Abstract
Background.
We hypothesized that metastatic colorectal cancer (mCRC) patients whose tumors had low thymidylate synthase (TS-L) expression would have a higher response rate with 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX)/bevacizumab (Bev) than patients with high TS (TS-H) tumors; and irinotecan, oxaliplatin (IROX)/Bev would be more effective than FOLFOX/Bev in TS-H tumors.
Methods.
TS protein expression was determined in mCRC tissue. Patients with TS-L tumors received FOLFOX/Bev. Patients with TS-H tumors were randomly assigned to FOLFOX/Bev or IROX/Bev. Primary endpoint was response rate (CR+PR).
Results.
211 of 247 patients (70% TS-H) were registered to the treatment phase. Efficacy analyses included eligible patients who started treatment (N=186). The CR+PR rates for IROX/Bev (TS-H), FOLFOX/Bev (TS-H) and FOLFOX/Bev (TS-L) were 33%, 38%, and 49%, respectively (P = NS). Median progression free survival (PFS) was 10 months (95% CI, 9–12): IROX/Bev (TS-H) 10, FOLFOX/Bev (TS-H) 9, and FOLFOX/Bev (TS-L) 13 months. The TS-L group had improved PFS compared with the TS-H group treated with FOLFOX/Bev: PFS HR was 1.6 (95% CI, 1.0–2.4) (P=0.04, Cox Regression). Median overall survival (OS) was 22 months (95% CI, 20 29): IROX/Bev (TS-H) 18, FOLFOX/Bev (TS-H) 21 and (TS-L) 32 months. The OS comparison for the two TS-H arms and the FOLFOX/Bev in TS-H vs TS-L was not significantly different.
Conclusions.
TS expression was prognostic: TS-L patients receiving FOLFOX/Bev had a longer PFS than TS-H patients, and a trend to longer OS. Patients with TS-H tumors did not benefit more with IROX/Bev than FOLFOX/Bev.
Keywords: Thymidylate synthase, 5-Fluorouracil, Prognostic factors, Predictive factors, Oxaliplatin, Irinotecan, Bevacizumab, Colorectal cancer
Condensed Abstract:
Colorectal cancer patients were assigned treatment based on thymidylate synthase protein (TS) expression (low versus high) in tumor tissue to a combination chemotherapy regimen that either contained 5-fluorouracil (5-FU) or not. Patients with TS-L tumors treated with 5-FU, leucovorin, oxaliplatin and bevacizumab (FOLFOX/bev) had a longer PFS than TS-H patients, and a trend to longer OS; patients with TS-H tumors did not benefit more with irinotecan, oxaliplatin and bevacizumab than FOLFOX/Bev.
Introduction
Thymidylate synthase (TS) plays a central role in providing thymidine triphosphate for DNA synthesis and repair and is an important intracellular target of 5-fluorouracil (5-FU). Inhibition of TS results in accumulation of intracellular deoxyuridine monophosphate; both deoxyuridine triphosphate and fluorodeoxyuridine triphosphate are misincorporated into DNA causing direct damage and interference with repair (1). Several studies reported that low tumor TS expression was associated with a higher response rate to 5-FU-based therapy than tumors with high TS (2–9). We hypothesized that metastatic colorectal cancer patients (mCRC) whose tumors had TS-H protein expression would have a lower overall response rate (CR+PR) to treatment with FOLFOX (5-FU, leucovorin, oxaliplatin)/bevacizumab (Bev) than TS-L tumors (10–11). In addition, we hypothesized that cancers with TS-H expression would have a higher response rate with a non-fluoropyrimidine regimen IROX (irinotecan, oxaliplatin) /Bev compared to FOLFOX/Bev (12). The objectives of the trial were to evaluate the response rates of patients with mCRC treated with FOLFOX/Bev or IROX/Bev; to compare the response rates, progression-free survival (PFS), overall survival (OS) and toxicity of patients with TS-H tumor expression treated with either FOLFOX/Bev or IROX/Bev; and to compare these parameters in TS-H tumor expression to those with TS-L expression treated with FOLFOX/Bev.
Patients and Methods
Eligibility
Eligibility criteria included mCRC patient age 18 or older with no prior therapy for metastatic or locally recurrent disease, measurable disease, ECOG performance status of 0–2, ANC > 1500/μL, platelets > 100,000/μL, total bilirubin of < 1.5 mg/dL, AST and ALT < 3 x upper limits of normal, normal serum creatinine, and an INR < 1.5, unless the patient was on full dose anticoagulation. No patient could have a myocardial infarction, New York Heart Association class III-IV myocardial disease, or unstable angina within 6 months of study entry. Patient could not have symptomatic arrhythmia, a history of significant peripheral artery disease, grade 2 or worse peripheral neuropathy. No history of stroke or transient ischemic attack within 6 months of study entry was permitted. Pregnant or breast-feeding females were excluded. Prior adjuvant therapy was permitted if it was completed 12 months prior to enrolling in the current study. IRB approval was obtained and written informed consent was required for all patients.
Design Summary
Tissue submission from metastatic tumor to the ECOG/ACRIN Pathology Coordinating Office (PCO) was mandatory for registration prior to treatment assignment: Step 1. Treatment assignment was based upon TS expression in recurrent or metastatic tumor measured by immunohistochemical staining, as previously described (13–15). Patients moved to step 2 once TS expression was determined. Those with TS-H expression (2+ or higher) were randomized equally using a permuted blocks algorithm to one of two arms: IROX/Bev or FOLFOX/Bev. Those with TS-L expression (0 or 1+) or indeterminate (the sample contained no tumor, had an insufficient cell number, or was deemed to be not evaluable) were assigned to FOLFOX/Bev. In all three arms, Bev 5 mg/kg was given IV over 30–90 min. Arm A: IROX/Bev involved oxaliplatin 65 mg/m2/120 min IV, then irinotecan 120 mg/m2/90 IV (10, 12). Arm B: FOLFOX/Bev consisted of oxaliplatin 85 mg/m2 given with leucovorin 400 mg/m2/120 min IV, then 5-FU bolus 400 mg/m2 and 5-FU infusion 2400 mg/m2/46 hr (11–12). Arm C: FOLFOX/Bev employed the same doses as Arm B. Each cycle consisted of treatment given on days 1 & 15 of a 28-day cycle. Dose modifications can be found in the Supplementary Material.
Immunohistochemical Method for TS Protein Expression
The specimens were analyzed in the ECOG central pathology office at Northwestern University Medical Center following the methodology described by Allegra et al (15). Details of the method can be found in the Supplementary Material.
Statistical Considerations
The sample size was determined based on the TS-L and TS-H expression patients. Patients with indeterminate TS expression were entered on Arm C but were excluded in the power calculations for the primary analysis. It was expected that 144 TS-H expression patients would be randomized equally to arms A or B with the expectation that 138 patients would be eligible (69 eligible per arm). It was expected that 98 low/indeterminate TS expression patients would be assigned to treatment (Arm C) with the expectation that 87 patients would be eligible with TS-L expression. A total of 246 patients (236 eligible, 69 on Arms A and B; 98 on Arm C) would be entered onto this trial in a two-stage design with 117 patients in the first stage. A planned interim analysis for response determination occurred after the accrual of 121 patients registered to the treatment phase. Additional information regarding the statistical design can be found in the Supplementary Material. Following this assessment, all 3 arms of the study were reopened in an effort to provide more precision on the estimates of response rates to narrow the confidence intervals.
Toxicities were assessed using the NCI Common Toxicity Criteria (CTC) Version 3.0. Objective tumor response, categorical patient characteristics, as well as toxicity, were compared using Fisher’s exact tests with a two-sided significance level of 0.05 (16). Continuous patient characteristics were compared using Kruskal-Wallis tests. OS was defined as the time from registration to step 2 of the study until death (event), or censored at last known date of survival. PFS was defined as the time from registration to step 2 of the study to progression or death without evidence of progression. For cases without documentation of progression, follow-up was censored at the date of last disease assessment without progression, unless death occurred within a short period of time (4 months) following the date last known progression-free, in which case the death was counted as an event. OS and PFS were estimated using the Kaplan-Meier method, with 95% confidence intervals calculated using Greenwood’s formula, and compared by the log rank test (16–17). Cox regression models of OS and PFS were utilized to provide hazard ratio estimates and associated inferences with use of the Wald test (18). All P-values reported were for two-sided significance tests and P-values less than 0.05 were considered significant.
Results
Patient Characteristics
This study was active between July, 2005 and April, 2012. Accrual was 247 patients to step 1, and 211 patients (70% TS-H; 30% TS-L/indeterminate) to the treatment phase, of whom 186 patients deemed eligible started treatment. 247 patients were registered to step 1. 36 patients did not go on to step 2: 9 were ineligible; 6 withdrew or were never registered; 3 had unknown TS status; 2 had either disease progression or decline in performance status; 15 patients with TS-L could not enter step 2 as arm C was suspended; 1 patient had no insurance. 211 patients were registered to step 2. Efficacy analyses included eligible patients who started treatment (N=186) and the safety analysis (toxicity) included all patients who started treatment (N=205). 73 patients were randomized to arm A; 70 of these received treatment; 9 were deemed ineligible for the following reasons: 6 had baseline scan > 4 weeks prior; 1 had non-measurable disease; 1 had an ANC below 1500/μL, and 1 had another reason. 3 patients did not get treatment due to ineligibility (2) or insurance denial (1). 70 patients were analyzed for toxicity, and 61 were analyzed for efficacy. Of 75 patients randomized to Arm B, 73 got treatment. Of these, 7 were deemed ineligible for the following reasons: 6 had baseline scans > 4 weeks prior; 1 had an elevated bilirubin. 2 patients refused treatment. 73 patients were analyzed for toxicity, and 66 were analyzed for efficacy. Of 63 patients allocated to arm C, 62 received therapy. 3 were deemed ineligible due to baseline scans > 4 weeks prior. 1 patient refused therapy. 62 patients were analyzed for toxicity, and 59 were analyzed for efficacy. There were 5 patients with indeterminate TS expression levels (2.4%).
Patient Characteristics
For all 186 eligible and treated patients, 127 patients had TS-H expression (68%) and 59 had TS-L/indeterminate expression (32%). The demographics are shown in Table 1. There was a higher proportion of PS 0 patients (59.1%) on TS-H (FOLFOX/Bev) (P=0.03), compared to 39.3% (TS-H, IROX/Bev) and 40.7% (TS-L, FOLFOX/Bev). Race, age, gender and disease status were not statistically different among the treatment arms. Fewer than 10% of patients received prior adjuvant therapy. Over 80% had liver metastasis, about 30% had lung or lymph node metastasis, and less than 10% had bone or peritoneal metastasis. Information on the location of the primary tumor was not recorded. The tumor characteristics were not statistically different among the treatment arms.
Table 1.
Baseline Clinical Characteristics of Eligible and Treated Patients
| Total (n=186) |
TS-High | TS-Low |
P-value |
||
|---|---|---|---|---|---|
| IROX/Bev (n-61) |
FOLFOX/Bev (n=66) |
FOLFOX/Bev (n=59) |
|||
| Age (years) Median (Min - Max) |
60 (29–85) |
61 (37–85) |
60 (31–79) |
59 (29–80) |
0.51 (K) |
| Gender n (%) Male Female |
111 (59.7) 75 (40.3) |
40 (65.6) 21 (34.4) |
36 (54.5) 30 (45.5) |
35 (59.3) 24 (40.7 |
0.45 (E) |
| Race n (%) Hispanic Non-Hispanic White Non-Hispanic Black Other Missing/Unknown |
8 (4/3) 148 (79.6) 25 (13.4) 4 (2.2) 1 (0.5) |
3 (4.9) 47 (77.0) 10 (16.4) 1 (1.6) 0 |
3 (4/5) 56 (84.8) 5 (7.6) 2 (3.0) 0 |
2 (3.4) 45 (76.3) 10 (16.9) 1 (1.7) 1 (1.7) |
0.69 (E) |
| PS n (%) 0 1 2 |
87 (46.8) 92 (49.5) 7 (3.8) |
24 (39.3) 32 (52.5) 1 (1.6) |
39 (59.1) 27 (40.9) 0 |
24 (40.7) 33 (55.9) 2 (3.4) |
0.03 (E) |
| Disease Status n (%) Initial Diagnosis Recurrence |
150 (80.6) 36 (19.4) |
50 (82.0) 11 (18.0) |
50 (75.8) 16 (24.2) |
50 (84.7) 9 (15.3) |
0.44 (E) |
| Prior Adjuvant Therapy n (%) Yes No Unknown |
16 (8.6) 168 (90.3) 2 (1.1) |
10 (16.4) 50 (82.0) 1 (1.6) |
4 (6.1) 61 (92.4) 1(1.5) |
2 (3.4) 57 (96.6) 0 (0) |
0.024 (C) |
| Metastasis to Liver n (%) Yes No unknown |
155 (83.3) 28 (15.1) 3 (1.6) |
50 (82.0) 10 (16.4) 1 (1.6) |
57 (86.4) 7 (10.6) 2 (3.0) |
48 (81.4) 11 (18.6) 0 (0) |
0.464 (C) |
| Metastasis to Lung n (%) Yes No unknown |
59 (31.7) 123 (66.1) 4 (2.2) |
20 (32.8) 39 (63.9) 2 (3.3) |
18 (27.3) 47 (71.2) 1 (1.5) |
21 (35.6) 37 (62.7) 1 (1.7) |
0.576 (C) |
| Metastasis to Bone n (%) Yes No unknown |
12 (6.5) 166 (89.2) 8 (4.3) |
3 (4.9) 55 (90.2) 3 (4.9) |
6 (9.1) 56 (84.8) 4 (6.1) |
3 (5.1) 55 (93.2) 1 (1.7) |
0.521 (C) |
| Metastasis to Peritoneum n (%) Yes No unknown |
14 (7.5) 166 (89.2) 6 (3.2) |
4 (6.6) 54 (88.5) 3 (4.9) |
4 (6.1) 60 (90.9) 2 (3.0) |
6 (10.2) 52 (88.1) 1 (1.7) |
0.669 (C) |
| Primary tumor / Direct extension Yes No unknown |
20 (10.8) 161 (86.6) 6 (3.2) |
5 (8.2) 43 (86.9) 3 (4.9) |
5 (7.6) 59 (89.4) 2 (3.0) |
10 (16.9) 49 (83.1) 0 |
0.210 (C) |
| Metastasis to Lymph nodes n (%) Yes No unknown |
63 (33.9) 115 (61.8) 8 (4.3) |
19 (31.1) 39 (63.9) 3 (4.9) |
26 (39.4) 38 (57.6) 2 (3.0) |
18 (30.5) 38 (64.4) 3 (5.1) |
0.549 (C) |
TS = thymidylate synthase; IROX = irinotecan and oxaliplatin; Bev = bevacizumab; FOLFOX = folinic acid (leucovorin), 5-Fluorouracil, and oxaliplatin; E = Exact Test for RxC Tables; K = Kruskal-Wallis Test; C = Chi-square analysis (unknowns excluded). The TS-Low cohort includes both TS-Low and TS-indeterminate expression
Clinical Outcomes
The median number of treatment cycles was 6 for all arms (range 1 −32). The distribution of the reasons for treatment termination varied significantly among the three arms (P =0.02). A higher proportion of patients stopped treatment due to progressive disease and died on study in the TS-H IROX/Bev arm (Table 2). A higher proportion of patients stopped for toxicity/side effects in the TS-H FOLFOX/Bev arm. Point estimates for the response rate favored the FOLFOX/Bev arms, with the highest response rate of 49% in the TS-L group (Table 2). However, no statistically-significant differences in pre-planned objective response rate comparisons were observed in the two comparisons TS-H (IROX/Bev vs FOLFOX/Bev) or FOLFOX/Bev (TS-H vs TS-L).
Table 2.
Clinical Outcomes among Treated and Eligible Patients (N = 186)
| TS-High Stratum | TS-Low Stratum | ||
|---|---|---|---|
| IROX/Bev | FOLFOX/Bev | FOLFOX/Bev | |
| (n=61) | (n= 66) | (n=59) | |
| Stopped for PD | 45.9% | 25.8% | 39% |
| Stopped for Toxicity | 13.1% | 25.8% | 13.6 |
| Death on Study | 9.8% | 1.5% | 0% |
| CR + PR (90% CI) |
33% (23–44) |
38% (28–49) |
49% (38–61) |
| CR | 0 | 2 (3.0%) | 1 (1.7%) |
| PR | 20 (32.8%) | 23 (34.8%) | 28 (47.5%) |
| SD | 25 (41.0%) | 36 (54.5%) | 23 (39.0%) |
| PD | 2 (3.3%) | 1 (1.5%) | 0 |
| Unevaluable | 14 (23.0%) | 4 (6.1%) | 7 (11.9%) |
| Subsequent Surgery | 5 (8.2%) | 7 (10.6%) | 15 (25.4%) |
TS = thymidylate synthase; IROX = irinotecan and oxaliplatin; Bev = bevacizumab; FOLFOX = folinic acid (leucovorin), 5-Fluorouracil, and oxaliplatin PD = progressive disease; CR = complete response; PR = partial response; SD = stable disease. The TS-Low cohort includes both TS-Low and TS-indeterminate expression
Table 3 shows the clinical toxicity by treatment arm for all 205 treated patients. Table 4 shows the comparison of cumulative grade 3–4 toxicities by treatment arm. No differences were found in the two comparisons of grade 3 non-hematologic toxicity or grade 4 hematologic toxicity: TS-H (IROX/Bev vs FOLFOX/Bev) and FOLFOX/Bev (TS-H vs TS-L). However, there were differences in the proportion of subjects with Grade 3+ diarrhea for the two comparisons, with higher toxicity seen in patients with TS-L tumors than TS-H tumors treated with FOLFOX/Bev; TS-H patients treated with IROX/bev also had a higher incidence of grade 3 diarrhea than the TS-H patients treated with FOLFOX/Bev. Eleven patients (5.9%) experienced lethal events, and 10 of 11 were felt to be related to underlying colorectal cancer, rather than treatment-associated toxicity. Twenty-six patients underwent subsequent surgery. A higher proportion of TS-L patients treated with FOLFOX/Bev had post-chemotherapy surgery (P = 0.030, Chi-square test). Because this was not a study endpoint, specific details on the nature and outcome of the surgeries are not available.
Table 3.
Percentage of Patients with Toxicity by Treatment Arm
| Treatment Arm | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TS-High Stratum | TS-Low Stratum | ||||||||
| Toxicity Type | IROX/Bev (n=70) | FOLFOX/Bev (n=73) | FOLFOX/Bev (n=70) | ||||||
| Grade | Grade | Grade | |||||||
| 1–2 | 3 | 4 | 1–2 | 3 | 4 | 1–2 | 3 | 4 | |
| Allergic reaction | 10 | 3 | - | 3 | 3 | - | - | 2 | - |
| Hemoglobin | 74 | 4 | - | 75 | 1 | - | 79 | 2 | 2 |
| Neutrophils | 27 | 13 | 10 | 23 | 27 | 11 | 26 | 18 | 16 |
| Platelets | 34 | 1 | - | 56 | - | - | 52 | - | 2 |
| Hypertension | 20 | 6 | - | 25 | 5 | - | 11 | 8 | - |
| Fatigue | 49 | 21 | - | 74 | 11 | 73 | 6 | - | |
| Hand-Foot syndrome | 1 | - | - | 12 | 1 | - | 13 | 6 | - |
| Anorexia | 47 | 3 | - | 40 | 1 | - | 44 | 2 | - |
| Constipation | 29 | - | - | 30 | 3 | - | 34 | 2 | - |
| Dehydration | 9 | 4 | 1 | 11 | 1 | - | 10 | 3 | - |
| Diarrhea w/o prior ostomy | 61 | 11 | 1 | 56 | 1 | 1 | 53 | 11 | - |
| Stomatitis | 20 | - | - | 32 | 3 | - | 34 | 2 | - |
| Nausea | 63 | 3 | - | 68 | 4 | - | 53 | 3 | - |
| Perforation of bowel | - | - | - | - | - | 3 | - | 3 | - |
| Taste disturbance | 20 | - | - | 32 | - | - | 23 | - | - |
| Vomiting | 49 | - | - | 23 | 5 | - | 18 | - | - |
| Hemorrhage: Nose | 20 | - | - | 32 | - | - | 26 | 2 | - |
| Hemorrhage: other site | 4 | - | - | 8 | 1 | - | 13 | - | - |
| Neuropathy-Sensory | 61 | 11 | - | 68 | 16 | - | 55 | 24 | - |
| Neuropathy-motor | 3 | 1 | - | 1 | - | - | 2 | 2 | - |
| Vascular access, thrombosis | - | - | 1 | - | - | 1 | 2 | - | - |
| Thrombosis, embolism | - | 1 | 4 | 3 | 7 | 3 | 2 | 6 | 2 |
IROX = irinotecan and oxaliplatin; Bev = bevacizumab; FOLFOX = folinic acid (leucovorin), 5-Fluorouracil, and oxaliplatin. The TS-Low cohort includes both TS-Low and TS-indeterminate expression.
Table 4.
Proportion of Toxicities by Treatment Arm
|
Toxicity Type |
TS-High Stratum | TS-Low Stratum | |||
|---|---|---|---|---|---|
| Arm A IROX/Bev (n=70) |
Arm B FOLFOX/Bev (n=73) |
Arm C FOLFOX/Bev (n=62) |
P-Value Fischer’s exact test |
||
| Proportion (90% CI) | Proportion (90% CI) | Proportion (90% CI) | A vs B | B vs C | |
| Grade 3+ non-hematologic | 61 (51–71) | 55 (44–65) | 58 (47–69) | 0.50 | 0.63 |
| Grade 4+ hematology | 10 (6–20) | 11 (6–19) | 26 (9–26) | 0.99 | 0.45 |
| Grade 3+ diarrhea | 11 (6–20) | 1 (0.1–6) | 11 (5–20) | 0.02 | 0.02 |
| Grade 5 toxicity | 13 (7–21) | 3 (0.5–8) | 0 (0–5) | 0.03 | 0.50 |
TS = thymidylate synthase; IROX = irinotecan and oxaliplatin; Bev = bevacizumab; FOLFOX = folinic acid (leucovorin), 5-Fluorouracil, and oxaliplatin; CI = confidence interval. The TS-Low cohort includes both TS-Low and TS-indeterminate expression.
Overall and Progression-Free Survival
Among 182 evaluable cases for the PFS analysis, there were a total of 142 events, including 125 deaths. The overall PFS and OS were 10 months (95% CI 9–12) and 22 months (95% CI 20–29). Median PFS and OS for the 3 arms are shown in Table 5. PFS was superior in TS-L patients compared to TS-H patients treated with either arm. Figure 1 shows the Kaplan-Meier PFS and OS curves, respectively, according to treatment arm.
Table 5.
Univariate Analysis of Progression-Free and Overall Survival among Eligible and Treated Patients
| PFS in months Median (95% CI) |
OS in months Median (95% CI) |
P-value Log-rank |
|||
|---|---|---|---|---|---|
| All patients | 10 (9–12) | 22 (20–29) | PFS | OS | |
| High TS | Arm A IROX/Bev | 10 (6–11) | 18 (14–23) | A vs B 0.41 B vs C 0.04 |
A vs B 0.15 B vs C 0.07 |
| Arm B FOLFOX/Bev |
9 (8–11) | 21 (16–32) | |||
| TS Low | Arm C FOLFOX/Bev |
13 (10–14) | 32 (23–44) | ||
TS = thymidylate synthase; IROX = irinotecan and oxaliplatin; Bev = bevacizumab; FOLFOX = folinic acid (leucovorin), 5-Fluorouracil, and oxaliplatin; CI = confidence interval. The TS-Low cohort includes both TS-Low and TS-indeterminate expression
Figure 1.
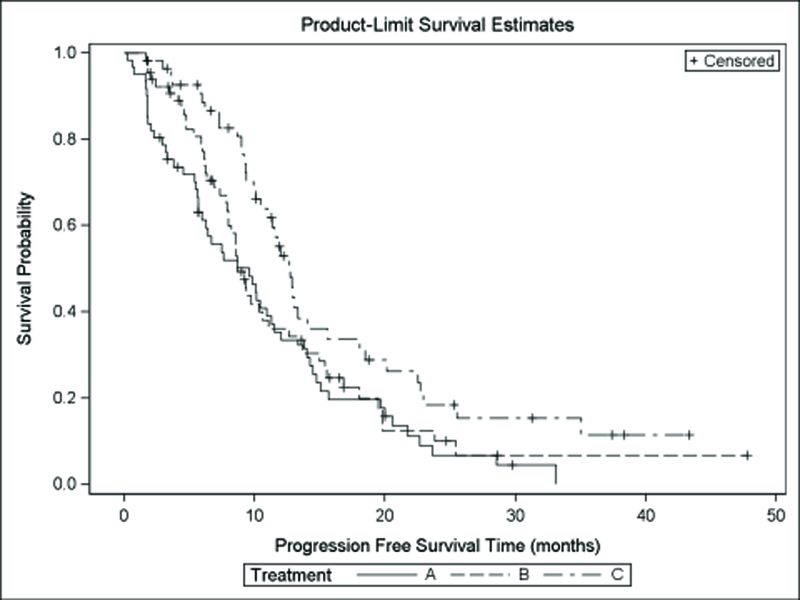
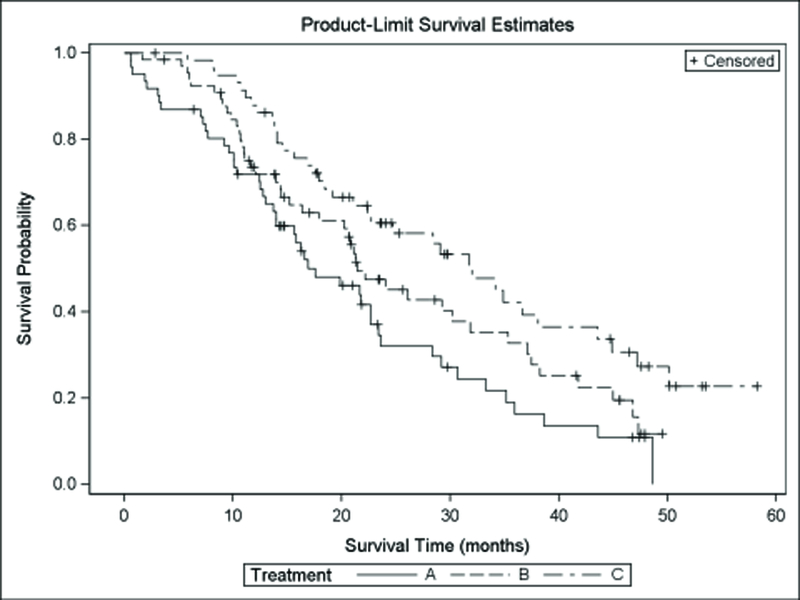
Kaplan-Meier Plots for Progression Free and Overall Survival by Treatment Arm, 1a. The Cox regression models for PFS for the two comparisons revealed the following hazard ratios: TS-H IROX/Bev (arm A) vs TS-H FOLFOX/Bev (arm B) 1.2 (95% CI 0.8–1.7, P=0.41) and TS-H FOLFOX/Bev (arm B) vs TS-L FOLFOX/Bev (arm C) 1.6 (95% CI 1.0–2.4, P=0.04). 1b. Cox regression models for OS revealed the following hazard ratios: TS-H IROX/Bev vs TS-H FOLFOX/Bev 1.4 (95% CI 0.9–2.1, P=0.15) and TS-H FOLFOX/Bev vs TS-L FOLFOX/Bev 1.6 (95% CI 1.0–2.4, P=0.07).
If the 5 patients with indeterminate TS evaluation are excluded from the TS-L cohort, the median PFS for TS-L was 13 months (95% CI 10–18); the two-sided log rank test indicated significant differences between the TS-L and TS-H groups treated with FOLFOX/Bev (P=0.02). Median OS for FOLFOX/Bev in the TS-L only patients was 35 months (95% CI 23–45); the two-sided long rank test indicated significant differences between the two TS groups receiving FOLFOX/Bev in favor of the TS-L patients. (P=0.03). The objective response rate (1 CR and 25 PR) among 54 TS-L only patients was 48% (95% CI 38–61). No differences in the FOLFOX/Bev arms were found according to TS-status (P=0.17, Fisher’s exact test).
Intent-to-treat analysis using all 211 patients, including the patients who were ineligible or refused treatment, are shown in Table 6. Among those treated with FOLFOX/Bev, the TS-L group had a significantly longer PFS by both log-rank and Cox analyses, while OS became significant using log-rank and marginally significant with Cox analysis.
Table 6.
Intention to Treat Analysis
|
|
PFS in months Median (95% CI) |
OS in months Median (95% CI) |
P-value | Hazard Ratio Cox Regression |
|||
|---|---|---|---|---|---|---|---|
| Log-rank | (95% CI) P-value |
||||||
| All patients (N-211) | 10 (9–12) | 23 (20–29) | PFS | OS | PFS | OS | |
| High TS | Arm A IROX/Bev |
9 |
20 | A vs B 0.55 |
A vs B 0.22 |
A vs B 1.1 (0.8–1.6) P=0.55 |
A vs B 1.3 (0.9–1.9) P=0.23 |
| Arm B FOLFOX/Bev |
9 |
21 | |||||
| TS Low | Arm C FOLFOX/Bev |
13 |
32 | B vs C 0.02 |
B vs C 0.04 |
B vs C 1.6 (1.1–2.4) 0.02 |
B vs C 1.3 (0.9–1.9) 0.23 |
TS = thymidylate synthase; IROX = irinotecan and oxaliplatin; Bev = bevacizumab; FOLFOX = folinic acid (leucovorin), 5-Fluorouracil, and oxaliplatin; CI = confidence interval; PFS = progression-free survival; OS = overall survival. The TS-Low cohort includes both TS-Low and TS-indeterminate expression
Discussion
Low TS protein expression was associated with a longer PFS among patients with TS-L vs TS-H tumors who were treated with FOLFOX/Bev. Point estimates for response rate and overall survival also favored patients with TS-L tumors, although these differences were not statistically significant in this small phase 2 trial. The hypothesis that resistance to 5-FU in tumors with TS-H would favor treatment with the non-5-FU containing regimen, IROX, was not supported. Formal testing of the hypothesis that TS-L is associated with prediction of response to 5-FU would have required an additional randomization of patients with TS-L tumors to a non-5FU regimen.
This study demonstrated the feasibility of determining TS protein expression in mCRC tissue in real time prior to treatment assignment. Protein expression, rather than mRNA expression, was used in the current study for the following reasons: protein expression is the final denominator in gene expression. The use of paraffin-embedded tissue was thought to ease the logistical considerations for this multicenter study, since routine methods of sample preparation, analysis and shipment could be employed. To enroll in this study, patients must either have had access to a previously obtained biopsy of a metastatic site (paraffin-embedded) or be willing to undergo a biopsy of a metastatic site, since treatment assignment was based upon intratumoral TS expression. Comparison of TS expression in primary versus metastatic tumor was not performed in this study. The design of this study does not take into account possible heterogeneity in TS protein expression in different sites of metastatic disease, but obtaining multiple biopsies from more than one site was not feasible. Kumamoto et al found a weak correlation between TS mRNA expression between primary and liver metastatic lesions ( r = 0.57 by Spearman rank correlation, n = 30) (23). The correlation was better for synchronous liver metastasis ( r = 0.71, n = 15) compared to metachronous liver metastasis ( r = 0.53, n =15); TS protein expression was not studied.
At the time this study was designed, we anticipated a 10% improvement in response rate with the addition of Bev to chemotherapy (19–20); however, not all Bev combinations exhibit such an increment. Subsequent trials comparing 3-drug combination regimens such as FOLFOX or FOLFIRI with Bev indicate no substantial increase in response rate with the addition of Bev (21–22). At interim analysis, the overall response rate for the study population was lower than anticipated. A modified study design allowed expanded accrual to all 3 arms. Patients with TS-L tumors had a trend for a higher CR+PR rate (49% vs 38%, P=0.07), longer PFS (13 vs 9 months, P=0.04), and a trend for longer OS (32 vs 21 months, P=0.07) with FOLFOX/bevacizumab compared with patients with TS-H tumors.
In a post-hoc analysis by intent to treat using all 211 patients, the median OS for the 3 arms were 20, 21 and 32 months, respectively. The two-sided log rank tests indicate no significant OS differences between the two TS-H arms (P=0.22), but a significant OS difference between the two FOLFOX/Bev arms in favor of TS-L patients (P=0.04). The intent-to-treat analysis was not pre-specified in the protocol, therefore the interpretation of the P-value must be viewed cautiously. However, the reason that 15 of 21 patients (71%) deemed on audit to be ineligible for stage 2 of the study was greater than a 28 day interval for baseline RECIST scan and initiation of study therapy. These 15 subjects received the chemotherapy as specified on the protocol. This issue may inform the design of future studies that employ real-time analysis of a biomarker for treatment assignment or randomization.
It is possible that with the addition of agents to 5-FU/LV, TS expression is less important as a predictive factor than when 5-FU is given alone. 5-FU also mediates cytotoxicity via mis-incorporation of fluorouridine triphosphate into all forms of RNA and interference with RNA function; these mechanisms do not rely on TS (6). The incidence of grade 3+ diarrhea (11%), was higher in those with TS-L tumors compared to those with TS-H tumors (1%) treated with FOLFOX/bev. This finding is consistent with a hypothesis that patients whose tumors have TS-L content may also have TS-L expression in normal organs, and thus may be at risk for increased host toxicity. Patients with TS-H tumors appeared to have comparable benefit with IROX/Bev and FOLFOX/Bev, although the incidence of grade 3+ diarrhea was higher with IROX/Bev. In CALGB study 9741, IROX was associated with a TTP of 6.7 months and OS of 17.3 months in CRC patients whose TS status was not determined (10). In comparison, patients with TS-H tumors treated with IROX in the current study appeared to have better outcomes (TTP = 9 months and OS = 20 months), although inter-study comparisons must be considered with caution. Our data do not support the routine use of IROX, and its role is likely limited as therapy in patients who do not tolerate fluoropyrimidines.
This was the first prospective trial using TS as a determinant of treatment assignment for patients with advanced colorectal cancer. The data support the value of TS as a prognostic biomarker of 5-FU benefit. Patients with TS-L tumors derive considerable benefit with FOLFOX/bev. However, the results indicate that mCRC patients with TS-H tumors need not avoid 5-FU-based therapy. 5-FU remains an integral component of systemic therapy for mCRC, and studies have shown clinical synergy of 5-FU combined with oxaliplatin and irinotecan. High TS levels in tumor tissue does not appear to predict for complete lack of benefit with fluoropyrimidine combination chemotherapy. It remains plausible that low TS tumors have increased sensitivity to fluoropyrimidine therapy compared with high TS tumors, but our study design did not permit definitive assessment of this hypothesis. TS may be also be an indicator for other key determinants of cancer biology, and has shown independent prognostic importance in patients with locally advanced colon and rectal cancer (15, 24–26). Pharmacodynamic and pharmacogenomic studies may also provide useful and complementary information to ultimately guide treatment selection.
With the increasing complexity of chemotherapy regimens, it may be more difficult to conduct prospective studies of biomarker-selected treatment unless the biomarker is associated with a clear-cut binary outcome (possibility of benefit vs no possibility of benefit). At the present time, such biomarkers include wild-type expanded RAS panel to select patients for anti-EGFR therapy, or microsatellite instability-high/mismatch repair deficient status to select patients for treatment with immune check-point inhibitors (26–27). Emerging clinical data further attest to the inhomogeneity of mCRC, such as location of the primary tumor (28). While biomarker development is often focused on newer “targeted” therapeutics, our data highlight the recognition that traditional cytotoxics, such as 5-FU, also have targets with variable expression that may influence treatment outcome and should be further developed for clinical use.
Supplementary Material
Acknowledgments
Funding: This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180794, CA180820, CA180853, CA180864, CA180847, CA189954, CA180795, CA189863, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Cooperative Group funding was provided by Sanofi-Aventis. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Disease Group: Gastrointestinal Diseases
Conflicts of Interest:
The following authors have no disclosures pertaining to this manuscript:
Y. Feng, J. Grem, M. Mulcahy, P. Catalano, J. Saltzman, T. George, J. Kauh; J. Zangmeister, P. Cheema; P. O’Dwyer
N. Meropol has funding from ECOG, NCI and Sanofi-Aventis
M. Hall has research collaborations (but no direct funding) with Invitae and Caris. The institution has funding from Myriad Genetics.
E. Chiorean serves on an Advisory Board for Pfizer, Genentech, Merrimack, Celgene, Novocure. She has a research grant from Boehringer Ingelheim. Research grants to her institution come from Celgene, Genentech, Prism, New Link Genetics, Igynta, Incyte, Stemline, and Spectrum.
Al B Benson has research grants from Genentech, and is a consultant with Genentech/Roche. Sanofi, Bnstol-Myers Squibb, Merck Serono, Merck/Schering Plough, Spectrum Pharmaceuticals, Lilly/lmClone,
Celgene, Genomic Health, National Cancer Institute, Vicus Therapeutics, Pharmacyclics ,Precision
Therapeutics, Taiho Pharmaceutical, Bayer, Alchemia, Infinity Pharmaceuticals, Boehringer lngelheim, Astellas Pharma. EMD Serono
References
- 1.Grem JL, Chabner BA, Ryan DP, Wadlow RC. 5-Fluoropyrimidines In: Chabner BA, Longo DL (Eds.), Cancer Chemotherapy and Biotherapy: Principles and Practice, Fifth Edition Lippincott-Williams & Wilkins, Philadelphia, 2011; 139–170. Grem, JL. Sequencing of Treatment in Advanced Unresectable Colorectal Cancer. J Natl Compr Canc Netw 2013; 11:S-28-S-37. [Google Scholar]
- 2.Leichman CG, Lenz HJ, Leichman L, et al. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol 1997; 15:3223–9. [DOI] [PubMed] [Google Scholar]
- 3.Kornmann M, Link KH, Lenz HJ, et al. Thymidylate synthase is a predictor for response and resistance in hepatic artery infusion chemotherapy. Cancer Lett 1997; 118:29–35. [DOI] [PubMed] [Google Scholar]
- 4.Aschele C, Debernardis D, Casazza S, et al. Immunohistochemical quantitation of thymidylate synthase expression in colorectal cancer metastases predicts for clinical outcome to fluorouracil-based chemotherapy. J Clin Oncol 1999; 17:1760–70. [DOI] [PubMed] [Google Scholar]
- 5.Davies MM, Johnston PG, Kaur S, Allen-Mersh TG. Colorectal liver metastasis thymidylate synthase staining correlates with response to hepatic arterial floxuridine. Clin Cancer Res 1999; 5:325–8. [PubMed] [Google Scholar]
- 6.Cascinu S, Aschele C, Barni S, et al. Thymidylate synthase protein expression in advanced colon cancer: correlation with the site of metastasis and the clinical response to leucovorin- modulated bolus 5-fluorouracil. Clin Cancer Res 1999; 5:1996–9. [PubMed] [Google Scholar]
- 7.Etienne MC, Chazal M, Laurent-Puig P, et al. Prognostic value of tumoral thymidylate synthase and p53 in metastatic colorectal cancer patients receiving fluorouracil-based chemotherapy: phenotypic and genotypic analyses. J Clin Oncol 2002; 20:2832–43. [DOI] [PubMed] [Google Scholar]
- 8.Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res 2000; 6:1322–7. [PubMed] [Google Scholar]
- 9.Gonen M, Hummer A, Zervoidakis A, et al. Thymidylate synthase expression in hepatic tumors is a predictor of survival and progression in patients with resectable metastatic colorectal cancer. J Clin Oncol 2003; 21:406–12. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RM, Sargent DJ, Morton RF, et al. : A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22:23–30. [DOI] [PubMed] [Google Scholar]
- 11.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study J Clin Oncol. 2004; 22:229–37. [DOI] [PubMed] [Google Scholar]
- 12.Scheithauer W, Kornek GV, Raderer M, et al. Randomized multicenter phase II trial of oxaliplatin plus irinotecan versus raltitrexed as first-line treatment in advanced colorectal cancer. J Clin Oncol 2002; 20:165–72. [DOI] [PubMed] [Google Scholar]
- 13.Johnston PG, Liang CM, Henry S, et al. The production and characterization of monoclonal antibodies that localize human thymidylate synthase in the cytoplasm of human cells and tissues. Cancer Res 1991; 51:6668–6676 [PubMed] [Google Scholar]
- 14.Johnston PG, Fisher ER, Rockette HE, et al. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol 1994; 12:2640–2647 [DOI] [PubMed] [Google Scholar]
- 15.Allegra CJ, Parr AL, Wold LE, et al. Investigation of the prognostic and predictive value of thymidylate synthase, p53, and Ki-67 in patients with locally advanced colon cancer. J Clin Oncol 2002;20(7):1735–43. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statistical Assoc 1958; 53:457–481. [Google Scholar]
- 17.Mantel N Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966; 50:163–170. [PubMed] [Google Scholar]
- 18.Cox DR Regression models and life tables. J Royal Statistical Soc 1972; B34:187–220. [Google Scholar]
- 19.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 250:2335–2342. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with FU and LV: An active regimen for fist-line metastatic colorectal cancer. J Clin Oncol 2005; 23:3502–3508. [DOI] [PubMed] [Google Scholar]
- 21.Saltz LB, Clarke S Diaz-Rubio, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized Phase III study. J Clin Oncol 2008; 26:2013–2019. [DOI] [PubMed] [Google Scholar]
- 22.Venook AP, Niedzwiecki D, Lenz H- J, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin or oxaliplatin/5-FU/leucovorin with bevacizumab or cetuximab for patients with KRAS wild-type untreated metastatic adenocarcinoma of the colon or rectum. J Clin Oncol Vol 32, No 15_suppl (May 20 Supplement), 2014: LBA3. [Google Scholar]
- 23.Kumamoto K, Kuwabara K, Tajima Y et al. Thymidylate synthase and thymidine phosphorylase mRNA expression in primary lesions using laser capture microdissection is useful for prediction of the efficacy of FOLFOX treatment in colorectal cancer patients with liver metastasis. Oncol Lett 2012; 3(5), 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edler D, Hallström M, Johnston PG, et al. Thymidylate synthase expression: an independent prognostic factor for local recurrence, distant metastasis, disease-free and overall survival in rectal cancer. Clin Cancer Res 2000; 6, 1378 −1384. [PubMed] [Google Scholar]
- 25.Allegra CJ, Paik S, Colangelo LH, et al. Prognostic value of thymidylate synthase, Ki-67, and p53 inpatients with Dukes’ B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol 2003; 21:241–250. [DOI] [PubMed] [Google Scholar]
- 26.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch repair deficiency. J Clin Oncol 2015; 33 (suppl; abstr LBA100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen R, Svrek M, Dreyer C, et al. New therapeutic opportunities based on DNA mismatch repair and BRAF status in metastatic colorectal cancer. Curr Oncol Rep 2016. 18: 18DOI: 10.1007/s11912-016-0504-2 [DOI] [PubMed] [Google Scholar]
- 28.Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary tumor locations on overall survival and progression-free survival in patients with metastatic colorectal cancer. Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016; 24 (suppl; abstr 3504). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


