Introduction
For most women, the unpredictable menstrual and hormonal events of the perimenopause follow decades of predictable cycles and hormone patterns. By a median age of 47 years, both the outward evidence of ‘normal’ reproductive function—a menstrual period every 25–35 days—and the internal hormonal milieu begin to demonstrate marked variability1. These changes cause disruption, which can be emotional/psychological, or can involve more somatic symptoms that are related to specific hormonal events or patterns. This article will review the hormonal changes within menstrual cycles and discuss within and between-cycle individual hormonal patterns that relate to changes in sleep, mood, and headache.
Studying Sex Hormones During the Menopausal Transition
Types of Studies
Challenges in studying sex hormones in perimenopausal women include study design, measurement, participant burden, incomplete knowledge around theoretical models, time, money, and complexities of statistical analysis. As such, large-scale efforts to characterize hormones in the research setting are infrequent. Studies that are interview/survey only without biological sampling can describe what women are experiencing, thinking, or feeling, but preclude linking that information to mechanistic evidence about hormonal levels or changes that are part of the experience. Designs that include one instance of biologic sampling allow for a snapshot of hormones and symptoms and their correlation. Sampling of women annually or at other intervals allows for a within-person picture of a trend over time. Both cross-sectional and longitudinal biospecimen sampling must indicate the phase of a menstrual cycle during which the sampling occurred, and this must be consistent for all women in the study protocol. Daily biologic sampling for an entire menstrual cycle allows for the fullest description of sex hormones. Herein we will discuss major contributing design features of studies that have informed the current knowledge of sex hormones during the years preceding menopause.
Longitudinal Biologic Sampling
A longitudinal study design includes the same person at multiple points over time. The Rotterdam study (described below) enrolled multiple cohorts over time, so while all participants are followed longitudinally, some participants have been followed longer than others. In contrast, in a cross-sectional study, information from each participant is obtained only once. While cross sectional studies are easier to operationalize and analyze, the data from such a study would not be useful in the study of sex hormones among women approaching menopause for a number of reasons. Sex hormones change within a person more consistently than across women: if averaging across women, the change over time could be obscured by between-women differences. In order to characterize a biologic sample measured on (for instance) the 4–6th day of a menstrual cycle by how far it precedes the final menstrual period (FMP), longitudinal follow-up is required, as it is not yet possible to know the time to FMP until a year after it has happened. Chronological age cannot substitute for reproductive age, as women reach menopause at different ages.
A commonly used way to characterize sex hormone change over time can be achieved with annual sampling of the same woman. Annual serum sampling per study protocol occurs early in the follicular phase for each study described. This timing will miss the large midcycle spikes in estradiol, luteinizing hormone (LH), and FSH that prompt ovulation. Sampling during the luteal phase of the cycle would be challenging to coordinate, as timing would require knowledge of when ovulation occurred, a far less obvious event than the start of a menstrual cycle. Luteal phase biologic sampling is necessary, however, to reliably characterize events like progesterone production, and the secondary estrogen surge.
Longitudinal biologic sampling is essential for full-cycle characterization of hormones measured in urine, such as in the Study of Women’s Health Across the Nation (SWAN) Daily Hormone Study (DHS, described below). The steepness with which hormones rise and fall can only be characterized when biologic sampling is obtained every day from the same woman during one cycle. How to best characterize hormones within a cycle is an evolving area of research (e.g. whole- cycle average, absolute peak, difference between peak and trough, percent change in days following peak). Characterizing (or parameterizing) sex hormones in a clinically meaningful manner requires pairing with other health information from the same individual, as the DHS, which included a daily diary during the month urine was collected, allowed researchers to do.
Statistical analysis of data collected in a longitudinal study can be carried out using only one observation per person. To fully utilize the longitudinal nature of the data, sophisticated statistical methods are required; consideration of the impact of missing data due to dropout or other reasons is critical.
Longitudinal, Epidemiological Studies of Reproductive Hormones Across the Menopause Transition
In the 1960s and 1970s, clinicians were informed by their experience in practice, rather than population-based data which was not yet available. By the 1980s, available studies of midlife women largely focused on alleviating symptoms rather than cultivating an understanding of the in vivo patterns and mechanisms of reproductive aging2. Methodological problems common to many, but not all, of these studies included an absence of baseline data, retrospective reporting, absent or inaccurate measurement of endogenous hormones, absence of appropriate control groups for studies of hormone therapy, and lack of diversity of study participants2.
The Rotterdam Study
The Rotterdam Study began in 1990 as a prospective study of men and women to determine causes of late-life diseases3. Recruitment occurred in four phases, 1990, 2000, (both of age 55 and older) and 2006 (age 45–54); by 2008, 14,926 persons were included. In 2016, a new cohort was added of persons age 40–55, with plans to enroll an additional 4000 by 2019. Additional inclusion criteria included residence in particular districts within the Netherlands. As part of the genomics, biomarker and microbiome studies, biological samples have been banked. As part of a Reproductive Traits study, age at menopause and sex hormones measured in serum will be investigated for their impact on health later in life. The Rotterdam Study remains open and revisits participants in all cohorts around every 4–5 years.
The Melbourne Women’s Midlife Health Project
Melbourne was a community-based study of Australian-born women, started in 1991, with 9 years of annual interviews of participants aged 45–55 at enrollment, all of whom had menstruated in the previous 3 months and were not using hormone therapy4. This was the first study to follow women annually, establish patterns of change in cardinal reproductive hormones (follicle stimulating hormone [FSH], estradiol, inhibins A and B, sex hormone binding globulin [SHBG], testosterone, free testosterone index [FTI] and dehydroepiandrosterone [DHEAS]), and to link them to symptoms. Of the 438 women initially enrolled, 88% were retained in the 9th year; only 8% were not yet menopausal at the end of follow-up. Blood was sampled between days 4 and 8 of the menstrual cycle to standardize them to the follicular phase of the menstrual cycle, and other measures included interviews, menstrual calendars, quality of life and measures of bone density.
The Seattle Midlife Women’s Health Study
The Seattle Midlife Women’s Health Study began enrollment in 1990, and was designed to illuminate women’s symptom experience during the menopausal transition5. In its second and third phases, a urinary hormone assay component was included. The Seattle study was community based, screening 1,428 women and following 390 longitudinally in all phases. Age upon enrollment was 35–55 years, and follow-up continued for 23 years. For a subset, first morning voided urine was collected on the 6th day of the cycle (follicular phase) at monthly intervals between July 1996 and 2001, quarterly between 2001 and 2005, and annually through 2006. Urine was assayed for estrone glucuronide, the chief urinary metabolite of estradiol, FSH, total testosterone, cortisol, epinephrine and norepinephrine. Other measures included yearly health questionnaires, health diaries, menstrual calendars and buccal smears. Follow-up for each participant ended up to five years after menopause, with 2013 the final year of follow-up for the study.
The Penn Ovarian Aging Study
The Penn Ovarian Aging Study (POAS)6 is a longitudinal cohort study of women recruited in Philadelphia County, PA. Eligible women had at least 1 ovary, a uterus, at least one menstrual period within the previous 3 months, a cycle duration of 22–35 days, and age between 35–47 years. Exclusions included some medications, medical conditions that might affect hormones, pregnancy, or non-English fluency. Of 1,426 women aged 35–47 screened, 436 of 578 eligible were enrolled over 18 months in 1996 and 1997. Enrolled women were of either black or white race. Blood samples were collected in the early follicular phase of the menstrual cycle (days 2–6) during all 28 study visits over 14 years, spaced as 2 visits 1 menstrual cycle apart, occurring every 9 months for the first five years of the study, then annually for years 6–15. After 14 years of follow-up, 67% of participants remained active. Phone-only interviews were done in years 15–17, with a full follow-up in year 18 (final year). A unique feature of the POAS design is back-to-back monthly samples, and 9-month spacing of the paired visits in the first 5 years.
The Study of Women’s Health Across the Nation (SWAN) and the Daily Hormone Study (DHS)
SWAN7 used a prospective, multicenter design, initiated to characterize the natural history of the menopausal transition. A screening interview of 16,065 US women during 1995–1996 constituted the sampling frame for the longitudinal study. Sites in Pittsburgh, PA, Boston, MA, Detroit, MI area, Chicago, IL, Los Angeles, CA, Oakland, CA area, and Newark, New Jersey recruited women of black and white races, and Japanese, Chinese, and Hispanic ethnicities8. Upon enrollment, women were aged 40–55 years; follow-up is ongoing. SWAN’s design allows for investigation of race/ethnicity, culture, or geographic region-based differences, or adjustment for these characteristics in other analyses. Sex hormones are available from serum drawn during annual visits. Timing of the fasting blood draw was targeted to the early follicular phase (days 2–5 after menses) among menstruating women, and within 90 days of baseline examination date anniversary.
The DHS included a subset of SWAN participants, with the first DHS visit coinciding with approximately the first annual SWAN follow-up. Eight-hundred forty-eight women completed the first DHS visit. Women collected a daily, first- morning-void urine beginning with the start of one menstrual cycle, and stopping with onset of the subsequent cycle (or after 50 days in the absence of a second cycle). During collection, a daily diary was used to record mood and menopausal symptoms, and a follicular phase blood draw after the menstrual period that ended the urine collection was done. DHS visits were offset from SWAN visits by half a year: there would be two instances of a blood draw from these women in each year of dual-participation, in addition to the urine samples from the DHS. The DHS was designed to continue for up to 10 years, or for a fixed number of days one year after the FMP; whichever came first. Whole cycle hormones allow for characterization of a cycle in terms of the timing of presumed ovulation9, and parameters within a cycle indicating longer-term patterns of hormonal change10.
Limitations of studies performed to date
Exclusions
With the exception of the Rotterdam Study, which does not seek to characterize cycle patterns, all of the studies described above excluded women who did not have at least somewhat regular menstrual cycles. While this design feature is essential to allow investigators to define the FMP, it eliminates women with irregular or absent menses. Polycystic ovary syndrome (PCOS) is prevalent in up to 20% of women11, and is diagnosed in part by irregular menses. Treatment for PCOS can involve birth control pills, which is an exclusion criterion for the studies presented herein. Among women age 35–45 years, an estimated 11% have undergone a hysterectomy12. Functional hypothalamic amenorrhea (FHA)13 is another cause of secondary amenorrhea, present in about 5% of adult women. Premature ovarian failure or insufficiency, the loss of ovarian function before age 40, is found in 1% of adult women14. Pregnancy and lactation were also exclusions for most studies, but this likely had a minor impact, as only 0.2% of women between ages 40 and 44 gave birth in 201215. Approximately 11% of women in the US between ages 40 and 44 use hormonal contraception16. Taken together, potentially more than a third of adult women have not contributed data to studies of the menopausal transition to date. It is important to keep this in mind when interpreting the data and applying it to patient care.
Geographic considerations
The Rotterdam, Melbourne, Seattle and Penn studies included women living in or near the eponymous region. SWAN intentionally expanded and over-recruited multiple underrepresented races and ethnicities in multiple locations. However, the southeast and southwest US have been missing from all studies; namely: Alabama, Arizona, Arkansas, Georgia, Florida, Kentucky, Louisiana, Mississippi, New Mexico, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia and West Virginia. As these regions of the country have different weather patterns, cultures, and structures, unmeasured regional differences in hormonal patterns could exist among women who live and are traversing menopause there.
Respondent burden
Collection of urine, completion of lengthy questionnaires, allowing research staff to swab for samples, disclosing personal information, and repeating this process year after year, is burdensome for participants. While the most frequent sampling possible would be ideal for precision of estimation, the less frequently a participant is contacted, the less burdensome their participation, and the more likely they will continue. Ethnic and racial minorities are frequently over-recruited for studies, thereby burdening this population for research more so than their counterparts of white race. Very detailed protocols such as the DHS, in tandem with its parent study, SWAN, could theoretically be used to determine the optimal frequency of sampling to ensure precision while minimizing respondent burden.
In summary, features of what would characterize a gold-standard study of hormones include: prospective, longitudinal design, diversity of the cohort, multiple visits in a year, herculean efforts to maximize retention, full-cycle characterization of hormones within a woman, and extensive biological sampling. The DHS is a high quality source of complete hormone characterization during the final years of reproductive function; however, like most studies designed to characterize mid-life women, its strict exclusion criteria precluded a substantial portion of women from participating. As such, and as should be the case with any study, care should be taken in extrapolating results to the appropriate population.
Changes in menstrual cycle patterns and hormones with progression to menopause
As women age, the follicle cohort shrinks along with the decline in the total oocyte pool17(See Quinn MM, Cedars MI: Declining Fertility with Reproductive Aging: How to Protect Your Patient’s Fertility by Knowing the Milestones; Gracia CR, Freeman EW: Onset of the Menopause Transition: The Earliest Signs and Symptoms; and Harlow SD: Menstrual Cycle Changes as Women Approach the Final Menses: What Matters?, in this issue for more detail). Briefly, anti-mullerian hormone (AMH), a stable product of very small, but growing follicles that reflects the available remaining follicle complement most accurately, declines throughout reproductive life18. The decline in AMH may directly release ovarian follicles from inhibition, allowing the less responsive follicles in the ovary to activate and to maintain the ovulatory capacity of the human menstrual cycle in the face of ever-dwindling gametes. Inhibin B, which is produced by later stage, growing follicles, also declines with reproductive aging, reflecting the reduced follicle cohort size over time. Secondary to these ovarian changes, follicle-stimulating hormone (FSH) rises19, especially in the early follicular phase20. This rise in FSH appears necessary to maintain normal folliculogenesis because the remaining follicles have a higher threshold for activation and stimulation. Finally, ultrasound assessment of small, antral follicles 2–9mm in size (antral follicle count) also provides a measure of ovarian reserve21. The process of follicle loss is initially compensated by lower AMH and inhibin B and intermittently rising FSH (STRAW −2: the early transition), and then becomes intermittently uncompensated (STRAW −1, the late transition), until follicle exhaustion is complete at menopause (STRAW stage 0)1. Investigators from the Penn Ovarian Aging Study (POAS) identified a stage that divides the STRAW Stage −2, the early transition, into a late premenopause (with only 1 irregular menstrual cycle event) and the early transition (2 or more cycle length changes)22. Menstrual cycle changes will be briefly reviewed for each of these stages.
The Early Transition (including POAS late premenopause): Compensated Follicle Failure
This first stage of the menopause transition can be conceptualized as a critical drop in follicle numbers that causes the first noticeable changes in a woman’s menstrual cycle. She then experiences one of two events: (a.) her cycles become noticeably less regular, or (b.) she skips a menstrual cycle, and then resumes her previous pattern. At least a decade of rising FSH and reduced inhibin A and B23 and AMH precede this event, but these hormonal adjustments are clinically silent, reflecting the compensatory mechanisms invoked to maintain regular cyclicity and potential fertility in the face of follicle loss. These changes are described in Figure 1.
Figure 1.
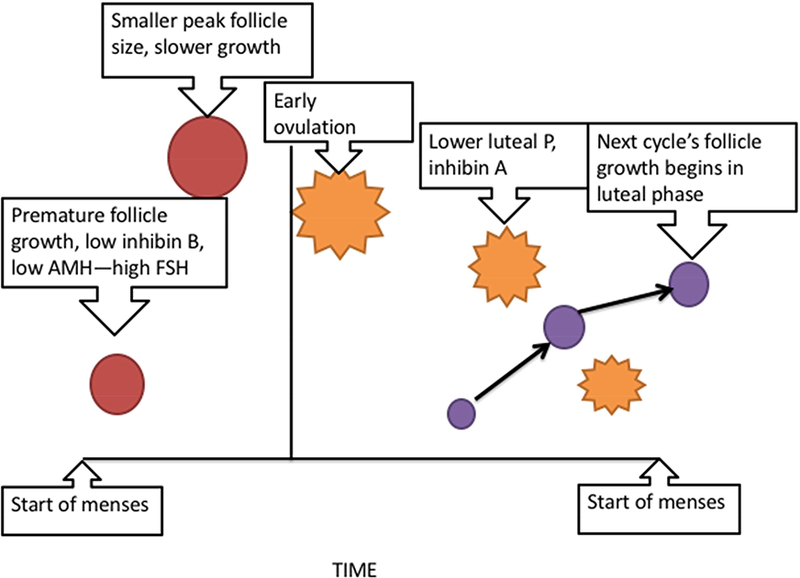
Summary of the events involved in the ‘compensated failure’ of follicle growth during the menstrual cycle in the early transition. Reduced inhibin B and AMH, reflective of the smaller follicle cohort, remove restraint on follicle activation and growth and allow FSH to rise in the early follicular phase. Elevated FSH and accelerated follicle development lead to earlier ovulation of a smaller follicle. Since the follicle has greater aromatase activity, normal estradiol levels are preserved and may even be higher than midreproductive aged women’s cycles. The earlier ovulation leads to a greater proportion of the cycle being spent in the luteal phase, and the reduced steroid and protein hormones from the corpus luteum fail to restrain FSH in the luteal phase, leading to recruitment of the next cycle’s dominant follicle prior to menses.
In addition to these changes in circulating hormones, the process of follicle maturation is altered and there are changes within oocytes. Follicles grow earlier in the cycle than ever before24, due to high FSH stimulation, and they grow more rapidly25,26. Increased follicular aromatase27 leads to maintenance of midreproductive, or even higher than midreproductive, estrogen production28. Follicle growth becomes dysregulated, in that ovulation occurs earlier in the menstrual cycle25 and follicle diameter at the time of ovulation is smaller26 than in midreproductive women’s cycles.
Oocytes of reproductively aging women are more prone to meiotic spindle abnormalities29 and to have a loss of mitochondrial DNA30. These deficits likely underlie the propensity for older reproductive aged women to undergo nondisjunction during gametogenesis and to have a greater risk of chromosomal abnormalities in conceived offspring. Whether these defects are correctable is not known, but certainly a topic of active investigation31.
The underlying physiologic changes described herein predict a hormonal environment that has more frequent and more variable menstrual periods, more variable hormone production, a lesser likelihood of consistent ovulation, and brief bouts of amenorrhea. Collectively, these changes can destabilize women in several ways.
The Late Transition: Longer Periods of Uncompensated Follicle Failure
As women enter the late transition, they are subject to longer periods of amenorrhea (>60 days). These bouts can contain a variety of patterns of hormone production, but eventually they become more uniformly hypoestrogenic and hypergonadotropic. When cycles do occur, they remain relatively likely to be ovulatory, with robust rises in progesterone production, and therefore potentially fertile.
Menstrual Cycle Patterns Throughout the Transition
The most common type of menstrual cycle observed in women as they transition appears to be an ovulatory cycle. These cycles have evidence of luteal activity (ELA), typically defined as a robust and sustained rise in progesterone or in its metabolite, pregnanediol glucuronide (Pdg), when urinary studies are performed. Cycles that do not have a robust rise in progesterone or Pdg may reflect a failed ovulation, or an inadequate luteal phase. Examples of these cycle types have been described in reproductively aging women26. It is important to keep in mind, however, that luteal phase progesterone production occurs across a spectrum, and lesser degrees of progesterone production, even though they might result in a cycle being classified as ‘anovulatory’, may not necessarily reflect a failure of follicle growth and estradiol production. Most studies have not combined hormonal assessments with follicle assessments and thus, it is impossible to know the sequence of ovarian events. An example of a completely anovulatory cycle accompanied by initially normal follicle growth, and a normal menstrual cycle length is provided in Figure 2.
Figure 2.
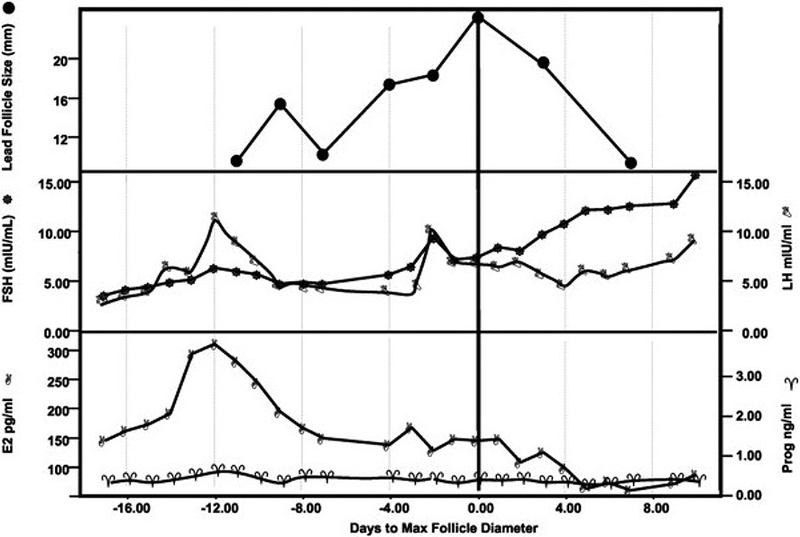
Follicle growth and estradiol production without ovulation in a perimenopausal woman. The participant had a 28 day intermenstrual interval. Note the early rise in estradiol to 300 pg/ml (1101 nmol/liter) without evidence of large follicle growth and no sign of subsequent progesterone production. Peak estradiol production is seen on cycle day 6 (Day 12 on the graph), and peak follicle growth to over 20 mm is apparent by cycle day 18 (Day 0 on the graph).
From Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab 2003;88(11):5506; with permission.
SWAN performed the most comprehensive assessment of menstrual cycles in perimenopausal women to date. In the early phases of the SWAN DHS, the FMP was not known for all participants, and thus cycles could not be organized by proximity to the FMP. However, the vast majority of cycles (most of which, in retrospect, were from the early transition) had an ovulatory pattern with a robust rise in Pdg (80.9%)32. As women progressed towards menopause, the amount of Pdg produced in the luteal phase declined33, but overall preservation of a robust, ovulatory pattern remained for the majority of participants until about 5 years before the FMP34. By the year before the FMP, only 23% of cycles retained this ovulatory pattern. Figure 3 indicates the dropoff in cycles with an ovulatory pattern as women approached the FMP.
Figure 3.
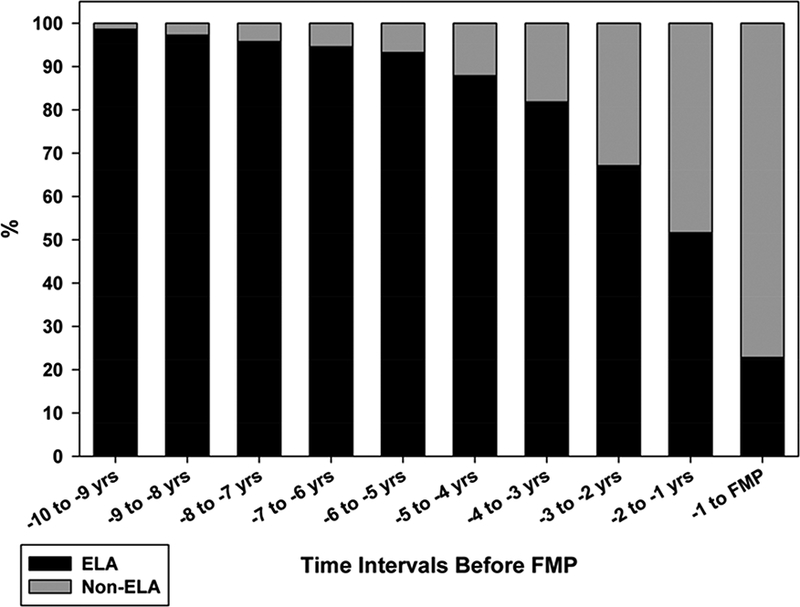
The proportion of cycles with robust progesterone production, called ELA (evidence of luteal activity) in the figure, declines as women approach the final menstrual period (FMP), but the change is most striking in the latter few years before FMP.
From Santoro N, Crawford SL, El Khoudary SR, et al. Menstrual Cycle Hormone Changes in Women Traversing Menopause: Study Of Women’s Health Across the Nation. J Clin Endocrinol Metab 2017; 102(7): 2222; with permission.
The Luteal-Out-of-Phase (LOOP) Cycle
Hale, et al, defined a type of menstrual cycle pattern unique to women in the menopausal transition35. The accelerated follicle growth that occurs in older reproductive aged women appears related to abnormally early growth of follicles from the previous luteal phase. The wave of follicles recruited during the luteal phase of the cycle are close to maturation at the time of menses, leading to a very rapid, very early second ovulatory event, hence the name ‘luteal-out-of-phase’ to characterize this cycle type (Figure 4). LOOP events are associated with higher luteal estradiol exposure, as well as rapidly successive progesterone excursions.
Figure 4.
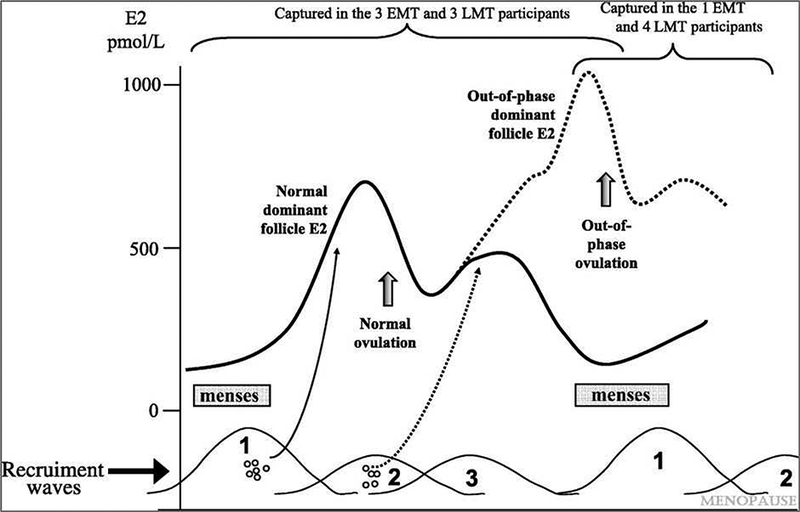
Luteal-out-of-phase (LOOP) cycle and its underlying mechanisms. A wave of follicle growth that is initiated in the prior luteal phase causes very early ovulation immediately after or concurrent with menses from the prior cycle. Higher estradiol levels and rapid re-exposure to progesterone result.
From Hale GE, Hughes CL, Burger HG, et al. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause 2009;16(1):50–9; with permission.
Cycles Without Evidence of Luteal Activity
The non-ELA cycles described in the SWAN DHS36 do not simply represent cycles without robust estrogen production. Non-ELA cycles may have a robust estrogen rise that mimics nearly exactly the estrogen rise seen in a clearly ovulatory cycle, or there may be an atypically large estrogen, rise, or they may be no estrogen production at all. Non-ELA cycles with an estrogen rise may or may not have an accompanying LH surge, and the amount of Pdg produced is variable but insufficient to meet the criteria for an ovulatory cycle. These cycle patterns appear to be associated with differing symptomatology. Table 1 indicates the classification of non-ELA cycles and their associated bleeding patterns. This work is ongoing, and further classifications may emerge.
Table 1.
Cycles without luteal activity identified in the SWAN DHS and their hormonal and expected menstrual correlates
| Hormonal Pattern |
LH surge | Pdg pattern | Bleeding pattern |
|---|---|---|---|
| Estrogen rise |
+ | Variable but low |
Menses when estrogen falls |
| Estrogen rise |
− | Variable but low |
Menses when estrogen falls |
| No estrogen rise |
N/A | None | No menses |
Linking Hormones to Symptoms
The menopausal transition has long been associated with a number of symptoms, with vasomotor symptoms recognized as the cardinal menopausal symptom (See Gracia CR, Freeman EW: Onset of the Menopause Transition: The Earliest Signs and Symptoms, in this issue). While other symptoms such as mood disorders, sleep disturbances and headaches have been long recognized to significantly affect quality of life in menopause, their recognition as being specifically related to reproductive hormones during the transition remains controversial. This controversy is due, in part, to a lack of studies targeting midlife women with non-vasomotor symptoms and conflicting methodologies for characterizing reproductive staging. There is also a scarcity of longitudinal studies including both hormone and symptoms data. Many studies relating hormones to changes in symptoms across the menopausal transition use only a single point of hormone and symptom assessment performed no more than annually. The prevailing hypothesis is that the erratic fluctuations in hormones (estrogen in particular) results in susceptibility to symptoms (mood changes, sleep disturbance, headache) and ultimately syndrome (depression, insomnia, migraine) presentation; but the knowledge of precise hormonal change and resulting biological mechanisms remains limited. The hormone and daily symptom diary data used by the SWAN DHS can provide information on trajectories of both hormonal and symptom changes (hot flashes, mood, headache, and others) across and within women.
Mood
A majority of women of perimenopausal age endorse symptoms of depression in cross-sectional studies (See Bromberger JT, Epperson CN: Depression During and After the Perimenopause: Impact of Hormones, Genetics, and Environmental Determinants of Disease, in this issue, for more details on mood and the perimenopause). An 1.5–3 fold increased risk of depressive symptoms has been seen in longitudinal studies of menopausal transition37. The effects of changing estrogen on perimenopausal mood reflect the prevailing opinion that, as the levels of estrogen vary widely and decline, susceptibility to mood symptoms and major depression increases38. Estrogen has long been implicated in the activity of neurotransmitters involved in depressive symptoms, namely serotonin (5-HT) and norepinephrine (NE). Estrogen exerts anti-depressant properties by regulating the synthesis, metabolism and receptor activity of 5-HT and NE. Wider fluctuations in estradiol (E2) levels and FSH are associated with worse mood symptoms37. Long-term trajectories of mood symptoms have also been examined in SWAN, but day to day changes in mood in association with menstrual cycles have not yet been reported.
Four distinct trajectories of depressive symptoms were identified in the Australian Longitudinal Study of Women’s Health over a 15-year follow up, with 80% of women having low scores, 9% with increasing scores, 8.5% with decreasing depression scores and 2.5% of women with stable high depression scores. A recent study used a mathematical model (latent class analyses) to group women going through the different stages of menopause into one of six symptom classes39. Women in the highest symptom class (LC1) reported a high intensity of most symptoms, including physical and psychological symptoms such as depression and anxiety, followed by women with moderate intensity of most symptoms (LC2). Lower symptom classes included women with moderate intensity of a subset of symptoms (vasomotor symptoms, pain, fatigue, sleep disturbances and physical health symptoms), women with numerous milder symptoms (LC3 - LC5), and women who were relatively asymptomatic (LC6). In pre-menopause, 10% of women were classified in LC1, 16% in LC2, 14% in LC3, 26% in LC4, 14% in LC5 and 20% in LC6 and the majority of women remained in the same latent class while transitioning through menopause, further supporting the need to study individual hormonal and symptom fingerprints of women by examining multiple years of follow up40.
Sleep
Poor sleep quality and sleep distruption are frequently observed in the menopause transition41 and have most typically been attributed to vasomotor symptoms which have been seen as the primary risk factor for sleep disruption in midlife women42,43(See Kravitz HM, Kazlauskaite R, Joffe H: Sleep, Health, and Metabolism in Midlife Women and Menopause: Food for Thought, in this issue, for more information on sleep and perimenopause). However, fluctuations in hormones have been associated with sleep disturbance during the menopausal transition independent of VMS, suggesting that not all perimenopausal sleep disturbances are explained by night sweats and that menopausal transition related hormonal changes can independently lead to sleep disturbance44–46. When day-to-day menstrual cycle hormones were assessed along with daily self-reported trouble sleeping, the periovulatory phase of the cycle was associated with the least trouble sleeping42. This implies that daily hormonal exposures influence sleep. Independent of VMS, night-time awakenings and difficulty falling and staying asleep during the menopausal transition and postmenopause have been associated with lower estradiol41,47. Difficulty falling asleep and remaining asleep has been associated with higher levels of FSH48, and more rapidly increasing levels of FSH41. In contrast to SWAN, there was no consistent change in estradiol, testosterone (T), and FSH in relation to sleep disruption in the Penn Ovarian Aging cohort49.
Headaches and pain
Pain symptoms are generally perceived as a common symptom of aging and not specifically transition-related, though women are at greater risk for developing pain disorders, and exhibit greater sensitivity to noxious stimuli compared to men. Past studies suggest that perimenopause is associated with increased prevalence of some pain disorders, such as headache50,51, and musculoskeletal pain52; however the effect of the stage of the menopausal transition on the frequency of subjective pain complaints has not been completely explored. Postmenopausal women report greater musculoskletal pain than premenopausal women52, but this was a study limited to a single annual follow-up and based on a one-time retrospective assesment of musculoskeletal pain complaints over the prior 4 weeks, rather than a daily, prospective assessment. Bodily pain (aches, joint pain and stiffness) have been associated with estradiol variability in the Penn Ovarian Aging Study53. In contrast, joint pain and back pain were not associated with urinary levels of estrone or FSH in the Seattle Midlife Women’s Health Study54.
Though migraine headache has long been recognized to be hormonally regulated in women, no study to date has focused on specific sex hormone changes over the menopausal transition. Overall, stable and rising estrogen levels, such as seen in pregnancy, have been associated with fewer attacks of migraine, while rapidly changing estrogen levels such as seen in perimenopause have been associated with more frequent migraine attacks. Migraine affects close to a quarter of perimenopausal aged women, and is a priority for further study using adequate methodology. The SWAN DHS sub-study has been the only study to date to compare within-woman change in hormones in migraineurs compared to controls. Women with history of migraine had a faster urinary E1c decline over the 2 days following the luteal peak than controls; this finding was independent of headache occurrence in the cycle of study, suggesting an endogenous difference in estrogen processing in women with migraine as compared to women without history of migraine10. DHS data are currently being used to further examine the day-to-day occurrence of headache in relation to daily hormone changes in women with migraine.
Conclusions
Current data from a number of longitudinal studies of perimenopausal women are being used to describe how reproductive hormone change over time affects women’s symptom experience. In addition to characterization of cycles and their eventual breakdown, longitudinal, day-to-day sampling indicates that both sleep and headache vary in relation to specific hormone patterns. Overall, hormonal instability appears to be associated with worse symptoms, but more research is needed. A better understanding of how hormone patterns may cause symptoms in midlife women will inform appropriate approaches to treatment.
Key Points:
As opposed to midreproductive life, perimenopausal cycles have less predictable physiologic features such as ovulation, and more variability in reproductive hormones over time.
Intensive, longitudinal study designs provide the clearest picture of the transition, but not all women seen by clinicians are represented in these samples.
Symptoms such as headache and trouble sleeping vary across the menstrual cycle, but others, such as hot flashes, are related to increased longer-term hormone variability.
Synopsis.
Key cycle changes occur as women transition from reproductive life to the menopause, and they can be roughly linked to menopausal staging. It is important to understand the types of studies that inform our current knowledge. Patterns of symptoms within menstrual cycles (sleep, headache) generally favor worsening in association with the perimenstrual phase of the cycle, and patterns of chronic symptoms, such as hot flashes and adverse mood, appear to be worse when hormones are more variable.
Acknowledgements
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Disclosure statement: Dr. Santoro is a consultant for Ogeda/Astellas Pharmaceuticals, and has stock options with Menogenix, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012;97:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rostosky SS, Travis CB. MENOPAUSE RESEARCH AND THE DOMINANCE OF THE BIOMEDICAL MODEL 1984–1994. Psychology of Women Quarterly 1996;20:285–312. [Google Scholar]
- 3.Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol 2017;32:807–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study. The Melbourne Women’s Midlife Health Project. Climacteric 2004;7:375–89. [DOI] [PubMed] [Google Scholar]
- 5.Woods NF, Mitchell ES. The Seattle Midlife Women’s Health Study: a longitudinal prospective study of women during the menopausal transition and early postmenopause. Women’s Midlife Health 2016;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman EW, Sammel MD. Methods in a longitudinal cohort study of late reproductive age women: the Penn Ovarian Aging Study (POAS). Women’s Midlife Health 2016;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobo RA, Kelsey J, Marcus R. Menopause: Biology and Pathobiology: Elsevier Science; 2000. [Google Scholar]
- 8.Sommer B, Avis N, Meyer P, et al. Attitudes toward menopause and aging across ethnic/racial groups. Psychosom Med 1999;61:868–75. [DOI] [PubMed] [Google Scholar]
- 9.Santoro N, Crawford SL, Allsworth JE, et al. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab 2003;284:E521–30. [DOI] [PubMed] [Google Scholar]
- 10.Pavlovic JM, Allshouse AA, Santoro NF, et al. Sex hormones in women with and without migraine: Evidence of migraine-specific hormone profiles. Neurology 2016;87:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical Epidemiology 2014;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010;65:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meczekalski B, Katulski K, Czyzyk A, Podfigurna-Stopa A, Maciejewska-Jeske M. Functional hypothalamic amenorrhea and its influence on women’s health. Journal of endocrinological investigation 2014;37:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray A, Schoemaker MJ, Bennett CE, et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genetics in Medicine 2014;16:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief 2014:1–8. [PubMed] [Google Scholar]
- 16.Allen RH, Cwiak CA, Kaunitz AM. Contraception in women over 40 years of age. CMAJ : Canadian Medical Association Journal 2013;185:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod 2008;23:699–708. [DOI] [PubMed] [Google Scholar]
- 18.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS One 2011;6:e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed Ebbiary NA, Lenton EA, Cooke ID. Hypothalamic-pituitary ageing: progressive increase in FSH and LH concentrations throughout the reproductive life in regularly menstruating women. Clin Endocrinol (Oxf) 1994;41:199–206. [DOI] [PubMed] [Google Scholar]
- 20.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res 2002;57:257–75. [DOI] [PubMed] [Google Scholar]
- 21.Hansen KR, Craig LB, Zavy MT, Klein NA, Soules MR. Ovarian primordial and nongrowing follicle counts according to the Stages of Reproductive Aging Workshop (STRAW) staging system. Menopause 2012;19:164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gracia CR, Sammel MD, Freeman EW, et al. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause 2005;12:128–35. [DOI] [PubMed] [Google Scholar]
- 23.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab 1999;84:105–11. [DOI] [PubMed] [Google Scholar]
- 24.Klein NA, Harper AJ, Houmard BS, Sluss PM, Soules MR. Is the short follicular phase in older women secondary to advanced or accelerated dominant follicle development? J Clin Endocrinol Metab 2002;87:5746–50. [DOI] [PubMed] [Google Scholar]
- 25.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab 1996;81:1038–45. [DOI] [PubMed] [Google Scholar]
- 26.Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab 2003;88:5502–9. [DOI] [PubMed] [Google Scholar]
- 27.Shaw ND, Srouji SS, Welt CK, et al. Compensatory Increase in Ovarian Aromatase in Older Regularly Cycling Women. J Clin Endocrinol Metab 2015;100:3539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996;81:1495–501. [DOI] [PubMed] [Google Scholar]
- 29.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 1996;11:2217–22. [DOI] [PubMed] [Google Scholar]
- 30.Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril 1995;64:577–83. [PubMed] [Google Scholar]
- 31.Bentov Y, Casper RF. The aging oocyte--can mitochondrial function be improved? Fertil Steril 2013;99:18–22. [DOI] [PubMed] [Google Scholar]
- 32.Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab 2004;89:2622–31. [DOI] [PubMed] [Google Scholar]
- 33.Santoro N, Crawford SL, Lasley WL, et al. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab 2008;93:1711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santoro N, Crawford SL, El Khoudary SR, et al. Menstrual Cycle Hormone Changes in Women Traversing the Menopause: Study Of Women’s Health Across the Nation. J Clin Endocrinol Metab 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause 2009;16:50–9. [DOI] [PubMed] [Google Scholar]
- 36.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA 2004;292:2991–6. [DOI] [PubMed] [Google Scholar]
- 37.Soares CN. Mood disorders in midlife women: understanding the critical window and its clinical implications. Menopause 2014;21:198–206. [DOI] [PubMed] [Google Scholar]
- 38.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006;63:375–82. [DOI] [PubMed] [Google Scholar]
- 39.Harlow SD, Karvonen-Gutierrez C, Elliott MR, et al. It is not just menopause: symptom clustering in the Study of Women’s Health Across the Nation. Womens Midlife Health 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bromberger JT, Kravitz HM, Youk A, Schott LL, Joffe H. Patterns of depressive disorders across 13 years and their determinants among midlife women: SWAN mental health study. J Affect Disord 2016;206:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 42.Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med 2005;165:2370–6. [DOI] [PubMed] [Google Scholar]
- 43.Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep 2013;36:1977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman EW, Sammel MD, Gross SA, Pien GW. Poor sleep in relation to natural menopause: a population-based 14-year follow-up of midlife women. Menopause 2015;22:719–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kravitz HM, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am 2011;38:567–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kravitz HM, Janssen I, Bromberger JT, et al. Sleep Trajectories Before and After the Final Menstrual Period in The Study of Women’s Health Across the Nation (SWAN). Curr Sleep Med Rep 2017;3:235–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep 2010;33:539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab 2015;100:1426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pien GW, Sammel MD, Freeman EW, Lin H, DeBlasis TL. Predictors of sleep quality in women in the menopausal transition. Sleep 2008;31:991–9. [PMC free article] [PubMed] [Google Scholar]
- 50.Wang SJ, Fuh JL, Lu SR, Juang KD, Wang PH. Migraine prevalence during menopausal transition. Headache 2003;43:470–8. [DOI] [PubMed] [Google Scholar]
- 51.Martin VT, Pavlovic J, Fanning KM, Buse DC, Reed ML, Lipton RB. Perimenopause and Menopause Are Associated With High Frequency Headache in Women With Migraine: Results of the American Migraine Prevalence and Prevention Study. Headache 2016;56:292–305. [DOI] [PubMed] [Google Scholar]
- 52.Dugan SA, Powell LH, Kravitz HM, Everson Rose SA, Karavolos K, Luborsky J. Musculoskeletal pain and menopausal status. Clin J Pain 2006;22:325–31. [DOI] [PubMed] [Google Scholar]
- 53.Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol 2007;110:230–40. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell ES, Woods NF. Pain symptoms during the menopausal transition and early postmenopause. Climacteric 2010;13:467–78. [DOI] [PubMed] [Google Scholar]


