Abstract
Probing the biophysical properties of the tumor niche offers a new perspective in cancer mechanobiology, and supports the development of next-generation diagnostics and therapeutics for cancer, in particular for metastasis.
Keywords: Biophysical cues, cancer, metastasis, mechanobiology, stem cell, microenvironment
Biophysical Cues in the Tumor Microenvironment
Research in cancer development and progression has focused in the past on the role of biochemical and molecular factors in the tumor. However, tumor cells are surrounded by a complex microenvironment of extracellular matrix (ECM) and subjected to a variety of biophysical cues including direct mechanical forces such as shear or compression, or the material properties of the ECM such as topography, porosity, and stiffness that can be just as important in influencing disease behavior. For example, the tumor stroma of breast cancers can be 10 times stiffer than the normal breast tissue [1], and increased breast density is seen in 30% of breast tumors, whereas well-known genetic mutations such as BRCA1 and BRCA2 only account for 5% of breast cancers [2]. Also, altered ECM states, such as increased stiffness, persist for long periods of time and have lower heterogeneity compared to protein or genetic markers, therefore being more reliable biomarkers. The process by which cells translate these mechanical signals into biological responses is called mechanotransduction.
The ECM biophysical homeostasis experienced by normal cells is perturbed in cancer [3]. The differences between normal and tumor ECMs are largely due to an increase in the deposition of fibrillar matrix components such as collagen or fibronectin, as well as an increase in ECM protein crosslinking and linearization [4,5]. The resulting matrix stiffness raises cell cytoskeletal tension, prompting the assembly of focal adhesions and the clustering of integrins, leading to increased integrin-dependent mechanotransduction in cancer cells that promotes growth, migration and metastatic behavior [5]. For instance, highly linearized collagen fibers at the boundaries of tumors contribute to the migration of cells away from the primary tumor, leading to more invasive cancers and to poorer patient outcomes [4]. Dense and crosslinked ECM proteins can also locally create pre-metastatic niches- sites that are favorable to cancer cell colonization and survival. Finally, an abnormal ECM also creates physical barriers that limit the permeability of drugs or immune cells, hindering the efficacy of therapies.
Targeting Biophysical Cues in Cancer
Differences in the physical properties of the tumor microenvironment can be used to study, to detect, and to better treat cancer (Figure 1). Technologies that measure and use high ECM strain and tumor stiffness as a biomarker in the early detection of tumors and pre-metastatic niches allow vital therapeutic interventions, since highly fibrotic and stiff tumor environments are linked to poorer patient prognosis and higher rates of recurrence [5].
Figure 1. Biophysical cues: interrogating, detecting and treating cancer and metastasis.
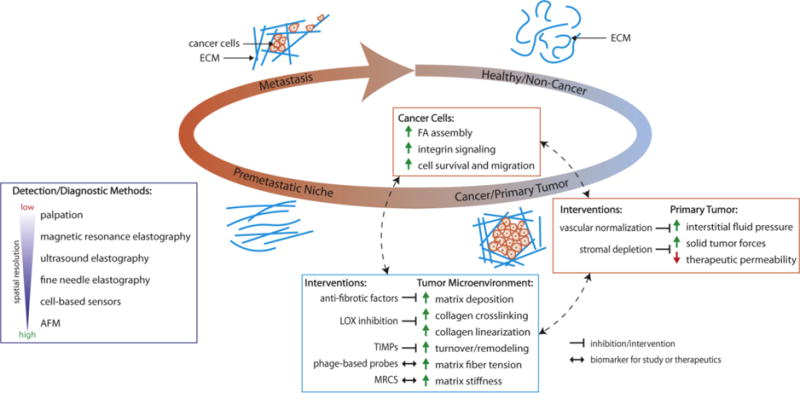
As cancer develops, the surrounding microenvironment undergoes biophysical changes which promote tumor proliferation and invasion, creating a feedback loop of cancer growth and metastasis. The unique biophysical properties of the tumor microenvironment present potential targets in the study, diagnosis, and treatment of cancer. Abbreviation: ECM, extracellular matrix; FA, focal adhesion; LOX, lysyl oxidase; TIMPs, tissue inhibitors of metalloproteinase; MRCS, mechano-responsive cell system; AFM, atomic force microscopy.
Shear-wave elastography, a technique which uses disturbances made by a focused ultrasound beam to mechanically interrogate tissues, provides quantitative and noninvasive measurement of local tissue stiffness and is used in the diagnosis of breast and other cancers. It remains difficult, however, to study the mechanics of cancer in vivo and at the cellular scale, since current physical measurements either require invasive biopsies or lack spatial resolution. Elastography techniques, for example, can only distinguish physical differences on the scale of millimeters, orders of magnitude larger than the microenvironment in contact with individual cells. Other technologies have been developed recently, such as phage-based probes that discriminate between relaxed or strained fibers of fibronectin [6], or Fine Needle Elastography that determines the local mechanical properties of tumor nodules with high precision and resolution down to 10 μm [7]. These higher resolution technologies provide new ways to study the effects of biophysical cues in the local microenvironment and on cell responses.
Current biophysical therapeutic strategies mostly focus on altering the ECM of tumors, following the premise that if increased ECM deposition and stiffness contribute to cancer growth, then inhibiting this process and restoring ECM homeostasis can potentially slow or prevent cancer progression. Such approaches include inhibition of lysyl oxidase (LOX) to prevent collagen crosslinking [5]; inhibition of angiotensin to reduce the production of collagen and hyaluronan [8]; and depletion of tumor stroma with drugs such as nab-paclitaxel [9]. Although these approaches show promise in reducing tumor stiffness and improving drug penetration, the exact molecular and cellular mechanisms of how these pathways affect ECM deposition are still unknown. Clinical trials targeting tissue inhibitors of metalloproteinases (TIMPs) show low specificity and unexpected toxicity [3], while ablating the tumor stroma completely by inhibiting fibroblast activation led to uncontrolled tumor growth [10].
A different approach relies on targeting directly the existing biophysical cues of the ECM, rather than targeting the pathways that modulate the matrix. To target stiffness in tumors, we developed a cell-based system – MRCS (mechano-responsive cell system), based on engineered mesenchymal stem cells (MSCs), which respond to high stiffness by driving the expression of cytosine deaminase (CD), and enzyme that locally converts the inactive chemotherapy drug (5-FC) into the active drug (5-FU) (Text Box 1, Figure I) [11].
Text Box 1. MRCS - a Mechano-Responsive Cell System that targets tumor stiffness.
The MRCS - mechano-responsive cell system -uses mesenchymal stem cells (MSCs) engineered with a mechanosensitive promoter to drive the expression of different reporters or therapeutics. MSCs are ideal vectors because they can differentiate in response to matrix elasticity [14]. When MRCS encounter high substrate stiffness, the transcriptional regulators YAP (Yes-associated protein) and TAZ (transcriptional co-activator with PDZ-binding motif) [15] localize to the nucleus and activate a mechanosensitive promoter that drives the downstream expression of desired genes. MRCS are only activated on tissues with increased stiffness, a hallmark of solid tumors, and can locally deliver cancer therapeutics while remaining inactive on healthy tissues, limiting off-target toxicity.
Figure I. Text Box 1. MRCS: local delivery of cancer therapeutics.
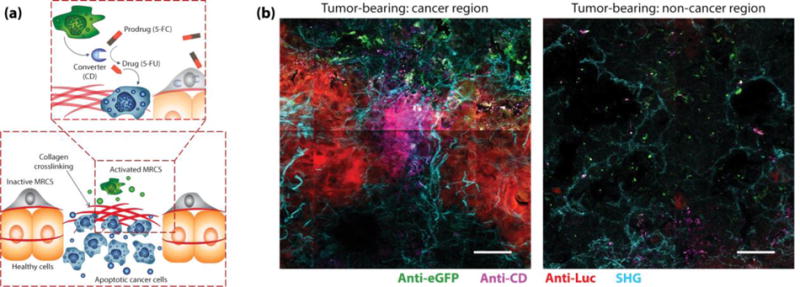
(a) In cancer tissues, the MRCS is selectively activated on stiff regions leading to production of cytosine deaminase (CD), an enzyme that converts the prodrug 5-Fluorocytosine (5-FC) into the active chemotherapy drug 5-Fluorouracil (5-FU). (b) Immunohistochemistry of lungs from tumor-bearing NOD scid gamma (NSG) mice 24 hours after infusion of MRCS. Selective release of CD (magenta) occurs in lung tumor regions (left), but not in regions without tumor (right). Breast cancer cells are labeled with firefly luciferase (red), and MRCS are labeled with eGFP (green). Second harmonic generation (SHG) imaging of collagen networks (cyan) is overlaid. Scale bar = 50 μm. Image adapted with permission from [11].
The MRCS attenuated the growth of metastatic cancer in murine models of human breast cancer lung metastasis without causing off-target normal tissue damage because the release of CD, production of 5-FU, and subsequent cell apoptosis occurred only locally in stiff crosslinked regions of the ECM (regions associated with cancer). Systems such as the MRCS represent a new paradigm in cancer therapy, in which biophysical cues of the tumor microenvironment act as a cancer biomarker, and targeting directly these cues increases the specificity of cancer treatment and lowers systemic toxicity and side effects. Next-generation combinatorial therapies could leverage targeting stiffness with MRCS or systems alike to improve the specificity of existing treatments.
Concluding Remarks and Future Perspectives
Biophysical cues are emerging as critical regulators of cancer progression, as detection biomarkers, and as therapeutic targets. Although the field has seen significant recent developments, many unexplored approaches and outstanding questions remain. For example, it is important to interrogate not only how these biophysical cues directly affect cancer cells, but also how do they affect tumor stromal cells, such as cancer associated fibroblasts or infiltrating immune cells such as macrophages. Cell-based stiffness sensors can reveal how cells actually “feel” in their native environment and dynamically interrogate the mechanobiology of primary tumors and metastases, and also the biophysical changes that occur during disease progression and in response to therapies at a cellular resolution in vivo. Platforms such as MRCS can be modified to express diagnostic reporters, for example the HSV-1-tk (herpes simplex virus type 1 thymidine kinase) gene, which can be readily introduced into cancer cells using herpes oncolytic viruses to catalyze phosphorylation of positron emission tomography (PET) radiotracers to improve imaging and detection of micrometastases [12]. Existing immune cell therapies, such as CAR-T cells, could also be further engineered to utilize the biophysical cues of tumors to improve targeting and specificity. Mechano-responsive CAR-T could, for example, induce cancer killing only in the presence of increased tissue stiffness in addition to a specific tumor antigen, such as HER2 for breast cancer, to reduce off-tumor activity. Further development of biomaterials may also provide a variety of new synthetic microenvironments in vitro for studying the effects of specific biophysical cues, such as stiffness or topography on cancer and cancer stromal cells. For example, 3D collagen gels in vitro have been used to show that increased stiffness and collagen crosslinking can induce malignancy in epithelium [13].
If pre-metastatic niches can be detected based on their distinct biophysical cues, then it may be possible to intervene early by restoring ECM homeostasis before cancer cells can engraft and successfully grow. Characterizing ECM environments after successful cancer therapy and associating the biophysical cues of these niches with cancer recurrence could lead to better predictions of patient outcomes. Monitoring biophysical changes that correlate with other types of signals, for example the correlation of oncogene expression with tissue stiffness, may lead to better combinatorial therapies. Finally, altered biophysical properties are also a hallmark of other disease states such as fibrosis, which correlates with higher incidence of cancer. If targeting biophysical cues can also treat fibrotic diseases, it may be an alternative way to mitigate the risk of developing cancer.
Acknowledgments
This work was supported by the NIH (1DP2CA195763-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Zhao is a co-founder of Baylx Inc that develops cell based therapeutics.
References
- 1.Lu P, et al. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sounni NE, Noel A. Targeting the Tumor Microenvironment for Cancer Therapy. Clin Chem. 2013;59:85–93. doi: 10.1373/clinchem.2012.185363. [DOI] [PubMed] [Google Scholar]
- 3.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Høye AM, Erler JT. Structural ECM components in the premetastatic and metastatic niche. Am J Physiol - Cell Physiol. 2016;310:C955–C967. doi: 10.1152/ajpcell.00326.2015. [DOI] [PubMed] [Google Scholar]
- 5.Levental KR, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao L, et al. Phage-based molecular probes that discriminate force-induced structural states of fibronectin in vivo. Proc Natl Acad Sci. 2012;109:7251–7256. doi: 10.1073/pnas.1118088109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickramaratne D, et al. Fine Needle Elastography (FNE) device for biomechanically determining local variations of tissue mechanical properties. J Biomech. 2015;48:81–88. doi: 10.1016/j.jbiomech.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 8.Jain RK, et al. The Role of Mechanical Forces in Tumor Growth and Therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garber K. Stromal Depletion Goes on Trial in. J Natl Cancer Inst. 2017;102:2008–2010. doi: 10.1093/jnci/djq113. [DOI] [PubMed] [Google Scholar]
- 10.Cox TR, Erler JT. Fibrosis and Cancer: Partners in Crime or Opposing Forces? Trends in Cancer. 2016;2:279–282. doi: 10.1016/j.trecan.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, et al. Mechanoresponsive stem cells to target cancer metastases through biophysical cues. Sci Transl Med. 2017;9:eaan2966. doi: 10.1126/scitranslmed.aan2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brader P, et al. Imaging of lymph node micrometastases using an oncolytic herpes virus and [18F]FEAU PET. PLoS One. 2009;4:e4789. doi: 10.1371/journal.pone.0004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu L, Mooney DJ. Biomaterials and emerging anticancer therapeutics: Engineering the microenvironment. Nat Rev Cancer. 2016;16:56–66. doi: 10.1038/nrc.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engler AJ, et al. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]


