ABSTRACT
NF-κB signaling pathway shows significant influence on wear particle-induced osteolysis, and this study aims to explore the underlying mechanism and the role of let-7f-5p in this process. A mouse calvarial osteolysis model was constructed with PMMA particles, and the bone marrow-derived macrophages (BMMs) were isolated from the osteolysis area. The expression of miRNA and protein was determined by qRT-PCR and western blot, respectively. The level of cytokines was evaluated with ELISA. Recombinant plasmids were transfected into cells for the endogenous expression of related genes. Dual-luciferase reporter assay was performed to determine the interaction between let-7f-5p and IL-10 in macrophage RAW264.7 cells. M1 macrophage polarization and expression of let-7f-5p were promoted in BMMs of osteolysis mouse model, compared with that in sham group. The expression of let-7f-5p was increased in the process of M1 macrophage polarization that induced by PMMA. Let-7f-5p was involved in M1 polarization in macrophages that treated with PMMA. IL-10 was negatively regulated by let-7f-5p. NF-κB regulated the expression of IL-10 through let-7f-5p. NF-κB participated in the PMMA-induced M1 macrophage polarization through let-7f-5p. Let-7f-5p contributed to PMMA-induced osteolysis by promoting M1 polarization of macrophages. The NF-κB/let-7f-5p/IL-10 pathway induces M1 macrophage polarization, and thus contributing to wear particle-induced osteolysis.
KEYWORDS: Osteolysis, NF-κB, let-7f-5p, IL-10, M1 macrophage polarization
Introduction
Periprosthetic osteolysis is a complication caused by wear particles after hip or knee joint replacement [1,2]. Wear particle-induced osteolysis has been considered as the leading cause of prosthesis aseptic loosening, prosthesis failure and joint revision surgery, seriously affecting the functional recovery of joint [3]. Activation of signaling pathway has been proven to be implicated in osteolysis induced by wear particle, among which is the nuclear factor kappa B (NF-κB) pathway [4,5]. NF-κB is a family of regulatory proteins, and p65 and p50 are the members that highly expressed in cells [6]. It is well known that activation of NF-κB signal channel triggers the activation of osteoclast, which leads to bone resorption and subsequent osteolysis [7]. It has been recently reported that anthocyanin suppressed CoCrMo particle-induced osteolysis by inhibiting NF-κB signaling in mouse calvarial model [8]. These findings implied that NF-κB pathway played a critical role in wear particle-induced osteolysis, while the action mechanism still remains unclear.
Wear particles generated around the prosthesis can cause inflammatory response, which is dominated by pro-inflammatory macrophage phenotype (M1) rather than the anti-inflammatory macrophage phenotype (M2). Polarization from M1 macrophage into M2 phenotype has been proposed as a strategy to relieve wear particle-induced periprosthetic osteolysis [9]. It implied that polarization of macrophage played a pivotal role in osteolysis caused by wear particle. Furthermore, IL-10 is one of the anti-inflammatory cytokines and it is produced by M2 macrophages, and the effect of IL-10 in ameliorating inflammation and osteolysis may be related to macrophage polarization [10]. In bfief, the IL-10/macrophage polarization axis may contribute to the process of wear particle-induce osteolysis, and decrease of IL-10 was considered as the symbol of M1 macrophage polarization.
MicroRNA (miRNA) let-7f is a member of the let-7 family that involved in cell growth, migration, invasion and angiogenesis in tumors [11], and over-expression of let-7f is associated with induction of immune tolerance [12]. It also has been demonstrated that let-7f targeted A20, a feed-back inhibitor of the NF-κB pathway, to modulate the immune response to mycobacterium tuberculosis infection [13], suggesting the tight relationship between let-7f and NF-κB pathway. In addition, bioinformatics analysis pointed out that the highly up-regulated miRNAs, including let-7f-5p miRNA, were correlated with colon cancer pathways and associated with cytokines expression, including IL-10 [14].
It’s a breakthrough that the complementary base pairs between let-7f-5p and IL-10 were predicted with bioinformatics method, indicating the potential binding site and interplay between them. Collectively, we speculated that the cascade of let-7f-5p/IL-10/macrophage polarization/osteolysis may exist in the process of wear particle-induced osteolysis, while the role of NF-κB in the pathway needs to be confirmed. We initiated this study to explore the impact and mechanism of let-7f-5p on wear particle-induced osteolysis, which may give some clues to prevent or alleviate osteolysis after joint replacement.
Materials and methods
Animals and grouping
All the animal related experiments were approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University, and were performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Eight weeks old and healthy male BALB/c mice were obtained from the Experimental Animal Center of the Affiliated Hospital of Xuzhou Medical University. Mice were randomly divided into two groups (n = 7 in each group): sham group (sham operation) and calvarial osteolysis model group (PMMA particle treatment).
Wear particle preparation
Polymethylmethacrylate (PMMA, Polysciences) particles used in this study have been identified with a mean diameter of 0.33 μm (0.33 ± 0.019 μm) and 90% of them have a diameter less than 1μm. The particles were disinfected by torrefaction at 180°C for 6h and then washed in 70% ethanol solution twice. For decontamination from endotoxins, a Limulus Amoebocyte Lysate assay (Endosafe) was performed on PMMA particles. PMMA particles were finally suspended in sterile phosphate-buffered saline (PBS) solutions and stored at 4°C.
Mouse calvarial osteolysis model
Mice in the sham group and calvarial osteolysis model group were anesthetized with 10% chloral hydrate solution by intraperitoneal injection. An incision with the length of 1cm was made on the head of mice from the midpoint of two ears to the eyes, and the periosteum was separated from the sagittal suture of calvarium to both sides. In the calvarial osteolysis model group, the mice received 2 mg of PMMA particles (100μL) on the surfaces of the calvarial bones and the incision was sutured. Mice in the sham group were treated with equivalent volume of PBS solution without particles. After two weeks, mice were sacrificed and calvariae were taken and used for bone marrow-derived macrophages isolation.
Isolation of bone marrow-derived macrophages (BMMs)
The BMMs were isolated from mouse calvariae at the osteolysis area. Briefly, each calvaria was cleaned in 70% alcohol, and the bone marrow flushed out with supplemented α-MEM (Gibco), using a 25G needle and syringe. Bone marrow cells were placed into one well of 24 wells/plate and cultured with 1ml α-MEM medium (Gibco) supplemented with 10% FBS (Gibco), M-CSF (30ng/ml), and 1% penicillin/streptomycin (Invitrogen) at 37°C in a humidified atmosphere with 5% CO2. 5 days later, adherent cells were then collected for genes expression analysis and assay of TNF-α and IL-10 secretion.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from BMMs and macrophage RAW264.7 cells using Trizol reagent (Invitrogen) according to its specification. The cDNA Reverse Transcription Kit (Applied Biosystems) was used for reverse transcription to synthesize cDNA. Subsequently, qRT-PCR was performed with SYBR Select Master Mix (Applied Biosystems) on an ABI 7300-fast Real Time PCR system. Primers were designed and synthesized by Sangon Biotech (Shanghai, China). The relative expression was calculated with the 2 −∆∆Ct method.
Western blot analysis
The BMMs and RAW264.7 cells were treated with RIPA lysis buffer (Beyotime) and the supernatants were collected. The concentration of proteins was measured with a BCA protein assay kit (Thermo Fisher). Proteins were separated with 10% SDS-PAGE with electrophoresis system and transferred into the polyvinylidene difluoride (PVDF) membrane (Bio-Rad). The membrane was then blocked with 5% skimmed milk for 1 hour at RT, and then incubated with primary antibodies including anti-np65 antibody (Abcam, 1:3000), anti-IL-10 antibody (1:500), anti-β-actin antibody (Abcam, 1:2000) and anti-Lamin B antibody (Abcam, 1:1000) at 4℃ for overnight. The membrane was incubated with HRP-bounded antibodies for 2h at room temperature and the target proteins were visualized by ECL Plus Western Blotting Substrate (Thermo Fisher). Lamin B and β-actin were served as control.
Cell culture and transfection
Macrophages RAW264.7 purchased from America type culture collection (ATCC) were cultured in the DMEM supplemented with 10% FBS (Hyclone), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen), at 37℃ in a humidified environment with 5% CO2. Then RAW264.7 cells were seeded in a 6-well plate and transfected with plasmids including let-7f-5p mimic and let-7f-5p inhibitor by Lipofectamine 2000 (Invitrogen).
Enzyme-linked immuno sorbent assay (ELISA)
RAW264.7 cells were treated with 100ng/ml LPS (Lipopolysaccharide) for 12h and the culture supernatants were used to determine the levels of cytokines. The concentrations of TNF-α and IL-10 were measured by ELISA system kits (R&D Systems) according to its instruction.
Dual luciferase reporter assay
Dual luciferase reporter assay was conducted to examine the interaction relationship between let-7f-5p and IL-10. The WT IL-10 3’-UTR or MUT IL-10 3’-UTR were inserted into pmirGLO vector (Promega). The vectors and pre-NC or let-7f-5p mimic were co-transfected into RAW264.7 cells by using Lipofectamine2000 (Invitrogen). The luciferase activity was detected by the dual luciferase reporter assay system (Promega) according to the manufacturer’s instructions.
Statistical analysis
Quantified data were represented as mean ± standard deviation, and all statistical analyzes in this study were carried out with SPSS 22.0 software (SPSS Inc.). Differences between groups were determined by Student’s t test and a value of P < 0.05 was considered statistically significant.
Results
M1 macrophage polarization and expression of let-7f-5p were promoted in BMMs of osteolysis mouse model
To evaluate the macrophage polarization states and let-7f-5p level in osteolysis, mouse osteolysis model (PMMA, n = 7) was constructed and BMMs were isolated, and the expression of np65, let-7f-5p and IL-10 was detected. Compared with the sham group (n = 7), the concentration of TNF-α was clearly increased in BMMS of mice treated with PMMA (70.07 ± 16.80 compared with 2193.91 ± 117.93 pg/ml, p < 0.05), while the concentration of IL-10 was markedly declined (79.97 ± 5.66 compared with 29.69 ± 3.18 pg/ml, p < 0.05); the expression of inducible NO synthase (iNOS) was dramatically raised in BMMS of mice treated with PMMA (1.00 ± 0.00 compared with 8.40 ± 0.22, p < 0.05), while expression of Arginase 1 (Arg1) was obviously decreased (1.00 ± 0.00 compared with 0.33 ± 0.03, p < 0.05) (Figure 1(a)). The expression of np65 was up-regulated in PMMA group (Figure 1(b)), and expression of let-7f-5p was also up-regulated (1.00 ± 0.00 compared with 16.13 ± 0.48, p < 0.05) (Figure 1(c)), while the expression of IL-10 mRNA (1.00 ± 0.00 compared with 0.37 ± 0.02, p < 0.05) and protein was significantly down-regulated (Figure 1(d)). These results revealed that M1 macrophage polarization and expression of let-7f-5p were promoted in osteolysis induced by wear particles.
Figure 1.
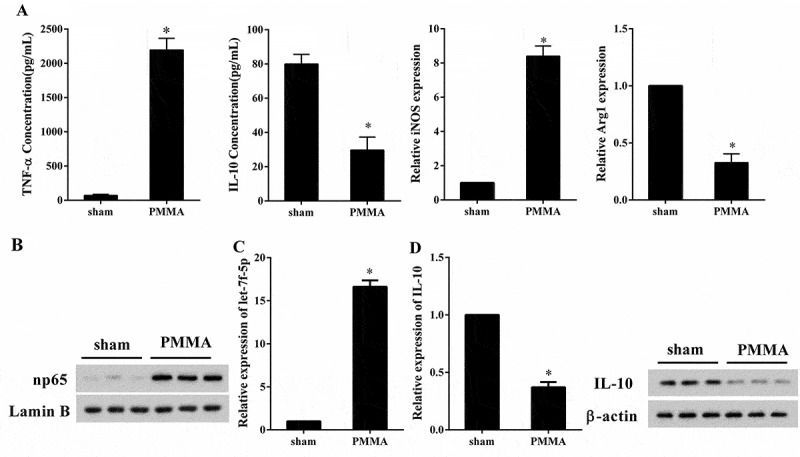
M1 macrophage polarization and expression of let-7f-5p were promoted in BMMs of osteolysis mouse model. Mouse osteolysis model (PMMA, n = 7) was constructed and BMMs were isolated, with the sham group (n = 7) served as control. (a) The concentration of TNF-α and IL-10 in BMMs was detected by ELISA, and the expression of iNOS and Arg1 in BMMs was quantified by qRT-PCR. *P < 0.05 vs. sham. (b) The expression of np65 and Lamin B protein in BMMs was analyzed by western blot. (c) The expression of let-7f-5p in BMMs was determined by qRT-PCR. *P < 0.05 vs. sham. (d) The expression of IL-10 mRNA and protein in BMMs was determined by qRT-PCR and western blot, respectively. *P < 0.05 vs. sham.
The expression of let-7f-5p was increased in the process of M1 macrophage polarization that induced by PMMA
To assess the effect of PMMA on macrophage polarization, RAW264.7 cells were assigned into control and PMMA group, and the expression of np65, let-7f-5p and IL-10 was determined. Compared with control group, the concentration of TNF-α was significantly higher in PMMA group (77.56 ± 8.08 compared with 2190.11 ± 176.81 pg/ml, p < 0.05), while the concentration of IL-10 was far lower in PMMA group (72.66 ± 4.01 compared with 26.75 ± 6.00 pg/ml, p < 0.05); the expression of iNOS was largely promoted in PMMA group (1.00 ± 0.00 compared with 8.96 ± 0.67, p < 0.05), while expression of Arg1 was notably suppressed (1.00 ± 0.00 compared with 0.33 ± 0.05, p < 0.05) (Figure 2(a)). The expression of np65 was boosted in PMMA group (Figure 2(b)), and expression of let-7f-5p was also elevated (1.00 ± 0.00 compared with 17.84 ± 1.32, p < 0.05) (Figure 2(c)), while the expression of IL-10 was reduced (1.00 ± 0.00 compared with 0.40 ± 0.02, p < 0.05) (Figure 2(d)). We demonstrated here that PMMA induced M1 macrophage polarization, meanwhile, the expression of let-7f-5p was promoted in this process.
Figure 2.
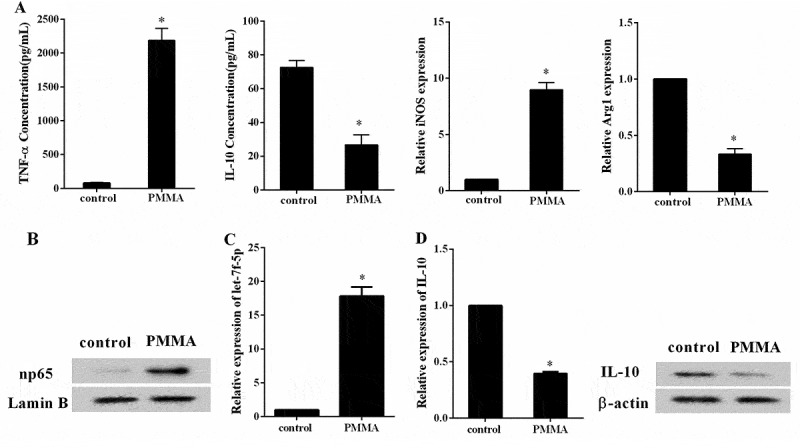
The expression of let-7f-5p was increased in the process of M1 macrophage polarization that induced by PMMA. RAW264.7 cells were assigned into control and PMMA group. (a) The concentration of TNF-α and IL-10 in RAW264.7 cells was detected by ELISA, and the expression of iNOS and Arg1 in RAW264.7 cells was quantified by qRT-PCR. *P < 0.05 vs. control. (b) The expression of np65 and Lamin B protein in RAW264.7 cells was analyzed by western blot. (c) The expression of let-7f-5p in RAW264.7 cells was determined by qRT-PCR. *P < 0.05 vs. control. (d) The expression of IL-10 mRNA and protein in RAW264.7 cells was determined by qRT-PCR and western blot, respectively. *P < 0.05 vs. control.
Let-7f-5p is involved in M1 polarization of macrophage induced by PMMA
Next, the impact of let-7f-5p on macrophage polarization was investigated. RAW264.7 cells were divided into four groups: control, PMMA, PMMA+NC (negative control of let-7f-5p inhibitor) and PMMA+let-7f-5p inhibitor. The PMMA treatment was performed after cell transfection for 48h. The concentration of TNF-α was extremely elevated by PMMA (58.55 ± 6.21 compared with 1853.85 ± 100.86 pg/ml, p < 0.05) but reversed by let-7f-5p inhibitor (1911.97 ± 21.21 compared with 471.66 ± 58.28 pg/ml, p < 0.05), while the concentration of IL-10 was greatly reduced with PMMA treatment (83.75 ± 7.47 compared with 35.88 ± 5.93 pg/ml, p < 0.05) but reversed by let-7f-5p inhibitor (38.58 ± 5.85 compared with 66.24 ± 6.34 pg/ml, p < 0.05) (Figure 3(a)). The expression of iNOS was obviously facilitated by PMMA (1.00 ± 0.00 compared with 9.75 ± 0.56, p < 0.05), while it was reversed by let-7f-5p inhibitor (9.43 ± 0.32 compared with 2.98 ± 0.44, p < 0.05); the expression of Arg1 was significantly attenuated with PMMA treatment (1.00 ± 0.00 compared with 0.26 ± 0.04, p < 0.05), which was reversed by let-7f-5p inhibitor (0.31 ± 0.04 compared with 0.83 ± 0.06, p < 0.05) (Figure 3(b)). It was illustrated that let-7f-5p further enhanced PMMA-induced M1 macrophage polarization.
Figure 3.
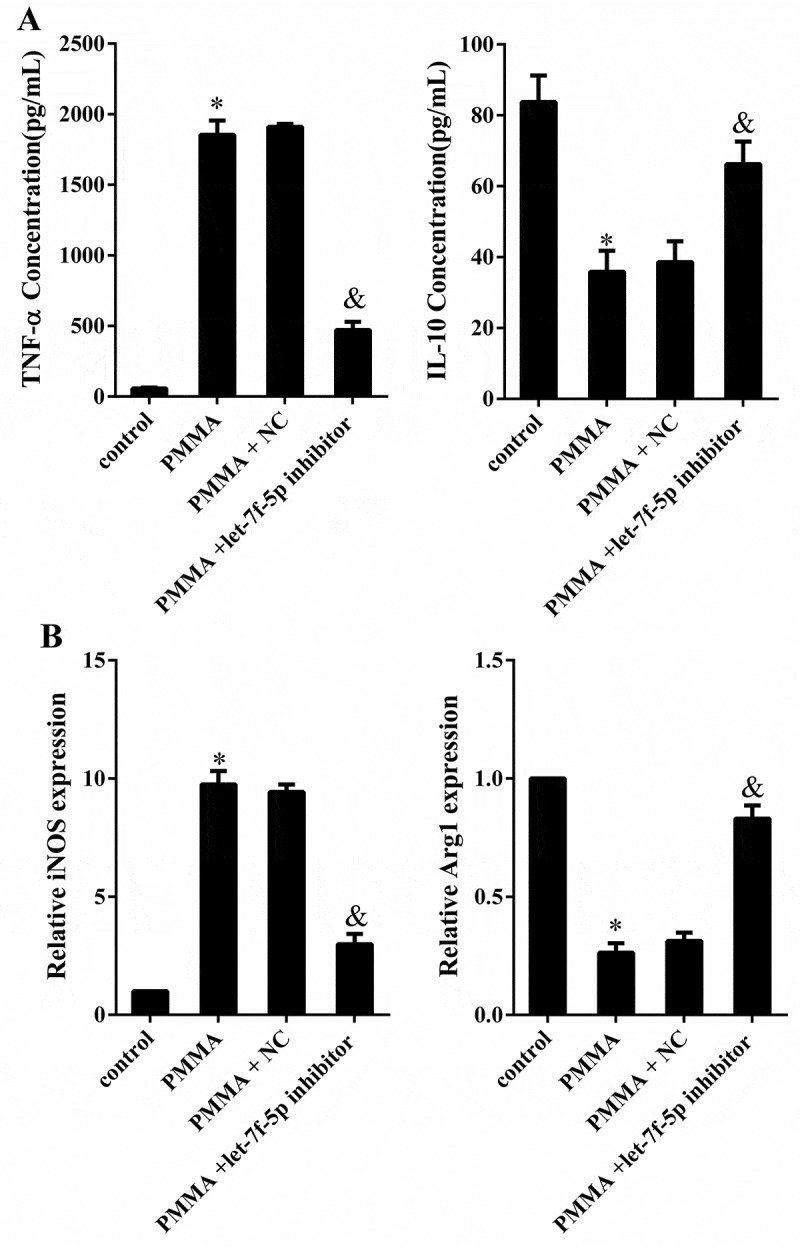
Let-7f-5p is involved in M1 polarization of macrophage induced by PMMA. RAW264.7 cells were divided into four groups: control, PMMA, PMMA+ NC (negative control of let-7f-5p inhibitor) and PMMA+ let-7f-5p inhibitor. (a) The concentration of TNF-α and IL-10 in RAW264.7 cells was detected by ELISA. *P < 0.05 vs. control. &P < 0.05 vs. PMMA+ NC. (b) The expression of iNOS and Arg1 in RAW264.7 cells was quantified by qRT-PCR. *P < 0.05 vs. control. &P < 0.05 vs. PMMA+ NC.
IL-10 was negatively regulated by let-7f-5p
Binding site between let-7f-5p and WT IL-10 3’ UTR predicted by TargetScan and microrna.org was displayed in Figure 4(a). Dual luciferase reporter assay was performed to validate the interaction between them. Compared with pre-NC (negative control of let-7f-5p mimic), over-expressed let-7f-5p inhibited the 3’UTR activity of WT IL-10 (1.01 ± 0.08 compared with 0.43 ± 0.04, p < 0.05), but not for the Mut IL-10 (0.98 ± 0.05 compared with 0.96 ± 0.06, p > 0.05); and over-expressed let-7f-5p inhibited the expression of IL-10 mRNA (1.00 ± 0.00 compared with 0.33 ± 0.02, p < 0.05) and protein (Figure 4(b)). Compared with NC (negative control of let-7f-5p inhibitor), low-expressed let-7f-5p up-regulated the 3’UTR activity of IL-10 (1.04 ± 0.03 compared with 2.13 ± 0.08, p < 0.05), but not for the Mut IL-10 (0.99 ± 0.10 compared with 1.05 ± 0.04, p > 0.05); and low-expressed let-7f-5p promoted the expression of IL-10 mRNA (1.00 ± 0.00 compared with 2.00 ± 0.09, p < 0.05) and protein (Figure 4(c)). The result indicated that let-7f-5p negatively regulated IL-10 by targeting its 3’ UTR.
Figure 4.
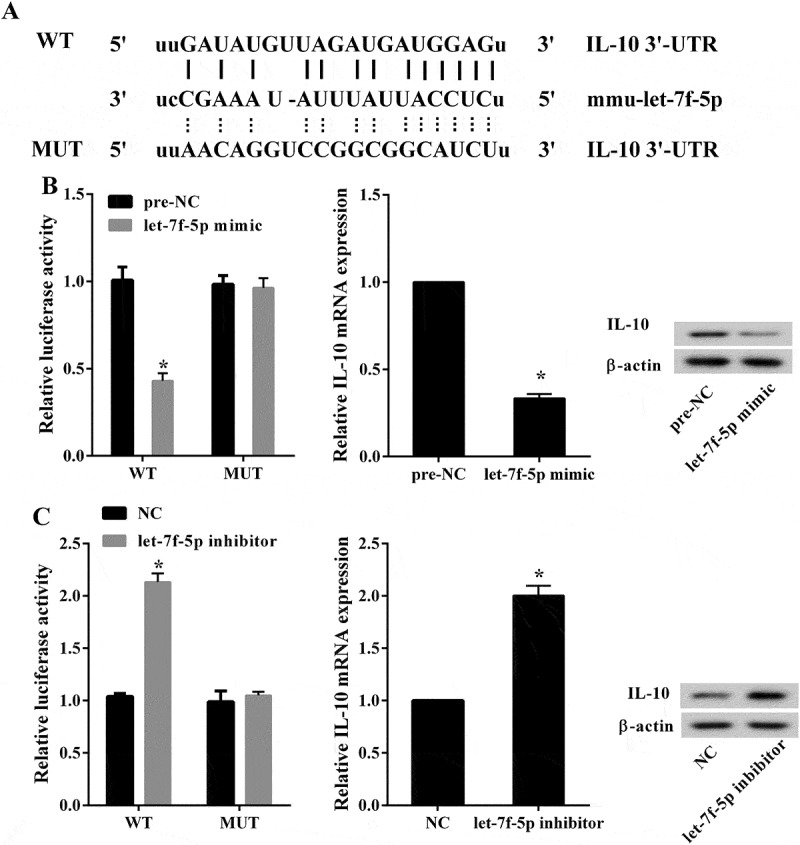
IL-10 was negatively regulated by let-7f-5p. (a) The binding site between let-7f-5p and WT IL-10 3’ UTR was predicted by TargetScan and microrna.org. (b) The luciferase activity was detected to show the interaction between let-7f-5p and IL-10. *P < 0.05 vs. pre-NC. The expression levels of IL-10 mRNA and protein in RAW264.7 cells were analyzed by qRT-PCR and western blot, respectively. *P < 0.05 vs. pre-NC. (c) The luciferase activity was detected to show the interaction between let-7f-5p and IL-10. *P < 0.05 vs. NC. The expression levels of IL-10 mRNA and protein in RAW264.7 cells were analyzed by qRT-PCR and western blot, respectively. *P < 0.05 vs. NC.
PMMA regulated the expression of IL-10 through the NF-κB/let-7f-5p pathway to affect the macrophage polarization
To elucidate the underlying mechanism of let-7f-5p influencing macrophage polarization induced by PMMA, RAW264.7 cells were assigned into five groups according to different treatment: control, PMMA, PMMA+BAY (10mM, the inhibitor of NF-κB), PMMA+BAY+pre-NC and PMMA+BAY+let-7f-5p mimic. Cells were incubated with PMMA after transfection for 48h. It was shown that the expression of IL-10 mRNA was notably down-regulated by PMMA (1.00 ± 0.00 compared with 0.41 ± 0.05, p < 0.05) but reversed by BAY (0.41 ± 0.05 compared with 0.87 ± 0.04, p < 0.05), which was further reversed by let-7f-5p mimic (0.90 ± 0.04 compared with 0.63 ± 0.05, p < 0.05) (Figure 5(a)); the expression of IL-10 protein was notably down-regulated by PMMA but reversed by BAY, which was further reversed by let-7f-5p mimic (Figure 5(b)). The concentration of TNF-α was significantly higher in PMMA group (81.51 ± 5.02 compared with 1835.63 ± 87.11 pg/ml, p < 0.05), but it was inverted by BAY treatment (1835.63 ± 87.11 compared with 720.66 ± 72.46 pg/ml, p < 0.05), which was further reversed by let-7f-5p mimic (750.84 ± 51.99 compared with 1547.94 ± 96.31 pg/ml, p < 0.05); while the concentration of IL-10 was just the opposite (66.99 ± 3.47 compared with 24.95 ± 4.96, 24.95 ± 4.96 compared with 57.44 ± 3.59, and 60.72 ± 2.81 compared with 41.33 ± 4.22 pg/ml, p < 0.05) (Figure 5(c)). In addition, the expression of iNOS was significantly promoted with PMMA treatment (1.00 ± 0.00 compared with 8.72 ± 0.30, p < 0.05), but it was reversed by BAY treatment (8.72 ± 0.30 compared with 3.99 ± 0.33, p < 0.05), which was finally reversed by let-7f-5p mimic (3.98 ± 0.16 compared with 7.72 ± 0.35, p < 0.05); while the expression of Arg1 was just the opposite (1.00 ± 0.00 compared with 0.33 ± 0.05, 0.33 ± 0.05 compared with 0.82 ± 0.04, and 0.79 ± 0.03 compared with 0.58 ± 0.06, p < 0.05) (Figure 5(d)).
Figure 5.

(Continued).
Figure 5.
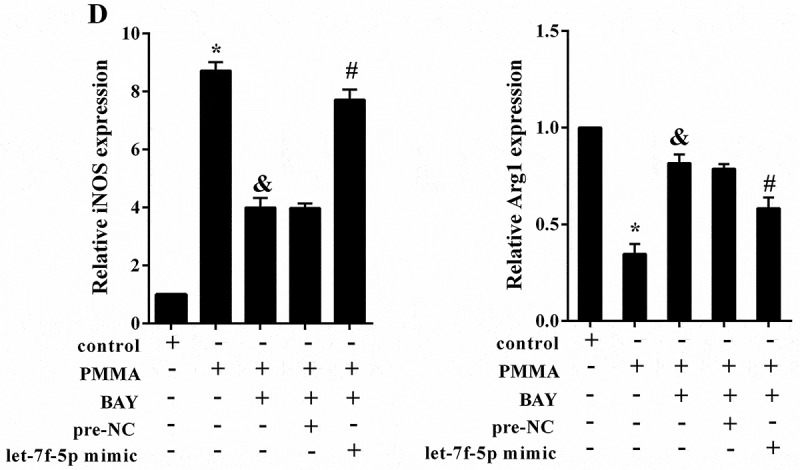
PMMA regulated the expression of IL-10 through the NF-κB/let-7f-5p pathway to affect the macrophage polarization. RAW264.7 cells were allocated into five groups according to different treatment: control, PMMA, PMMA+BAY (10mM, the inhibitor of NF-κB), PMMA+BAY+pre-NC and PMMA+BAY+let-7f-5p mimic. (a) The expression of IL-10 mRNA in RAW264.7 cells was determined by qRT-PCR. *P < 0.05 vs. control. &P < 0.05 vs. PMMA. #P < 0.05 vs. PMMA+BAY+pre-NC. (b) The expression of IL-10 protein in RAW264.7 cells was determined by western blot. (c) The concentration of TNF-α and IL-10 in RAW264.7 cells was detected by ELISA. *P < 0.05 vs. control. &P < 0.05 vs. PMMA. #P < 0.05 vs. PMMA+BAY+pre-NC. (d) The expression of iNOS and Arg1 in RAW264.7 cells was quantified by qRT-PCR. *P < 0.05 vs. control. &P < 0.05 vs. PMMA. #P < 0.05 vs. PMMA+BAY+pre-NC.
Let-7f-5p contributed to osteolysis by inducing M1 macrophage polarization
With the mouse calvarial osteolysis model established, the BMMs were isolated and divided into five groups: control+NC、PMMA+NC、PMMA+let-7f-5p inhibitor、control+let-7f-5p inhibitor. Cells were incubated with PMMA after transfection for 48h. Figure 6(a) showed that the expression of IL-10 mRNA was greatly suppressed by PMMA (1.00 ± 0.00 compared with 0.35 ± 0.06, p < 0.05) but reversed by let-7f-5p inhibitor (0.66 ± 0.05 compared with 1.03 ± 0.06, p < 0.05). The variation trend of IL-10 concentration (78.33 ± 10.50, 30.33 ± 9.50, 60.00 ± 6.00, and 76.23 ± 8.22 pg/ml) was same with the IL-10 mRNA, while the concentration of TNF-α (90.03 ± 14.53, 1825.67 ± 147.36, 540.87 ± 84.71, and 111.70 ± 15.60 pg/ml) was just the opposite (Figure 6(b)). Besides, the expression of iNOS was significantly promoted with PMMA treatment (1.00 ± 0.00 compared with 7.50 ± 0.50, p < 0.05), but it was reversed by let-7f-5p inhibitor (2.00 ± 0.60 compared with 1.13 ± 0.35, p < 0.05); while the expression of Arg1 was just on the contrary (1.00 ± 0.00 compared with 0.34 ± 0.07, and 0.76 ± 0.03 compared with 0.94 ± 0.06, p < 0.05) (Figure 6(c)).
Figure 6.
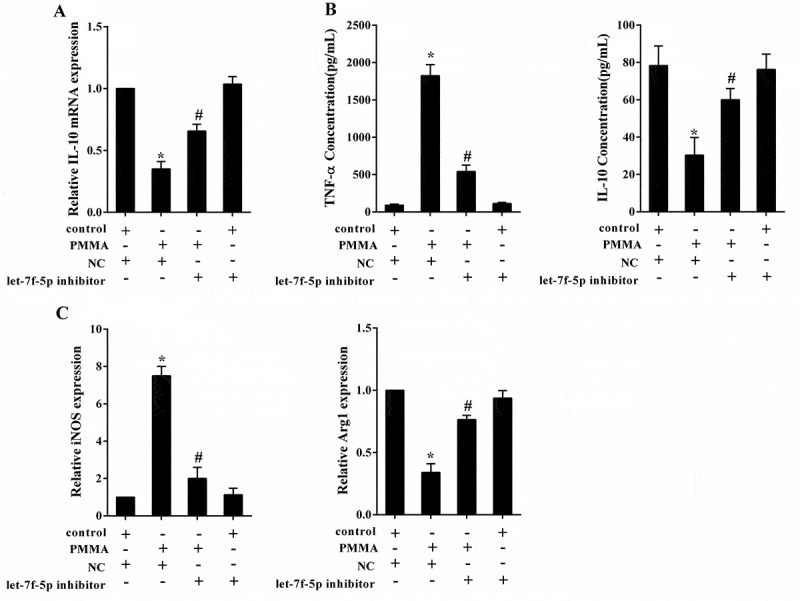
Let-7f-5p contributed to osteolysis by inducing M1 polarization of macrophage. With the mouse calvarial osteolysis model established, the BMMs were isolated and divided into four groups: control+NC、PMMA+NC、PMMA+let-7f-5p inhibitor、control+let-7f-5p inhibitor. (a) The expression of IL-10 mRNA in RAW264.7 cells was determined by qRT-PCR. *P < 0.05 vs. control+NC. &P < 0.05 vs. PMMA+control. (b) The concentration of TNF-α and IL-10 in RAW264.7 cells was detected by ELISA. *P < 0.05 vs. control+NC. &P < 0.05 vs. PMMA+ ontrol. (c) The expression of iNOS and Arg1 in RAW264.7 cells was quantified by qRT-PCR. *P < 0.05 vs. control+NC. &P < 0.05 vs. PMMA+control.
Discussion
Based on the study that anthocyanin suppressed CoCrMo particle-induced osteolysis by inhibiting NF-κB signaling in mouse calvarial model [8], we revealed the interplay between let-7f-5p and NF-κB pathway in influencing macrophage polarization and wear particle-induced osteolysis. In this study, the impact and mechanism of NF-κB, let-7f-5p, and IL-10 on affecting M1 macrophage polarization in wear particle-induced osteolysis were clarified, and it can be concluded that let-7f-5p induces macrophage M1 polarization via targeting IL-10, and thus contributing to wear particle-induced osteolysis. Th present study provided novel therapeutic targets for preventing and improving wear particle-induced osteolysis and prosthesis aseptic loosening in clinical.
With increasing joint replacement used in clinical for patients with knees or hips problems, wear particle-induced osteolysis and subsequent aseptic loosening is becoming the leading cause for prosthesis failure that demands prompt solution [15,16]. It has been reported that particulates from polymeric implants (such as PMMA) evoked an immune response mediated by macrophage/monocyte activation [17], while metallic implants both innate and adaptive (T lymphocyte-mediated) immune response [18]; both of them play a significant role in the pathogenesis of debris-associated peri-prosthetic osteolysis. In our previous study, titanium wear particles were also used to stimulate macrophages, and the pro-inflammatory reaction was also noted and was similar with that of PMMA treatment (data not shown). Moreover, it has been claimed that PMMA particle-induced inhibition of osteoprogenitor differentiation and proliferation was not due to secreted inhibitory factors [19]. In the current study, not only the underlying mechanism of PMMA particles inducing osteolysis was illuminated, but also the effect of NF-κB/let-7f-5p/IL-10 signaling pathway on M1 macrophage polarization was underlined in osteolysis after joint replacement.
In addition, our study confirmed that NF-κB pathway played a crucial role in pathological process of wear particle-induced osteolysis. NF-κB is a transcription factor family that is composed of NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, and c-Rel, and each of them has individual role in bone resorption and inflammatory osteolysis [20]. In our study, the abnormal activation of NF-κB p65 contributed in the occurrence of wear particle-induced osteolysis. Activation of NF-κB occurs in most cells upon stimulation with kinds of stimuli including cytokines, immune modulators and other stresses [21,22]. It can be inferred that activation of NF-κB in wear particle-induced osteolysis may attribute to the cytokines generated from inflammatory response around the prosthesis.
It’s a new discovery that the expression of let-7f-5p was markedly up-regulated in BMMs of osteolysis mouse model and macrophages treated with PMMA, and the role of let-7f-5p in wear particle-induced osteolysis was first clarified. Identification of the NF-κB/let-7f-5p/IL-10 cascade illuminated the targeting relationship of NF-κB to let-7f-5p, which was contrary to the previous study that let-7f targeted A20, the feed-back inhibitor of the NF-κB pathway, in immune response to mycobacterium tuberculosis infection [13]. The conflict results may be caused by different internal environment or other factors related to the both, which needs further research. Besides, IL-10 was identified as a novel target of let-7f-5p in the study, which mediated polarization of macrophage, highlighting the role of let-7f-5p and IL-10 in wear particle-induced osteolysis. IL-10 is one of the anti-inflammatory cytokines that produced by M2 macrophages, which plays a critical role in deactivating M1 macrophages and modulating macrophage phenotype [23].
Wear particle-induced osteolysis is largely influenced by the state of macrophage polarization [24]. With different stimulus, macrophages usually polarize into the pro-inflammatory M1 phenotype and anti-inflammatory M2 phenotype, and they are differed from cytokine productions [25]. Induced by LPS, M1 macrophage produces cytokines including TNF-α, IL-1, IL-6 and so on, together with high expression of iNOS [17]. M2 macrophage is characterized with release of IL-4, IL-10 and IL-3, along with expression of Arg1 [26]. Wear particles around the prosthesis can active macrophages to produce pro-inflammatory factors and cytokines, which contributes to inflammatory response and subsequent osteolysis [17]. With these indicators evaluated, our study suggested that M1 macrophage polarization was promoted in osteolysis induced by wear particle, confirming that wear particle-induced osteolysis is dominated by pro-inflammatory M1 macrophage phenotype.
Taken together, we conclude that the NF-κB/let-7f-5p/IL-10 pathway induces M1 macrophage polarization, and thus contributing to wear particle-induced osteolysis. This study explained the mechanism of wear particle-induced osteolysis mediated by let-7f-5p, and highlighted its pivotal role in regulating macrophage polarization, implying great potential of NF-κB/let-7f pathway as a therapeutic target for the treatment of periprosthetic osteolysis.
Funding Statement
This study was supported by Foundation of President of Xuzhou Medical University [No. 2011KJZ05]; Foundation of Xuzhou Science and Technology Bureau (2018); Jiangsu Planned Projects for Postdoctoral Research Funds (2018); and Foundation for the Returned Overseas Chinese Scholars of the Affiliated Hospital of Xuzhou Medical University [2017].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Howie DW, Neale SD, Haynes DR, et al. Periprosthetic osteolysis after total hip replacement: molecular pathology and clinical management. Inflammopharmacology. 2013;21(6):389–396. [DOI] [PubMed] [Google Scholar]
- [2].Dalling JG, Math K, Scuderi GR.. Evaluating the progression of osteolysis after total knee arthroplasty. J Am Acad Orthop Surg. 2015;23(3):173–180. [DOI] [PubMed] [Google Scholar]
- [3].Ries MD, Link TM. Monitoring and risk of progression of osteolysis after total hip arthroplasty. Instr Course Lect. 2013;62:207–214. [PubMed] [Google Scholar]
- [4].Ouyang Z, Zhai Z, Li H, et al. Hypericin suppresses osteoclast formation and wear particle-induced osteolysis via modulating ERK signalling pathway. Biochem Pharmacol. 2014;90(3):276–287. [DOI] [PubMed] [Google Scholar]
- [5].Liu X, Qu X, Wu C, et al The effect of enoxacin on osteoclastogenesis and reduction of titanium particle-induced osteolysis via suppression of JNK signaling pathway. Biomaterials. 2014;35(22):5721–5730. [DOI] [PubMed] [Google Scholar]
- [6].Sirotkin AV, Alexa R, Kisova G, et al. MicroRNAs control transcription factor NF-kB (p65) expression in human ovarian cells. Funct Integr Genomics. 2015;15(3):271–275. [DOI] [PubMed] [Google Scholar]
- [7].Goodman SB, Gibon E, Pajarinen J, et al Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. J R Soc Interface. 2014;11(93):20130962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li Y, Li J, Li B, et al. Anthocyanin suppresses CoCrMo particle-induced osteolysis by inhibiting IKKalpha/beta mediated NF-kappaB signaling in a mouse calvarial model. Mol Immunol. 2017;85:27–34. [DOI] [PubMed] [Google Scholar]
- [9].Antonios JK, Yao Z, Li C, et al. Macrophage polarization in response to wear particles in vitro. Cell Mol Immunol. 2013;10(6):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang J, Jia T, Gong W, et al. Macrophage polarization in IL-10 treatment of particle-induced inflammation and osteolysis. Am J Pathol. 2016;186(1):57–66. [DOI] [PubMed] [Google Scholar]
- [11].Liang S, He L, Zhao X, et al MicroRNA let-7f inhibits tumor invasion and metastasis by targeting MYH9 in human gastric cancer. PLoS One. 2011;6(4):e18409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sathe A, Patgaonkar MS, Bashir T, et al. MicroRNA let-7f: a novel regulator of innate immune response in human endocervical cells. Am J Reprod Immunol. 2014;71(2):137–153. [DOI] [PubMed] [Google Scholar]
- [13].Kumar M, Sahu SK, Kumar R, et al MicroRNA let-7 modulates the immune response to mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-kappaB pathway. Cell Host Microbe. 2015;17(3):345–356. [DOI] [PubMed] [Google Scholar]
- [14].Pathak S, Meng WJ, Nandy SK, et al. Radiation and SN38 treatments modulate the expression of microRNAs, cytokines and chemokines in colon cancer cells in a p53-directed manner. Oncotarget. 2015;6(42):44758–44780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yoon HS, Lee J, Choi WJ, et al. Periprosthetic osteolysis after total ankle arthroplasty. Foot Ankle Int. 2014;35(1):14–21. [DOI] [PubMed] [Google Scholar]
- [16].Hozack WJ, Nelson CL. Management of instability and osteolysis after total hip arthroplasty. Orthopedics. 2013;36(12):941–943. [DOI] [PubMed] [Google Scholar]
- [17].Rao AJ, Gibon E, Ma T, et al. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8(7):2815–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hallab NJ, Caicedo M, Finnegan A, et al. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J Orthop Surg Res. 2008;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chiu R, Ma T, Smith RL, et al. Kinetics of polymethylmethacrylate particle-induced inhibition of osteoprogenitor differentiation and proliferation. J Orthop Res. 2007;25(4):450–457. [DOI] [PubMed] [Google Scholar]
- [20].Abu-Amer Y. NF-kappaB signaling and bone resorption. Osteoporos Int. 2013;24(9):2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shaked H, Guma M, Karin M. Analysis of NF-kappaB activation in mouse intestinal epithelial cells. Methods Mol Biol. 2015;1280:593–606. [DOI] [PubMed] [Google Scholar]
- [22].Kaidashev IP. [NF-kB activation as a molecular basis of pathological process by metabolic syndrome]. Fiziol Zh. 2012;58(1):93–101. [PubMed] [Google Scholar]
- [23].Villalta SA, Rinaldi C, Deng B, et al. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet. 2011;20(4):790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pajarinen J, Kouri VP, Jamsen E, et al. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 2013;9(11):9229–9240. [DOI] [PubMed] [Google Scholar]
- [25].Mills CD, Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun. 2014;6(6):716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gao S, Zhou J, Liu N, et al Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol. 2015;85:131–139. [DOI] [PubMed] [Google Scholar]


