Abstract
Purpose
To confirm the findings from a previous single-institution study of 572 patients from Memorial Sloan Kettering Cancer Center, in which we found that a significant proportion (49%) of patients recovered to their preoperative estimated glomerular filtration rate (eGFR) within 2 years following radical nephrectomy for renal cell carcinoma.
Materials and Methods
A multi-center retrospective study was conducted among 1928 patients using data contributed by three independent centers. The outcome of interest was postoperative recovery to preoperative eGFR. Data were analyzed using cumulative incidence and competing risks regression, with death from any cause treated as a competing event.
Results
This study demonstrated that 45% of patients recovered to their preoperative eGFR by 2 years following radical nephrectomy. Furthermore, this study confirmed that recovery of renal function differs according to preoperative renal function, such that patients with lower preoperative eGFR have an increased chance of recovery. This study also suggested that larger tumor size and female sex are significantly associated with increased chance of renal functional recovery.
Conclusions
In this multi-center retrospective study, we confirmed that over the long-term, a large proportion of patients recover to their preoperative renal function following radical nephrectomy for kidney tumors, and that recovery is more likely among those with lower preoperative eGFR.
Keywords: creatinine, estimated glomerular filtration rate, kidney cancer, radical nephrectomy, renal cell carcinoma, renal function
Introduction
Patients undergoing radical nephrectomy for renal tumors are at risk of a postoperative reduction in renal function due to loss of renal mass. Previous studies have shown that lower preoperative estimated glomerular filtration rate (eGFR), older age, and higher comorbidity are associated with lower postoperative eGFR and new onset chronic kidney disease following radical nephrectomy1–6. It is of interest to characterize the natural history of eGFR following radical nephrectomy for renal tumors in order to better understand long-term trends in renal functional recovery and to identify patient characteristics associated with postoperative renal functional recovery. We recently reported results from a study investigating the postoperative natural history of eGFR in patients who underwent radical nephrectomy for kidney cancer at Memorial Sloan Kettering Cancer (MSKCC) and found that nearly half (49%) of all patients recovered to their preoperative eGFR within 2 years following surgery7. Additionally, we found that eGFR recovery differed according to preoperative eGFR. In patients with preoperative eGFR < 60, younger age and female sex were also associated with higher chance of eGFR recovery, whereas in patients with preoperative eGFR ≥ 60, hypertension was associated with a lower chance of eGFR recovery and increased tumor size was associated with a higher chance of eGFR recovery7. In order to confirm these single center findings, a multi-center retrospective study was conducted from 3 centers performing a high volume of kidney surgery.
Materials and Methods
Data were contributed by Spectrum Health, Cleveland Clinic, and Mayo Clinic after institutional review board approval for retrospective data analysis. Patients from the same contemporary time period and meeting the same inclusion and exclusion criteria as the previous study7 were selected, specifically including patients with non-metastatic renal cell carcinoma who underwent radical nephrectomy between 2006 and 2013 and had not received systemic therapy. Patients were excluded if missing preoperative creatinine (n=62), race (n=47), age (n=1), tumor size (n=45), diabetes (n=9), or no postoperative creatinine (n=7), resulting in a final sample size for this analysis that includes 1928 patients with 24,066 serum creatinine measurements. The final sample included 323 patients from Spectrum Health, 932 patients from Cleveland Clinic, and 673 patients from Mayo Clinic. Serum creatinine values were used to calculate eGFR using the CKD-Epidemiology Collaboration formula as follows: eGFR (ml/min per 1.73m2) = 141 × min(SCr/k, 1)a × max(SCr/k, 1)−1.209 × 0.993Age × 1.018[if female] × 1.159[if black], where SCr is serum creatinine (mg/dl), k is 0.7 for female patients and 0.9 for male patients, a is −0.329 for female patients and −0.411 for male patients, min indicates the minimum of SCR/k or 1, and max indicates the maximum of SCr/k or 18.
The statistical methods in this study mirror those of the previous study7, as is common in a study attempting to confirm a previous finding. Rather than undertaking any variable selection or model building, we are simply including variables from the multivariable analysis from the prior study. Preoperative eGFR was dichotomized as ≥ 60 versus < 60 ml/min per 1.73 m2. We plotted the trajectory of each patient's eGFR over time from the immediate preoperative measurement through three years postoperatively, and used locally weighted scatterplot smoothing (LOWESS) to explore trends both overall and according to dichotomous preoperative eGFR. The association between patient and disease characteristics with preoperative eGFR was analyzed using logistic regression adjusted for study center, to account for possible differences across centers.
The outcome of interest in this study was postoperative recovery to preoperative eGFR, within a 5% margin of error. A competing risks analysis framework was used, with eGFR recovery as the primary event of interest and death from any cause as the competing event. Follow-up times were calculated from the date of radical nephrectomy, and patients alive and without eGFR recovery were censored at either their last eGFR measurement or 36 months, whichever came first. The cumulative incidence of eGFR recovery was estimated. Between-groups comparisons were made using competing risks regression adjusted for study center. Multivariable competing risks regression was stratified by dichotomous preoperative eGFR and incorporated factors identified in our prior study7, including age at surgery, sex, diabetes, hypertension, and tumor size7, with additional adjustment for study center.
A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using R software, version 3.2.5 (R Core Development Team, Vienna, Austria) including the ‘cmprsk’ package.
Results
Among the 1928 patients, 64.6% were male and median age at surgery was 64 (interquartile range (IQR): 54-72). Median preoperative eGFR was 71.9 ml/min/1.73m2 (IQR: 56.6 – 87.5). 70.1% of patients had preoperative eGFR ≥ 60 whereas 29.9% of patients had preoperative eGFR < 60. Patients with preoperative eGFR < 60 were older (median age at surgery 70 versus 61, p < .001) and more frequently had diabetes (27.6% versus 21.4%, p < .001) and hypertension (75.6% versus 59.2%, p < .001) as compared to patients with preoperative eGFR ≥ 60 (Table 1). Line plots with LOWESS trends revealed that all patients experienced a drop in eGFR immediately postoperatively, followed by a generally flat trend over time among those with preoperative eGFR ≥ 60 and a slightly upwards trend among those with preoperative eGFR < 60 (Figure 1A). These trends are broadly similar to what was seen in our prior study (Figure 1B), though in the MSKCC data we saw a more pronounced upward trend in both groups in the later part of follow-up7.
Table 1.
Patient characteristics by preoperative eGFR
| Overall (N = 1928) |
eGFR≥60 (N = 1351) |
eGFR<60 (N = 577) |
p-value* | Orignal MSKCC study (N = 572) |
|
|---|---|---|---|---|---|
| Age at surgery, median (IQR) | 64 (54, 72) | 61 (53, 69) | 70 (61, 77) | <.001 | 61 (53, 69) |
| Tumor size (cm), median (IQR) | 6.9 (4.8, 9.5) | 6.8 (4.7, 9.4) | 7.1 (5.0, 9.8) | 0.528 | 7.7 (5.5, 10.4) |
| Sex, N (%) | 0.731 | ||||
| Female | 683 (35.4) | 482 (35.7) | 201 (34.8) | 185 (32.3) | |
| Male | 1245 (64.6) | 869 (64.3) | 376 (65.2) | 387 (67.7) | |
| Race, N (%) | 0.414 | ||||
| Black | 117 (6.1) | 93 (6.9) | 24 (4.2) | 37 (6.5) | |
| Other | 54 (2.8) | 36 (2.7) | 18 (3.1) | 29 (5.1) | |
| White | 1757 (91.1) | 1222 (90.5) | 535 (92.7) | 506 (88.5) | |
| Diabetes, N (%) | <.001 | ||||
| No | 1480 (76.8) | 1062 (78.6) | 418 (72.4) | 476 (83.2) | |
| Yes | 448 (23.2) | 289 (21.4) | 159 (27.6) | 96 (16.8) | |
| Hypertension, N (%) | <.001 | ||||
| No | 692 (35.9) | 551 (40.8) | 141 (24.4) | 215 (37.6) | |
| Yes | 1236 (64.1) | 800 (59.2) | 436 (75.6) | 357 (62.4) |
p-value from logistic regression adjusted for study center
Figure 1.
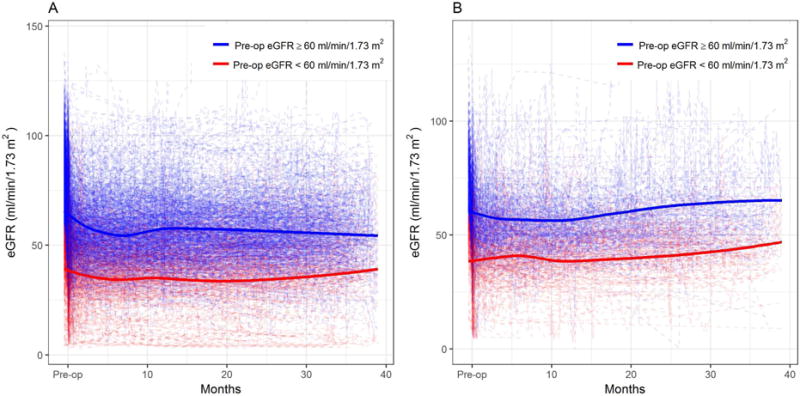
Postoperative eGFR trajectories according to preoperative eGFR in (A) the current multi-center study population and (B) the original MSKCC study (dotted lines represent individual patient data whereas solid lines represent LOWESS)
Median follow-up time among survivors was 3.7 years (IQR: 1.8 – 6.1). During follow-up, 883 patients experienced recovery to preoperative eGFR whereas 95 patients died without eGFR recovery. Whereas 499 patients recovered to within 5% of their preoperative eGFR, 384 recovered to an eGFR >5% higher than their preoperative level. Among these 384 patients, the median increase above their preoperative level was 8.8 ml/min/1.73 m2 (IQR: 5.6 – 13.8). To examine the time to eGFR recovery, we estimated the cumulative incidence of eGFR recovery, with death from any cause treated as a competing event, according to preoperative eGFR (Figure 2). We find significant differences in eGFR recovery according to preoperative eGFR, such that patients with higher preoperative eGFR were less likely to fully recover function (p < .001). Compared to the original result in the MSK data (dashed lines), the observed result is quite similar among those with preoperative eGFR >= 60 whereas the slope of the cumulative incidence of eGFR among those with preoperative eGFR < 60 is less steep.
Figure 2.
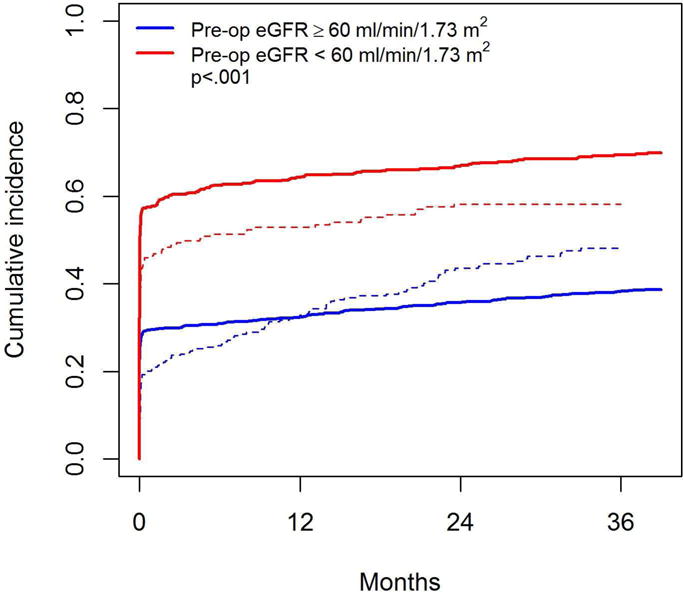
Cumulative incidence of eGFR recovery according to preoperative eGFR (solid lines represent results from the current multi-center study data; dashed lines represent results from the previous study using MSKCC data). The p-value is from competing risks regression adjusted for study center.
Overall, 42% and 45% of patients recovered to their baseline eGFR by 1 and 2 years postoperatively. The 1-year cumulative incidences of eGFR recovery were 32% and 64% and the 2-year cumulative incidences of eGFR recovery were 36% and 67%, among those with preoperative eGFR ≥ 60 and < 60, respectively. This validates the finding from our original study that nearly half of all patients have eGFR recovery long-term, and that frequency of recovery differs according to preoperative eGFR7. Unlike the previous study, median time to eGFR recovery was not reached in this study, as those in the preoperative eGFR ≥ 60 group did not experience as much late recovery so the cumulative incidence curve flattened off.
Multivariable competing risks regression incorporated age at surgery, sex, diabetes, hypertension, and tumor size, was stratified by preoperative eGFR, and additionally adjusted for study center (Table 2). Stratification was performed after identifying significant interaction effects with preoperative eGFR in the original study7. We observed that female sex is associated with a significantly increased chance of eGFR recovery among those with preoperative eGFR ≥ 60 (hazard ratio (HR): 1.33, 95% confidence interval (CI): 1.13 – 1.57). We also found that increasing tumor size is significantly associated with increased chance of eGFR recovery among both those with preoperative eGFR ≥ 60 (HR: 1.06, 95% CI: 1.04 – 1.08) and with preoperative eGFR < 60 (HR: 1.04, 95% CI: 1.02 – 1.06). These results differ somewhat from the original MSKCC study in that we did not find that younger age or female sex are associated with increased chance of eGFR recovery in those with preoperative eGFR < 60. We also did not find that hypertension is associated with decreased chance of eGFR recovery in those with preoperative eGFR ≥ 60.
Table 2.
Multivariable competing risks regression incorporating all factors shown in the table as well as study center
| Preoperative eGFR ≥ 60 | Preoperative eGFR < 60 | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age at surgery | 1.00 (0.99-1.00) | 0.400 | 0.99 (0.99-1.00) | 0.170 |
| Sex | 0.001 | 0.730 | ||
| Male | 1.00 | 1.00 | ||
| Female | 1.33 (1.13–1.57) | 1.03 (0.86–1.25) | ||
| Diabetes | 0.90 (0.71–1.14) | 0.390 | 0.95 (0.76–1.19) | 0.670 |
| Hypertension | 1.09 (0.91–1.30) | 0.370 | 1.12 (0.90–1.41) | 0.310 |
| Tumor size (cm) | 1.06 (1.04–1.08) | <.001 | 1.04 (1.02–1.06) | 0.001 |
Discussion
Overall this study confirms that a substantial proportion of patients experience eGFR recovery following radical nephrectomy, that this recovery differs according to preoperative eGFR, and that tumor size and patient sex are important factors associated with eGFR recovery. Patients with low preoperative eGFR and patients with larger tumors were more likely to experience renal functional recovery. This finding suggests that low eGFR should not be seen as a contraindication for a radical nephrectomy when such a procedure is otherwise indicated since the 1-year cumulative incidence of recovery was 64% in patients with preoperative eGFR < 60 in this study.
It is of course important to understand that renal functional recovery differs between patients undergoing radical nephrectomy versus partial nephrectomy, a debate that was initially begun by investigators comparing renal functional outcomes in patients with small renal tumors (T1a) undergoing partial nephrectomy to radical nephrectomy. In a recent review article, Li et al9 found that in both single-center retrospective studies as well as population-based studies, worse renal functional outcomes in small renal tumors (T1a,b) have been reported in patients undergoing radical versus partial nephrectomy, including higher postoperative mean serum creatinine, increased cumulative incidence of renal insufficiency, and increased rates of new onset chronic kidney disease. The centers in this study have a long established commitment to kidney sparing operations for patients with small renal tumors. The situation changes when urologists are confronted with large and locally advanced tumors for which radical nephrectomy is indicated. The focus of the current confirmatory study was to understand the renal functional impact radical nephrectomy and it was encouraging to find that many patients indeed recover their preoperative renal function, and sometimes even experience improved renal function, postoperatively.
A novel finding of our previous study, confirmed here, was that increased tumor size was significantly associated with increased chance of eGFR recovery. It is possible that the normal contralateral kidney is the major contributor to total eGFR in patients with large tumors and was already in the process of enhancing its contribution to overall renal function long before the index tumor was removed by radical nephrectomy. In a study of parenchymal volume and function of the contralateral kidney, Takagi et al10 found that the median increase in eGFR in the contralateral kidney was 2.3% in patients undergoing partial nephrectomy and 21.1% in patients undergoing radical nephrectomy. A study by Choi et al11 found that preoperative volume of both the affected and contralateral kidneys were higher among patients with lower CKD stage. The phenomenon of hyperfiltration and recovery of renal function as shown in donor nephrectomies12,13 as well as in animal studies14 is due to a decrease in functional renal volume, which reduces the afferent arteriolar resistance and increases the effective plasma flow. However, the biological mechanisms underlying this renal functional compensation in the contralateral kidney, and the patient and disease factors that may affect this compensation, are not well understood and further study is needed. Assuming that radical nephrectomy is being performed at high volume centers in patients with large tumors not amenable to kidney sparing approaches, research questions regarding the degree to which contralateral kidney functional compensation occurs prior to radical nephrectomy and continues after radical nephrectomy is of great interest as are the underlying physiological mechanisms that lead to these results.
A limitation of this study is the retrospective nature of the data; however, it is promising to see a similar pattern of results in this large, multi-center study as we found in our original single-center study. Creation of a binary time-to- event endpoint does not allow for detailed investigation of patterns over time, and there is clearly intra-patient variation over time as demonstrated in Figure 1. Originally, interest was in time to initial recovery of renal function, and this current study simply sought to confirm the previous findings. Future studies could rather categorize the measurement at each time point as having returned to baseline or not and look at longitudinal trends in renal functional recovery, or could examine the recovery status at the last measured timepoint. Furthermore, we acknowledge that renal function recovery is not the only significant outcome in patients following radical nephrectomy, and treatment decisions must also consider the impact of radical nephrectomy on cardiovascular and pulmonary function, which are outside the scope of the current study. Nevertheless, it is important to confirm our previously reported novel findings from a single-institution retrospective study and we have done so in a rigorous and hypothesis-driven manner, which lends strength to these results.
Conclusions
In this multi-center retrospective study, we confirmed that over the long-term, a substantial proportion of patients recover to their preoperative renal function following radical nephrectomy for kidney tumors. Renal function recovery is more likely among patients with lower preoperative eGFR and among patients with larger tumors. The biological mechanisms underlying this affect are not well understood, and further study, especially prospective study, is needed.
Acknowledgments
This work was supported by funding from the Core Grant (P30 CA008748). We acknowledge the contribution of Jessica L. Parker to this work.
Glossary
- eGFR
estimated glomerular filtration rate
- MSKCC
Memorial Sloan Kettering Cancer Center
- SCr
serum creatinine
- IQR
interquartile range
- HR
hazard ratio
- CI
confidence interval
- CKD
chronic kidney disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung JS, Son NH, Byun SS, et al. Trends in renal function after radical nephrectomy: a multicentre analysis. BJU international. 2014;113(3):408–415. doi: 10.1111/bju.12277. [DOI] [PubMed] [Google Scholar]
- 2.Jeon HG, Choo SH, Sung HH, et al. Small tumour size is associated with new-onset chronic kidney disease after radical nephrectomy in patients with renal cell carcinoma. European journal of cancer (Oxford, England : 1990) 2014;50(1):64–69. doi: 10.1016/j.ejca.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J. Predicting renal functional outcomes after surgery for renal cortical tumours: a multifactorial analysis. BJU international. 2010;106(4):489–492. doi: 10.1111/j.1464-410X.2009.09147.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark MA, Shikanov S, Raman JD, et al. Chronic kidney disease before and after partial nephrectomy. The Journal of urology. 2011;185(1):43–48. doi: 10.1016/j.juro.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Klarenbach S, Moore RB, Chapman DW, Dong J, Braam B. Adverse renal outcomes in subjects undergoing nephrectomy for renal tumors: a population-based analysis. European urology. 2011;59(3):333–339. doi: 10.1016/j.eururo.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Malcolm JB, Bagrodia A, Derweesh IH, et al. Comparison of rates and risk factors for developing chronic renal insufficiency, proteinuria and metabolic acidosis after radical or partial nephrectomy. BJU international. 2009;104(4):476–481. doi: 10.1111/j.1464-410X.2009.08376.x. [DOI] [PubMed] [Google Scholar]
- 7.Zabor EC, Furberg H, Mashni J, Lee B, Jaimes EA, Russo P. Factors Associated with Recovery of Renal Function following Radical Nephrectomy for Kidney Neoplasms. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(1):101–107. doi: 10.2215/CJN.04070415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Lau WL, Rhee CM, et al. Risk of chronic kidney disease after cancer nephrectomy. Nature reviews Nephrology. 2014;10(3):135–145. doi: 10.1038/nrneph.2013.273. [DOI] [PubMed] [Google Scholar]
- 10.Takagi T, Mir MC, Sharma N, et al. Compensatory hypertrophy after partial and radical nephrectomy in adults. The Journal of urology. 2014;192(6):1612–1618. doi: 10.1016/j.juro.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Choi DK, Jung SB, Park BH, et al. Compensatory Structural and Functional Adaptation after Radical Nephrectomy for Renal Cell Carcinoma According to Preoperative Stage of Chronic Kidney Disease. The Journal of urology. 2015;194(4):910–915. doi: 10.1016/j.juro.2015.04.093. [DOI] [PubMed] [Google Scholar]
- 12.Pabico RC, McKenna BA, Freeman RB. Renal function before and after unilateral nephrectomy in renal donors. Kidney international. 1975;8(3):166–175. doi: 10.1038/ki.1975.96. [DOI] [PubMed] [Google Scholar]
- 13.Strandgaard S, Kamper A, Skaarup P, Holstein-Rathlou NH, Leyssac PP, Munck O. Changes in glomerular filtration rate, lithium clearance and plasma protein clearances in the early phase after unilateral nephrectomy in living healthy renal transplant donors. Clinical science (London, England : 1979) 1988;75(6):655–659. doi: 10.1042/cs0750655. [DOI] [PubMed] [Google Scholar]
- 14.Shirley DG, Walter SJ. Acute and chronic changes in renal function following unilateral nephrectomy. Kidney international. 1991;40(1):62–68. doi: 10.1038/ki.1991.180. [DOI] [PubMed] [Google Scholar]


