Abstract
The goal of this study is to examine the extent to which child temperament predicts the adiposity rebound, a steep increase in the body mass index (BMI) between ages 5 and 7 years. If this increase occurs at an earlier age, the risk for later obesity is elevated. To improve the accuracy of the examination, we use a genetically informed design, a sibling-control study, to control for genetic and familial confounding. We hypothesize that temperament traits tapping negative emotionality, approach and avoidance are associated with the adiposity rebound.
Methods
We repeatedly examined 25889 siblings within the Norwegian Mother and Child Cohort Study, following them from the mothers’ pregnancy through child age 8 years. Information on the children’s height and weight was collected by means of health registries and maternal reports. Information on the siblings’ temperament was collected by questionnaires completed when they were 1.5, 3, and 5 years old. We examined the associations of temperament at different child ages with the timing of the adiposity rebound among siblings and controls by means of growth curve and multilevel analyses.
Results
Within siblings, high scores on the approach trait of sociability predicted an earlier adiposity rebound and high scores on the avoidance trait of shyness predicted a later adiposity rebound with timing differences ranging between 6 and 16 weeks. Surprisingly, negative emotionality did not predict the adiposity rebound. The associations between temperament and the adiposity rebound increased with increasing child age. The results within controls—comparing siblings with the population, broadly paralleled those within siblings,
Conclusions
The findings encourage the notion that child temperament functions as an early marker for the adiposity rebound. Future studies may advance our knowledge by including measures of child personality along the taxonomy of the adult Five Personality Factors.
Introduction
Obesity in adulthood is a major risk factor for chronic diseases and death worldwide [1], and being obese in childhood has been regarded as an important stepping stone on this path. Yet, obesity during the earlier years of childhood is a poor predictor of adult obesity, with low relative risk rates. Moreover, 70% of obese adults were not obese in childhood [2, 3]. A better predictor than a specific weight status in childhood, is the BMI-for-age curve. This curve shows ascending and descending phases, reflecting stages of body fat development in children. After infancy, this curve falls, then starts to rise again. The age corresponding to the lowest point of that curve is the Adiposity Rebound [4, 5]. Usually, the adiposity rebound occurs between the age of 5 to 7 years, and the earlier it occurs, the higher is the risk for adult obesity [4–7].
Risk factors for an early timing of the adiposity rebound are the fetal environment [8], parental feeding strategies [9], and early nutrition and diet [10, 11]. Another risk factor may be temperament, i.e. children’s biologically-based differences in reactivity and self-regulation [12]. Temperament traits comprise negative emotionality, activity, extraversion, regularity or restraint, and shyness or inhibition [12, 13]. Negative emotionality involves high reactivity to stress expressed as fearfulness or anger. Activity involves motor activity as well as restlessness. Extraversion (or surgency, sociability) manifests itself as seeking out and enjoying social activities and relations. Restraint and regularity are characterized by perseverance and low impulsiveness. Shyness reflects fear and embarrassment vis-a-vis strangers.
At present, the extent of the association of children’s temperament with the adiposity rebound is still unknown. To generate hypotheses about potential associations, we consulted the literature on the associations between child temperament on the one hand and children’s weight, diet and eating on the other hand. Regarding weight status and early growth, associations with temperament have been established. The trait of negative emotionality is a key predictor of overweight in children and adolescents [14–17]. Self-regulation, low impulsiveness and restraint appear to protect from obesity [14, 15, 17–20]. Temperamental activity has shown an association with lower body weight in children [21–23]. Extraversion (or its subtraits surgency and sociability) is a risk factor for rapid growth and later obesity [15, 20, 24–26]. Some studies also showed positive relations between social anxiety, a construct similar to shyness, and higher body weight [27–29].
Temperament is also related to children’s appetite [30, 31], food enjoyment [32], satiety responsiveness, external eating [33–37], and preferences for sweet food and drinks [19, 38]. Several mechanisms may underlie these relations. Savory, palatable foods and eating in the absence of hunger serve as means to dampen negative emotions such as anxiety, depression, and anger, and this may explain why greater consumption of sweet foods and external eating are related to child negative emotionality [39–45]. Restraint and self-control are related to self-regulation of diet and eating [34, 45–49]. Food has strong intrinsic reward and punishment potentials [50], whereby savory foods trigger approach motivations, and unpleasant foods engender avoidance motivations. This mechanism may explain why extraverted children, having higher sensitivity to rewards [51], show greater enjoyment of food, and preference for palatable foods [47, 48]. Shy, anxious children, in contrast, show food neophobia, i.e. fear of tasting new foods, and are slow, fussy eaters [33, 36, 52].
A general problem when studying the association of any predictor with weight and growth is confounding, the presence of common causes for the exposure and the outcome. In behavioural genetics, one distinguishes between genetic confounding and environmental confounding. Genetic confounding refers to the heritability of the outcome and the predictors. The BMI is highly heritable [53], with estimates ranging from 50% to 70%, and sibling correlations over r = 0.86 [54]. Weight for height growth rates during childhood are heritable as well [55]. High heritability is also found for temperament or personality, with an average estimate of 47% across 1 774 twin studies [56]. Heritability also plays a role for potential mediators of the association between temperament and BMI, for instance caloric intake, appetite, and physical activity [57, 58]. Environmental confounding refers to wide array of circumstances during childhood creating an obesogenic environment, such as socio-economic status, parental feeding styles, health-related attitudes, nutrition, as well as the family’s activity habits [59–63].
Twin studies are the best method to parse genetic, shared environmental and unique environmental effects and thus control for confounding. However, suitable twin samples are rare. Sibling studies offer a partial solution to confounding, as they control for genetic and shared environmental effects. This is because siblings share 50% of their genes, and 100% of their familial environment [64]. For example, siblings live in the same household, in the same neighbourhood, share the family’s meals, and partake in family activities, while also attending the same kindergarten and school. In adults, a sibling control study for instance showed that the family environment attenuated the effects of conscientiousness on overweight [65].
The goal of the present study is to examine the associations of children’s temperament with the adiposity rebound. We will use a prospective sibling-control study featuring three assessments of temperament between the ages of 18 months and five years, and seven assessments of weight between birth and age eight years. By investigating the effects of temperament at three time points, we will be able to determine how early this marker can foretell the adiposity rebound. The temperamental traits considered in this study are negative emotionality, activity, sociability, and shyness. We expect, based on the outlined literature, that children scoring high in negative emotionality and sociability will be more likely to have an earlier adiposity rebound, translating into a higher risk for later obesity. More tentatively, we expect that children scoring high in activity and shyness have a later adiposity rebound, translating into a lower risk for later obesity.
Material and methods
Design and participants
Siblings were culled from The Norwegian Mother and Child Study (MoBa), an ongoing prospective study with repeated assessments of mothers and children starting in pregnancy. This study is conducted by the Norwegian Institute of Public Health, for details see [66]. In brief, between 1999 and 2008, pregnant women in the catchment area of 50 hospitals and maternity units across Norway received a postal invitation to attend their first free ultrasound scan scheduled between week 17 and 18 of the pregnancy. This invitation also included a letter inviting the pregnant women to participate in the MoBa study. The letter also contained a consent form, the first MoBa questionnaire, and an information brochure. In all, 44% of the invited women participated and provided written consent. Today, the MoBa study includes 95 200 mothers, and their 114 247 living children. Among these, there are 25 889 living biological siblings born to 12 550 mothers, 48.5% girls and 51.5% boys. To assure that all siblings resulted from different pregnancies, we excluded co-twins, co-triplets, and co-quadruplets and included only the first-borns.
MoBa has all necessary concessions from the Norwegian Data Protection Authority and ethical clearance from the responsible ethical committees across the counties in Norway [66]. This specific article received ethical clearance from the Regional Ethics Committee in South East Norway (Ref. 2016/398).
Assessments
To assess the children’s temperament, mothers completed a validated 12-item short form of the Emotionality Activity and Sociability (EAS) temperament survey for children [67], when their children were ages 1.5, 3 and 5 years old. This questionnaire yields four scales: 1. Negative emotionality, characterized by unregulated negative emotions; 2. Activity, a proxy for involuntary activity in children; 3. Sociability, the tendency to enjoy spending time with others rather than alone; 4. Shyness, the tendency to feel distressed by and avoid unfamiliar persons and novel situations. Each item is scored on 5-point response categories (from untypical to very typical). Valid scores were available for 92.5%, 76.5%, and 53.7% of the sample at 1.5, 3, and 5 years, respectively. Unfortunately, the EAS does not include a self-regulation or restraint scale.
Information on the child’s weight and length was available for seven time−points between the child’s birth and the child’s 8th birthday. At birth, this data was obtained from the Medical Birth Registry of Norway [66, 68]. Through age 4 years, mothers reported from records of objective measurements provided to them by national health-stations for children. For the assessments at 5 years, 7 years, and 8 years, mothers self-reported the children’s height and weight. The children’s BMI was calculated as kg/m2. Valid measures of BMI were available for 100%, 79.5%, 49.0%, 61.4%, 47.5%, 55.7%, and 36.5% of the sample at 0, 1.5, 2, 3, 5, 7, and 8 years, respectively.
Socio-demographic familial confounders such as parents’ socio-economic status, education, place of residence, and the familial dietary and activity environment are constant when comparing siblings. The same is true for genetic confounders such as the parents’ BMI, health, and temperament. However, individual confounders need to be adjusted for, so all analyses were controlled for the child’s gender, gestational age, standardized weight and height at birth, and the mothers’ parity.
Statistical analysis
The sibling control analysis compares siblings with each other (“within siblings”) and siblings with other children in the same sample (“sibling-controls” or “within population”). The outcome is the expected change in adiposity rebound when a child differs in temperament compared to his or her siblings. By comparing siblings within the same family, 100% of the familial environment (e.g. the parents’ SES) and 50% of the siblings’ genes are adjusted for by design.
For multiple pregnancies (twins, triplets), only the first-born from each pregnancy was included in the analyses. Within siblings, temperament was centered on the average temperament score of the siblings of the same mother (mean temperament score of siblings minus individual temperament score of each sibling). Thus, for example, a positive score means that a specific sibling has a higher score than the average score of the siblings within that family. In the sibling-control analysis, individual scores are compared to those of other children in that family. Subsequently, each child’s temperament score was kept as a continuous variable, but scaled along ± 2 standard deviations.
The timing of the children’s adiposity rebound was estimated based on their individual BMI trajectories using a quadratic curve (parabola) where the lowest point (vertex) corresponds to the adiposity rebound. The mathematical characteristics of these trajectories (i.e. their slopes and intercept) were allowed to vary across children rendering three random latent variables: an intercept, a linear growth effect, and a quadratic growth effect. We regressed the random effects of the growth curve on the temperament categories and the covariates. Dependency between siblings was accounted for by using a standard sandwich estimator in Mplus 7.1; a method rendering correct standard errors. We estimated all models using full information maximum likelihood (FIML). FIML allows estimating data missing due to attrition or other forms of non-response, hence we could include all children with valid BMI data on one or more occasions. This means that our analyses based on the missing at random assumption and accounts for all missing data covarying with observed covariates. An example would be that mothers of obese children drop out of the study.
Results
We estimated the mean adiposity rebound to occur at age 64.3 months (standard error = 0.02), corresponding to age 5 years, 4 months and 1 week. The average BMI at the adiposity rebound was 15.6. Furthermore, the adiposity rebound of boys occurred on average 16 weeks earlier than the adiposity rebound in girls.
Table 1 shows that the mean gestational age of the siblings was 277.57 days, which is 2.5 days longer than the 275.1 days than the average gestational age of all children born in Norway between the years 2001 and 2008 (Table 1). Table 1 also shows a stable BMI development across 18 months through 3 years, with a slight drop between 5 and 7 years.
Table 1. Child birthweight, gestational age, and BMIs at different ages.
| Mean | Standard deviation | |
|---|---|---|
| Gestational age (days) | 277.57 | 14.41 |
| Birth weight (g) | 3532.51 | 624.01 |
| BMI 18 months | 16.74 | 1.34 |
| BMI 2 years | 16.48 | 1.41 |
| BMI 3 years | 16.13 | 1.48 |
| BMI 5 years | 15.57 | 1.59 |
| BMI 7 years | 15.80 | 1.85 |
| BMI 8 years | 16.24 | 2.02 |
| Maternal age at birth (years) | 30.25 | 4.11 |
BMI = Body Mass Index
The correlations between the temperamental scales of the EAS across all three measurements, adjusted for age, are presented in Table 2, with controls (the entire sample) above the diagonal, and siblings (adjusted for age and dependency) below the diagonal. The correlations for controls and siblings were very similar, with siblings showing slightly lower correlations. The correlations between activity and sociability were very high, suggesting a common core of both traits. Emotionality showed small correlations with the three other temperament traits. There were negative correlations of approximately equal size between shyness and sociability and shyness and activity.
Table 2. Age-adjusted correlations between EAS temperament scales among controls and within siblings.
| Controls | Siblings | |||
|---|---|---|---|---|
| Emotionality | Activity | Sociability | Shyness | |
| Emotionality | — | 0.07 | 0.05 | 0.12 |
| Activity | 0.06 | — | 0.69 | -0.21 |
| Sociability | 0.05 | 0.75 | — | -0.24 |
| Shyness | 0.16 | -0.23 | -0.26 | — |
Note. All correlations are signifikant at p < .001. Siblings above the diagonal, correlations corrected for dependency; controls below the diagonal.
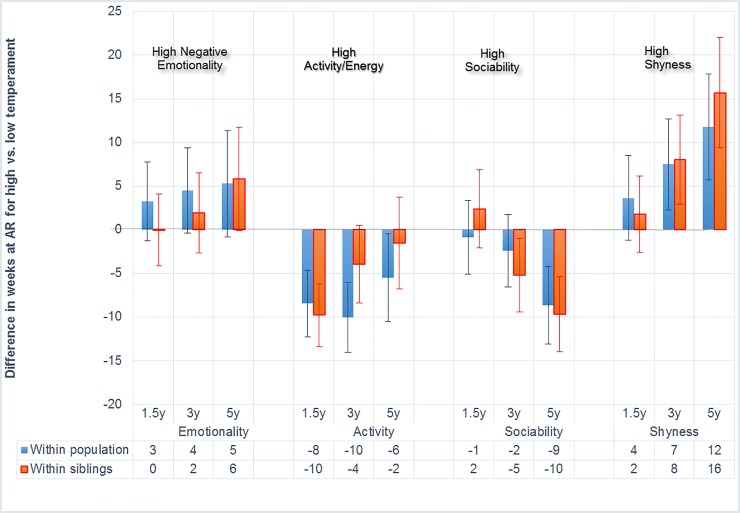
Fig 1 contains three types of information: 1. it shows differences of the timing of the adiposity rebound in weeks for children scoring lower or higher on each temperament trait (along ±2 standard deviations). 2. It shows differences in the timing of the adiposity rebound for each trait a three time points. 3. It shows differences in the timing of the adiposity rebound within siblings and within the population.
Fig 1. Earlier and later adiposity rebound according to temperament differences.
Vertical axis: AR = adiposity rebound differences scaled in weeks. Positive numbers represent later adiposity rebound (lower obesity risk) for those scoring higher than 2 standard deviations on the temperament dimension. Negative numbers represent earlier adiposity rebound (higher obesity risk) for children scoring higher than 2 standard deviations. Red columns: within siblings = comparison of the high scoring siblings’timing of the adiposity rebound with that of the other sibling. Blue columns: within population = comparison of the high scoring siblings’ timing of the adiposity rebound to that of controls (unrelated children). Numbers in the data table represent weeks. Vertical bars represent the width of the 95% confidence interval. Confidence intervals staying within one end of the bars indicate significance p ≤ 0.05. y = age in years at temperament assessment.
Negative emotionality, the trait capturing children’s moodiness and irritability, was not associated with the adiposity rebound at any age, neither within siblings nor within the population. Activity, the trait indicating children’s energy and vigor, was associated with an earlier adiposity rebound at 1.5 years. Sociability, the trait indicating pleasure, gregariousness and reward orientation, was not associated with the adiposity rebound within the population at 1.5 and 3 years. Within siblings, however, sociability was associated with a 5 weeks earlier adiposity rebound at 3 years and a 10 weeks earlier rebound at 5 years. Shyness, the trait capturing fear of strangers and novelty, as well as avoidance of social contacts, was associated with a later adiposity rebound both within the population and within siblings at child ages 3 and 5 years. Within siblings, the timing differences were 8 and 16 weeks, respectively.
Discussion and conclusion
The present study adds new information to the child obesity literature by examining the association of early childhood temperament with the timing of the adiposity rebound. Sociability, a temperament trait representing approach motivation was associated with an earlier adiposity rebound, i.e. a higher risk for later obesity. In contrast, shyness, a temperament trait representing social anxiety, avoidance and inhibition, was associated with a later adiposity rebound, signifying a lower risk for later obesity. Temperamental activity at 1.5 years was associated with an earlier adiposity rebound, but activity at 3 years and 5 years was no longer significantly associated with the adiposity rebound. Surprisingly, there were no associations between negative emotionality and the adiposity rebound. By using the conservative design of sibling control, these findings are far less confounded by shared genes or shared environment than classical studies.
Contrary to our expectations, negative emotionality was not associated with the adiposity rebound. This null finding was not limited to the siblings in our sample, where lower associations are to be expected. The few and relatively small classical studies examining the relation of the EAS negative emotionality scale with eating yielded contradictory results, showing associations with both picky eating and overeating [69–71]. Activity was not related to the adiposity rebound, except when measured at age 1.5 years. The association of activity with weight is equivocal, this being the result of the wide divergence of assessment methods, ranging from global temperament scales to the highly sophisticated doubly labeled water methods [72]. Sociability showed associations with an earlier adiposity rebound, which is compatible with mechanisms of higher approach motivation, greater sensitivity to rewards and greater enjoyment of food [47, 48, 73]. The remarkable association of shyness with an earlier timing of the adiposity rebound surprised us, because there are few relevant studies with which to compare our findings. However, shyness has been linked to food neophobia—fear of tasting new foods—and picky eating in a few earlier studies [33, 35, 36, 52].
We observed that associations between temperament and the adiposity rebound increased with increasing child age, probably because growing up implies that children assume greater control of all aspects of their diet and eating behaviors.
This study has a strong design, including a very large number of siblings, extensive longitudinal assessments reaching back to the pregnancy and up to the children’s 8th birthday. The homogeneity of the sample with respect to socio-economic status, ethnicity, education, availability of antenatal health care, and other social background factors was an advantage because it reduces variability. There were many assessment points for both temperament and weight, which strengthens the reliability and validity of the assessments. Most importantly, comparing sibling rather than unrelated participants allowed distilling the unique effects of each sibling’s temperament while adjusting for half of the genetic influences and all of the shared family environment.
At the same time, there are limitations. Importantly, the EAS did not assess children’s self-regulation skills and impulse control. Today, temperament research in children is being superseded by the Five Factor personality research, an approach that has been standardized and refined across decades in adults.[74] The five factor personality taxonomy captures aspects of negative emotionality including self-worth, interpersonal behavior, conscientiousness (self-control, dutifulness), extraversion (positive emotions, excitement seeking), and intellect. Conscientiousness is the essential personality trait predicting health-beneficial behavior in adults[75]. With respect to the children’s height and weight, this information was mother-reported from age 4–5 years onwards, resulting in lower reliability and bias in direction of desired weight. Moreover, the data collection was not completed at 8 years, thus needed a higher proportion of imputation. This, in turn inflated the width of the confidence intervals. As a whole, sibling samples are not representative of the entire population, nor are all mothers willing to participate in time-consuming surveys.
In conclusion, our findings underline the importance of temperament in early childhood as risk factor for the timing of the adiposity rebound. Further research regarding children’s self-control and the entire range of the Big Five personality traits in relation to the adiposity rebound is necessary. Factors mediating the association between temperament and age at adiposity fact rebound may help understand the mechanism involved in the risk of obesity and can be useful to improve prevention.
Data Availability
Data are available from the Norwegian Institute of Public Health Data Access Server for researchers who meet the criteria for access to confidential data. This requires the consent of the Regional Ethical Committee of Southwest Norway, the signing of a confidentiality agreement and the consent of the Board of Directors of the Norwegian Mother and Child Cohort Study. Data will not be accessible at any Public URL. Further information is available from datatilgang@fhi.no.
Funding Statement
Grant funding supporting the Norwegian Mother and Child Cohort Study was provided by the Norwegian Ministry of Health and the Ministry of Education and Research, the National Institute of Health, NIH/NIEHS (contract no NO1-ES-75558), NIH/NINDS (grant no.1 UO1 NS 047537-01 and grant no. 2 UO1 NS047537-06A1), and the Norwegian Research Council/FUGE (grant no. 151918/S10). The funders had no role in the study design but funded the data collection. Grant funding supporting Sarah E. Hampson is from the National Institutes of Health (USA), National Institute on Aging R01020048. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Media centre, obesity and overweight, fact sheet number 311 2015. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 2.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of growth trajectories of childhood obesity into adulthood. The New England journal of medicine. 2017;377(22):2145–53. Epub 2017/11/25. 10.1056/NEJMoa1703860 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev. 2016;17(2):95–107. Epub 2015/12/24. 10.1111/obr.12334 . [DOI] [PubMed] [Google Scholar]
- 4.Rolland-Cachera MF, Deheeger M, Bellisle F, Sempe M, Guilloud-Bataille M, Patois E. Adiposity rebound in children: a simple indicator for predicting obesity. The American journal of clinical nutrition. 1984;39(1):129–35. Epub 1984/01/01. 10.1093/ajcn/39.1.129 . [DOI] [PubMed] [Google Scholar]
- 5.Rolland-Cachera MF, Cole TJ. Does the age at adiposity rebound reflect a critical period? Pediatr Obes. 2018. 10.1111/ijpo.12467 . [DOI] [PubMed] [Google Scholar]
- 6.Taylor RW, Grant AM, Goulding A, Williams SM. Early adiposity rebound: review of papers linking this to subsequent obesity in children and adults. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8(6):607–12. 10.1097/01.mco.0000168391.60884.93 WOS:000233527000004. [DOI] [PubMed] [Google Scholar]
- 7.Dulloo AG, Jacquet J, Seydoux J, Montani JP. The thrifty 'catch-up fat' phenotype: its impact on insulin sensitivity during growth trajectories to obesity and metabolic syndrome. Int J Obes 2006;30 Suppl 4:S23–35. 10.1038/sj.ijo.0803516 . [DOI] [PubMed] [Google Scholar]
- 8.Barker DJP, editor. Fetal and infant origins of adult disease London, UK: BMJ Publishing Group; 1992. [Google Scholar]
- 9.Russell CG, Russell A. Biological and Psychosocial Processes in the Development of Children's Appetitive Traits: Insights from Developmental Theory and Research. Nutrients. 2018;10(6). Epub 2018/05/31. 10.3390/nu10060692 ; PubMed Central PMCID: PMCPMC6024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes. 2006;30:11–7. 10.1038/sj.ijo.0803514 [DOI] [PubMed] [Google Scholar]
- 11.Péneau S, González-Carrascosa R, Gusto G, Goxe D, Lantieri O, Fezeu L, et al. Age at adiposity rebound: determinants and association with nutritional status and the metabolic syndrome at adulthood. Int J Obes 2016;40(7):1150–6. [DOI] [PubMed] [Google Scholar]
- 12.Rothbart MK. Becoming who we are: Temperament and personality in development. New York, NY: Guilford Press; 2011. [Google Scholar]
- 13.Thomas A, & Chess S. Temperament and development. New York, NY: Brunner/Mazel; 1977. [Google Scholar]
- 14.Sutin AR, Kerr JA, Terracciano A. Temperament and Body Weight from ages 4 to 15. Int J Obes. 2017;41(7):1056–61. 10.1038/ijo.2017.62 PMC5496782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmeier H, Skouteris H, Horwood S, Hooley M, Richardson B. Associations between child temperament, maternal feeding practices and child body mass index during the preschool years: a systematic review of the literature. Obes Rev. 2014;15(1):9–18. WOS:000328293700004. 10.1111/obr.12066 [DOI] [PubMed] [Google Scholar]
- 16.Amaro H, Flores Peña Y. Effect of infant temperament on the weight of preschool children: A systematic review. Enfermeria Global. 2017;16(2):610–23. 10.6018/eglobal.16.2.262231 [DOI] [Google Scholar]
- 17.Anzman-Frasca S, Stifter CA, Birch LL. Temperament and childhood obesity risk: a review of the literature. Journal of developmental and behavioral pediatrics: JDBP. 2012;33(9):732–45. 10.1097/DBP.0b013e31826a119f . [DOI] [PubMed] [Google Scholar]
- 18.Braet C, Claus L, Verbeken S, Van Vlierberghe L. Impulsivity in overweight children. Eur Child & Adolesc Psychiatry. 2007;.16(8):pp. 10.1007/s00787-007-0623-217876511 2008-00242–001. [DOI] [PubMed] [Google Scholar]
- 19.Vollrath ME, Hampson SE, Júlíusson PB. Children and eating. Personality and gender are associated with obesogenic food consumption and overweight in 6- to 12-year-olds. Appetite. 2012;58(3):1113–7. 10.1016/j.appet.2012.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graziano PA, Kelleher R, Calkins SD, Keane SP, Brien MO. Predicting weight outcomes in preadolescence: the role of toddlers' self-regulation skills and the temperament dimension of pleasure. International journal of obesity (2005). 2013;37(7):937–42. 10.1038/ijo.2012.165 ; PubMed Central PMCID: PMCPMC3543516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koziel S, Chakraborty R, Bose K. Relationship between temperament and fatness in 11-year-old children and 17-year-old adolescents from Wroclaw, Poland. Homo. 2017;68(6):479–86. Epub 2017/11/28. 10.1016/j.jchb.2017.10.002 . [DOI] [PubMed] [Google Scholar]
- 22.Anderson SE, Bandini LG, Dietz WH, Must A. Relationship between temperament, nonresting energy expenditure, body composition, and physical activity in girls. Int J Obes. 2004;28(2):300–6. [DOI] [PubMed] [Google Scholar]
- 23.Chi DL, Luu M, Chu F. A scoping review of epidemiologic risk factors for pediatric obesity: Implications for future childhood obesity and dental caries prevention research. J Public Health Dent. 2017;77 Suppl 1:S8–s31. Epub 2017/06/11. 10.1111/jphd.12221 . [DOI] [PubMed] [Google Scholar]
- 24.Cheng H, Furnham A. Personality traits, education, physical exercise, and childhood neurological function as independent predictors of adult obesity. PLoS ONE. 2013;8(11). 10.1371/journal.pone.0079586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lahti M, Raikkonen K, Lemola S, Lahti J, Heinonen K, Kajantie E, et al. Trajectories of physical growth and personality dimensions of the Five-Factor Model. J Pers Soc Psychol. 2013;105(1):154–69. Epub 2013/05/30. 10.1037/a0032300 . [DOI] [PubMed] [Google Scholar]
- 26.Stifter CA, Moding KJ. Infant temperament and parent use of food to soothe predict change in weight-for-length across infancy: early risk factors for childhood obesity. International journal of obesity (2005). 2018. 10.1038/s41366-018-0006-4 ; PubMed Central PMCID: PMCPMC6066452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson LM, Wong N, Lanciers S, Lim CS. The relative importance of social anxiety facets on disordered eating in pediatric obesity. Eat Weight Disord. 2018. 10.1007/s40519-018-0526-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartmann T, Zahner L, Puhse U, Schneider S, Puder JJ, Kriemler S. Physical activity, bodyweight, health and fear of negative evaluation in primary school children. Scand J Med Sci Sports. 2010;20(1):e27–34. Epub 2009/05/09. 10.1111/j.1600-0838.2009.00888.x . [DOI] [PubMed] [Google Scholar]
- 29.Liu LF, Bian QT, Zhai JG. Analysis of psychological characteristics of obese children. Eur Rev Med Pharmacol Sci. 2017;21(11):2665–70. . [PubMed] [Google Scholar]
- 30.Leung CY, Lumeng JC, Kaciroti NA, Chen YP, Rosenblum K, Miller AL. Surgency and negative affectivity, but not effortful control, are uniquely associated with obesogenic eating behaviors among low-income preschoolers. Appetite. 2014;78:139–46. Epub 2014/04/02. 10.1016/j.appet.2014.03.025 ; PubMed Central PMCID: PMCPMC4039349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoeckel LE, Birch LL, Heatherton T, Mann T, Hunter C, Czajkowski S, et al. Psychological and neural contributions to appetite self-regulation. Obesity. 2017;25:S17–S25. 10.1002/oby.21789 WOS:000394975000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung CY, Miller AL, Kaciroti NA, Chen YP, Rosenblum K, Lumeng JC. Low-income pre-schoolers with higher temperamental surgency enjoy and respond more to food, mediating the path to higher body mass index. Pediatr Obes. 2016;11(3):181–6. Epub 2015/06/18. 10.1111/ijpo.12042 ; PubMed Central PMCID: PMCPMC4683115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandeweghe L, Vervoort L, Verbeken S, Moens E, Braet C. Food approach and food Avoidance in young children: Relation with reward sensitivity and punishment sensitivity. Front Psychol [Internet]. 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: The role of impulsivity. Eating Behaviors. 2006;.7(4):pp. 10.1016/j.eatbeh.2005.11.00517056407 2006-20501-004. [DOI] [PubMed] [Google Scholar]
- 35.Pliner P, Loewen ER. Temperament and food neophobia in children and their mothers. Appetite. 1997;28(3):239–54. 10.1006/appe.1996.0078 [DOI] [PubMed] [Google Scholar]
- 36.Moding KJ, Stifter CA. Temperamental approach/withdrawal and food neophobia in early childhood: Concurrent and longitudinal associations. Appetite. 2016;107:654–62. 10.1016/j.appet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matton A, Goossens L, Vervaet M, Braet C, Id, Goossens LOhoo, et al. Effortful control as a moderator in the association between punishment and reward sensitivity and eating styles in adolescent boys and girls. Appetite. 2017:177–86. 10.1016/j.appet.2017.01.00228065593 2017-06546-022. [DOI] [PubMed] [Google Scholar]
- 38.Vollrath ME, Stene-Larsen K, Tonstad S, Rothbart MK, Hampson SE. Associations between temperament at age 1.5 years and obesogenic diet at ages 3 and 7 years. J Dev & Behav Pediatrics. 2012;33(9):721–7. 10.1097/dbp.0b013e31826bac0d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hampson SE, Vollrath ME, Júlíusson PB. Personality and overweight in 6-12-year-old children. Pediatr Obes. 2015;10(5):5–7. 10.1111/ijpo.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niegel S, Ystrom E., & Vollrath M. E. Is difficult temperament related to overweight and rapid early weight gain in infants? A prospective cohort study. J Dev Behav Pediatr. 2007;28(6):462–6. 10.1097/DBP.0b013e31811431e8 [DOI] [PubMed] [Google Scholar]
- 41.Vollrath ME, Tonstad S., Rothbart M. K., & Hampson S. E. Infant temperament is associated with potentially obesogenic diet at 18 months. Int J Pediatric Obes. 2011;6(2–2):408–14. 10.3109/17477166.2010.518240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vollrath ME, Hampson S. E., & Júlíusson P. B. Children and eating. Personality and gender are associated with obesogenic food consumption and overweight in 6- to 12-year-olds. Appetite. 2012;58(3):1113–7. 10.1016/j.appet.2012.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anzman-Frasca S, Stifter C. A., & Birch L. L. Temperament and childhood obesity risk: A review of the literature. J Dev Behav Pediatr. 2012;33(9):732–45. 10.1097/DBP.0b013e31826a119f [DOI] [PubMed] [Google Scholar]
- 44.Kong KL, Anzman-Frasca S, Feda DM, Eiden RD, Sharma NN, Stier CL, et al. Infant temperament is associated with relative food reinforcement. Child Obes. 2016;12(6):411–7. WOS:000388194200001. 10.1089/chi.2016.0001 [DOI] [PubMed] [Google Scholar]
- 45.Stifter CA, Anzman-Frasca S., Birch L. L., & Voegtline K. Parent use of food to soothe infant/toddler distress and child weight status. An exploratory study. Appetite. 2011;57(3):693–9. 10.1016/j.appet.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 46.Vainik U, Dagher A, Dube L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: A systematic review. Neuroscience and Biobehavioral Reviews. 2013;37(3):279–99. 10.1016/j.neubiorev.2012.11.008 WOS:000317548100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vollrath ME, Torgersen S, Torgersen L. Associations of children’s Big Five Personality with eating behaviors BMC Res Notes. 2018. 10.1186/s13104-018-3768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keller C, Siegrist M. Does personality influence eating styles and food choices? Direct and indirect effects. Appetite. 2015;84:128–38. 10.1016/j.appet.2014.10.003 WOS:000347267000018. [DOI] [PubMed] [Google Scholar]
- 49.Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. International journal of obesity (2005). 2014;38(4):494–506. Epub 2013/08/06. 10.1038/ijo.2013.142 ; PubMed Central PMCID: PMCPMC4456183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Jaarsveld CH, Boniface D, Llewellyn CH, Wardle J. Appetite and growth: a longitudinal sibling analysis. JAMA Pediatr. 2014;168(4):345–50. 10.1001/jamapediatrics.2013.4951 . [DOI] [PubMed] [Google Scholar]
- 51.Michaud A, Vainik U, Garcia-Garcia I, Dagher A. Overlapping neural endophenotypes in addiction and obesity. Front Endocrinol. 2017;8:15 10.3389/fendo.2017.00127 WOS:000403223300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matton A, Goossens L, Braet C, Vervaet M. Punishment and reward sensitivity: Are naturally occurring clusters in these traits related to eating and weight problems in adolescents? Eur Eat Dis Rev. 2013;21(3):pp. 10.1002/erv.222623426856 2013-12345-002. [DOI] [PubMed] [Google Scholar]
- 53.Silventoinen K, Jelenkovic A, Sund R, Yokoyama Y, Hur YM, Cozen W, et al. Differences in genetic and environmental variation in adult BMI by sex, age, time period, and region: an individual-based pooled analysis of 40 twin cohorts. The American journal of clinical nutrition. 2017;106(2):457–66. Epub 2017/07/07. 10.3945/ajcn.117.153643 ; PubMed Central PMCID: PMCPMC5525120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown HW, Roberts J. Exploring the factors contributing to sibling correlations in BMI: a study using the Panel Study of Income Dynamics. Obesity (Silver Spring). 2012;20(5):978–84. 10.1038/oby.2011.351 ; PubMed Central PMCID: PMCPMC3346928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warrington NM, Howe LD, Wu YY, Timpson NJ, Tilling K, Pennell CE, et al. Association of a body mass index genetic risk score with growth throughout childhood and adolescence. PLoS One. 2013;8(11):e79547 10.1371/journal.pone.0079547 ; PubMed Central PMCID: PMCPMC3823612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 2015;47(7):702–9. 10.1038/ng.3285 . [DOI] [PubMed] [Google Scholar]
- 57.Smith AD, Herle M, Fildes A, Cooke L, Steinsbekk S, Llewellyn CH. Food fussiness and food neophobia share a common etiology in early childhood. J Child Psychol Psychiatry. 2017;58(2):189–96. Epub 2016/10/16. 10.1111/jcpp.12647 ; PubMed Central PMCID: PMCPMC5298015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Llewellyn CH, Trzaskowski M, van Jaarsveld CH, Plomin R, Wardle J. Satiety mechanisms in genetic risk of obesity. JAMA Pediatr. 2014;168(4):338–44. 10.1001/jamapediatrics.2013.4944 ; PubMed Central PMCID: PMCPMC3981891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin A, Booth JN, Laird Y, Sproule J, Reilly JJ, Saunders DH. Physical activity, diet and other behavioural interventions for improving cognition and school achievement in children and adolescents with obesity or overweight. The Cochrane database of systematic reviews. 2018;1:Cd009728 Epub 2018/01/30. 10.1002/14651858.CD009728.pub3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemmingsson E. Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. Curr Obes Rep. 2018;7(2):204–9. Epub 2018/04/29. 10.1007/s13679-018-0310-2 ; PubMed Central PMCID: PMCPMC5958160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scaglioni S, Salvioni M, Galimberti C. Influence of parental attitudes in the development of children eating behaviour. Br J Nutr. 2008;99 Suppl 1:S22–5. Epub 2008/04/09. 10.1017/s0007114508892471 . [DOI] [PubMed] [Google Scholar]
- 62.Birch LL, Davison KK. Family environmental factors influencing the developing behavioral controls of food intake and childhood overweight. Pediatr Clin North Am. 2001;48(4):893–907. Epub 2001/08/10. . [DOI] [PubMed] [Google Scholar]
- 63.Scaglioni S, Arrizza C., Vecchi F., & Tedeschi S. Determinants of children’s eating behavior. Am J Clin Nutr. 2011;94(6):2006–11. 10.3945/ajcn.110.001685 [DOI] [PubMed] [Google Scholar]
- 64.Hopper JL, Bishop DT, Easton DF. Population-based family studies in genetic epidemiology. Lancet. 2005;366(9494):1397–406. 10.1016/S0140-6736(05)67570-8 . [DOI] [PubMed] [Google Scholar]
- 65.Kim J. Personality traits and body weight: Evidence using sibling comparisons. Soc Scien Med 2016;163:54–62. 10.1016/j.socscimed.2016.06.054 PMC4970915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norwegian Institute of Public Health. Norwegian Mother and Child Cohort Study: revised protocol: Norwegian Institute of Public Health; 2012 [cited 2016 05.2016]. Available from: http://www.fhi.no/dokumenter/6d6187a561.pdf.
- 67.Mathiesen KS, Tambs K. The EAS Temperament questionnaire—Factor structure, age trends, reliability, and stability in a Norwegian sample. J Child Psychol Psychiatry Allied Discip. 1999;40(3):431–9. ISI:000079621500013. [PubMed] [Google Scholar]
- 68.Norwegian Institute of Public Health. The Medical Birth Registry of Norway [Webpage]. Norwegian Institute of Public Health; 2017 [cited 2017 10/09]. Available from: https://www.fhi.no/en/hn/health-registries/medical-birth-registry-of-norway/medical-birth-registry-of-norway/.
- 69.Haycraft E, Farrow C, Meyer C, Powell F, Blissett J. Relationships between temperament and eating behaviours in young children. Appetite. 2011;56(3):689–92. 10.1016/j.appet.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 70.Haycraft E, Blissett J. Predictors of paternal and maternal controlling feeding practices with 2- to 5-year-old children. J Nutr Educ Behav. 2012;44(5):390–7. 10.1016/j.jneb.2010.03.001 . [DOI] [PubMed] [Google Scholar]
- 71.Hafstad GS, Abebe DS, Torgersen L, von Soest T. Picky eating in preschool children: The predictive role of the child's temperament and mother's negative affectivity. Eating Behaviors. 2013;14(3):274–7. 10.1016/j.eatbeh.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Worobey J. Physical activity in infancy: developmental aspects, measurement, and importance. Am J Clin Nutr. 2014;99(3):729s–33s. WOS:000332144000008. 10.3945/ajcn.113.072397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zelenski JM, Blouin-Hudon EM. Broad Traits Need Simple Process Explanations. European Journal of Personality. 2017;31(5):576–7. WOS:000413761600070. [Google Scholar]
- 74.Mervielde I, De Clercq B., De Fruyt F., & Van Leeuwen K. Temperament, Personality, and Developmental Psychopathology as Childhood Antecedents of Personality Disorders. J Personal Disord. 2005;19(2): 171–201. 10.1521/pedi.19.2.171.62627 [DOI] [PubMed] [Google Scholar]
- 75.Bogg T, Roberts BW. Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychol Bull. 2004;130(6):887–919. 10.1037/0033-2909.130.6.887 WOS:000224778300003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Norwegian Institute of Public Health Data Access Server for researchers who meet the criteria for access to confidential data. This requires the consent of the Regional Ethical Committee of Southwest Norway, the signing of a confidentiality agreement and the consent of the Board of Directors of the Norwegian Mother and Child Cohort Study. Data will not be accessible at any Public URL. Further information is available from datatilgang@fhi.no.