Introduction
Regular participation in physical activity (PA) has many health benefits, perhaps none more important to midlife women than combatting the decline in physical functioning (PF) accompanying aging. This article reviews the existing literature relevant to PA and PF, including definitions, measurement approaches, and descriptive and intervention studies, drawing on findings from the Study of Women’s Health Across the Nation (SWAN) and other relevant studies. Evidence supports the conclusion that the physical disablement process starts earlier than previously thought, with many limitations beginning in midlife rather than old age, when women still have many years to live. Understanding that restrictions in PF begin in the midlife for women is a strong argument for changing one’s behavior to include regular PA.
Physical Activity
Physical activity is defined as a behavior that involves human movement, resulting in physiological attributes including increased energy expenditure and improved physical fitness.1 Evidence gleaned from several decades of exercise training and public health studies has demonstrated the benefit of PA on a wide-range of health outcomes in adults.2 Yet, despite the well-established benefits, the majority of midlife women (defined as ages 45–64 years) are not sufficiently physically activity to meet 2008 Physical Activity Guidelines for Americans (henceforth: Guidelines). 2 Current aerobic Guidelines encourage adults to engage in at least 150 minutes per week of moderate intensity (activity types between 3 and 6 metabolic equivalent of task (METs), such as a brisk walk), or at least 75 minutes per week of vigorous intensity (activity types ≥6 METs, such as jogging), or an equivalent combination of moderate to vigorous intensity PA. In addition, all adults should incorporate moderate or high intensity muscle strengthening activities that involve all major muscle groups on two or more days of the week. The Guidelines also encourage adults to avoid inactivity, and directly state that some PA is better than none. Finally, Guideline targets for aerobic and muscle strengthening activities have no specific requirements for:
-
(1)
activity mode or type,
-
(2)
minimum duration per session, or
-
(3)
frequency of sessions per week.
This flexibility in the behavioral targets included in the Guidelines may particularly resonate with women who aspire to become more physically active.
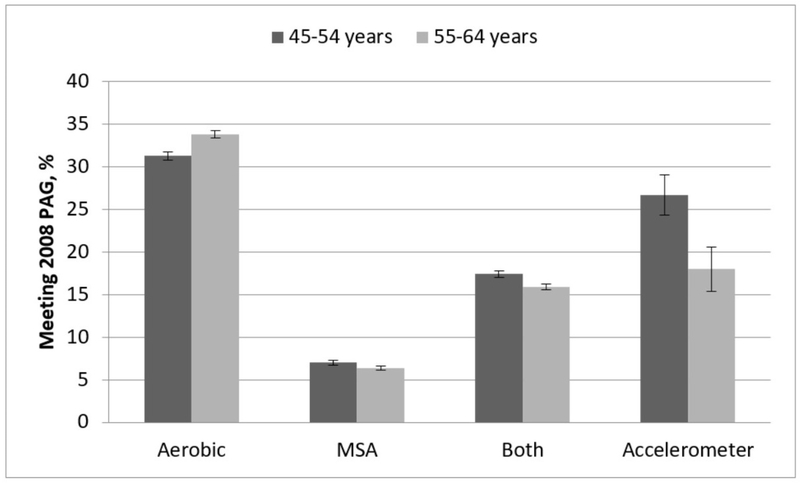
Public health surveillance data are particularly useful for monitoring trends in PA behaviors, including examining differences in prevalence estimates by various population sub-groups. This can provide important information on which groups of U.S. adults are in greatest need of targeted health promotion efforts. Based on 2015 Behavioral Risk Factor Surveillance System (BRFSS) data3, 31.1% [standard error (SE) ± 0.47] of women aged 45–54 years met aerobic Guidelines only, 7.0% (SE ± 0.25) met muscle strengthening Guidelines only, and just 17.4% (SE ± 0.37) met both aerobic and muscle strengthening Guidelines. Strikingly, 44.4% (SE ± 0.50) of women aged 45 to 54 years did not meet Guidelines for either aerobic or muscle strengthening, which is a higher proportion than similarly aged men [41.2% (SE ± 0.55)]. Among older midlife women (ages 55–64 years), the prevalence estimates for meeting Guidelines were similar. More specifically, 33.8% (SE ± 0.44), 6.4% (SE ± 0.22), and 15.9% (SE ± 0.31) met aerobic guidelines only, muscle-strengthening guidelines only, or met both guidelines, respectively. The prevalence of women aged 55–64 years that did not meet Guidelines for either the aerobic or muscle strengthening was slightly lower than women aged 45–54 years [43.8% (SE ± 0.46)]. Yet, similar to younger midlife women, this was a slightly higher proportion compared to similarly aged men [41.3% (SE ± 0.51)] (Figure 1).
Figure 1:
Prevalence of meeting 2008 Physical Activity Guidelines (PAG) for midlife American women (aged 45–54 years and 55–64 years) based on self-reported data from the 2015 Behavioral Risk Factor Surveillance System (i.e., aerobic guidelines, muscle strengthening (MSA) guidelines, and both aerobic and muscle strengthening guidelines) and accelerometer data from 2003–06 National Health and Nutrition Examination Survey (NHANES).
In the National Health and Nutrition Examination Survey (NHANES), PA levels of a U.S. representative sample were also directly measured via accelerometers.4 Because behavioral targets included in the Guidelines were established with a body of literature that primarily utilized participant reported measures, there is some controversy among PA experts about operationalizing accelerometer-derived data using the same intensitybased dose thresholds. Nonetheless, prevalence estimates for meeting Guidelines are strikingly lower with accelerometers, compared to those based upon self-report. Using the most current, publically available accelerometer data from the NHANES 2003–06, 26.7% (SE ± 2.4) and 18.0% (SE ± 2.6) of midlife women, aged 45–54 and 55–64 years, respectively, met aerobic Guidelines (Figure 1).
Yet, data from surveillance systems only provide a snapshot view or point prevalence estimates reflecting meeting or not meeting Guidelines. Further, estimates generalize the entire sub-population, with no acknowledgement that there may be different levels of PA within a defined population, and individual-level change in PA with trigger points that correspond to certain life events that characterize various adult life-course transitions. In women, major life events that coincide with midlife that could potentially increase PA levels including change in employment status or family structure. 5,6 Yet, these events may not always lead to increased PA. A woman may opt to engage in more sedentary pursuits to fill the added discretionary hours of the day due to retirement or may have reduced PA due to changes in health status. However, for some, a recent diagnosis may serve as a prompt for behavioral modification (e.g., joining a fitness club) to manage symptoms or progression of disease or disability. Finally, other life events that are not necessarily rooted in midlife may also result in positive or negative changes to PA behaviors. These include changes in relationship status or changes in residence.
The availability of longitudinal data from cohort studies provides the necessary evidence to characterize patterns of PA change during a period of life, rather than during a single point in time. While the availability of prospective cohort studies focused on midlife women’s health is limited, the Australian Longitudinal Study on Women’s Health (ALSWH) and the U.S.-based SWAN are two examples of studies that are poised to make significant contributions to improve our understanding of the patterns of PA change across the life-course.
The ALSWH is an ongoing longitudinal survey of over 58,000 women recruited in 1996 and enrolled as part of three age cohorts, including: 18–23, 45–50, or 70–75 years, using a mail-based survey once every three years to assess physical and mental health.7 In a 2009 study by Brown et al 8, the associations between life events and PA change were examined. Based on reported walking and moderate- and vigorous-intensity PA during leisure (or discretionary) time, 33.4% of the midlife cohort were classified as “active”, 15.6% as “low active”, and 7.0% as “none”, consistently over 3 years of follow-up, while 17.6% moved into a lower PA category (decreasing) and 26.5% moved into a higher category (increasing). Life events associated with a higher odds of “increasing” PA in midlife women, after adjustment for area of residence and potential confounders, included retirement, changing conditions at work, major personal achievement, death of a spouse or partner, and decreased income (all p<0.05). Yet, birth of a grandchild had a lower odds of membership in the “increasing” PA group. Conversely, after full adjustment, life events associated with a higher odds of “decreasing” PA in midlife women (17.6%) included: being pushed, grabbed or shoved and having a family member being arrested or jailed (both p<0.05). Women reporting infidelity of their spouse/partner or a major personal achievement had a lower odds of being classified in the “decreasing” PA group (both p<0.05).
Established at a similar time as the ALSWH, the SWAN study included 3,302 midlife women aged 42 to 52 years who were recruited and enrolled between 1996–97 into one of seven clinical sites across the U.S.9 The primary objective of SWAN is to evaluate the impact of reproductive aging on the health outcomes of women during midlife. In SWAN, reported PA data (i.e., moderate- and vigorous-intensity activity during leisure-time) were collected from baseline through the most recent visit in 2015/16. Using latent class growth modeling10 to examine patterns of PA change, five major patterns of PA emerged from SWAN data including: maintain low (26.2%), increasing (13.4%), decreasing (22.4%), maintain middle or moderate (23.9%), and maintain high (14.1%) PA. 11 Hispanic and black women were more likely to be in the maintain low PA group, and white women were least likely. Other characteristics associated with the maintain low PA group include income <$35,000, single/never married, fair/poor overall health status, obesity, current cigarette smoker, severe/very severe bodily pain, reported physical difficulties and osteoarthritis (all p<0.01).
Adherence to PA guidelines in midlife
Since midlife women are not adhering to recommended PA guidelines, there is room for improvement when it comes to health promotion messaging. One strategy is to link more immediate health benefits when one establishes a new PA routine, including favorable relationships to body composition, sleep, pain, and physical functioning. More distal outcomes like chronic disease reduction and changing the trajectory of physical and cognitive functioning decline may also be more relevant when viewed through the lens of midlife but may be perceived as an insufficient or delayed consequence given midlife women’s lack of discretionary hours.
Vasomotor symptoms and PA
While PA is recommended to address vasomotor symptoms of menopause, the impact on menopausal symptoms is not conclusive. A review by Pettee Gabriel et al 12 presents the evidence on the relationship between PA and vasomotor symptoms of menopause, primarily hot flashes and night sweats. While evidence from several cross sectional studies reported a significant association between PA (measured by self-report and accelerometry) and self-reported vasomotor symptoms, more recent prospective cohort studies showed variable results. 13–15 Randomized controlled trials, including MsFlash, show no association between PA, including both yoga and aerobic exercise, and vasomotor symptoms. 16,17 Daley et al18 in the 2014 Cochrane Report conclude there was insufficient evidence to demonstrate that PA is effective in managing vasomotor symptoms. The review noted variability among the observational studies with smaller studies reporting negative or no associations and larger studies reporting positive associations.
Sleep and PA
Given the prevalence of sleep disturbance in midlife19, informing menopausal women about the conclusive evidence linking PA to improved sleep quality may be an effective behavior change message. The Dose-response to Exercise in postmenopausal Women (DREW) Study found significant improvements in sleep quality in women randomized to all three exercise groups compared to the control group with a dose-response effect.20 In the MsFlash trial, exercise group participants had greater subjective sleep quality and reduced insomnia but differences were small and no longer statistically significant in adjusted analyses.17
Body composition and PA
More positively, multiple studies support an inverse association between PA and weight gain. In the Biobehavioral Health in Diverse Midlife Women Study, women in the increase in PA group had significantly less weight and waist circumference gain than those in the decrease PA group.21 An increase in leisure time PA including walking and cycling over six years was inversely associated with weight gain in the Nurses’ Health Study II.22 In the Women’s Health Initiative, in women aged 50–59, the moderate PA group had significant weight loss compared to the sedentary group while women in the 70–79 age group with higher PA showed attenuation of the expected age-related weight loss from sarcopenia and loss of lean body mass.23
Mental health and PA
Additionally, PA has an inverse relationship with adverse psychological symptoms including depression and anxiety. The Gabriel et al review noted that a significant positive impact of PA on psychological symptoms was consistently observed across most studies.12 In SWAN, participants meeting guidelines for moderate intensity exercise had lower odds of clinically significant depressive symptoms, and the finding persisted over ten years.24 Karacan et al reported positive benefits with reduced depressive mood, irritability, anxiety and exhaustion after 3 and 6 months of an intervention that included an aerobic exercise program.25 In a randomized controlled intervention of Finnish women, psychological symptoms were collected twice daily using a mobile phone.26 The prevalence of mood swings, but not irritability or depressive moods, was reduced pre- to post-intervention.
PA and PF
A key message to midlife women is the role of regular PA on PF. In the longitudinal SWAN analyses of PA trajectories, the participants that maintained high or moderate PA demonstrated significantly better PF than those who maintained low PA.11 In a previous longitudinal SWAN analysis, higher baseline PA was independently associated with a 7% higher likelihood of high SF-36 physical role functioning and 10% greater likelihood of low SF-36 bodily bodily pain scores.27 The association between PA and physical role functioning was mediated by level of pain. Women may be motivated by pain to seek medical care, providing a teachable moment to talk about the association between PA and PF.
Physical Functioning
Physical functioning is assessed in a variety of ways, including self-reported and objective, performance-based measures, though the common goal is to measure the degree that one is able to complete tasks related to independent living or tasks that impact quality of life.28 Self-reported measures of PF are an assessment of an individual’s perception of limitations in functioning given the context of their own environment.29 Objective measures of PF, such as grip strength or gait speed measures, are less vulnerable to the impact of individual-level factors.. As such, self-reported and performance-based assessments of PF measure distinct, yet related domains. 30,31
Physical functioning has long been studied in geriatric populations, with functional limitations being highly predictive of future poor outcomes, including disability, hospitalizations, nursing home admission and mortality.32,33 Although impairments in PF are most prevalent at older ages, the midlife period is an important life stage with respect to the onset of poor functioning, though risk factors for impairments can accumulate across the lifespan. Findings from an analysis conducted from 2000–2008 using data from five national U.S. surveys including NHANES, the National Health Interview Survey (NHIS), the Health and Retirement Study, the Medicare Current Beneficiary Survey, and the National Long Term Care Survey, suggest that the prevalence of limitations in activities of daily living (ADLs) is quite stable among older adults, and even slightly decreasing among those age 85+ years, but that the burden is increasing for midlife adults.34 In NHIS, the number of 50–64 year old individuals reporting difficulty with mobility tasks increased by approximately 10% from the 1997–99 data collection cycle versus the 2005–07 cycle.35 Similarly, among 40–64 year olds in NHIS, the odds of reporting PF limitations or limitations in ADLs increased by 0.9% annually and the increases were independent of increases in obesity.36
Those who have impairments at midlife have a high likelihood of developing further impairments or death within ten years; however, regaining function and maintaining independence is also common.37 SWAN has been instrumental in illustrating the prevalence of impairments during this life stage, and importantly describing the development of functional limitations during the menopause transition. At age 40–55, 81% of women in the SWAN cross-sectional study (N=14,427) reported having no PF limitations, however, 10% reported some limitations, and an additional 9% reported substantial limitations.38 These estimates were in line with those in the NHIS from a similar timeframe (midlife during the mid-1990s) which indicated that 15% of persons aged 45–64 reported having some functional limitations39 and from a British cohort of adults age 43–53 years in which 28% of participants had difficulty walking or stair climbing.40 By the fourth SWAN visit when the cohort was between the ages of 45–57 years, 11% reported substantial limitations and approximately 30% reported moderate limitations.41 At age 56–66 (SWAN visit 12), nearly 50% of women reported having at least some limitations.42 The high prevalence of self-reported limitations during midlife was substantiated by findings from performance-based PF obtained from the Southeast Michigan cohort. Thirty-one-percent of women from this cohort (includes 366 women from the Michigan SWAN site and 514 women the Michigan Bone Health and Metabolism Study, which followed the same protocol, mean age 47 years) walked at gait speeds below federal standards set for safely crossing pedestrian intersections (<1.22 m/sec), and an additional 12% walked at speeds indicative of frailty in older adults (<1 m/sec).43
In the U.S. women have a longer life expectancy compared to men, but often live longer with disability.44 Women have poorer levels of PF compared to men45,46 and experience a more rapid decline in functioning.47,48 While the prevalence of PF limitations are positively correlated with age in both men and women, the emergence of declines in functioning often coincide with the timing of the menopausal transition.49 While poorer PF among peri- as compared to premenopausal women can be attributed to health conditions and menopause-related symptoms50, the menopause-associated differences among late peri- and postmenopausal women are largely independent of age and other health factors.41,49 In SWAN, postmenopausal women and those with surgical menopause were three times more likely to report severe limitations in PF as compared to premenopausal women.51 Postmenopausal and surgically menopausal women also have poorer performance-based PF and more rapid declines in functioning as compared to pre- or perimenopausal women.51 Thus, reproductive aging and the associated hormonal and metabolic changes characteristic of the menopausal transition may play a role in this process, independent of chronological aging.
Reproductive aging and PF
Work from SWAN demonstrated that greater reductions in estradiol and testosterone during this time were significantly associated with greater risk of PF limitations.52 Further, loss of muscle strength during the menopause 48,53–55 has been associated with the physiologic declines in estradiol.47,55 In some studies, muscle strength has improved following exogenous hormone therapy.47,56,57 In SWAN, women with poorer PF were more less likely to initiate use of hormone therapy and the initiation of hormone therapy was associated with poorer subsequent PF.58
Body composition changes during midlife and PF
While overall weight gain is primarily a product of chronological aging, changes in body composition, or the relative proportion of fat mass to lean mass, is a function of both chronological and reproductive aging.59 Marked changes in body composition occur during the menopausal transition60 and greater fat mass and less skeletal muscle mass is associated with poorer performance-based PF measures including gait speed and stair climb.43 Further, in longitudinal analyses, loss of lean mass across the midlife is predictive of slower gait speed and less leg strength.61
Inflammation changes during midlife and PF
Women experience an exacerbated inflammatory response and alterations in homeostasis due to decreased levels of estrogen and the redistribution of adipose tissue that occurs during the menopausal transition.62–64 In a SWAN analysis of black and white women, higher concentrations of C-reactive protein (CRP) and fibrinogen were associated with poorer self-reported PF.65 In longitudinal analyses, higher concentrations of the pro-inflammatory biomarker CRP and hemostatic markers plasminogen activator inhibitor-1, tissue plasminogen activator-antigen, and Factor VIIc predicted poorer PF.66
One potential mechanism linking changes in body composition and inflammatory changes with PF may be the endocrine activity of adipose tissue through the secretion of adipokines, including leptin and adiponectin. Concentrations of the pro-inflammatory adipokines leptin and adiponectin are positively correlated with insulin resistance, lipid accumulation and decreased oxidation of fatty acids in skeletal muscle.67,68 In a longitudinal analysis of black and white SWAN women, higher baseline concentrations of leptin predicted poorer performance-based measures of PF mobility including stair climb, sit-to-rise, 2-pound lift and forward reach but leptin was not associated with strength measures69, findings that persisted after adjustment for CRP. In the same analysis, baseline concentrations of adiponectin predicted quadriceps muscle strength but was not associated with any mobility-based measures of PF.
Midlife mental and physical health and PF
Approximately one-quarter of midlife women experience depressive symptoms70,71. In analyses of black and white women, higher scores (i.e., more depressive symptoms) on the Center for Epidemiologic Studies-Depression scale (CES-D) was significantly associated with poorer performance-based PF including sit-to-stand times and timed walk.72 Further, women who had incident depressive symptoms during follow-up experienced subsequent declines in performance-based PF including 2-pound lift and stair climb times.
In terms of physical health conditions, a high prevalence of both peripheral nerve impairment and knee osteoarthritis has been reported among midlife women. Among black and white SWAN women from the Michigan site, the prevalence of peripheral nerve impairment was 14–19%.73 In that same sample, the prevalence of moderate to severe radiographic knee osteoarthritis was 28%.74 Women with peripheral nerve impairment had poorer PF performance including stair climb and sit-to-stand.75 Further, age-related declines in stair climb time were twice as rapid for women with as compared to women without peripheral nerve impairment. Similarly, the presence of moderate to severe knee osteoarthritis was associated with 20–35% poorer performance in PF including the timed walk and stair climb times.76 Associations between mental and physical health measures and PF are likely bidirectional, as maintenance of PA and ability to continue functioning may be protective to long-term health outcomes.
Midlife sociodemographic factors and PF
In addition to an understanding of psychological and physiologic predictors of poor PF, examination of sociodemographic and lifestyle correlates of functioning impairments is needed to help us identify appropriate interventions to support healthy aging and prevent functioning impairments. One of the most notable sociodemographic measures associated with differences in PF is race/ethnicity. In SWAN, black women were more likely to report substantial PF limitations in cross-sectional analyses38 whereas Chinese and Japanese women had the greatest declines in PF over time.42
Modifiable lifestyle factors have shown to be predictive of better midlife PF. In SWAN, better diet quality was associated with less PF limitations.77 Greater intake of cholesterol, fat or saturated fat was associated with 40–60% greater odds of having substantial PF limitations and lower fruit, vegetable and fiber intakes were inversely associated with self-reported PF. In SWAN, a healthy lifestyle score based upon selfreported measures of smoking, PA and diet was predictive of better PF including faster timed walk and shorter chair stand times.78 When considering each measure individually, the association between healthy lifestyle and PF was largely driven by PA.
Measures of PF are important as markers of healthy aging, and are valuable to our understanding of future poor health outcomes. Poorer PF is associated with unfavorable cardiometabolic biomarkers79,80 and worse vascular health indicators.81 In older women, poor PF may be prognostic for cardiovascular disease.82 In SWAN, women with poorer self-reported PF were more likely to experience incident metabolic syndrome in the next 10 years.83
Summary
Functional limitations become more prevalent with age, but PF is a dynamic aspect of health, and recovery from limitations is possible.37 PA interventions have been successful for preventing major mobility disability in older adults84, and may be promising for maintaining function at younger ages. Arguably, interventions are needed to help women maintain functional status through midlife and into older ages. The rich longitudinal information in SWAN will continue to allow researchers to further investigate predictors at midlife that influence health well into old age. In addition, as the complete cohort reaches post-menopausal status, researchers can better define the role of reproductive aging on PA and PF.
Key Points:
Midlife women do not meet guidelines for physical activity participation for a variety of reasons, missing out on the proven physical and mental health benefits.
Evidence suggests that the disablement process begins in midlife when women have multiple decades to live.
Recent studies link physical activity to reduced declines in physical functioning in midlife women.
Providing messages about the benefits of moving may shift the needle on participation, changing the trajectory not only for disablement but chronic disease.
Future work should expand on exploring the role of reproductive aging beyond solely chronological aging in midlife.
Synopsis.
Evidence supports that the physical disablement process starts earlier than previously thought, in midlife when women still have many years to live. Physical activity participation and interventions have been successful in preventing disability in older adults and may be promising for maintaining function at younger ages. Changing the conversation to more relevant topics in midlife, like positive changes in body composition, sleep and improved mood, may move the dial on participation, as midlife women do not meet guidelines for physical activity. Exploring the role of reproductive aging beyond chronological aging may provide gender-specific insights on both disablement and participation.
Acknowledgements
Funding: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
The authors would also like to thank Allen M. Hallet, MS for computing the physical activity BRFSS (based on self-report) prevalence estimates and Eun Me Cha, MPH for computing the physical activity NHANES (based on accelerometry) prevalence estimates.
Footnotes
Disclosure Statement: The authors have no commercial or financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pettee Gabriel KK, Morrow JR Jr, Woolsey AL. Framework for physical activity as a complex and multidimensional behavior. J Phys Act Health 2012;Suppl 1:S11–18. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services. 2008. Physical Activity Guidelines for Americans. www.health.gov/paguidelines. Accessed November 10, 2017.
- 3.CDC 2015. Behavioral Risk Factor Surveillance System. https://www.cdc.gov/brfss/annual_data/annual_data.html Accessed November 10, 2017.
- 4.CDC National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.html. Accessed November 10, 2017.
- 5.Allender S, Hutchinson L, Foster C. Life-change events and participation in physical activity: a systematic review. Health Promot Int. 2008;23(2):160–172. [DOI] [PubMed] [Google Scholar]
- 6.Corder K, Ogilvie D, van Sluijs EM. Invited commentary: Physical activity over the life course--whose behavior changes, when, and why? Am J Epidemiol. 2009;170(9):1078–1081; discussion 1082–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Australian Longitudinal Study on Women’s Health. http://ALSWH.org/
- 8.Brown WJ, Heesch KC, Miller YD. Life events and changing physical activity patterns in women at different life stages. Ann Behav Med. 2009;37(3):294–305. [DOI] [PubMed] [Google Scholar]
- 9.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition In: Lobo R, Marcus R, Kelsey J, editors. Menopause: biology and pathobiology. San Diego: Academic Press; 2000. p. 175–88. [Google Scholar]
- 10.Andruff H, Carraro N, Thompson A, et al. Latent class growth modelling: A tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5(1):11–24. [Google Scholar]
- 11.Pettee Gabriel K, Sternfeld B, Colvin A, et al. Physical activity trajectories during midlife and subsequent risk of physical functioning decline in late mid-life: The Study of Women’s Health Across the Nation (SWAN). Prev Med. 2017;105:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettee Gabriel K, Mason JM, Sternfeld B. Recent evidence exploring the association between physical activity and menopausal symptoms in midlife women: perceived risks and possible health benefits. Women’s Midlife Health. 2015;1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson C, Matthews K, Thurston R. Daily physical activity and hot flashes in the Study of Women’s Health Across the Nation (SWAN) Flashes Study. Fertil Steril. 2014;101(4):1110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gjelsvik B, Rosvold EO, Straand J, et al. Symptom prevalence during menopause and factors associated with symptoms and menopausal age. Results from the Norwegian Hordaland Women’s Cohort study. Maturitas. 2011;70(4):383–90. [DOI] [PubMed] [Google Scholar]
- 15.de Azevedo Guimaraes AC, Baptista F. Influence of habitual physical activity on the symptoms of climacterium/menopause and the quality of life of middle-aged women. Int J Womens Health. 2011;3:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton KM, Reed SD, Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21(4):339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sternfeld B, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause. 2014;21(4):330–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142(12 Pt 1):1003–13. [PubMed] [Google Scholar]
- 20.Kline CE, Sui X, Hall MH, et al. Dose–response effects of exercise training on the subjective sleep quality of postmenopausal women: exploratory analyses of a randomised controlled trial. BMJ Open. 2012;2(4):e001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J, Guiterrez Y, Gilliss C, et al. Physical activity, weight, and waist circumference in midlife women. Health Care Women Int. 2012;33(12):1086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lusk AC, Mekary RA, Feskanich D, et al. Bicycle riding, walking, and weight gain in premenopausal women. Arch Intern Med. 2010;170(12):1050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sims ST, Larson JC, Lamonte MJ, et al. Physical activity and body mass: changes in younger versus older postmenopausal women. Med Sci Sports Exerc. 2012;44(1):89–97. [DOI] [PubMed] [Google Scholar]
- 24.Dugan SA, Bromberger JT, Segawa E, et al. Association between physical activity and depressive symptoms: midlife women in SWAN. Med Sci Sports Exerc. 2015;47(2):335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karacan S Effects of a long-term aerobic exercise on physical fitness and postmenopausal symptoms with menopausal rating scale. Sci Sports. 2010;25(1):39–46. [Google Scholar]
- 26.Moilanen JM, Mikkola TS, Raitanen JA, et al. Effect of aerobic training on menopausal symptoms--a randomized controlled trial. Menopause. 2012;19(6):691–6. [DOI] [PubMed] [Google Scholar]
- 27.Dugan SA, Everson-Rose SA, Karavolos K, et al. The impact of physical activity level on SF-36 role-physical and bodily pain indices in midlife women. J Phys Act Health. 2009;6:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Painter P, Stewart AL, Caery S. Physical Functioning: Definitions, Measurement and Expectations. Adv Ren Replace Ther. 1999;6(2):110–23. [DOI] [PubMed] [Google Scholar]
- 29.Tomey KM, Sowers MR. Assessment of Physical Functioning: A Conceptual Model Encompassing Environmental Factors and Individual Compensation Strategies. Phys Ther. 2009;89(7):705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittink H, Rogers W, Sukiennik A,et al. Physical functioning: self-report and performance measures are related but distinct. Spine. 2003;15;28(20):2407–13. [DOI] [PubMed] [Google Scholar]
- 31.Bean JF, Olveczky DD, Kiely DK, et al. Performance-based versus patientreported physical function: what are the underlying predictors? Phys Ther. 2011;91(12):1804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J Gerontol. 1994;49(2):M85. [DOI] [PubMed] [Google Scholar]
- 33.Pennix BW, Ferrucci L, Leveille SG, et al. Lower Extremity Performance in Nondisabled Older Persons as a Predictor of Subsequent Hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55(11):M691–7. [DOI] [PubMed] [Google Scholar]
- 34.Freedman VA, Spillman BC, Andreski PM, et al. Trends in late life activity limitations in the United States: an update from five national surveys. Demography. 2013. April;50(2):661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin LG, Freedman VA, Schoeni RF et al. Trends in disability and related chronic conditions among people ages fifty to sixty four. Health Aff (Millwood). 2010. 29:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin LG, Schoeni RF. Trends in disability and related chronic conditions among the forty-and-over population: 1997–2010. Disabil Health J 2014;7(1 Suppl):S4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown RT, Diaz-Ramirez LG, Boscardin WJ, et al. Functional impairment and decline in middle age: A cohort study. Ann Intern Med. 2017;167(11):761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowers M, Pope S, Welch G, et al. The Association of Menopause and physical Functioning in Women at Midlife. J Am Geriatr Soc. 2001;49(11):1485–92. [DOI] [PubMed] [Google Scholar]
- 39.Adams PF, Marano MA. Current estimates from the National Health Interview Survey, 1994. Vital Health Stat 10 1995;(193 Pt 1):1–260. [PubMed] [Google Scholar]
- 40.Murray ET, Hardy R, Strand BH, et al. Gender and life course occupational social class differences in trajectories of functional and limitations in midlife: findings from the 1946 British birth cohort. J Gerontol A Biol Sci Med Sci. 2011;66(12):1350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng LA, El Khoudary SR, Young EA, et al. The association of menopause status with physical function: the Study of Women’s Health Across the Nation. Menopause.2012;19(11):1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ylitalo KR, Karvonen-Gutierrez CA, Fitzgerald N, et al. Relationship of race-ethnicity, body mass index, and economic strain with longitudinal self-report of physical functioning: the Study of Women’s Health Across the Nation. Ann Epidemiol. 2013;23(7):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sowers M, Jannausch ML, Gross M, et al. Performance-based physical functioning in African-American and Caucasian women at midlife: Considering body composition, quadriceps strength, and knee osteoarthritis. Am J Epidemiol. 2006;163(10):950–8. [DOI] [PubMed] [Google Scholar]
- 44.Freedman VA, Wolf DA, Spillman BC. Disability-Free Life Expectancy Over 30 Years: A Growing Female Disadvantage in the US Population. Am J Public Health. 2016;106(6):1079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danneskiold-Samsoe B, Bartels EM, Bülow PM, et al. Isokinetic and isometric muscle strength in a healthy population with special reference to age and gender. Acta Physiol (Oxf). 2009;197 Suppl 673:1–68. [DOI] [PubMed] [Google Scholar]
- 46.Kuh D, Bassey EJ, Butterworth S, et al. Musculoskeletal Study Team. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol A Biol Sci Med Sci. 2005;60(2):224–31. [DOI] [PubMed] [Google Scholar]
- 47.Phillips SK, Rook KM, Siddle NC, et al. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond). 1993;84(1):95–8. [DOI] [PubMed] [Google Scholar]
- 48.Samson MM, Meeuwsen IB, Crowe A, et al. Relationships between physical performance measures, age, height, and body weight in healthy adults. Age and Ageing 2000; 29: 235–42. [DOI] [PubMed] [Google Scholar]
- 49.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multi-ethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16(5):860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avis NE, Ory M, Matthews KA, et al. Health-related quality of life in a multiethnic sample of middle aged women: Study of Women’s Health Across the Nation (SWAN). Med Care 2003;41:1262–76. [DOI] [PubMed] [Google Scholar]
- 51.Sowers M, Tomey K, Jannausch M, et al. Physical functioning and menopause states. Obstet Gynecol. 2007;110(6):1290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Khoudary SR, McClure CK, VoPham T, et al. Longitudinal assessment of the menopausal transition, endogenous estradiol, and perception of physical functioning: The Study of Women’s Health Across the Nation. J Gerontol A Biol Sci Med Sci. 2014;69(8):1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carville SF, Rutherford OM, Newham DJ. Power output, isometric strength and steadiness in the leg muscles of pre- and postmenopausal women; the effects of hormone replacement therapy. Eur J Appl Physiol. 2006;96(3):292–8. [DOI] [PubMed] [Google Scholar]
- 54.Cooper R, Mishra G, Clennell S, et al. Menopausal status and physical performance in midlife: findings from a British birth cohort study. Menopause. 2008;15(6):1079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cauley JA, Gutai JP, Kuller LH, et al. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. 1989;129(6):1120–31. [DOI] [PubMed] [Google Scholar]
- 56.Greeves JP, Cable NT, Reilly T, et al. Changes in muscle strength in women following the menopause: a longitudinal assessment of the efficacy of hormone replacement therapy. Clin Sci (Lond). 1999;97(1):79–84. [PubMed] [Google Scholar]
- 57.Skelton DA, Phillips SK, Bruce SA, et al. Hormone replacememt therapy increases isometric muscle strength of adductor pollicis in post-menopausal women. Clin Sci (Lond). 1999;96(4):357–64. [PubMed] [Google Scholar]
- 58.Hess R, Colvin A, Avis NE, et al. The impact of hormone therapy on health-related quality of life: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008;15(3):422–8. [DOI] [PubMed] [Google Scholar]
- 59.Karvonen-Gutierrez C, Kim C. Association of mid-life changes in body size, body composition and obesity status with the menopausal transition. Healthcare (Basel). 2016;4(3). pii: E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at mid-life: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sowers MR, Crutchfield M, Richards K, et al. Sarcopenia is related to physical functioning and leg strength in middle-aged women. J Gerontol A Biol Sci Med Sci. 2005;60(4):486–90. [DOI] [PubMed] [Google Scholar]
- 62.Gebara OC, Mittleman MA, Sutherland P, et al. Association between increased estrogen status and increased fibrinolytic potential in the Framingham Offspring Study. Circulation. 1995;91(7):1952–8. [DOI] [PubMed] [Google Scholar]
- 63.Pfeilschifter J, Köditz R, Pfohl M, et al. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. [DOI] [PubMed] [Google Scholar]
- 64.Teede HJ, McGrath BP, Smolich JJ, et al. Postmenopausal hormone replacement therapy increases coagulation activity and fibrinolysis. Arterioscler Thromb Vasc Biol. 2000;;20(5):1404–9. [DOI] [PubMed] [Google Scholar]
- 65.Tomey K, Sowers M, Zheng H, et al. Physical functioning related to C-reactive protein and fibrinogen levels in mid-life women. Exp Gerontol. 2009;44(12):799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McClure CK, El Khoudary SR, Karvonen-Gutierrez C. Prospective Associations between Inflammatory and Hemostatic Markers and Physical Functioning Limitations in Mid-life Women: Longitudinal Results of the Study of Women’s Health Across the Nation (SWAN). Exp Gerontol. 2014;49:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf). 2006;186(1):5–16. [DOI] [PubMed] [Google Scholar]
- 68.Yang J Enhanced skeletal muscle for effective glucose homeostasis. Prog Mol Biol Transl Sci. 2014;121:133–63. [DOI] [PubMed] [Google Scholar]
- 69.Karvonen-Gutierrez CA, Zheng H, Mancuso P, et al. Higher leptin and adiponectin concentrations predict poorer performance-based physical functioning in midlife women: the Michigan Study of Women’s Health Across the Nation. J Gerontol A Biol Sci Med Sci. 2016;71(4):508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woods NF, Mitchell ES. Pathways to depressed mood for midlife women: Observations from the Seattle Midlife Women’s Health Study. Res. Nurs. Health, 20: 119–129. [DOI] [PubMed] [Google Scholar]
- 71.Bromberger JT, Kravitz HM, Matthews K, et al. Predictors of first lifetime episodes of major depression in midlife women. Psychol Med. 2009;39(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tomey S, Sowers MR, Harlow S, et al. Physical functioning among mid-life women: associations with trajectory of depressive symptoms. Soc Sci Med. 2010;71(7):1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ylitalo KR, Herman WH, Harlow SD. Monofilament insensitivity and small and large nerve fiber symptoms in impaired fasting glucose. Prim Care Diabetes. 2013;7(4):309–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karvonen-Gutierrez C, Harlow SD, Mancuso P, et al. Association of leptin levels with radiographic knee osteoarthritis among a cohort of midlife women. Arthritis Care Res (Hoboken). 2013. June;65(6):936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ylitalo KR, Herman WH, Harlow SD. Performance-based physical functioning and peripheral neuropathy in a population-based cohort of mid-life women. Am J Epidemiol. 2013;177(8): 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sowers M, Karvonen-Gutierrez CA, Jacobson JA, et al. Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am. 2011;93(3):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomey S, Sowers MR, Crandall C, et al. Dietary Intake Related to Prevalent Functional Limitations in Midlife Women. Am J Epidemiol. 2008;167(8):935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sternfeld B, Colvin A, Stewart A, et al. The Impact of a Healthy Lifestyle on Future Physical Functioning in Midlife Women. Med Sci Sports Exerc. 2017;49(2):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amiri P, Hosseinpanah F, Rambod M, et al. Metabolic syndrome predicts poor health-related quality of life in women but not in men: Tehran Lipid and Glucose Study. J Womens Health (Larchmt). 2010;19(6):1201–7. [DOI] [PubMed] [Google Scholar]
- 80.Sowers, 2009. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum. 2009;61(10):1328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El Khoudary SR, Chen HY, Barinas-Mitchell E, et al. Simple physical performance measures and vascular health in late midlife women: The Study of Women’s Health Across the Nation. Int J Cardiol. 2015;182:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–26. [DOI] [PubMed] [Google Scholar]
- 83.Ylitalo KR, Karvonen-Gutierrez C, McClure C, et al. Is self-reported physical functioning associated with incident cardiometabolic abnormalities or the metabolic syndrome? Diabetes Metab Res Rev. 2016. May;32(4):413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE Study randomized clinical trial. JAMA. 2014;311(23):2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]