Abstract
Academic drug discovery is a vital component to current drug discovery and development environments. In this study, we investigated 798 drug discovery projects that took place between 1991 and 2015 at 36 academic institutions in the United States. The observed success rates of academic drug discovery and development were 75% at phase I, 50% at phase II, 59% at phase III, and 88% at the new drug application/biologics license application (NDA/BLA) phase. These results were similar to the corresponding success rates of the pharmaceutical industry. Collaboration between academic institutions and the pharmaceutical industry seemed more important at later stages than earlier ones; all projects that succeeded at phase III or the NDA/BLA stage involved academic‐industrial collaboration. Many academic research projects involved neoplasms and infectious diseases, and were focused on small molecules and biologics. The success rates and possible effects of academic‐industrial collaboration seemed to vary depending on disease domains and drug modalities.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Although the number of successful projects from the pharmaceutical industry has been stagnant for decades, more drugs originating from academia are being brought to market through academic‐industrial collaboration.
what question did this study address?
In most similar studies, the questionnaire surveys were conducted only on approved drugs. On the other hand, we desired to focus on not only the successful portions of drugs but on all drug candidates and programs during a specific period.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This study contributes to our understanding of drug R&D in academia, including the early research stages, and presents a new basis for quantitative evidence regarding current academic drug discovery.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
Academic‐industrial collaboration during the drug discovery and development process seems to be of significant importance. With further substantial evidence, our study may suggest the need to propose a more efficient mechanism for academic drug discovery and development that involves collaboration with the industry.
Stagnation in new drug research and development has been a focal issue in past decades. Although the number of US Food and Drug Administration (FDA) approvals of new molecular entities (NMEs) hit a 21‐year high in 2017, such approvals have remained at low levels since the 2000s, after reaching a peak in the mid‐1990s.1, 2 Despite various initiatives and efforts to improve success rates in both the public and private sectors, the likelihood of approvals (LOAs) from phase I clinical trials remains at ~10%.3, 4 Challenging environmental conditions in research and development (R&D) have inevitably led the pharmaceutical industry to spend more on new drug development operations; R&D investment in 2014 tripled that of 1995, and doubled the amount spent in 2000.5
The decreasing numbers of NME approvals and increasing R&D costs have often been discussed in relation to the exhaustion of obvious drug targets. Hopkins and Groom6 showed that there were 600–1500 “drug targets” that could potentially receive industrial research. The creation of innovative new drugs is predicated on the discovery and development of new technologies in addition to the search for new targets outside the existing sphere, which is often achieved by linking industrial drug discovery to basic academic research. Basic research in the context of pharmaceutical R&D, which typically entails target‐screening and in vitro studies based on novel concepts, is a strength of academic institutions.7 The process of drug discovery in academia also differs from that of the industry in that academic researchers tend to pursue higher‐risk targets and undertake more in‐depth research than industrial researchers.8
Kneller9 analyzed the origins of 252 drugs that received FDA approval between 1998 and 2007. Of the 252 compounds, 191 originated from pharmaceutical companies, whereas the remaining 61 originated from universities and biotechnology companies before being transferred to the industry.9 The analysis showed that universities and biotechnology companies substantially contributed to the current discovery of innovative drugs during the period of study, and discussed how drug “seeds” that originated from universities and biotechnology companies were transferred to the industry.
Given the concerns about possible stagnation regarding the successful drug discovery and development projects that originate from within the industry, pharmaceutical companies around the world have been pursuing open innovation in an attempt to acquire drug discovery “seeds” that originate in academia.10, 11 It is of research interest whether such strategic options have actually resulted in new drug development successes. There have been various studies on the historical performance of drug discovery and development in both academia and the public sector.7, 12, 13, 14 Most of these studies involved questionnaire surveys that were only conducted for the examination of approved drugs. This is because it is practically difficult to grasp the details of projects that failed or were dropped at some stage of the R&D process. Considering the important role of academia in finding and/or creating seeds through fundamental research in the early stages of development, we need to focus not only on the successful portions of drugs, but on all drug candidates and programs during a specific period.
The objective of our study was to provide a broad overview of performance in addition to the characteristics of current academic drug discovery and development in the United States. In this study, academic drug discovery and development was defined as the drug discovery and development projects involving compounds that originate in academia. We conducted an analysis on projects conducted by major research universities in the United States and examined how academic‐industrial collaboration was associated with success rates in each clinical trial stage.
Methods
We chose projects for which we could confirm the success or failure of nonclinical trials at 36 universities in the United States. The universities were selected according to their rankings in “The 2014–2015 Times Higher Education World University Rankings' clinical, preclinical, and health.”15 The names of the 36 universities are provided in Table S1.
Information on candidate compounds was extracted from the Cortellis Competitive Intelligence (Clarivate Analytics) as of September 9 and September 10, 2015. We found 798 projects for which nonclinical research was begun between 1991 and 2010, and for which information on the success or suspension of nonclinical research was available by 2015. The beginning year of nonclinical research was estimated based on the methods used by Paul et al.16. We excluded compounds originally discovered by universities, but for which biotechnology companies were specified as the originator. We included all types and modalities of drugs in this study.
The results of clinical trials conducted in the United States, Europe, and Japan were obtained from the same database. The stages of drug discovery and development (i.e., preclinical, phase I, phase II, phase III, new drug application (NDA), biologics license application (BLA), and approval) used in this study were the same as those used by Hay et al.3. Following previous studies, we defined phase success rate as the number of drugs that progressed from one phase to the next divided by the sum of the number of drugs that progressed to the next phase and the number of drugs that were suspended.3 The decision on whether a project was suspended or not was made according to the database. The LOA was shown as the cumulative success rate from phase I to approval.3, 17 The LOA was obtained by multiplying the success rate of each phase. Diseases were classified according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD‐10). When projects had multiple indications, the first classification that appeared in ICD‐10 was designated as the indication. Drug modalities were classified into small molecules, biologics, diagnostic drugs, and other modalities. We used Stata 14 (StataCorp, College Station, TX) as the statistical tool for this study.
Results
Table 1 shows the overview of academic drug discovery and development projects conducted by major US universities during this study's observation period. Six compounds reached approval from the 245 projects that were begun between 1991 and 1995, whereas 7 compounds reached approval from the 154 projects that were begun between 1996 and 2000.
Table 1.
The current status of drug discovery and development as originated by academia in the United States
| Preclinical | Phase I | Phase II | Phase III | NDA/BLA | Approval | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of projects | Success rate | No. of projects | Success rate | No. of projects | Success rate | No. of projects | Success rate | No. of projects | Success rate | No. of projects | LOA from Phase 1 | |
| All | 798 (100%) | 32% (254/798) | 185 (100%) | 75% (139/185) | 76 (100%) | 50% (38/76) | 29 (100%) | 59% (17/29) | 16 (100%) | 88% (14/16) | 14 (100%) | 19% |
| 1991–1995 | 245 (31%) | 36% (89/245) | 83 (45%) | 71% (59/83) | 40 (53%) | 48% (19/40) | 17 (59%) | 41% (7/17) | 6 (38%) | 100% (6/6) | 6 (43%) | 14% |
| 1996–2000 | 154 (19%) | 44% (67/154) | 55 (30%) | 80% (44/55) | 23 (30%) | 52% (12/23) | 10 (34%) | 80% (8/10) | 8 (50%) | 88% (7/8) | 7 (50%) | 29% |
| 2001–2005 | 191 (24%) | 36% (69/191) | 41 (22%) | 76% (31/41) | 12 (16%) | 50% (6/12) | 2 (7%) | 100% (2/2) | 2 (13%) | 50% (1/2) | 1 (7%) | 19% |
| 2006–2010 | 208 (26%) | 14% (29/208) | 6 (3%) | 83% (5/6) | 1 (1%) | 100% (1/1) | 0 (0%) | NR | 0 (0%) | NR | 0 (0%) | NR |
| Collaboration | ||||||||||||
| No collaboration with the industry | 539 (68%) | 30% (159/539) | 84 (45%) | 71% (60/84) | 18 (24%) | 37% (7/18) | 2 (7%) | 0% (0/2) | 0 (0%) | NR | 0 (0%) | NR |
| Collaboration with the industry | 259 (32%) | 37% (95/259) | 101 (55%) | 78% (79/101) | 58 (76%) | 54% (32/58) | 27 (93%) | 63% (17/27) | 16 (100%) | 88% (14/16) | 14 (100%) | 23% |
| ICD‐10 | ||||||||||||
| Certain infectious and parasitic diseases | 146 (18%) | 23% (34/146) | 24 (13%) | 88% (21/24) | 13 (17%) | 39% (5/13) | 5 (17%) | 40% (2/5) | 2 (13%) | 100% (2/2) | 2 (14%) | 14% |
| Neoplasms | 338 (42%) | 35% (118/338) | 81 (44%) | 79% (64/81) | 32 (42%) | 44% (14/32) | 9 (31%) | 22% (2/9) | 2 (13%) | 100% (2/2) | 2 (14%) | 8% |
| No collaboration with the industry | 246 (73%) | 35% (85/246) | 49 (60%) | 76% (37/49) | 11 (34%) | 36%(4/11) | 2 (29%) | 0% (0/2) | 0 (0%) | NR | 0 (0%) | NR |
| Collaboration with the industry | 92 (27%) | 36% (33/92) | 32 (40%) | 84% (27/32) | 21 (66%) | 48% (10/21) | 5 (71%) | 29% (2/5) | 2 (100%) | 100% (2/2) | 2 (100%) | 12% |
| Diseases of the nervous system | 65 (8%) | 23% (15/65) | 10 (5%) | 60% (6/10) | 3 (4%) | 33% (1/3) | 1 (3%) | 100% (1/1) | 1 (6%) | 100% (1/1) | 1 (7%) | 20% |
| Diseases of the circulatory system | 46 (6%) | 28% (13/46) | 12 (6%) | 67% (8/12) | 4 (5%) | 25% (1/4) | 1 (3%) | 100% (1/1) | 1 (6%) | 0% (0/1) | 0 (0%) | 0% |
| Mental and behavioral disorders | 45 (6%) | 38% (17/45) | 12 (6%) | 75% (9/12) | 5 (7%) | 80% (4/5) | 2 (7%) | 100% (2/2) | 2 (13%) | 100% (2/2) | 2 (14%) | 60% |
| Endocrine, nutritional, and metabolic diseases | 43 (5%) | 44% (19/43) | 16 (9%) | 69% (11/16) | 6 (8%) | 83% (5/6) | 3 (10%) | 100% (3/3) | 3 (19%) | 100% (3/3) | 3 (21%) | 57% |
| Other diseases | 115 (14%) | 33% (38/115) | 30 (16%) | 67% (20/30) | 13 (17%) | 62% (8/13) | 8 (28%) | 75% (6/8) | 5 (31%) | 80% (4/5) | 4 (29%) | 25% |
| Modality | ||||||||||||
| Small molecule | 449 (56%) | 25% (113/449) | 85 (46%) | 80% (68/85) | 42 (55%) | 48% (20/42) | 15 (52%) | 67% (10/15) | 9 (56%) | 78% (7/9) | 7 (50%) | 20% |
| Biologics | 310 (39%) | 37% (116/310) | 84 (45%) | 70% (59/84) | 27 (36%) | 41% (11/27) | 8 (28%) | 38% (3/8) | 3 (19%) | 100% (3/3) | 3 (21%) | 11% |
| Diagnostic drug | 30 (4%) | 70% (21/30) | 13 (7%) | 85% (10/13) | 6 (8%) | 100% (6/6) | 5 (17%) | 80% (4/5) | 4 (25%) | 100% (4/4) | 4 (29%) | 68% |
| Other modalities | 9 (1%) | 44% (4/9) | 3 (2%) | 33% (1/3) | 1 (1%) | 100% (1/1) | 1 (3%) | 0% (0/1) | 0 (0%) | NR | 0 (0%) | NR |
ICD‐10, International Statistical Classification of Diseases and Related Health Problems 10th revision; NDA/BLA, new drug application and biologics license application; NR, not reportable.
Phase success rate is defined as the number of drugs that progressed from one phase to the next divided by the sum of the number of drugs that progressed to the next phase and the number of drugs that were suspended.
Among the projects that were begun after 2001, only one compound reached approval and only a few projects reached clinical trial phases. It is difficult to discuss the trend in the 2000s based solely on these examples because drug R&D generally takes >10 years.16 Thus, our observation period did not seem sufficient for evaluating the recent trend.
Success rates and collaboration with private companies
The success rate of each drug discovery stage in academia was 31.8% for preclinical, 75.1% for phase I, 50.0% for phase II, 58.6% for phase III, and 87.5% for NDA and BLA. The LOA from phase I to approval was 19.3% (Figure 1).
Figure 1.
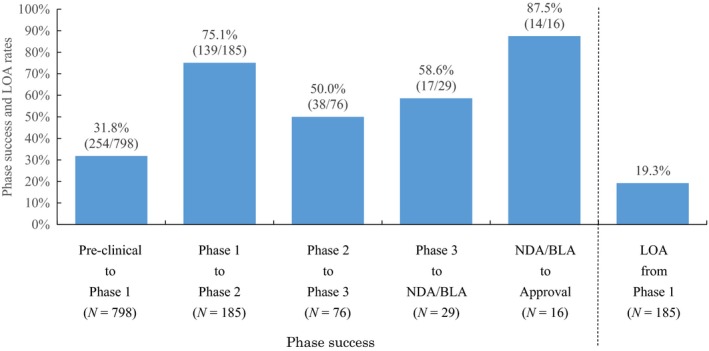
Phase success and likelihood of approvals (LOA) rates of academic drug discovery and development that was begun between 1991 and 2010. NDA/BLA, new drug application/biologics license application.
Academic‐industrial collaboration seemed to have some positive impact on academic drug discovery; the nonclinical success rate was 36.7% for collaborating projects and 29.5% for noncollaborating projects (Figure 2 ). Higher success rates for collaborating projects were evident in later stages. The respective success rates of collaborating projects compared with noncollaborating projects was 78.2% vs. 71.4% in phase I, 54.4% vs. 36.8% in phase II, 63.0% vs. 0% in phase III, and 87.5% vs. 0% at NDA/BLA. No projects in the database were successful at either phase III or NDA/BLA without engaging in industrial collaboration.
Figure 2.
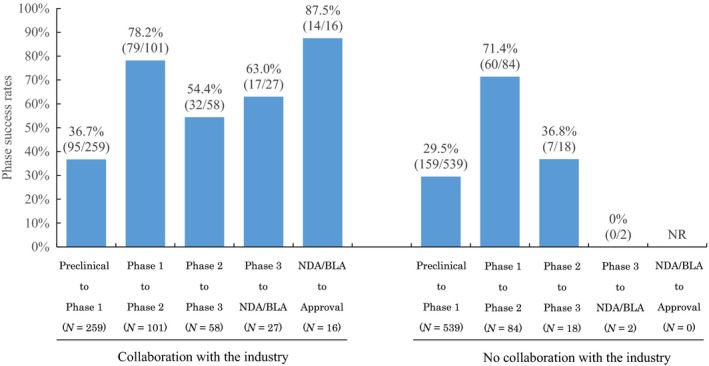
The impact of academic‐industrial collaboration on academic drug discovery and development projects that were begun between 1991 and 2010. NDA/BLA, new drug application/biologics license application; NR, not reportable.
Disease classifications
The most common research domain in academia was neoplasms, followed by infectious and parasitic diseases, diseases of the nervous system, diseases of the circulatory system, mental and behavioral disorders, and endocrine, nutritional, and metabolic diseases (Figure 3).
Figure 3.
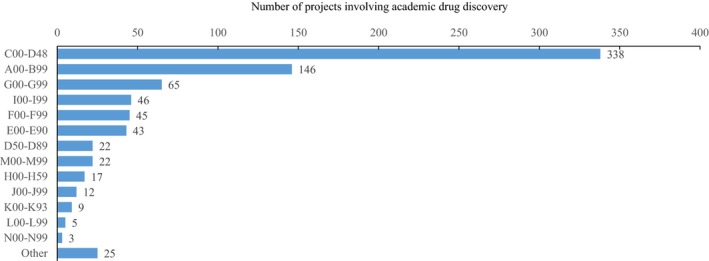
The classifications of academic drug discovery projects that were begun between 1991 and 2010 by International Statistical Classification of Diseases and Related Health Problems 10th Revision.
For neoplasms and infectious diseases (the two top research disease domains in academia), projects in both domains had a higher phase I success rate, but lower phase II and phase III success rates than those involving other diseases (Figure 4). We observed a lower rate of academic‐industrial collaboration for neoplasm projects compared with projects involving other diseases in most phases. The rate of academic‐industrial collaboration for neoplasm drugs was 27% at preclinical, 40% at phase I, 66% at phase II, 78% at phase III, and 100% at NDA/BLA, whereas the overall academic‐industrial collaboration rate was 33% at preclinical, 55% at phase I, 76% at phase II, 93% at phase III, and 100% at NDA/BLA.
Figure 4.
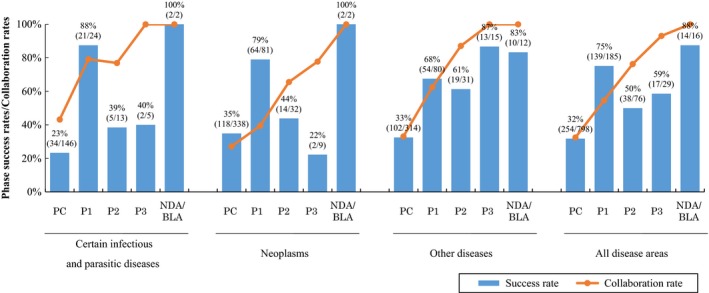
The differences in success and collaboration rates for academic drug discovery and development projects that were begun between 1991 and 2010, presented by disease areas. NDA/BLA, new drug application/biologics license application.
The number of projects involving the nervous and circulatory systems were relatively small, and phase II success rates seemed lower in these two domains (i.e., 33% for nervous system projects and 25% for circulatory system projects). Although the number of projects that reached phase III trials was higher for those in infectious diseases and neoplasms, the success rate of phase III for these two diseases was lower than for those in other disease areas.
Modalities
There were 56% small molecules, 39% biologics, 4% diagnostics, and 1% other modalities (Figure 5). The proportion of biologics was particularly high in projects related to diseases of the blood and blood‐forming organs as well as for certain disorders involving the immune mechanism (77.3%), which is a classification that often involves antibody drugs. Biologics also accounted for a large proportion in neoplasms (40.8%), where many biologics have already been brought to market. The proportion of biologics was also high in diseases of the eyes and adnexa (47.1%), diseases of the respiratory system (43.5%), and diseases of the musculoskeletal system and connective tissue (50.0%).
Figure 5.
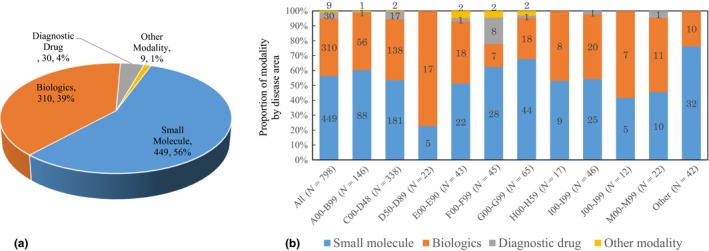
The current status of modalities in academic drug discovery and development projects that were begun between 1991 and 2010. (a) Proportion of modalities in academic drug discovery (N = 798) (b) Proportion of modality by disease area in academic drug discovery.
The success and collaboration rates of small molecule and biologics are shown in Figure 6. The success rates in late‐phase clinical trials were lower in biologics than in small molecules.
Figure 6.
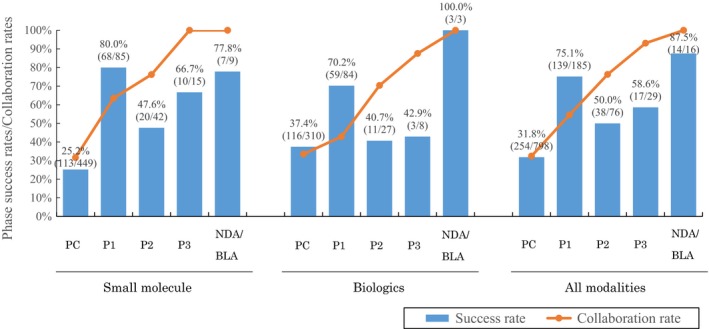
Success and collaboration rates by modality in academic drug discovery and development projects. NDA/BLA, new drug application/biologics license application.
Discussion
According to a report by the Pharmaceutical Research and Manufacturers of America (PhRMA), the average cost associated with developing a drug to the approval stage has increased since the 2000s, reaching US $2.6 billion in the first half of the 2010s.18 This is ~14.5 times the cost of development in the 1970s and 6.3 times the cost in the 1980s. The cost also increased 2.6 times between the 1990s and the first half of the 2000s. The PhRMA explains that possible reasons for this influx include increased trial complexity and regulatory burdens, increased focus on areas where failure risks are high, and expanded research burdens to meet payer demands.18 The number of NMEs approved by the FDA has remained constant since the 1950s,1 except for a temporary increase in the second half of the 1990s due to the Prescription Drug User Fee Act. Although we have seen a high number of new drug approvals in 2017, we are still concerned whether the long‐lasting stagnation has really been overcome.
This background has resulted in recent attention regarding the collaboration between universities and the biotechnological industry.9 Given stagnation in the number of successful projects originating from within the pharmaceutical industry, more drugs originating from academia are being brought to market through collaboration with industry.
In our survey focusing on major US universities, we identified 798 academic projects that were in preclinical stages between 1991 and 2010. Although it fluctuates every 5 years, the number of academic projects as shown in Table 1 indicates that academic drug discovery has gained momentum in the United States. Historical changes in regulation, research funding, and the pharmaceutical industry over the past decades concur with this trend. First, the Bayh‐Dole Act of 1980 allowed scientists, universities, and small businesses to patent and profit from achievements made through federally funded research, but its impact is not limited to drug discovery. That is, the act has made a substantial contribution to patenting in academia. The percentage and total number of patents awarded to academic entities have increased in tandem.19, 20 A previous report estimated that between 1996 and 2007, university‐based research‐licensing agreements rose in contribution to the gross domestic product, increasing from US $47 billion to $187 billion.20 Second, academia now requires licensing revenues in order to continue conducting research. Fierce competition has recently arisen over National Institutes of Health (NIH) research grants,21 and drug discovery research aimed at practical applications and licensing revenue has become a requirement to obtain industry funding. Third, industry efforts to seek out drug seeds from within academia have increasingly intensified. Academia has basic research advantages, including target screening and in vitro studies based on novel ideas and concepts,7 and a variety of strategies are used to mitigate risk in preclinical studies conducted in an academic context.22 Furthermore, academic researchers provide innovative research and new mechanisms in clinical practice because hospital‐based clinical researchers identify medical problems and trends within the patient population and collaborate closely with academic scientist.23 Translational science plays a role of returning these researches from bench to the bed again. In return, the industry provides commercial and pharmaceutical expertise. Open innovation plays a critical role in goal achievement. In the harsh R&D environments, proactive collaboration with academia as a source of drug seeds has become an effective means of contributing to drug discovery and development.
Several reports have shown that the success rates of drug development projects have recently been dwindling;3, 4, 17, 24, 25 drug discovery and development in academia also faces the same level of difficulty. Using the average success rates estimated from our sample, we can discuss the performance of drug discovery and development projects that have originated in academic settings. Previous reports focusing mainly on overall drug discovery and development projects (e.g., those of pharmaceutical companies, biotechnology companies, nonprofit organizations, and academia) have indicated that the success rates of phase II trials, in which investigators attempt to find optimal target populations and treatment regimens, tend to be lower than those of phase I and phase III trials.3, 4, 17, 22 Our results suggest that this issue is also evident in academic drug discovery. The success rates of academic projects in phases I and II (most of which involving the investigation of compounds that originated in academia) were higher than those common success rates. For example, although the success rates reported by the Biotechnology Innovation Organization (BIO) were 63% for phase I and 31% for phase II,4 our results for academic success rates indicated 75% for phase I and 50% for phase II. The success rates of phase III trials and NDA/BLA in academia were close to those achieved by pharmaceutical companies. The success rates reported by BIO (2016) were 58% for phase III and 85% for NDA/BLA,4 whereas our results for success rates in academia were 59% for phase III and 88% for NDA/BLA. Our results suggest that the success rates for phase I and phase II trials that originated in academia were somewhat higher than the overall success rates seen in both academia and the industry. This may lead to academia's overall dominance regarding LOAs (i.e., 19.3% vs. the industry's 9.6%).4 Of the 14 approved drugs, 6 drugs are priority review and orphan drugs, which indicates that discovery and development of academic drugs may have important meanings.
With many unmet medical needs still remaining, a number of reports have emphasized both the importance and challenges of academic‐industrial collaboration.26, 27 Smietana et al.28 (2016) demonstrated that partnering has recently had a positive influence on the success of clinical trials regardless of whether it involved academia or the industry. Our results revealed that, on average, academic projects that collaborated with pharmaceutical companies showed higher success rates than those that did not (Table 1 and Figure 2). By collaborating with the industry, the success rates for projects that originated in academia seemed to be even higher than those seen in overall drug discovery and development.3, 4, 22
Previous studies have shown that the majority of funding for clinical trials and drug approval process is supported by the pharmaceutical industry.29, 30 Clinical trials (especially in phases II and III) result in huge costs and come with variable success rates depending on the disease area and modality.3, 4, 31 All projects in phase III and NDA/BLA in our data involved some form of collaboration, which suggests that economic assistance and industry‐acumen are needed in these stages.
The success rates of academic phase I and II trials that involved collaboration also surpassed the success rates of academic projects undertaken without collaboration in addition to those undertaken by the industry.3, 4 Cook et al 32. discussed the results of a comprehensive longitudinal review of AstraZeneca's small‐molecule drug projects and showed that safety concerns (i.e., toxicology or clinical safety) were the most common reason for project closure in phase I, and failure to achieve sufficient efficacy was the most reason in phase II.33 In this survey, we were not able to obtain information on how drug companies contributed to academic projects or specific reasons for project closure due to the limitations in the commercial database. The observed higher success rates of collaborative projects may be ascribed to synergy in some form between the strengths of universities in scientific innovation and disease expertise and those of industries in investment and late‐phase development,7 and further studies need to be done to explore specific mechanism(s) that have caused these findings. Although collaborative projects seem to have higher success rates, only a small proportion of projects are actually undertaken with industry collaboration during the early R&D stages, including the preclinical stage. The industry is reportedly cautious about the reproducibility of academic research at the preclinical stage,34 which may partly explain the above observation. Another possibility is that academic institutions, especially the top universities in this analysis, are self‐sufficient in leading early phase preclinical research without an obligated requirement of an industry collaborator. In later stages, collaborative projects tend to be more successful because the partnerships are necessary as the scale of clinical research exceeds the infrastructural and financial capabilities of the nonprofit sector.
The disease areas mainly focused on during academic drug discovery and development (Figure 3) were similar to those of general drug discovery and development projects in the United States.3, 4, 5, 17Neoplasms and infectious diseases appear at the top of the list in a number of other surveys conducted on drug discovery research at universities, nonprofit research institutions, and in the public sector.7, 14 Stevens et al.14 examined the budget of each research center at the NIH and discussed possible associations between availability of research grants and disease domains that appeared in R&D activities. Similarly, our survey revealed that academic research has been undertaken in disease areas with large NIH budgets.35 This correlation may indicate that many academic projects are undertaken in line with current R&D trends and budget allocations in the United States public sector.
It has been reported that drugs for neoplasms generally have lower success rates during the late clinical development stages compared with those for other diseases.3, 4 Our survey of academic drug discovery and development, similarly, shows very low success rates in projects for neoplasms; among the 338 neoplasm projects from this study's database, only diagnostic drugs passed the phase III stage. To date, it has become increasingly harder to clinically develop oncologic drugs as a result of challenging indicators, including requirements for progression‐free survival and/or overall survival in most efficacy‐showing trials. Both success and collaboration rates are low in neoplasms, which is probably related to the high risk of later‐stage clinical trials. Furthermore, due to the low collaboration rate, it may be more difficult to conduct academic drug discovery and development in neoplasms. Indeed, our results indicated that the success rates of neoplasm projects involving academic‐industrial collaboration were higher than those without such collaboration (Table 1). However, we need to be careful about the implications of these results. Although academia can technically conduct phase I clinical research without an industry partner for anticancer drugs, the reverse is nearly impossible because the industry always relies on a network of academia‐based clinical research sites to conduct early phase clinical research that needs to be done in patients with cancer. These technical and practical dependencies should be considered when we interpret the association of collaboration and success rates in sound perspectives.
Although it was reported that drugs for infectious diseases generally had higher success rate during later stage clinical trials than those for other diseases,3, 4 we observed the opposite result in our survey (Figure 4). In academic drug discovery, the category not only includes traditional antimicrobial treatments, but also other types of infectious diseases, of which pandemics (e.g., influenza, human immunodeficiency virus, and hepatitis C virus) are a current global concern. The lower success rates for infectious diseases observed in academia may reflect such differences in the targets of diseases.
A previous survey targeting public‐sector research institutions reported the modalities of drug products brought to market as follows: 60.8% small molecules; 33.3% biologics; 5.3% diagnostic drugs; and 1% over‐the‐counter drugs.14 We found that the modalities of compounds in academic research were similar to these (Figure 5). The success rates for biologics are known to be higher than those for small molecules.28 However, the success rates for biologics indicated by this study's survey were somewhat lower than other recently published results, especially in phases II and III. This is probably due to a large proportion of phases II and III biologics projects being targeted at neoplasms (phase II: 67% and phase III: 63%). Indeed, no phase III anticancer drug projects in the biologic modality progressed to the next stage. This contributed to the low success rates of biologics, which had lower collaboration rates at every stage compared with small molecules. The low collaboration rates for biologic projects may be associated with the low success rates observed in our survey, although the causalities behind this association are difficult for us to establish.
A limitation of this study is that it only covered projects for which universities were specified as originators. This excluded compounds originally discovered by universities but for which biotechnology companies were specified as the originator. This study only examined projects with completed stages. Thus, sampling biases were inevitable because recent projects were under‐represented. Considering the expansion of clinical trial “offshoring,”36 we included projects for which clinical trials had been performed in the United States, Europe, or Japan, but we did not examine the effects of regional and/or national differences on project progress. Many of the universities in this study have global networks, and some clinical trials for the observed projects have been done outside the United States. Collaboration with the industry can take on a variety of forms. However, this survey did not distinguish between industry input type (e.g., financial or technological). We cannot accurately determine the nature of industrial contributions. We were not able to explore specific causes of failure or reasons for closure of each project due to the limitation of the database. Finally, we observed success or failure of a project and not of a target indication. Further studies that focus on indications are necessary because drug companies create development plans considering complicated interactions at the level of indication to achieve optimal outcomes in R&D success and also to meet various business and regulatory needs.
In conclusion, academic drug discoveries exhibited characteristics similar to those of industrial drug discoveries. That is, there were high success rates for phases I and III, but low success rates for phase II. Academic‐industrial collaboration was shown to contribute substantially to the success of later stage clinical trials. The most common disease domains subject to academic drug discovery were neoplasms, viral infectious diseases, and nervous system diseases. About 40% of the academic drug discovery projects involved biologics, which indicates that academia takes on leading‐edge and challenging projects in terms of both drug modality and disease domain. This study contributes to our understanding of drug R&D in academia, including the early research stages, and presents a new basis for quantitative evidence regarding current academic drug discovery.
Funding
No funding was received for this work.
Conflict of Interest
T.T. is an employee of the Mitsubishi Tanabe Pharma Corporation, while R.I. is an employee of the Institute for Health Economics and Policy. S.O. has no conflicts of interest to declare.
Author Contributions
T.T., R.I., and S.O. wrote the manuscript. T.T., R.I., and S.O. designed the research. T.T., R.I., and S.O. performed the research. T.T., R.I., and S.O. analyzed the data. T.T., R.I., and S.O. contributed new reagents/analytical tools.
Supporting information
Table S1. 36 US universities extracted from the 2014–2015 Times Higher Education World University Rankings (Clinical, Pre‐Clinical and Health).
Table S2. 14 drugs approved from the 798 projects begun between 1991 and 2010.
Acknowledgments
This study was carried out following the “Survey on the environment surrounding translational research in Japan and the United States: Structure of university drug discovery for practical use,” which was conducted at the Institute for Health Economics and Policy (IHEP, Tokyo, Japan). We thank Takafumi Akabane and Mitsuhiro Kondo (IHEP) for their thoughtful advice. We thank Editage for English editing.
References
- 1. Munos, B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. Drug Discov. 8, 959–968 (2009). [DOI] [PubMed] [Google Scholar]
- 2. Mullard, A. 2012 FDA drug approvals. Nat. Rev. Drug Discov. 12, 87–90 (2013). [DOI] [PubMed] [Google Scholar]
- 3. Hay, M. , Thomas, D.W. , Craighead, J.L. , Economides, C. & Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 32, 40–51 (2014). [DOI] [PubMed] [Google Scholar]
- 4. Biotechnology Innovation organization, Biomedtracker, Amplion Clinical Development Success Rates 2006‐2015 (BIO, Washington, DC, BioMedTracker, CA, Ampion, OR, 2016) <https://www.bio.org/sites/default/files/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf>.
- 5. Pharmaceutical Research and Manufacturers of America 2015 biopharmaceutical research industry profile. PhRMA, Washington, DC: (2015). [Google Scholar]
- 6. Hopkins, A.L. & Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 (2002). [DOI] [PubMed] [Google Scholar]
- 7. Frye, S. , Crosby, M. , Edwards, T. & Juliano, R. US academic drug discovery. Nat. Rev. Drug Discov. 10, 409–410 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huryn, D.M. Drug discovery in an academic setting: Playing to the strengths. Med. Chem. Lett. 4, 313–315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kneller, R. The importance of new companies for drug discovery: origins of a decade of new drugs. Nat. Rev. Drug Discov. 9, 867–882 (2010). [DOI] [PubMed] [Google Scholar]
- 10. Khanna, I. Drug discovery in pharmaceutical industry: productivity challenges and trends. Drug Discov. Today 17, 1088–1102 (2012). [DOI] [PubMed] [Google Scholar]
- 11. Wang, L. , Plump, A. & Rindel, M. Racing to define pharmaceutical R&D external innovation models. Drug Discov. Today 20, 361–370 (2015). [DOI] [PubMed] [Google Scholar]
- 12. Zycher, B. , DiMasi, J.A. & Milne, C.P. Private sector contributions to pharmaceutical science: thirty‐five summary case histories. Am. J. Ther. 17, 101–120 (2010). [DOI] [PubMed] [Google Scholar]
- 13. Sampat, B.N. Academic patents and access to medicines in developing countries. Am. J. Public Health 99, 1–17 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stevens, A.J. et al The role of public‐sector research in the discovery of drugs and vaccines. N. Engl. J. Med. 364, 535–541 (2011). [DOI] [PubMed] [Google Scholar]
- 15. Times Higher Education Website. Subject ranking 2014–15: clinical, pre‐clinical & health. <https://www.timeshighereducation.com/world-university-rankings/2015/subject-ranking/clinical-pre-clinical-health#!/page/0/length/25/sort_by/rank/sort_order/asc/cols/scores>. Accessed September 9, 2015.
- 16. Paul, S.M. et al Schacht How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat. Rev. Drug Discov. 9, 203–214 (2010). [DOI] [PubMed] [Google Scholar]
- 17. DiMasi, J.A. , Feldman, L. , Seckler, A. & Wilson, A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin. Pharmacol. Ther. 87, 272–277 (2010). [DOI] [PubMed] [Google Scholar]
- 18. Pharmaceutical Research and Manufacturers of America Website. Prescription medicine: costs in context. <http://phrma-docs.phrma.org/sites/default/files/pdf/prescription-medicines-costs-in-context-extended.pdf>. Updated August 2016. Accessed July 6, 2017.
- 19. Cohen, F.J. Macro trends in pharmaceutical innovation. Nat. Rev. Drug Discov. 4, 78–84 (2005). [DOI] [PubMed] [Google Scholar]
- 20. Markel, H. Patents, profits, and the American people — the Bayh‐Dole act of 1980. N. Engl. J. Med. 369, 794–796 (2013). [DOI] [PubMed] [Google Scholar]
- 21. National Institute of Health . Research Portfolio Online Reporting Tools (RePORT) Web site. Research and training grants: success rates by mechanism and selected activity codes. <https://report.nih.gov/nihdatabook/index.aspx>. Accessed July 6, 2017.
- 22. Dahlin, J.L. , Inglese, J. & Walters, M.A. Walters Mitigating risk in academic preclinical drug discovery. Nat. Rev. Drug Discov. 14, 279–294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fishburn, C.S. Translational research: the changing landscape of drug discovery. Drug Discov. Today 18, 487–494 (2013). [DOI] [PubMed] [Google Scholar]
- 24. Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–715 (2004). [DOI] [PubMed] [Google Scholar]
- 25. Abrantes‐Metz, R. , Adams, C. & Metz, A . Pharmaceutical development phases: a duration analysis. Working paper no. 274. <http:/www.ftc.gov/be/workpapers/wp274.pdf> (US Federal Trade Commission: Bureau of Economics, 2004).
- 26. Tralau‐Stewart, C.J. , Wyatt, C.A. , Kleyn, D.E. & Ayad, A. Drug discovery: new models for industry–academic partnerships. Drug Discov. Today 14, 95–101 (2009). [DOI] [PubMed] [Google Scholar]
- 27. Stewart, S.R. et al Leveraging industry‐academia collaborations in adaptive biomedical innovation. Clin. Pharmacol. Ther. 100, 647–653 (2016). [DOI] [PubMed] [Google Scholar]
- 28. Smietana, K. , Siatkowski, M. & Møller, M. Trends in clinical success rates. Nat. Rev. Drug Discov. 15, 379–380 (2016). [DOI] [PubMed] [Google Scholar]
- 29. Dorsey, E.R. et al Funding of US biomedical research, 2003–2008. JAMA 303, 137–143 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moses, H. III , Dorsey, E.R. , Matheson, D.H.M. & Thier, S.O. Financial anatomy of biomedical research. JAMA 294, 1333–1342 (2005). [DOI] [PubMed] [Google Scholar]
- 31. DiMasi, J.A. , Hansen, R.W. & Grabowski, H.G. The price of innovation: new estimates of drug development costs. J. Health Econ. 22, 151–185 (2003). [DOI] [PubMed] [Google Scholar]
- 32. Cook, D. et al Lessons learned from the fate of AstraZeneca's drug pipeline: a five‐dimensional framework. Nat. Rev. Drug Discov. 13, 419–431 (2014). [DOI] [PubMed] [Google Scholar]
- 33. Suter, L. , Babiss, L.E. & Wheeldon, E.B. Toxicogenomics in predictive toxicology in drug development. Chem. Biol. 11, 161–171 (2004). [DOI] [PubMed] [Google Scholar]
- 34. Prinz, F. , Schlange, T. & Asadullah, K. Believe it or not: how much can we rely on published data on potential drug targets? Nat. Rev. Drug Discov. 10, 712–713 (2011). [DOI] [PubMed] [Google Scholar]
- 35. National Institute of Health . Research Portfolio Online Reporting Tools (RePORT) Website. Research grants: Awards by institute/center. <https://report.nih.gov/nihdatabook/index.aspx>. Accessed July 6, 2017.
- 36. Rosenblatt, M. How academia and the pharmaceutical industry can work together. Ann. Am. Thorac. Soc. 10, 31–38 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. 36 US universities extracted from the 2014–2015 Times Higher Education World University Rankings (Clinical, Pre‐Clinical and Health).
Table S2. 14 drugs approved from the 798 projects begun between 1991 and 2010.


