VMS: Primary Symptoms Associated with Menopause
Vasomotor symptoms (VMS) or hot flashes and night sweats, are hallmarks of the menopausal transition (MT) and can significantly affect quality of life.1–4 Up to 80% of women experience VMS during menopause 5,6 and a majority of women rate them as moderate-to-severe.7 Recent research from the Study of Women’s Health Across the Nation (SWAN) found that frequent VMS last a median of 7.4 years, which is longer than previously thought.8 The Penn Ovarian Aging Study has shown that the mean duration for any VMS is about 10 years.9 VMS are one of the chief menopause-related complaints for which US women seek medical treatment.10,11 VMS are also independently associated with multiple indicators of elevated cardiovascular risk,12,13 greater bone loss,14 and higher bone turnover.14
The cause of hot flashes is not fully understood and is likely multifactorial. It is generally thought that hot flashes result from a narrowing of the thermoneutral zone in perimenopausal women.15 Reproductive hormones play an important role in this narrowing, given that the onset of VMS corresponds to changes in reproductive hormones at the menopausal transition and the therapeutic effect of exogenous estrogen. Although lower levels of estrogen and higher FSH are associated with VMS reporting, not all women who experience hormonal changes have VMS.16 Longitudinal analyses from SWAN found that FSH is more strongly associated with VMS than E2.17 SWAN further found that neither hormone levels nor bleeding changes entirely explained VMS prevalence or frequency, thus suggesting the importance of other factors such as lifestyle and psychosocial characteristics.
Although symptoms such as depression, difficulty concentrating, and moodiness are often thought to be associated with menopause, VMS are the only symptom clearly and directly associated with menopause.18,19 Research using large lists of symptoms to look at how symptoms aggregate has found that hot flashes and night sweats do not “track” with these other symptoms.18,20,21 Further, the Stages of Reproductive Aging Workshop (STRAW) also concluded that these other symptoms do not track closely with menstrual cycle or endocrine changes during the menopausal transition.19 This was later confirmed in a follow-up consensus workshop referred to as STRAW+10 (Table 1).22
Table 1:
Prevalence of VMS by stage of menopausal transition
| Stages of menopause transition as defined by the STRAW + 10 staging system22 | VMS prevalence estimates5 |
|---|---|
| Late reproductive stage: possible subtle changes in menstrual cycle length or flow | 6 – 13% |
| Early menopausal transition: change in menstrual cycle regularity | 4 – 46% |
| Late menopausal transition: skipped menstrual periods | 33 – 63% |
| Postmenopause: 1+ year with no menstrual flow | 41 – 79% |
Prevalence, Frequency, and Severity
Vasomotor symptoms occur during the MT for up to 80% of US women,16,23 but the daily frequency varies. On average, women report 4–5 hot flashes per day,24,25 although some women have as many as 20 per day.26 One in four women report having VMS every day.24 Daytime hot flashes are reported more often than night sweats,24–27 although this may reflect difficulty in perceiving or recording nighttime symptoms.28 Night sweats are generally considered more bothersome than daytime symptoms.24,26 Greater frequency of VMS also is linked to higher bother.26 Overall, about half of symptomatic women report only mild severity or bother.26,29
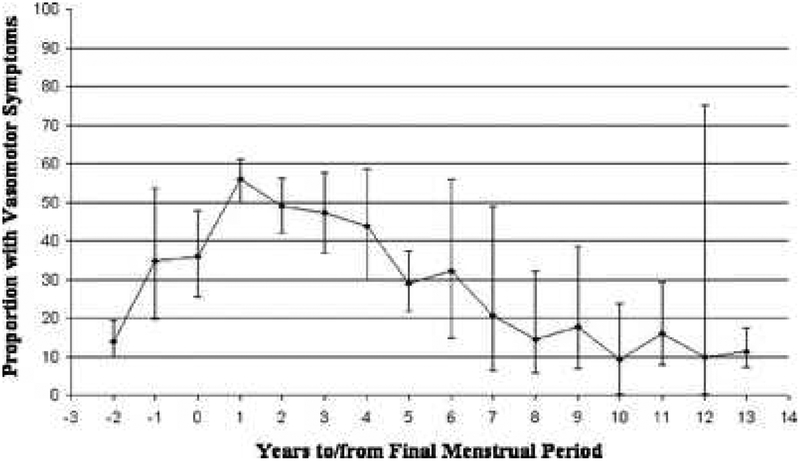
For women undergoing a natural MT – not due to hysterectomy/oophorectomy or other medical intervention – occurrence of VMS varies widely by MT stage.5–7,24,30 Occurrence is lowest prior to entering the menopausal transition, increasing in the early transition and higher still in the late transition near the final menstrual period (FMP).5 Postmenopause, VMS occur in as many as 3 out of 4 women in the first two years post-FMP, and decline slowly afterward, taking 8–10 years to return to pre-FMP levels (Figure 1).7,31 Patterns for severe VMS, defined variously across studies in terms of higher frequency, severity, or bother, are generally similar, with a peak prevalence of 50% near the FMP.6,7,25,31
Figure 1:
Pooled estimates from six studies of proportion of vasomotor symptoms by years to/from final menstrual period. One study was longitudinal, and five were cross-sectional. From Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med 2008;23(9):1510; with permission.
Although menopausal hormone therapy (HT) is a highly effective treatment for VMS,32 VMS often recur after HT discontinuation.33–38 In one recent study, over 90% of women discontinuing HT had a recurrence, with severe VMS in two-thirds of women.38 Recurrence after HT discontinuation is more common in women with VMS prior to HT initiation33,35 and in women who initiated HT for symptom relief,33 although even previously asymptomatic women may have new VMS after HT discontinuation, estimated in one study at 7%.34 VMS recurrence also is more likely in younger women.37
How long do they last?
Despite their pervasiveness, negative influence on quality of life, and association with adverse health indicators, we have lacked robust estimates of how long VMS last. In part this is because, until recently, few studies had sufficient follow-up of individual participants, and thus within-woman duration was inferred indirectly by comparing different women at varying stages of the menopause transition. Previous clinical guidelines suggested a typical duration of VMS between 6 months and 2 years.39 Recent findings, however, indicate that VMS last much longer. Any VMS – i.e., regardless of frequency or severity – have been found to last a total of 10.2 years on average, and average duration after the FMP is 4.9 years among those who continue to have symptoms.7 Average or median durations for frequent or moderate/severe VMS are somewhat shorter at 7.4 – 8.8 years in total, and 4.5 – 4.6 years after the FMP.7,8 VMS last longer in women whose symptoms begin earlier in the menopause transition. Frequent or moderate/severe VMS have a median duration of approximately 3.5 years in women whose symptoms begin postmenopause, compared with more than 11.5 years in women with an onset of VMS near the start of the menopause transition.7,8
Risk Factors for VMS
Many studies have identified characteristics of women who are most likely to get hot flashes (Box 1). Researchers have looked at health behaviors, psychosocial characteristics, and sociodemographic factors. Studies have also looked at racial and ethnic differences and these are discussed in a separate section.
Box 1: Risk factors for Hot Flashes.
Good evidence for:
Menopause status
Anxiety or depression prior to menopause
Generally more sensitive to symptoms
Black race
Smoking
Anti-endocrine (estrogen or androgen) therapy
Mixed or no evidence:
Physical activity
Diet
Alcohol consumption
An early myth about hot flashes is that being overweight can be protective. Early observations from postmenopausal women found that greater estrone production in peripheral fat from aromatization of androstenedione was associated with less symptom reporting.40,41 Later longitudinal and cross-sectional studies of women during the menopause transition indicated that greater body mass index (BMI) was associated with worse VMS.6,30,42 Recent data from SWAN has helped address this apparent contradiction.43 SWAN data show that while greater BMI is related to less frequent VMS in the late menopause, BMI is positively related to VMS in early menopause.43 Thus, BMI appears to have a different relationship with VMS before and after menopause.
Smoking is the primary health behavior that has been associated with VMS. SWAN has shown that both active smoking and passive smoke exposure are related to greater likelihood of VMS.44 Current smokers have an over 60% increased likelihood of reporting VMS, even adjusting for confounding factors such as education and race/ethnicity.6 Although it has been hypothesized that this relationship is due to the anti-estrogenic effects of cigarette smoking, differences in endogenous E2 levels do not account for this association in SWAN.44
Physical activity, diet, and alcohol consumption are other health behaviors people have studied, but these results are weak and inconsistent. About half of the observational studies report no association between physical activity and VMS (e.g., 6,45,46) while others report a protective association (e.g.,47,48). In a randomized aerobic exercise intervention trial, The MsFLASH study (Menopause Strategies: Finding Lasting Answers for Symptoms and Health) found no benefit of exercise on frequency or bothersomeness of VMS.49
Although alcohol can serve as a trigger for hot flashes, research has shown that alcohol consumption and VMS have either no 44,50 or modest association.51 SWAN found that less alcohol consumption was related to more frequent VMS in unadjusted analyses and showed no relationship when analyses controlled for other variables.6
Similar to physical activity and alcohol, research has not found a consistent relationship between diet and VMS. Phytoestrogens, a group of plant-derived chemicals found in foods such as soy, red clover, and alfalfa, have been thought to be protective against VMS due to their structural resemblance to E2 and the lower prevalence of VMS among Asian women. However, a meta-analysis of randomized studies found no indication that phytoestrogens show a beneficial effect on VMS.52 In SWAN, baseline genistein (one type of phytoestrogen) intake was not related longitudinally to VMS and did not account for reduced symptom reporting in Asian women.6
In contrast to the inconsistent role of lifestyle factors, studies have shown that psychosocial factors such as anxiety and depression are more consistently associated with VMS.5,6,53,54 Although in cross-sectional studies, it is not possible to determine whether VMS or psychosocial distress comes first, longitudinal studies suggest that psychological factors may impact subsequent VMS. The Penn Ovarian Aging Study found that anxiety and depression preceded hot flashes.54,55 In SWAN, depressive symptoms and anxiety at the first visit with frequent VMS were related to longer duration of VMS.8 SWAN has also shown that stress and generally being sensitive to symptoms are related to longer duration of VMS.8 Pathways connecting negative affective factors and VMS are likely complex and bidirectional.26,56,57 A direct physiologic link, through the hypothalamic-pituitary-gonadal axis, between negative affect and VMS has been proposed,58 but is not well-tested.
Research has also shown that older age,6 lower education level6,23,50 and premenstrual symptoms6 are related to VMS. The association between lower educational attainment and VMS does not seem to be explained by confounding factors such as smoking, higher BMI, higher perceived stress or higher negative affect.6
Most studies investigating the time course of VMS and the risk factors for VMS focus on population averages and do not consider variation in VMS patterns. However, not all women experience the same pattern of VMS. The Australian Longitudinal Study on Women’s Health (ALSWH) followed 695 white women over 14 years and identified four patterns of VMS severity over the menopause transition.59 Most women (42%) had moderate symptoms, peaking at menopause. Other women had early and severe VMS that began prior to menopause, but steadily declined post menopause (11% of women), while some had late and severe VMS (28%) or late moderate VMS (18%) that peaked post menopause and slowly declined but continued for more than a decade.
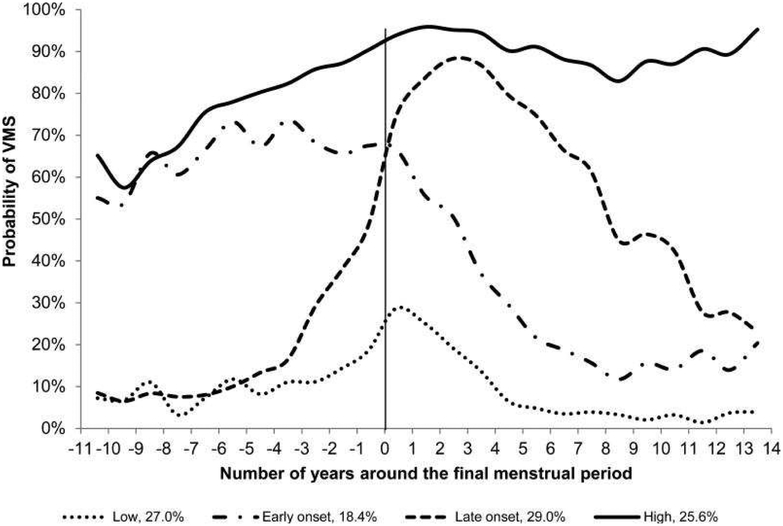
SWAN followed 1,455 women from 5 racial/ethnic groups who experienced natural menopause over 15 years and also found four distinct trajectories of VMS frequency (Figure 2).60 Similar to the ALSWH study, SWAN found a group (18.4%) that had early onset of VMS 11 years before the FMP, with later decline. A larger group had a later onset nearer the FMP with later decline (29%). However, somewhat different from the ALSWH study, SWAN found groups with persistently high frequency (25.6%) and persistently low frequency of VMS (29%). These differences could be due to racial/ethnic compositions of samples or other sample differences. In SWAN, women who had either persistently high or early onset VMS had higher baseline anxiety and depressive symptoms relative to women with persistently low frequency of VMS. SWAN also found that black women were overrepresented in the late onset and high VMS groups. There was a trend for the consistently high groups to have low levels of E2 across the menopause transition. However, the dynamics of E2 and VMS were correlated but not perfectly consistent, reinforcing the evidence that E2 alone is not the complete mechanistic explanation for VMS.61,62
Figure 2:
Trajectories of Vasomotor Symptoms over the Menopause Transition VMS indicates vasomotor symptoms. Probability of VMS represents the average observed probability of VMS at each time point within each trajectory subgroup. No factors were included in the model. From Tepper PG, Brooks MM, Randolph JF, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause 2016;23(10):1067–74; with permission.
Two groups of women deserve special attention: those who undergo a hysterectomy or oophorectomy and those who experience VMS as a result of breast cancer treatment. VMS are more likely in women with a hysterectomy--even with ovarian conservation--than in women with a natural menopause25,63,64, possibly due to disruption of blood flow to the ovaries (which in turn affects hormone levels) from abdominal surgery. Bilateral salpingo ovariectomy (BSO) without concurrent exogenous hormone therapy (HT) is also linked to more frequent and severe VMS compared with a natural menopause.65–67 This may be due to a more abrupt decline in gonadal steroids, which prevents a downward regulation of hormone receptors in the hypothalamus.68
VMS are a particular problem for breast cancer survivors.69,70 Hot flashes affect approximately 65% of women following treatment for breast cancer69,71,72 with many women rating them as severe.71,72 Hot flashes are even more prevalent among tamoxifen users, women taking aromatase inhibitors, or those who experience treatment-induced menopause.73–75 Among premenopausal women who receive both chemotherapy and anti-estrogen hormone therapy, the prevalence of vasomotor symptoms is as high as 90%76 and may lead to discontinuation of endocrine therapy. Nonadherence to endocrine therapy has been reported to range from 25% to 55%, with the development of adverse effects being the primary reason for non-adherence.77 Relief from hot flashes is a common request from breast cancer survivors.78
Racial/ethnic and cross-cultural differences
Racial/ethnic
Several studies have shown that black, or African-American, women experience more hot flashes compared to white women.6,18,30,51,79 Longitudinal data from SWAN show that a higher percentage of African-American women reported both any and frequent VMS across all stages of the menopause transition compared to other racial/ethnic groups.6 For example, in the transition from early to late perimenopause, approximately 80% of African-American women reported any hot flashes compared to about 65% of white women. Research suggests that the higher rates among African-Americans may in part be due to lower levels of E2 and higher BMI.51,79 However, racial differences may also be due to differences in perception and tolerance of temperature change. Several experimental studies have shown that African-Americans have lower levels of tolerance to cold80 and heat81,82 than Whites and that more African-Americans than Whites rate heat as being unpleasant.81
VMS symptom reporting has also been shown to vary by ethnicity.6,18,51,83 SWAN found that Hispanic women as a group tend to report somewhat more hot flashes compared to white women.6 This was also found in a study conducted in Texas.84 Multivariable analyses in SWAN suggest that the higher occurrence of VMS in Hispanics compared with non-Hispanic whites is largely due to factors such as education, anxiety, and depression.6 Additional SWAN analyses, however, have shown a marked variation across Hispanics of different origin.85 These analyses found that Central American women had the highest rates of VMS reporting and that Cuban women had the lowest rates relative to non-Hispanic Caucasians.85 These results suggest that, despite a shared language, Hispanic/Latina women from diverse racial/ethnic and cultural groups should not be considered a single group. These groups vary in terms of education, financial strain, health habits, and acculturation, all of which have been related to VMS.6
Women of Asian ethnicity consistently report fewer hot flashes and a shorter duration of VMS than other groups.30,51,86,87 SWAN data show that Chinese and Japanese women are the least likely to report any VMS or report them as bothersome when compared with other racial/ethnic groups.6,26,88 Some studies have suggested that higher soy intake among Asian women might account for these differences,89 but this was not the case in SWAN.6,44 Reasons for these differences are not fully understood and likely result from a combination of factors including lifestyle, genetics, psychosocial, and perceptual.
Cross-cultural
VMS reporting varies widely across different countries and cultures, with the lowest reporting in Asian countries and the highest in Europe and the United States.90 Despite the variation in prevalence, VMS are linked to stage of the menopausal transition consistently across numerous racial/ethnic and cultural groups.18,91 So what accounts for these differences? They appear to stem from both biologic factors, which likely affect occurrence of VMS, and nonbiologic factors, which likely affect perception or reporting of VMS.92 Examples of biologic factors related to both VMS and culture include:
Estrogen levels and genes involved in estrogen metabolism and receptors
Lifestyle behaviors such as smoking, diet (including phytoestrogens), and body composition
Gynecologic surgeries such as tubal ligation, hysterectomy, and oophorectomy
Examples of relevant non-biologic factors include:
Socioeconomic status indicators such as education
Symptom sensitivity
Attitudes towards menopause and VMS, e.g., “natural” versus bothersome or requiring medical intervention
Language and acculturation, which may affect specific words used for VMS
Conversational norms or acceptability of the topic
Comparisons of VMS across multiple cultures should be done based on consistent measurement methods, ideally within the same study. The changing racial and ethnic composition of the U.S. population requires greater awareness of ethnic diversities and variations in VMS reporting for optimal healthcare delivery.
Summary
VMS are the primary menopausal symptom, peaking around the final menstrual period and occurring in up to 80% of women during the menopausal transition. On average, they last for 10 years, with a longer duration in women with an earlier onset of symptoms. However, women show different patterns of experiencing VMS in terms of timing of onset and frequency level. Compared to non-Hispanic white women, black and Hispanic women are more likely and Asian women are less likely to report VMS, perhaps reflecting racial/differences in characteristics such as weight, smoking, and gynecological surgeries. Additional risk factors for VMS include body composition – although it appears to be protective in later stages of the menopausal transition – as well as smoking, prior and concurrent anxiety and depression, sensitivity to symptoms, PMS, education, and medical treatments such as hysterectomy, bilateral oophorectomy, and use of breast cancer-related endocrine therapies. It is important to keep in mind that while VMS are common during the menopausal transition, their patterns over time and within higher-risk subgroups are heterogeneous.
Synopsis.
Vasomotor symptoms (VMS) are the primary menopausal symptoms, occurring in up 80% of women and peaking around the final menstrual period. Average duration is 10 years, longer in women with an earlier onset. Compared to non-Hispanic white women, black and Hispanic women are more likely and Asian women are less likely to report VMS. Risk factors include body composition (in the early stage of the menopausal transition), smoking, anxiety, depression, sensitivity to symptoms, PMS, education, and medical treatments such as hysterectomy, oophorectomy, and breast cancer-related therapies. VMS patterns over time and within higher-risk subgroups are heterogeneous across women.
Key Points:
Vasomotor symptoms (VMS) occur in up to 80% of women during the menopausal transition, peaking near the final menstrual period.
On average, VMS last approximately 10 years, with a longer duration for women with an earlier onset.
Black (or African-American) and Hispanic women tend to report more hot flashes while Asian women report fewer hot flashes compared to non-Hispanic white women.
Cigarette smoking, higher levels of anxiety and depression, lower educational attainment, and premenstrual symptoms are risk factors for VMS. Data on physical activity, alcohol, and diet are inconsistent.
Women show several different patterns of timing and frequency of VMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to disclose.
References
- 1.Avis NE, Ory M, Matthews KA, et al. Health-related quality of life in a multiethnic sample of middle-aged women: Study of Women’s Health Across the Nation (SWAN). Med Care 2003;41(11):1262–76. Doi: 10.1097/01.MLR.0000093479.39115.AF. [DOI] [PubMed] [Google Scholar]
- 2.Avis NE, Colvin A, Bromberger JT, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause 2009;16(5):860–9. Doi: 10.1097/gme.0b013e3181a3cdaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumel JE, Chedraui P, Baron G, et al. A large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women. Menopause 2011;18(7):778–85. Doi: 10.1097/gme.0b013e318207851d. [DOI] [PubMed] [Google Scholar]
- 4.Williams RE, Levine KB, Kalilani L, et al. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas 2009;62(2):153–9. Doi: 10.1016/j.maturitas.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Woods NF, Mitchell ES. Symptoms during the perimenopause: prevalence, severity, trajectory, and significance in women’s lives. Am J Med 2005;118 Suppl 12B:14–24. Doi: 10.1016/j.amjmed.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women’s health across the nation. Am J Public Health 2006;96(7):1226–35. Doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn Ovarian Aging Study cohort. Menopause 2014;21(9). Doi: 10.1097/GME.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015;175(4):531–9. Doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman EW, Sammel MD, Lin H, et al. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol 2011;117(5):1095–104. Doi: 10.1097/AOG.0b013e318214f0de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams RE, Kalilani L, DiBenedetti DB, et al. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas 2007;58(4):348–58. Doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson WK, Ellison SA, Grason H, et al. Patterns of ambulatory care use for gynecologic conditions: A national study. Am J Obstet Gynecol 2001;184(4):523–30. Doi: 10.1067/mob.2001.111795. [DOI] [PubMed] [Google Scholar]
- 12.Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: The Study of Women’s Health Across the Nation. Am J Epidemiol 2009;170(6):766–74. Doi: 10.1093/aje/kwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, et al. Hot flashes and carotid intima media thickness among midlife women. Menopause 2011;18(12):352–8. Doi: 10.1161/STROKEAHA.116.014674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crandall CJ, Tseng CH, Crawford SL, et al. Association of menopausal vasomotor symptoms with increased bone turnover during the menopausal transition. J Bone Miner Res 2011;26(4):840–9. Doi: 10.1002/jbmr.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol 1999;181(1):66–70. [DOI] [PubMed] [Google Scholar]
- 16.Kronenberg F. Hot flashes: Epidemiology and physiology. Ann N Y Acad Sci 1990;592:52–86. [DOI] [PubMed] [Google Scholar]
- 17.Randolph JF Jr, Sowers M, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab 2005;90(11):6106–12. Doi: 10.1210/jc.2005-1374. [DOI] [PubMed] [Google Scholar]
- 18.Avis NE, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc Sci Med 2001;52(3):345–56. [DOI] [PubMed] [Google Scholar]
- 19.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril 2001;76:874–8. [DOI] [PubMed] [Google Scholar]
- 20.Harlow SD, Karvonen-Gutierrez C, Elliott MR, et al. It is not just menopause: symptom clustering in the Study of Women’s Health Across the Nation. Womens Midlife Health 2017;3(2):1–13. Doi: 10.1186/s40695-017-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cray LA, Woods NF, Herting JR, et al. Symptom clusters during the late reproductive stage through the early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause 2012;19(8):864–9. Doi: 10.1097/gme.0b013e31824790a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertil Steril 2012;97(4):843–51. Doi: 10.1016/j.fertnstert.2012.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avis NE, Crawford SL, McKinlay SM. Psychosocial, behavioral, and health factors related to menopause symptomatology. Womens Health 1997;3(2):103–20. [PubMed] [Google Scholar]
- 24.Williams RE, Kalilani L, DiBenedetti DB, et al. Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric 2008;11(1):32–43. Doi: 10.1080/13697130701744696. [DOI] [PubMed] [Google Scholar]
- 25.Hunter M, Gentry-Maharaj A, Ryan A, et al. Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10 418 British women aged 54–65. BJOG Int J Obstet Gynaecol 2012;119(1):40–50. Doi: 10.1111/j.1471-0528.2011.03166.x. [DOI] [PubMed] [Google Scholar]
- 26.Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: who is most bothered by vasomotor symptoms? Menopause 2008;15(5):841–7. Doi: 10.1097/gme.0b013e318168f09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievert L, Makhlouf Obermeyer C, Price K. Determinants of hot flashes and night sweats. Ann Hum Biol 2006;33(1):4–16. Doi: 10.1080/03014460500421338. [DOI] [PubMed] [Google Scholar]
- 28.Sievert LL. Subjective and objective measures of hot flashes. Am J Hum Biol 2013;25(5):573–80. Doi: 10.1002/ajhb.22415. [DOI] [PubMed] [Google Scholar]
- 29.Whiteley J, Wagner J-S, Bushmakin A, et al. Impact of the severity of vasomotor symptoms on health status, resource use, and productivity: Menopause 2013;20(5):1 Doi: 10.1097/GME.0b013e31827d38a5. [DOI] [PubMed] [Google Scholar]
- 30.Gold EB, Sternfeld B, Kelsey JL, et al. The relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol 2000;152:463–73. [DOI] [PubMed] [Google Scholar]
- 31.Politi MC, Schleinitz MD, Col NF Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med 2008;23(9):1507–13. Doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017;24(7):728–53. Doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 33.Grady D, Ettinger B, Tosteson ANA, et al. Predictors of difficulty when discontinuing postmenopausal hormone therapy. Obstet Gynecol 2003;102(6):1233–9. [DOI] [PubMed] [Google Scholar]
- 34.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial: Menopause 2010;17(5):946–54. Doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. J Am Med Assoc 2005;294(2):183–93. Doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 36.Espen Gjelsvik B, Straand J, Hunskaar S, et al. Use and discontinued use of menopausal hormone therapy by healthy women in Norway: the Hordaland Women’s Cohort study. Menopause 2014;21(5):459–68. Doi: 10.1097/GME.0b013e3182a11f2d. [DOI] [PubMed] [Google Scholar]
- 37.Ness J, Aronow WS, Beck G. Menopausal symptoms after cessation of hormone replacement therapy. Maturitas 2006;53(3):356–61. Doi: 10.1016/j.maturitas.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Gentry-Maharaj A, Karpinskyj C, Glazer C, et al. Use and perceived efficacy of complementary and alternative medicines after discontinuation of hormone therapy: a nested United Kingdom Collaborative Trial of Ovarian Cancer Screening cohort study. Menopause 2015;22(4):384–90. Doi: 10.1097/GME.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American College of Obstetricians and Gynecologists Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol 2014;123(1):202–16. Doi: 10.1097/01.AOG.0000441353.20693.78. [DOI] [PubMed] [Google Scholar]
- 40.Soules MR, Bremner WJ. The menopause and climacteric: Endocrinologic basis and associated symptomatology. J Am Geriatr Soc 1982;30(9):547–61. Doi: 10.1111/j.1532-5415.1982.tb05661.x. [DOI] [PubMed] [Google Scholar]
- 41.Nimrod A, Ryan KJ. Aromatization of androgens by human abdominal and breast fat tissue. J Clin Endocrinol Metab 1975;40(3):367–72. Doi: 10.1210/jcem-40-3-367. [DOI] [PubMed] [Google Scholar]
- 42.Herber-Gast G, Mishra GD, van der Schouw YT, et al. Risk factors for night sweats and hot flushes in midlife: results from a prospective cohort study. Menopause 2013;20(9):953–9. Doi: 10.1097/GME.0b013e3182844a7c. [DOI] [PubMed] [Google Scholar]
- 43.Gold EB, Crawford SL, Shelton JF, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: the Study of Womenʼs Health Across the Nation (SWAN). Menopause 2017;24(1):9–26. Doi: 10.1097/GME.0000000000000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gold EB, Block G, Crawford S, et al. Lifestyle and demographic factors in relation to vasomotor symptoms: Baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol 2004;159(12):1189–99. Doi: 10.1093/aje/kwh168. [DOI] [PubMed] [Google Scholar]
- 45.Guthrie JR, Smith AM, Dennerstein L, et al. Physical activity and the menopause experience: a cross-sectional study. Maturitas 1994;20:71–80. [DOI] [PubMed] [Google Scholar]
- 46.Sternfeld B, Quesenberry CP, Husson G. Habitual physical activity and menopausal symptoms: a case-control study. J Womens Health 1999;8(1):115–23. [DOI] [PubMed] [Google Scholar]
- 47.Elavsky S, McAuley E. Physical activity, symptoms, esteem, and life satisfaction during menopause. Maturitas 2005;52(3–4):374–85. Doi: 10.1016/j.maturitas.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Moilanen J, Aalto A-M, Hemminki E, et al. Prevalence of menopause symptoms and their association with lifestyle among Finnish middle-aged women. Maturitas 2010;67(4):368–74. Doi: 10.1016/j.maturitas.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Sternfeld B, Guthrie KA, Ensrud KE, et al. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause 2014;21(4):330–8. Doi: 10.1097/GME.0b013e31829e4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwingl P, Hulka B, Harlow S. Risk factors for menopausal hot flashes. Obstet Gynecol 1994;84(1):29–34. [PubMed] [Google Scholar]
- 51.Freeman EW, Sammel MD, Grisso JA, et al. Hot flashes in the late reproductive years: Risk factors for African American and Caucasian women. J Womens Health Gend Based Med 2001;10(1):67–76. Doi: 10.1089/152460901750067133. [DOI] [PubMed] [Google Scholar]
- 52.Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev 2007;17(4):CD001395 Doi: 10.1002/14651858.CD001395.pub3. [DOI] [PubMed] [Google Scholar]
- 53.Lermer MA, Morra A, Moineddin R, et al. Somatic and affective anxiety symptoms and menopausal hot flashes. Menopause 2011;18(2):129–32. Doi: 10.1097/gme.0b013e3181ec58f8. [DOI] [PubMed] [Google Scholar]
- 54.Freeman EW, Sammel MD, Lin H, et al. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause 2005;12(3):258–66. [DOI] [PubMed] [Google Scholar]
- 55.Freeman EW, Sammel MD, Lin H. Temporal associations of hot flashes and depression in the transition to menopause: Menopause 2009;16(4):728–34. Doi: 10.1097/gme.0b013e3181967e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunter MS, Chilcot J. Testing a cognitive model of menopausal hot flushes and night sweats. J Psychosom Res 2013;74(4):307–12. Doi: 10.1016/j.jpsychores.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Thurston RC, Blumenthal JA, Babyak MA, et al. Emotional antecedents of hot flashes during daily life. Psychosom Med 2005;67(1):137–46. Doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 58.Hanisch LJ, Hantsoo L, Freeman EW, et al. Hot flashes and panic attacks: a comparison of symptomatology, neurobiology, treatment, and a role for cognition. Psychol Bull 2008;134(2):247–69. Doi: 10.1037/0033-2909.134.2.247. [DOI] [PubMed] [Google Scholar]
- 59.Mishra GD, Dobson AJ. Using longitudinal profiles to characterize women’s symptoms through midlife: results from a large prospective study. Menopause 2012;19(5):549–55. Doi: 10.1097/gme.0b013e3182358d7c. [DOI] [PubMed] [Google Scholar]
- 60.Tepper PG, Brooks MM, Randolph JF, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition: Menopause 2016;23(10):1067–74. Doi: 10.1097/GME.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lasley BL, Santoro N, Randolf JF, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab 2002;87(8):3760–7. Doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 62.Lasley BL, Chen J, Stanczyk FZ, et al. Androstenediol complements estrogenic bioactivity during the menopausal transition: Menopause 2012;19(6):650–7. Doi: 10.1097/gme.0b013e31823df577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson LF, Pandeya N, Byles J, et al. Hot flushes and night sweats symptom profiles over a 17-year period in mid-aged women: The role of hysterectomy with ovarian conservation. Maturitas 2016;91:1–7. Doi: 10.1016/j.maturitas.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Zeleke BM, Bell RJ, Billah B, et al. Vasomotor and sexual symptoms in older Australian women: a cross-sectional study. Fertil Steril 2016;105(1):149–55. Doi: 10.1016/j.fertnstert.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 65.Benshushan A, Rojansky N, Chaviv M, et al. Climacteric symptoms in women undergoing risk-reducing bilateral salpingo-oophorectomy. Climacteric 2009;12(5):404–9. Doi: 10.1080/13697130902780846. [DOI] [PubMed] [Google Scholar]
- 66.Özdemir S, Çelik Ç, Görkemli H, et al. Compared effects of surgical and natural menopause on climacteric symptoms, osteoporosis, and metabolic syndrome. Int J Gynaecol Obstet 2009;106(1):57–61. Doi: 10.1016/j.ijgo.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 67.Hendrix SL. Bilateral oophorectomy and premature menopause. Am J Med 2005;118(12):131–5. Doi: 10.1016/j.amjmed.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 68.Bachmann GA. Vasomotor flushes in menopausal women. Am J Obstet Gynecol 1999;180:S312–316. [DOI] [PubMed] [Google Scholar]
- 69.Mom CH, Buijs C, Willemse PH, et al. Hot flushes in breast cancer patients. Crit Rev Oncol Hematol 2006;57(1):63–77. Doi: 10.1016/j.critrevonc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Davis SR, Panjari M, Robinson PJ, et al. Menopausal symptoms in breast cancer survivors nearly 6 years after diagnosis: Menopause 2014;21(10):1075–81. Doi: 10.1097/GME.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 71.Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol 1995;13(11):2737–44. Doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- 72.Carpenter JS, Andrykowski MA, Cordova M, et al. Hot flashes in postmenopausal women treated for breast carcinoma: prevalence, severity, correlates, management, and relation to quality of life. Cancer 1998;82:1682–91. [PubMed] [Google Scholar]
- 73.Vincent AJ, Ranasinha S, Sayakhot P, et al. Sleep difficulty mediates effects of vasomotor symptoms on mood in younger breast cancer survivors. Climacteric 2014;17(5):598–604. Doi: 10.3109/13697137.2014.900745. [DOI] [PubMed] [Google Scholar]
- 74.Olin JL, St PM. Aromatase inhibitors in breast cancer prevention. Ann Pharmacother 2014;48(12):1605–10. Doi: 10.1177/1060028014548416. [DOI] [PubMed] [Google Scholar]
- 75.Maunsell E, Goss PE, Chlebowski RT, et al. Quality of life in MAP.3 (Mammary Prevention 3): A randomized, placebo-controlled trial evaluating exemestane for prevention of breast cancer. J Clin Oncol 2014;32(14):1427–36. Doi: 10.1200/JCO.2013.51.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Biglia N, Cozzarella M, Cacciari F, et al. Menopause after breast cancer: a survey on breast cancer survivors. Maturitas 2003;45(1):29–38. [DOI] [PubMed] [Google Scholar]
- 77.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2007;107(2):167–80. Doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- 78.Hickey M, Saunders CM, Stuckey BG. Management of menopausal symptoms in patients with breast cancer: an evidence-based approach. Lancet Oncol 2005;6(1470–2045 (Print)):687–95. [DOI] [PubMed] [Google Scholar]
- 79.Miller SR, Gallicchio LM, Lewis LM, et al. Association between race and hot flashes in midlife women. Maturitas 2006;54(3):260–9. Doi: 10.1016/j.maturitas.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Walsh NE, Schoenfeld L, Ramamurthy S, et al. Normative model for cold pressor test. Am J Phys Med Rehabil 1989;68(1):6–11. [DOI] [PubMed] [Google Scholar]
- 81.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med 1999;61(3):346–54. [DOI] [PubMed] [Google Scholar]
- 82.Chapman WP, Jones CM. Variations in cutaneous and visceral pain sensitivity in normal subjects. J Clin Invest 1944;23(1):81–91. Doi: 10.1172/JCI101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adler SR, Fosket JR, Kagawa-Singer M, et al. Conceptualizing menopause and midlife: Chinese American and Chinese women in the US. Maturitas 2000;35(0378–5122):11–23. [DOI] [PubMed] [Google Scholar]
- 84.Simpkins JW, Brown K, Bae S, et al. Role of ethnicity in the expression of features of hot flashes. Maturitas 2009;63(4):341–6. Doi: 10.1016/j.maturitas.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green R, Polotsky AJ, Wildman RP, et al. Menopausal symptoms within a Hispanic cohort: SWAN, the Study of Women’s Health Across the Nation. Climacteric 2010;13(4):376–84. Doi: 10.3109/13697130903528272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avis NE, Kaufert PA, Lock M, et al. The evolution of menopausal symptoms. Baillières Clin Endocrinol Metab 1993;7(1):17–32. [DOI] [PubMed] [Google Scholar]
- 87.Haines CJ, Chung TK, Leung DH. A prospective study of the frequency of acute menopausal symptoms in Hong Kong Chinese women. Maturitas 1994;18(3):175–81. [DOI] [PubMed] [Google Scholar]
- 88.Green R, Santoro N. Menopausal symptoms and ethnicity: The Study of Women’s Health across the Nation. Womens Health 2009;5(2):127–33. Doi: 10.2217/17455057.5.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reed SD, Lampe JW, Qu C, et al. Premenopausal vasomotor symptoms in an ethnically diverse population: Menopause 2014;21(2):153–8. Doi: 10.1097/GME.0b013e3182952228. [DOI] [PubMed] [Google Scholar]
- 90.Obermeyer CM. Menopause across cultures: a review of the evidence. Menopause 2000;7(3):184–92. [DOI] [PubMed] [Google Scholar]
- 91.Obermeyer CM, Reher D, Saliba M. Symptoms, menopause status, and country differences: a comparative analysis from DAMES. Menopause 2007;14(4):788–97. Doi: 10.1097/gme.0b013e318046eb4a. [DOI] [PubMed] [Google Scholar]
- 92.Crawford SL. The roles of biologic and nonbiologic factors in cultural differences in vasomotor symptoms measured by surveys. Menopause 2007;14(4):725–33. Doi: 10.1097/GME.0b013e31802efbb2. [DOI] [PubMed] [Google Scholar]