecting decreased secretion rates of salivary IgA (SIgA) to increased URTI 20 . This finding, in turn, has led to decades of study and thousands of publications examining the relationship between SIgA and URTI in athletes. For over 30 years now, studies examining the effects of exercise, URTI and SIgA have continued across multiple populations. The high level of interest in this field stems mainly from the fact that URTI results in decreased performance; further, it can not only interrupt training, but if it occurs at the time of an Olympic event, can potentially undo years of training 34 .
The majority of studies record symptoms as URTI, but this label is problematic for a variety of reasons. First, pathological identifications of organisms are rarely made; more importantly, symptoms can be caused by a number of non-pathologies such as asthma, allergies or drying of airways 16 . There is tremendous confusion as to the exact cause of symptoms that athletes report. Therefore, unless an actual URTI diagnosis is made by a physician, the position of the International Society of Exercise Immunology is that researchers use the term “upper respiratory symptoms” (URS) 40 . Hence, the term URS will be used throughout this paper.
The extensive body of research that examines the relationship between exercise, SIgA and URS in athletic populations has led to the belief that the most useful outcome measure from a clinical viewpoint is URS 24 , and the immune variable most closely associated with URS is SIgA 16 . The mucosal immune system represents one of the first lines of defense against URS. One of its principle components, SIgA, assembled in the epithelial cells and found in saliva, has the capacity to bind antigens, neutralize viruses and inhibit the colonization of pathogens 19 . Years of research and thousands of studies have illuminated many aspects regarding the impact of exercise on immune function, but there are still myriad unanswered questions. For example, the most common reason that athletes report to a physician during a competitive season is URS 5 ; yet the direct relationship between exercise, URS and SIgA remains equivocal. For a period of time, studies pointed to the conclusion that moderate exercise improves the immune response 40 , whereas intense exercise leads to a decline 28 . However, subsequent study has demonstrated that the immune system and the symptom responses can be different for acute bouts of exercise 34 , chronic exercise 34 , and the competitive level of the athlete 41 . There may also be differences based on the particular sport in which the athlete is engaged.
The decades-long research on swimmers has provided extensive support for the idea that URS is more likely to occur in swimmers when they are engaged in intense training 27 35 . Other studies have shown that this is also true for sports like American football 8 . On the other hand, distance running has been shown to cause a URS episode post-competitive event 35 . To our knowledge, there is no research that follows the URS and SIgA across a competitive season for runners, yet these athletes provide a prime opportunity to examine the relationships between exercise, SIgA and URS from multiple perspectives, including moderate-intensity exercise, prolonged high-intensity exercise and post-competitive event responses. Additionally, these athletes undergo maximal oxygen consumption tests that afford the opportunity to investigate the study parameters after a brief, maximal effort. The purpose of this study was: (a) to evaluate salivary Immunoglobulin A (SIgA) and incidence of upper respiratory syndrome (URS) over a four-month time period in college cross-country runners, and (b) to assess the acute response of SIgA to a maximal exercise test in both cross-country runners and controls. We hypothesize that the secretion rate of SIgA will decrease across the competitive season for runners and not controls and that secretion rates below 40 μg. min −1 will be associated with increased risk of URS. We also predict that an acute bout of maximal exercise will result in a decrease in the secretion rate of SIgA post-exercise.
Methods
Subjects
This was a non-randomized, controlled, quasi-experimental study. The subject pool consisted of a convenience sample of 45 college-aged men and women who were non-tobacco users and free from any signs or symptoms of URS at the beginning of data collection. Twenty-two subjects (XC) were members of a large Midwestern university, NCAA Division II, cross-country team and 23 additional subjects served as controls (C). Controls were non-varsity, full time (defined as taking at least 12 semester hours of credit) university students who reported being physically active (defined as engaging in any type of activity that induces sweat for at least 30 min 3×wk −1 ) 22 . To be included, students needed to be a member of one of the above groups. Students were excluded if they were current tobacco users, currently experiencing any signs or symptoms of URS, or had recent dental work. An explanation of the research, including potential risks, was given to all subjects and they provided written informed consent before being allowed to participate. The study procedures were approved by the Institutional Review Board at the university and this research meets the ethical standards of the journal 17 .
Data collection
Data collection occurred monthly in the last week of each month and took place across the time period of one season, i.e., four months, from August through November. Baseline values were collected in late August (Pre) when subjects reported to practice for the beginning of the season. Vo 2max pre/post-tests were also run during this time on both athletes and controls. Data point 2 (September) represents the first month of in-season high-intensity training; data point 3 (October) represents the most extensive, intensive training they undertake during the season; data point 4 (November) represents continued training in preparation for the final culminating race of the season.
Signs and symptoms
Subjects were required to complete a weekly log in which they documented any sign or symptom consistent with URS (including cough, runny nose and nasal congestion) as well as the number of days that symptoms occurred. The illness symptoms listed on the weekly log were: sore throat, runny nose, cough, fever, and other (persistent muscle soreness, joint aches and pains, weakness, headache and loss of sleep). The non-numerical ratings of light, moderate or severe (L, M or S, respectively) of severity of symptoms were then scored as 1, 2 or 3, respectively, to provide a quantitative means of data analysis 11 , and the total symptom score (TSS) for every subject each week was calculated by multiplying the total number of days each symptom was experienced by the numerical symptom severity ratings.
In any given week, a total symptom score ≥12 was taken to indicate that a URS was present. In order to achieve it, a subject would have to record at least three moderate symptoms lasting for 2 days, or two moderate symptoms lasting for at least 3 days in a given week. A single URS episode was defined as a period during which the weekly total symptom score was ≥12. This URS scoring system was chosen for consistency with previous work in this area 12 .
Subjects were also asked to rate the impact of illness symptoms on their ability to train (normal training maintained, training reduced or training discontinued; L, M or S, respectively). They were instructed in include intensity, duration and frequency in their ratings. The coach collected the logs weekly from cross-country (XC) and the principal investigator (PI) collected them from the controls. The PI checked them immediately to make sure the URS were being classified correctly and to make referrals to the team physician as soon as possible after incident reporting. If the subject reported other symptoms or a decrease in daily activity, they were referred to a physician who then made the diagnosis of URS or “other illness.” If the PI was uncertain as to the nature of the illness, the physician made the diagnosis. This method of classifying URS is consistent with studies similar to the current one and recommended by the International Society of Exercise Immunology 40 . Other illnesses that subjects encountered throughout the season included influenza and mononucleosis.
Saliva collection
All saliva samples were collected between 1200 and 1400 h. Subjects reported at the same time for each collection period after fasting and refraining from any strenuous physical activity for two hours. After thoroughly rinsing their mouths with water, unstimulated saliva samples were collected for 4 min into 15 mL polypropylene tubes. Similar to previous studies, 6 7 8 9 20 25 , saliva was measured for volume and then stored at –70°C until analysis.
Saliva analysis
Saliva samples were analyzed for salivary IgA (SIgA) using an ELISA kit (Salimetrics, Philadelphia, PA, USA). Samples were run in duplicate and all samples for the same subject were run on the same plate. The intra-assay coefficient of variation for SIgA was 3.7. The secretion rate of SIgA, or the total amount of SIgA appearing on the mucosal surface per unit time, was calculated by multiplying the SIgA concentration (μg · ml −1 ) times saliva flow rate (ml . min −1 ). The saliva flow rate was calculated by dividing the total volume of saliva obtained in each sample (ml) by the time taken to produce each sample (4 min).
Maximal oxygen uptake
Maximal oxygen uptake (VO 2max ) was determined using the standard, graded, maximal, Bruce exercise protocol on a calibrated treadmill (Model 65, Quinton, Seattle, WA, USA). In this protocol, the treadmill speed and incline starts at 1.7 MPH at 10% grade and is increased every 3 min: Stage 2: 2.5 MPH at 12% grade; Stage 3: 3.4 MPH at 14% grade; Stage 5: 5.0 MPH at 18% grade, all the way up to Stage 9: 7.0 MPH at 26% grade. 2 . Throughout each test, respiratory gas exchange (VO 2 , VCO 2 , VE) was measured using open-circuit spirometry and indirect calorimetry methods (OCM2, Physiodyne, Farmingdale, NY, USA). Heart rate responses throughout exercise were monitored using an electrocardiographic telemetry system (G-2400T, EatonCare Telemetry, Ann Arbor, MI, USA) and bipolar V 5 lead configuration. In addition, a subject’s rating of perceived exertion (RPE) during exercise was assessed using the original, “6–20” category rating scale developed by Borg 1 . The use of handrails during exercise was not allowed, and a treadmill test was always terminated when a subject indicated she or he had reached volitional fatigue. In addition to the researchers’ subjective observations of marked dyspnea, facial flushing, unsteady gait, etc., each subject was required to demonstrate at least two of the following criteria at the end of each test: evidence of a plateau in VO 2max , a respiratory exchange ratio of ≥1.00; a heart rate of ≥90% of age predicted HR max (220 minus age); and RPE values of ≥18 32 33 .
Statistical analysis
The dependent variables of SIgA, saliva flow rate, the secretion rate of SIgA, URS infection and duration were analyzed separately using a 2-group (XC v. C)×4 times (pre, which was taken in August, September, October and November) analysis of variance (ANOVA) with repeated measures on the time factor. To determine the response to an acute bout of intense exercise, the dependent variables of SIgA, saliva flow rate and the secretion rate of SIgA were analyzed separately using a paired samples t-test. Partial η2 (eta squared) was presented as an index of effect size (i.e., small effect size, η2=0.04; moderate effect size, η 2 =0.25, and large effect size, η2=0.64) 10 . For XC, four separate forced-entry multiple linear regression analyses were conducted to predict the dependent variable “total symptom score” by the independent variables, SIgA, saliva flow rate, secretion rate of SIgA, and average miles run at each data collection point. An a priori power analysis for repeated measures ANOVA was calculated using η2=0.04, alpha=0.05, and power of.80. The calculation revealed that 32 subjects were needed to appropriately address the research questions. All p values of.05 or less were considered statistically significant and follow-up analyses on main effects were performed using Bonferroni’s post hoc procedure. The statistical package used to run all analyses was SPSS (Ver. 22.0), Chicago, IL, USA.
Results
The sample population consisted of twenty-two members of a large Midwestern university cross-country team (XC) (20.7±0.3 yr, 63.2±2.0 kg, and 1.7±0.2 m) and 23 additional subjects served as controls (C); (20.4±0.2 yr, 66.5±3.0 kg, and 1.7±0.2 m) ( Table 1 ).
Table 1 Subject characteristics (mean±SEM).
| Cross-country N=22 | Control N=23 | |
|---|---|---|
| Age (yr) | 20.7±0.3 | 20.4±0.2 |
| Height (m) | 1.7±0.2 | 1.7±0.2 |
| Weight (kg) | 63.2±2.0 | 66.5±3.0 |
| VO 2 max (ml/kg) | 62.6±1.8 | 49.8±1.9 |
Analysis of SIgA data revealed significant main effects for SIgA, [F(1,43)=10.742, p<0.001] with a moderate effect size (.266) as well as a significant group x time interaction, [F(3,41)=6.386, p=0.001 with a moderate effect size (.318)]. Post hoc analysis revealed the group main effect was the result of significantly lower SIgA values in XC compared to C at time points in Sept, Oct and Nov. Post hoc analysis of the within-subject time factor revealed decreased SIgA for XC at time points in Sept, Oct and Nov compared to Pre. A simple main effects analysis of the interaction revealed that groups differed with respect to SIgA values at time points in Sept, Oct and Nov (see Fig. 1 ).
Fig. 1.
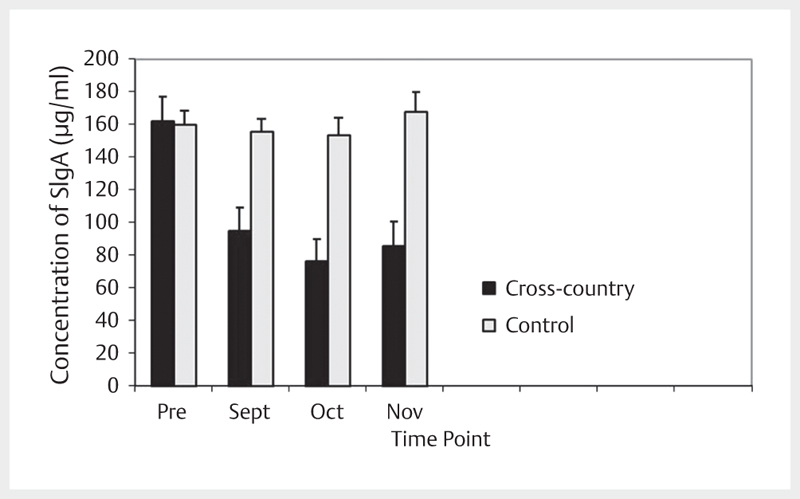
Concentration of SIgA across time.
Analysis of saliva flow data revealed no main effect of group, time or group x time interaction [F(3,41)=1.719, p=0.178].
Analysis of the secretion rate of SIgA data revealed significant main effects for the secretion rate of SIgA, [F(1,43)=15.617, p<0.001] with a moderate effect size (.223) as well as a significant group x time interaction, [F(3,41)=5.998, p=0.002] with a moderate effect size (.305). Post hoc analysis revealed the group main effect was the result of significantly lower secretion rate of SIgA values in XC compared to C at time points in Sept, Oct and Nov. Post hoc analysis of the within-subject time factor revealed decreased secretion rate of SIgA for XC at time points in Sept, Oct and Nov compared to Pre. A simple main effects analysis of the interaction revealed that groups differed with respect to SIgA values at time points in Sept, Oct and Nov ( Fig. 2 ).
Fig. 2.
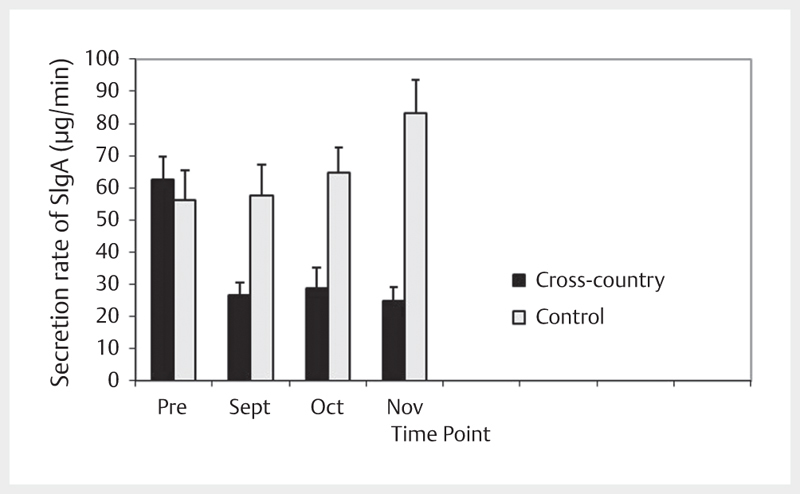
Concentration of the secretion rate of SIgA across time.
There was no significant difference in the Pre to Post values for an acute bout of maximal exercise for the following variables: saliva flow rate pre (M=0.350, SEM=0.05) and post (M=0.284, SEM=0.043), t(1,21)=2.035, p=0.055; SIgA pre (M=83.3, SEM=15.9) and post (M=84.8, SEM=17.0), t(1,21)=–0.101, p=0.920. There was a significant post-exercise decrease in the secretion rate of SIgA, pre (M=21.44, SEM=3.95) and post (M=14.5, SEM=3.0), t(1,21)=2.185, p=0.039 ( Table 2 ).
Table 2 Acute response to maximal exercise (mean±SEM).
| PRE | 95% LCL | 95% UCL | POST | 95% LCL | 95% UCL | p | |
|---|---|---|---|---|---|---|---|
| Saliva flow rate (ml . min −1 | 0.350±0.05 | 0.273 | 0.410 | .284±.043 | 0.231 | 0.357 | 0.055 |
| SIgA (μg . ml −1 ) | 83.3±15.9 | 62.4 | 102 | 84.8±17.0 | 67.4 | 104 | 0.920 |
| Secretion rate of SIgA (μg . min −1 ) | 21.2±3.9* | 15.3 | 26.1 | 14.5±3.0* | 11.1 | 18.2 | 0.039 |
Table 3 presents the results of the significant positive correlation between average miles run and a significant negative correlation between the secretion rate of SIgA at the “November” time point, indicating that those who ran more miles and those with a decreased secretion rate of SIgA were more likely to have a higher total symptom score for URS. At that time point, the regression model with all four predictors produced R 2 =0.443, [F(4,22)=26.9, p=0.001]. In contrast, there were no significant relationships at the August, R 2 =–0.004, [F(4,22)=0.979, p=0.447]; September, R 2 =0.195, [F(4,22)=0.753, p=0.396] or October, R 2 =0.443, [F(4,22)=26.9, p=0.001] time points.
Table 3 Summary statistics, correlations and results from the regression analysis on higher total symptom score for URS.
| “November” Time point | |||||||
|---|---|---|---|---|---|---|---|
| Multiple regression weights | |||||||
| Mean | SEM | 95% LCL | 95% UCL | Correlation with severity of URS | b | β | |
| SIgA (μg . ml −1 ) | 85.3 | 13.3 | 72.3 | 100.4 | 0.209 | 0.0086 | 0.055 |
| Saliva flow rate (ml . min −1 ) | 0.291 | 0.07 | 0.201 | 0.372 | 0.136 | 0.1224 | 0.20 |
| Secretion rate of SIgA (μg min −1 ) mean±SEM | 24.8 | 7.9 | 15.9 | 33.7 | –0.514* | –0.0034* | –0.324 |
| Average km/week | 67.1 | 3.7 | 63.4 | 72.6 | 0.486* | 0.0055* | 0.459 |
Table 4 presents the results of the training (km/wk), URS (number of subjects with a URS) and TSS (mean±SEM) of total symptom score for those subjects who reported URS data. XC had a significantly higher number of URS than C at the “November” time point.
Table 4 Training and URS data (mean±SEM).
| August | September | October | November | |||||
|---|---|---|---|---|---|---|---|---|
| XC (n=22) | C (n=23) | XC (n=22) | C (n=23) | XC (n=22) | C (n=23) | XC (n=22) | C (n=23) | |
| URS (#) | 0 | 0 | 1 | 0 | 4 | 1 | 8* | 2* |
| TSS | NA | NA | 14 | NA | 13±2 | 13 | 13±4 | 13±3 |
| Training (km/week) | 67.5±8.5 | NA | 80.5±7.1 | NA | 86.1±6.6 | NA | 67.1±8.9 | NA |
Discussion
This paper adds to the literature on the response of the mucosal immune system to intense acute exercise and exercise across a training season in highly competitive athletes. The major findings of this paper are that SIgA and the secretion rate of SIgA decreased within the first month upon the commencement of prolonged training and remained in a decreased state throughout the season, and that an acute bout of high-intensity exercise resulted in a significant decrease in the secretion rate of SIgA. This discussion will examine two items: the response to acute, high-intensity exercise, and the response to increased training with its connection between URS and the mucosal immune response.
Acute, high-intensity bouts of exercise
Training for many sports, and especially for cross-country runners, includes the incorporation of acute bouts of high-intensity exercise into the weekly training regimen. In the present study, an acute bout of high-intensity exercise resulted in a 31.6% decrease in the secretion rate of SIgA. This finding is consistent with earlier studies where the secretion rate of SIgA levels was decreased 29.3% after an ultra-endurance race 36 and 33% after a skating race 23 . Other tests of high-intensity exercise also produced decreased secretion rates of SIgA such as a 23.3% decrease after a rugby game 18 and decreases after repeated Wingate tests of 27.8% in women 9 and 38.8% in men 6 .
The mechanism for this appears to be two-fold. First, the sympathetic response to increased exercise results in arteriole vasoconstriction, which in turn decreases the volume of saliva; and second, there is an inhibitory effect on SIgA synthesis caused by the hypothalamic-pituitary-adrenal axis that occurs during intense exercise 39 . The secretion rate of SIgA represents the amount of SIgA available on the mucosal surfaces for protection against pathogens, and is thought to be the variable most closely linked with URS 39 . However, the decrease in the secretion rate of SIgA after acute intense exercise did not result in increased URS for the subjects in this study. Only two athletes exhibited signs of URS in the two weeks post maximal exercise. This is consistent with other studies of maximal exercise tests 6 7 9 . Given that there are multiple variables that contribute to infection, including availability of the pathogen, exposure to available pathogens, virulence of the pathogen and the ability of the individual to mount a response to the pathogen, further research examining the period of vulnerability is warranted.
Increased training response and its connection to both urs and the mucosal immune system
In the present study, individual athletes described URS at various times throughout the season, but there was no point at which more than 50% of the team reported symptoms. This is an unusual finding in athletes across a training season. Pyne 31 reports infection rates higher than 50% in swimmers, Fahlman reports rates of up to 75% in American football players 8 and Bury reports rates as high as 80% in competitive football players 3 . Although it is possible that the nature of team sports like swimming and football put players in closer contact with one another and thus increases the likelihood that they will share pathogens more than those in an individual sport like cross-country, further study is required to confirm this. It is more likely that due to lack of effective differential diagnostic criteria for URS and the fact that athletes are more likely to seek medical attention for illness, the number of URS in some previous studies was overstated 15 . Implementing the current system of incorporating symptoms with severity to create a total symptom score (TSS) may help procure measures of URS in athletes that are more accurate 11 .
The athletes in this study were followed over the course of their four-month season. SIgA and the secretion rate of SIgA decreased within the first month of the commencement of prolonged training and remained in a decreased state as the season progressed and the training intensified. Although there are no studies of cross-country runners across a season, this finding is in line with studies on other competitive athletes. There is a large body of literature that shows a decrease in SIgA across a season in swimmers. Gleeson and colleagues report a downward trend in SIgA levels across a seven-month training period in elite swimmers 13 , and Tharp and colleagues report a decrease in SIgA across a three-month training period in swimmers 37 . Further evidence for the connection between URS and the mucosal immune function comes from a study of one year of competitive American football. Fahlman and colleagues found that the secretion rate of SIgA decreased across periods of intense training, which was a unique predictor of URS in those athletes, and that the greater the reduction in the secretion rate of SIgA, the higher the incidence of URS 8 .
There is a long-standing conviction that regular, moderate physical activity offers a protective effect on immune function, whereas excessive physical activity increases susceptibility to infection 29 . In the current study, the athletes began with a slightly higher level of exercise than the moderately exercising controls ( Table 4 ), and within four weeks of training had decreased their secretion rate of SIgA to levels below 40 μg. min −1 , the level that is associated with an increase in URS throughout a training season 14 . Other research puts forth a similar, but slightly different, hypothesis. Neville and colleagues 26 reported that SIgA values under 40% of an athlete’s normal value were associated with a 50% chance of contracting a URS.
In the present study, the secretion rate of SIgA decreased below 40 μg. min −1 after the first month of training, but it was not until the fourth time period (November) that the decrease declined to 40% of baseline values and was related to URS. Setting aside all of the variables that must be present for infection to occur, there is another possibility for the lack of connection between mucosal immunity and increased training found in this study. The fact that these athletes were highly trained even when they were not in the competitive season raises the option that the immune parameters for the cross-country runners fall more in line with Malm’s 21 work. He proposes that there is a positive correlation between training distance and infection rate in sub-elite athletes, but in elite athletes the relationship is more likely to resemble that of an S-shaped relationship between training load and infection rate. The definition of elite varies across studies but there is consensus that athletes who are able to function at a high level have some innate ability to remain infection-free even when under the influence of stressors that would normally weaken the immune system 21 . Two studies were used to classify the performance level (PL) of the subjects in this study 4 30 . With VO 2max scores ranging from 55.0–64.9 mL. kg −1 , the majority of the XC athletes would be classified at a PL of 3. Likewise, the controls in the study, with VO 2max scores ranging from 45.0–54.9 mL. kg −1 would be classified at a PL of 2. It is possible that cross-country runners who cannot withstand the physiological and psychological stressors linked to their levels of training never make it to the college level in a manner similar to those on the elite level.
One limitation of this study is that, although every attempt was made to correctly classify URS, viral load was not measured and the possibility exists that some infections may have been misclassified. Additionally, it is possible that the infections experienced by the athletes were the result of exposure to other ill athletes, a unique, unidentifiable stress posed by being a varsity athlete or some other unknown factor related to varsity participation.
The hypotheses that an acute bout of maximal exercise will result in a decrease in the secretion rate of SIgA post-exercise is supported by this study as is the hypothesis that the secretion rate of SIgA will decrease across the competitive season for runners and not controls. However, we are unable to support the hypotheses or corroborate findings from other researchers that levels of the secretion rate of SIgA below 40 μg. min −1 are associated with an increased risk of URS. Rather, these results are more in line with the findings of Neville that the secretion rate of SIgA values under 40% of an athlete’s normal value are associated with URS. It also raises the question as to whether the S-curved response may be more prevalent in sub-elite athletes than previously reported, and if the method of reporting URS is inflated. This research adds to the body of literature that exercise above a moderate level is linked to decreased secretion rates of SIgA. It also adds another finding to the literature regarding what levels of SIgA may be associated with an increased risk of URS and adds to the discussion on the S-curve and manner of reporting URS.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Borg G. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 2.Bruce R, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 3.Bury T, Marechal R, Mahieu P, Pirnay F. Immunological status of competitive football players during the training season. Int J Sports Med. 1998;19:364–368. doi: 10.1055/s-2007-971931. [DOI] [PubMed] [Google Scholar]
- 4.Decroix L, DePauw K, Foster C, Meeusen R. Guidelines to classify female subject groups in sport-science research. Int J Sports Physiol Perform. 2016;11:204–213. doi: 10.1123/ijspp.2015-0153. [DOI] [PubMed] [Google Scholar]
- 5.Engebretsen L, Steffen K, Alonso J M, Aubry M, Dvorak J, Junge A, Meeuwisse W, Mountjoy M, Renstrom P, Wilkinson M. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med 2010. 44:772–780. doi: 10.1136/bjsm.2010.076992. [DOI] [PubMed] [Google Scholar]
- 6.Engels H J, Fahlman M M, Morgan A L, Formolo L R. Mucosal IgA response to intense intermittent exercise in healthy male and female adults. International Society of Exercise Physiologists. 2007;7:21–26. [Google Scholar]
- 7.Engels H J, Fahlman M M, Wirth J C. Effects of ginseng on secretory IgA, performance, and recovery from interval exercise. Med Sci Sports Exerc. 2003;35:690–696. doi: 10.1249/01.MSS.0000058363.23986.D2. [DOI] [PubMed] [Google Scholar]
- 8.Fahlman M M, Engels H J. Mucosal IgA and URTI in American college football players: A year longitudinal study. Med Sci Sports Exerc. 2005;37:374–380. doi: 10.1249/01.mss.0000155432.67020.88. [DOI] [PubMed] [Google Scholar]
- 9.Fahlman M M, Engels H J, Morgan A L, Kolokouri I. Mucosal IgA response to repeated Wingate tests in females. Int J Sports Med. 2001;22:127–131. doi: 10.1055/s-2001-11339. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson C J. An effect size primer: A guide for clinicians and researchers. Professional Psychology: Research and Practice. 2009;40:532–538. [Google Scholar]
- 11.Fricker P A, Pyne D B, Saunders P U, Cox A J, Gleeson M, Telford R D. Influence of training loads on patterns of illness in elite distance runners. Clin J Sport Med. 2005;15:246–252. doi: 10.1097/01.jsm.0000168075.66874.3e. [DOI] [PubMed] [Google Scholar]
- 12.Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P, Muhamad A S. Respiratory infection risk in athletes: Association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2012;22:410–417. doi: 10.1111/j.1600-0838.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson M, McDonald W A, Cripps A W, Pyne D B, Clancy R L, Fricker P A, Wlodarczyk J H. Exercise, stress and mucosal immunity in elite swimmers. Adv Exp Med Biol. 1995;371A:571–574. doi: 10.1007/978-1-4615-1941-6_120. [DOI] [PubMed] [Google Scholar]
- 14.Gleeson M, McDonald W A, Pyne D B, Cripps A W, Francis J L, Fricker P A, Clancy R L. Salivary IgA levels and infection risk in elite swimmers. Med Sci Sports Exerc. 1999;31:67–73. doi: 10.1097/00005768-199901000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Gleeson M, Pyne D B, Callister R. The missing links in exercise effects on mucosal immunity. Exerc Immunol Rev. 2004;10:107–128. [PubMed] [Google Scholar]
- 16.Gleeson M, Pyne D B, Elkington L J, Hall S T, Attia J R, Oldmeadow C, Wood L G, Callister R. Developing a multi-component immune model for evaluating the risk of respiratory illness in athletes. Exerc Immunol Rev. 2017;23:52–64. [PubMed] [Google Scholar]
- 17.Harriss D, Atkinson G. Ethical standards in sport and exercise science research: 2016 update. Int J Sports Med. 2015;36:1121–1124. doi: 10.1055/s-0035-1565186. [DOI] [PubMed] [Google Scholar]
- 18.Koch A J, Wherry A D, Petersen M C, Johnson J C, Stuart M K, Sexton W L. Salivary immunoglobulin A response to a collegiate rugby game. J Strength Cond Res. 2007;21:86–90. doi: 10.1519/00124278-200702000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Lamm M E. Current concepts in mucosal immunity. IV. How epithelial transport of IgA antibodies relates to host defense. Am J Physiol. 1998;274:G614–G617. doi: 10.1152/ajpgi.1998.274.4.g614. [DOI] [PubMed] [Google Scholar]
- 20.Mackinnon L T, Chick T W, van As A, Tomasi T B. The effect of exercise on secretory and natural immunity. Adv Exp Med Biol. 1987;216A:869–876. doi: 10.1007/978-1-4684-5344-7_102. [DOI] [PubMed] [Google Scholar]
- 21.Malm C. Susceptibility to infections in elite athletes: the S-curve. Scand J Med Sci Sports. 2006;16:1–6. doi: 10.1111/j.1600-0838.2005.00499.x. [DOI] [PubMed] [Google Scholar]
- 22.American College of Sports Medicine. Physical Activity in Children and Adolescents In 2016;
- 23.Michihiro K, Iizuka T, Maegawa T, Hashimoto E, Yuda J, Aoyanagi T, Akimoto T, Tajahashi H. Salivary secretory Immunoglobulin A response to elite speed skaters during a competition period. J Strength Cond Res. 2010;24:2249–2254. doi: 10.1519/JSC.0b013e3181aff28b. [DOI] [PubMed] [Google Scholar]
- 24.Moreira A, Delgado L, Moreira P, Haahtela T. Does exercise increase the risk of upper respiratory tract infections? Br Med Bull. 2009;90:111–131. doi: 10.1093/bmb/ldp010. [DOI] [PubMed] [Google Scholar]
- 25.Mylona E, Fahlman M M, Morgan A L, Boardley D, Tsivitse S K. s-IgA response in females following a single bout of moderate intensity exercise in cold and thermoneutral environments. Int J Sports Med. 2002;23:453–456. doi: 10.1055/s-2002-33744. [DOI] [PubMed] [Google Scholar]
- 26.Neville V, Gleeson M, Folland J P. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc. 2008;40:1228–1236. doi: 10.1249/MSS.0b013e31816be9c3. [DOI] [PubMed] [Google Scholar]
- 27.Nieman D C, Henson D A, Dumke C L, Lind R H, Shooter L R, Gross S J. Relationship between salivary IgA secretion and upper respiratory tract infection following a 160-km race. J Sports Med Phys Fitness. 2006;46:158–162. [PubMed] [Google Scholar]
- 28.Nieman D C, Johanssen L M, Lee J W, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness. 1990;30:316–328. [PubMed] [Google Scholar]
- 29.Nieman D C, Nehlsen-Cannarella S L, Markoff P A, Balk-Lamberton A J, Yang H, Chritton D B, Lee J W, Arabatzis K. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990;11:467–473. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- 30.Pauw K, Roelands B, Cheung S, DeGeus B, Rietjens G, Meeusen R. Guidelines to classify subject groups in sport-science research. Int J Sports Physiol Perform. 2013;8:111–122. doi: 10.1123/ijspp.8.2.111. [DOI] [PubMed] [Google Scholar]
- 31.Pyne D B, Gleeson M, McDonald W A, Clancy R L, Perry C, Jr., Fricker P A. Training strategies to maintain immunocompetence in athletes. Int J Sports Med. 2000;21 01:S51–S60. doi: 10.1055/s-2000-1452. [DOI] [PubMed] [Google Scholar]
- 32.Robben K, Poole D, Harms C. Maximal oxygen uptake validation in children with expiratory flow limitation. Pediatr Exerc Sci. 2013;25:84–100. doi: 10.1123/pes.25.1.84. [DOI] [PubMed] [Google Scholar]
- 33.Rowland T. Developmental Exercise Physiology: Human Kinetics 1996;
- 34.Schwellnus M, Soligard T, Alonso J M, Bahr R, Clarsen B, Dijkstra H P, Gabbett T J, Gleeson M, Hagglund M, Hutchinson M R, Janse Van Rensburg C, Meeusen R, Orchard J W, Pluim B M, Raftery M, Budgett R, Engebretsen L. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br J Sports Med. 2016;50:1043–1052. doi: 10.1136/bjsports-2016-096572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soligard T, Schwellnus M, Alonso J M, Bahr R, Clarsen B, Dijkstra H P, Gabbett T, Gleeson M, Hagglund M, Hutchinson M R, Janse van Rensburg C, Khan K M, Meeusen R, Orchard J W, Pluim B M, Raftery M, Budgett R, Engebretsen L. How much is too much? (Part 1) International Olympic Committee consensus statement on load in sport and risk of injury. Br J Sports Med. 2016;50:1030–1041. doi: 10.1136/bjsports-2016-096581. [DOI] [PubMed] [Google Scholar]
- 36.Tauler P, Martinez S, Moreno C, Martinez P, Aguilo A. Changes in salivary hormones, immunoglobulin A, and C-reactive protein in response to ultra-endurance exercises. Appl Physiol Nutr Metab. 2014;39:560–565. doi: 10.1139/apnm-2013-0466. [DOI] [PubMed] [Google Scholar]
- 37.Tharp G D, Barnes M W. Reduction of saliva immunoglobulin levels by swim training. Eur J Appl Physiol Occup Physiol. 1990;60:61–64. doi: 10.1007/BF00572187. [DOI] [PubMed] [Google Scholar]
- 38.Tomasi T B, Trudeau F B, Czerwinski D, Erredge S. Immune parameters in athletes before and after strenuous exercise. J Clin Immunol. 1982;2:173–178. doi: 10.1007/BF00915219. [DOI] [PubMed] [Google Scholar]
- 39.Walsh N P, Gleeson M, Pyne D B, Nieman D C, Dhabhar F S, Shephard R J, Oliver S J, Bermon S, Kajeniene A. Position statement. Part two: Maintaining immune health. Exerc Immunol Rev. 2011;17:64–103. [PubMed] [Google Scholar]
- 40.Walsh N P, Gleeson M, Shephard R J, Gleeson M, Woods J A, Bishop N C, Fleshner M, Green C, Pedersen B K, Hoffman-Goetz L, Rogers C J, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 41.Walsh N P, Oliver S J. Exercise, immune function and respiratory infection: An update on the influence of training and environmental stress. Immunol Cell Biol. 2016;94:132–139. doi: 10.1038/icb.2015.99. [DOI] [PubMed] [Google Scholar]


