Abstract
Synapses are intercellular junctions specialized for fast, point-to-point information transfer from a presynaptic neuron to a postsynaptic cell. At a synapse, a presynaptic terminal secretes neurotransmitters via a canonical release machinery, while a postsynaptic specialization senses neurotransmitters via diverse receptors. Synaptic junctions are likely organized by trans-synaptic cell-adhesion molecules (CAMs) that bidirectionally orchestrate synapse formation, restructuring, and elimination. Many candidate synaptic CAMs were described, but which CAMs are central actors and which are bystanders remains unclear. Moreover, multiple genes encoding synaptic CAMs were linked to neuropsychiatric disorders, but the mechanisms involved are unresolved. Here, I propose that engagement of multifarious synaptic CAMs produces parallel trans-synaptic signals that mediate the establishment, organization, and plasticity of synapses, thereby controlling information processing by neural circuits. Among others, this hypothesis implies that synapse formation can be understood in terms of inter- and intracellular signaling, and that neuropsychiatric disorders involve an impairment in such signaling.
Keywords: Synapse, cell-adhesion molecules, neurexins, neuroligins, cerebellins, latrophilins, BAIs, synaptic plasticity, synaptogenesis, teneurins
IN BRIEF
Südhof’s review proposes that multifarious trans-synaptic cell-adhesion molecules organize synapses by signaling bidirectionally to orchestrate synapse formation, thereby enabling synapses to function as the fundamental computational elements of the brain and to mediate information processing by neural circuits.
SYNAPSES ARE INTERCELLULAR SIGNALING JUNCTIONS
Neurons communicate by two basic mechanisms: Fast point-to-point information transfer mediated by synaptic transmission, and slower more widespread signaling mediated by a range of messengers, such as neuropeptides, endocannabinoids, and monoamines. Both mechanisms are used not only to transfer information from one neuron to the next, but also to send signals from brain to other cells in the body.
Synaptic transmission is effected by presynaptic exocytosis of synaptic vesicles containing neurotransmitters, and detection of these neurotransmitters by postsynaptic receptors. Fast, point-to-point synaptic transmission is enabled by the temporal and spatial restriction of neurotransmitter release and reception, and by the precise alignment of pre- and postsynaptic structures at a synaptic junction (Figure 1; Biederer et al., 2017). We now have a deep understanding of neurotransmitter release and of neurotransmitter receptors, resulting in an initial description of the protein complexes that constitute the presynaptic release machinery and the postsynaptic neurotransmitter signal transduction apparatus (reviewed in Sheng and Kim, 2011; Südhof, 2012 and 2013; Frank and Grant, 2017). How synapses are generated and dynamically maintained, however, remains largely unknown.
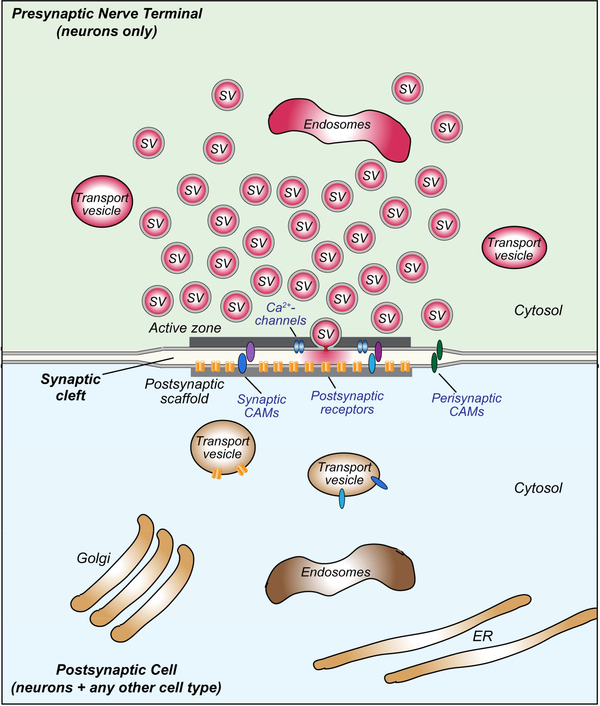
Figure 1: Canonical design of a central synapse.
Schematic drawing of a synaptic junction with a cluster of synaptic vesicles (SV) on the presynaptic side, and an array of neurotransmitter receptors on the postsynaptic side. Pre- and postsynaptic transport vesicles for receptors, active zone components, and transsynaptic cell adhesion molecules are indicated, as well as presynaptic endosomes and postsynaptic organelles (Golgi apparatus, endosomes, and endoplasmic reticulum [ER]). Note that in brain, the synaptic cleft is usually wider than the surrounding interstitial space. All synapses contain similar presynaptic components independent of type, although the specific isoforms of various proteins (synaptotagmins, neurotransmitter transporters, RIMs and Munc13, Ca2+-channels etc) vary. In contrast, postsynaptic components of excitatory and inhibitory synapses exhibit no homology, neither at the level of receptors nor in the postsynaptic scaffolding proteins. Moreover, presynaptic specializations are formed exclusively by neurons, but postsynaptic specializations can likely be formed by any cell in the body.
In cell-biological terms, synapses are asymmetric intercellular junctions. Synapses are diverse, with large differences in properties, such as their neurotransmitter types, release probability, postsynaptic receptor composition, and short- and long-term plasticity. The major activity of synapses is to transfer neurotransmitter signals unidirectionally from the pre- to the postsynaptic sides and to computationally change the information encoded in these signals. An understanding of synaptic transmission is essential for any assessment of how circuits process information because synapses not only transfer information between cells, but also process this information and do so in a very different, synapse-specific and plastic manner on a millisecond time scale (Costa et al., 2017; Yee et al., 2017). The properties of a given synapse – the manner in which it computes information – are coordinately regulated by bi-directional signals produced by the pre- and postsynaptic compartments, which shape the computation of synaptically transmitted neurotransmitter signals in a circuit. Thus, synapses are not only minimal computational unit in brain that process information during transfer between neurons, they are also bi-directional signaling devices that organize the transfer and computation of synaptic information.
As intercellular junctions, synapses contain cell-adhesion molecules (CAMs) that mediate the bidirectional organization of their pre- and postsynaptic compartments. CAMs primarily function in trans-cellular signaling and only secondarily in cell adhesion. Multifarious synaptic CAMs have been described that may initiate the formation of synapses, hold the pre- and postsynaptic sides of a synapse together, coordinate the precise alignment of pre- and postsynaptic sides, and enable short- and long-term synaptic plasticity of synaptic transmission (Figure 2). However, which of these candidate CAMs are actually important, how they work, and what principles guide the endowment of synapses with specific properties continues to be unclear. In the present review, I will not try to present an encyclopedic description of synapse formation and specification, but instead aim for a conceptual discussion of our present understanding of synaptic junctions. In addition, I propose to raise questions that may have to be addressed for further insight into how synapses are constructed and reconstructed throughout life. I apologize beforehand that my discussion will focus on mammalian synapses since space concerns do not allow an adequate consideration of the outstanding invertebrate literature.
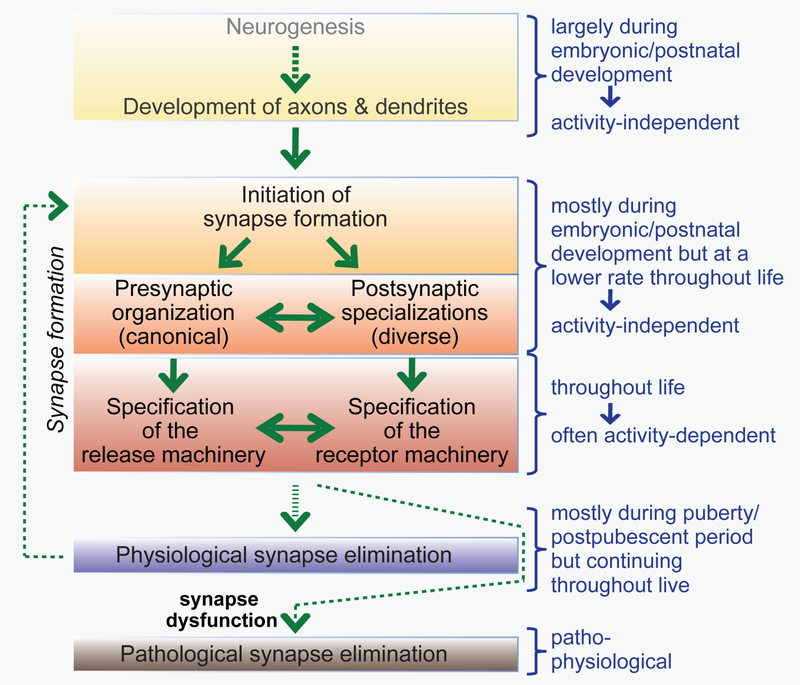
Figure 2: Flow diagram of synapse formation.
During development, neurons are generated, migrate, and grow short and long-range axons and extensive dendritic trees. Axons and dendrites establish initial synaptic contacts mostly during development and the early postnatal period, although synapse formation continues throughout life. Synapse formation is represented as a multicomponent process whereby an initial synaptic contact nucleates organization of pre- and postsynaptic specializations that are subsequently specified, i.e., become endowed with specific properties. Synapse specification is likely an activity-dependent process that takes place continuously for most synapses in brain as synapse are being restructured during synaptic plasticity. Most physiological synapse elimination occurs during the first decades of life, but a low level of synapse elimination, like synapse formation, continues throughout life. Pathological synapse elimination may be induced by synapse dysfunction and is a hallmark of neurodegenerative disorders (e.g., Alzheimer’s disease).
In animals, presynaptic terminals are uniquely neuronal – they may be the only cellular feature that is exclusively neuronal and present in all neurons. Postsynaptic specializations in brain are also mostly neuronal, but glia such as non-neuronal oligodendrocyte precursor cells can also receive synaptic inputs (Bergles et al., 2000; Lin and Bergles, 2004). Outside of brain, postsynaptic specializations can be elaborated by virtually any cell type. Axons that form presynaptic terminals differ from other cellular specialization in that they represent thin extensions of the soma that can span long distances and lack typical cellular organelles, such as a Golgi complex and a rough endoplasmic reticulum (Quassollo et al., 2015). Although all bona fide neurons form presynaptic terminals, not all neurons have axons; retinal amacrine cells or olfactory bulb granule cells, for example, lack axons and elaborate dendritic presynaptic specializations. Note that although it is possible that mature nerve terminals on axons are capable of protein synthesis (Shigeoka et al., 2016; Younts et al., 2016), such events are rare since axons lack a rough endoplasmic reticulum and a Golgi apparatus, and ribosomes are transported down the axon rarely, if at all, as judged from electron micrographs. Thus, all presynaptic protein synthesis would be restricted to soluble cytosolic proteins, and all membrane and secreted proteins have to be produced in the neuronal soma and axonally transported to presynaptic terminals, again a unique property of neuronal organization.
In humans, most synapses assemble during pre- and postnatal development. Approximately half of all synapses are subsequently ‘pruned’ during the following two decades. In the human prefrontal cortex, for example, the spine density is 2–3 fold higher in prepubescent individuals than in mature individuals, with synaptic pruning lasting well into the third decade of life (Petanjek et al., 2011). Most synapses surviving adolescent pruning in adulthood are stably maintained, although a subset of synapses continues to be eliminated and formed throughout life. Developmental synapse formation is largely activity-independent, whereas synaptic pruning may mostly be activity-dependent (see discussion below). Studies using knockout mice in which neurotransmitter release is blocked showed that the formation of a vast majority of synapses occurs normally in the absence of a neurotransmitter signal (Verhage et al., 2000; Sando et al., 2017; Sigler et al., 2017). This finding remarkably rules out a role for neurotransmitter signals in guiding axons, spinogenesis, or synapse specificity, suggesting that synapse formation in itself is ‘hardwired’. Experiments showing that a massive local release of glutamate or GABA can induce formation of postsynaptic specializations indicated that presynaptic release of neurotransmitters can trigger synapse formation and that synapse formation is thus activity-dependent (Kwon and Sabatini, 2011; Oh et al., 2016). However, this phenomenon may be more similar to the process of artificial synapse formation in which multifarious signals induce synaptic specializations (see discussion below) than to a physiological event since high concentrations of neurotransmitters are not normally released without a presynaptic specialization. The observation of glutamate- and GABA-induced postsynaptic specializations is interesting, however, because it suggests that neurotransmitter signals can have an effect on postsynaptic organization.
For the purpose of our discussion, we refer to synapse formation broadly as the aggregate of all processes that initiate and establish a synaptic junction, mediate the assembly of its pre- and postsynaptic specializations, and determine its specific properties that can differ dramatically between synapses (Figure 2). An expansive literature deals with synapse formation, but fundamental questions remain unanswered, while many myths continue. For example, it is unclear if pre- and postsynaptic specializations can form independently. The observation of ‘naked’ spines lacking presynaptic components in the cerebellar cortex of GluRδ2 (GluD2) knockout mice led to the notion that under certain conditions, postsynaptic specializations may develop independent of a presynaptic input signal (Kashiwabuchi et al., 1995). However, careful studies revealed that these spines become ‘naked’ only secondarily (Kurihara et al., 1997). Initially, typical synapses develop in GluD2 knockout mice, but presynaptic specializations are subsequently disassembled in a subset of synapses, leading to naked spines. The same was found for synapses in mice lacking cerebellins, the ligands for GluRδ2 (Seigneur and Südhof, 2018). Thus, the naked spines in the cerebellum of GluD2 knockout mice are a graveyard for dead synapses, and do not provide evidence for the independent generation of pre- and postsynaptic specializations.
METHODOLOGICAL CONTEXT FOR UNDERSTANDING SYNAPSE FORMATION
Progress in science depends on methods; paradigmatic advances are often fueled by applications of new techniques. Revolutionary new approaches, such as single-cell RNAseq, super-resolution microscopy, and CRISPR-mediated gene manipulations, now offer new avenues to understanding the brain. We live in a time of unprecedented opportunities for insight into synapse formation, although pitfalls remain.
In a perfect world, a CAM or other molecule could be considered a key player in synapse formation if its characterization had shown (i) that the CAM or molecule, or a family of redundant CAMs or molecules, is required for synapse formation, broadly defined, using well-controlled loss-of-function experiments; (ii) that the CAM or molecule is localized at synapses using high-resolution localization techniques, with a developmental expression profile that correlates with synapse formation; and (iii) that the CAM or molecule physically binds to other proteins essential for the construction and/or operation of synapses, such as other CAMs that operate in synapse formation. New techniques have facilitated meeting these criteria, but major issues remain, and some general considerations of techniques may be useful. I believe that we may need to pay more attention to technical details than customry because the pressures on investigators have increased the tendency to publish technically preliminary results, especially results obtained with new methods whose limitations are not yet clear. Thus, discussion of our current understanding of synapse formation requires assessment of the methodological context on which this understanding is based. Below, I will briefly outline aspects of emerging techniques that are central for our understanding of synapses.
The lure of transcriptomics.
Techniques for measuring cellular gene expression are evolving at breathtaking speed. Transcriptome analyses by single-cell RNAseq have changed our views of neuronal cell types and gene expression profiles. These methods are vastly superior to older micro-array based approaches. They have produced large amounts of expression data that are publicly available (e.g., see Saunders et al., 2018; Zeisel et al., 2018), and provide a guide for which molecules may be involved in synapse formation. However, significant problems remain that are sometimes not immediately apparent. Most studies use methods such as ‘drop seq’ or ‘10x genomics’, which enable high throughput with a relatively shallow sequencing depth, thus monitoring only abundant mRNAs. However, there is no reason to assume that abundant mRNAs are more important than rare mRNAs, suggesting that for functional analyses we need a higher sequencing depth. Moreover, computation of mRNA abundance from primary data differs between studies, which may explain why various studies report distinct expression profiles for the same cell types (Saunders et al., 2018; Zeisel et al., 2018). Even when more indepth methods for single-cell RNAseq are used, the finite efficacy of reverse transcription and amplification methods of the small amounts of mRNA per cell means that single-cell RNAseq is unable to reliably assess alternative splicing of mRNAs. Given the central importance of alternative splicing, this limitation makes it difficult to use only single-cell RNAseq for a complete assessment of a transcriptome. Furthermore, mRNA levels do not necessarily predict protein levels, but methods of proteomics by mass spectrometry are more difficult than transcriptome studies, making it hard to assess how much of an mRNA is actually translated.
Some of these limitations can be overcome by complementary low-throughput, old-school methods that are expensive and laborious. In situ hybridization with fluorescent probes is experiencing a renaissance because it allows quantitative assessment of gene expression with cellular resolution and can potentially measure alternative splicing of mRNAs. Immunocytochemistry can measure protein levels at subcellular resolution, but is hampered by the lack of reliable antibodies (see discussion below). Single-cell quantitative RT-PCR can assess alternative splicing of individual mRNAs but is limited by the primer-dependent inefficiencies of PCR amplifications whose effect has probably been underestimated (Fuccillo et al., 2015). In other words, nothing is perfect!
The enduring crux of antibodies.
Although one of the oldest tools in biology, antibodies remain one of biology’s most important reagents and biggest challenges. The fundamental problem is that each individual antibody only has a finite, although often high, affinity for its target, but also exhibits a finite, although often lower, affinity for ‘cross-reacting’ antigens. No antibody is ever completely specific. Each antibody preparation has to be separately analyzed in detail in a laborious set of experiments, with knockout (KO) controls being essential. Purchasing commercial antibodies is dangerous because many of these antibodies – possibly most – have not really been characterized well, and variations in lots of antibodies introduce additional artifacts. It is amazing how many studies are being published showing immunoblots of proteins with a wrong size, or reporting immunocytochemistry pictures that have no controls! Apart from tool antibodies to well-established marker proteins, it is thus difficult to trust results from any study using antibodies that have not been characterized by testing KO samples with the same technique as used for analysis (e.g., immunoblotting or immunocytochemistry).
A potential solution to the antibody problem are nanobodies. These single-chain antibodies can be produced in bacteria as recombinant proteins, and thus represent a renewable and reproducible resource (Schumacher et al., 2018; De Meyer et al., 2014). However, highaffinity nanobodies are scarce. Obtaining such nanobodies is more challenging than generating high-affinity monoclonal antibodies. In the absence of a commercial interest, it will be necessary to make a major public investment to achieve this.
The touchstone: Gain- and loss-of-function approaches.
Genetic procedures allow specific gene expression manipulations that remain the gold standard for functional analyses. Arguably most reliable are conditional manipulations that enable spatially and temporally defined ablation of a gene, or that make it possible to express conditional knock-in mutations that change specific features of a gene. Constitutive genetic manipulations (KO or knock-in animals) are a useful counterpart that better mimic a human disease condition but potentially elicit compensatory developmental processes. CRISPR not only facilitates the generation of genetically altered animals, but also enables acute genetic manipulations in wild-type mice, although it is still unclear how efficient these procedures are and whether they will entail significant off-target effects. Antisense and RNAi-dependent knockdown approaches suffer from significant and often uncontrollable potential off-target effects that limit their utility, but are useful if validated by genetic KOs. Note that rescue experiments do not really validate RNAi-dependent knockdown results because rescue invariably involves overexpression, which in itself causes often major effects.
One handicap of conditional genetic manipulations that equally applies to RNAi-dependent knockdowns and CRISPR-mediated procedures is that they require at least a week to become effective. This limitation is not only due to the actual gene manipulation (which is invariably enzymatic, and operates on the same time scale for conditional KOs, RNAi and CRISPR), but also due to the half-time of already synthesized proteins. Thus, a better and faster but more challenging genetic manipulation would be to render a protein drugdependent, allowing a more rapid manipulation of its function. This has been beautifully performed for protein kinases by changing their ATP-binding sites (Bishop et al., 2000), and can potentially be instituted for any proteins by adding a drug-dependent degradation tag (Sando et al., 2013). Such manipulations could be of key importance for analyzing central proteins in biological processes.
The challenge of protein-protein interactions.
Much of biology depends on assessing protein interactions, both in stable complexes with high-affinity binding and in more transient encounters that involve lower affinities. Analysis of stable protein complexes that can be isolated from tissues or reconstituted from recombinant proteins is reliably achieved using affinity measurements by surface-plasmon resonance, isothermal calorimetry or related techniques, and crystallography. Insight into low affinity interactions, however, is still difficult, and even conclusions about interactions of medium affinity pose technical challenges. Immunoprecipitations or pulldowns examined by immunoblotting are nonquantitative and inconclusive. Given the abundance of papers reporting non-validated protein interactions that cannot possibly be all correct, it seems that confidence in a possible protein-protein interaction requires either isolation of a stable complex or biophysical measurements of interactions using recombinant purified proteins.
Seeing is believing: Imaging.
Three very different imaging advances are revolutionizing neuroscience. The development of genetically encoded Ca2+-indicators such as gCaMP6 has allowed visualization of neuronal activity in hundreds of neurons at high temporal and spatial resolution (Tian et al., 2009), the discovery of super-resolution microscopy has enabled visualization of molecules at sub-micrometer resolution (Tønnesen and Nägerl, 2013), and the progress in cryo-electron microscopy has made it possible to examine the atomic structure of large molecular complexes (Henderson, 2018). These technologies greatly enhance what can be done at all levels of analysis, ranging from neuronal ensembles to the atomic architecture of macromolecular machines. Of particular relevance to the present discussion, super-resolution microscopy makes it possible to localize a CAM in a synapse. Owing to the sheer size of presynaptic terminals, it is impossible to tell by confocal microscopy whether a particular CAM is intra- or perisynaptic, let alone whether it is pre- or postsynaptic. For the first time, super-resolution microscopy will allow us to actually visualize where an antigen is under physiological conditions and possibly better than by immuno-electron microscopy because of its higher labeling efficiency.
Promises by stem cells and organoids.
The discovery of iPS cells enabled analysis of neurons derived from patients. In addition, since stem cells are a renewable resource subject to facile genetic manipulations, it is now possible to examine conditional gene mutations mimicking a disease in human neurons (Patzke and Südhof, 2015). Recent advances have, moreover, increased the utility of stem cell-derived neurons in the development of organoids (Lancaster and Knoblich, 2014; Chen et al., 2018). A major concern both with stem cell-derived neurons cultured in vitro and with organoids is that they do not appear to become fully mature. Although the development of organoids promises to revolutionize analysis of human brain development, it may be difficult to generate artificial brains in which normal synaptic connections with physiological properties are formed. Thus, the utility of human stem cell-derived neurons may be more in addressing fundamental questions such as the effect of disease-linked mutations on specific neuronal properties than in investigating connectivity mechanisms.
LESSONS FROM ARTIFICIAL SYNAPSE FORMATION ASSAYS
Pre- or postsynaptic specializations are surprisingly easy to induce by diverse signals. This was first shown in pioneering studies demonstrating that polylysine beads induce formation of presynaptic nerve terminals in cultured neurons and in brain in vivo (Burry, 1982; Burry and Hayes, 1986). When polylysine-coated beads were added to dissociated neurons, presynaptic specializations formed onto the beads within 3 hours. The synapses covering the beads were larger and more numerous than those formed between neurons, suggesting the beads successfully competed with postsynaptic neurons for synapse formation (Burry, 1982). After prolonged culture, however, glia started to phagocytose the bead synapses and their numbers declined. In vivo, presynaptic terminals were elaborated on implanted beads within 3 days, but also started to be eliminated after 2–3 weeks (Burry and Hayes, 1986). Looking back, these amazing studies illustrate important features of the induction of presynaptic specializations: Its non-specificity, its speed, its activity-independence, and the potential elimination of presynaptic terminals by phagocytic cells.
Subsequent work greatly elaborated these initial findings. Scheiffele and colleagues discovered that similar to polylysine-coated beads, non-neuronal HEK293 cells could trigger formation of presynaptic specializations from co-cultured neurons if the postsynaptic CAM neuroligin-1 was expressed in the HEK293 cells (Scheiffele et al., 2000; Figure 3). This finding was extended by the observation that other candidate synaptic CAMs were equally active in this assay (Biederer et al., 2001), and that neurexin-1, the presynaptic binding partner of neuroligin-1, induced the formation of postsynaptic specializations in co-cultured neurons when expressed in HEK293 cells (Graf et al., 2004; Nam and Chen, 2005). Strikingly, artificial synapses that are induced for example by neuroligin-1 resemble real synapses in electron micrographs, with even the appearance of a postsynaptic specialization in the non-neuronal cell (Figure 3). Moreover, the presynaptic components of such artificial synapses are functional (Biederer et al., 2002). At present, a large number of CAMs have been shown to induce either pre- or postsynaptic specializations in co-cultured neurons when expressed in a non-neuronal cell. A recent study showed that non-neuronal cells employed in artificial synapse formation assays express endogenous N-cadherin, and that this endogenous N-cadherin is essential for various synaptic CAMs to be able to induce pre- or postsynaptic specializations in cocultured neurons (Yamagata et al., 2018). These observations suggest that multiple celladhesion signals are required for artificial synapse formation, an exciting avenue to agrees well with the concept of multiple signaling pathways in synapse formation. It does, however, raise the question of how polylysine-coated beads could possibly induce synapses – one would have to speculate that such beads activate multiple parallel signaling pathways.
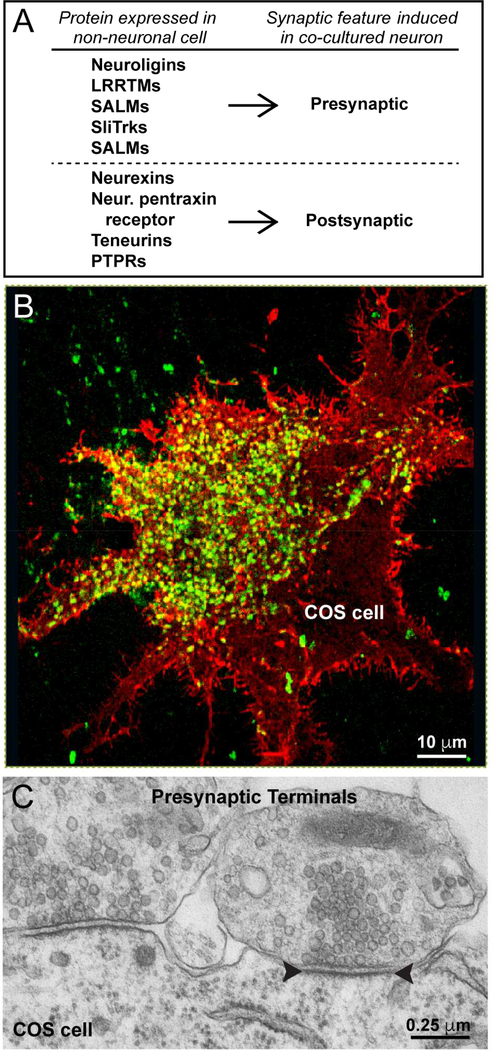
Figure 3: Artificial synapse formation assay.
A, Exemplary list of synaptic CAMs that induce either pre- or postsynaptic specializations during artificial synapse formation assays, which involve expression of a candidate synaptic CAM in a non-neuronal cell, such as a HEK293 or COS cell, and co-culture of this non-neuronal cell with dissociated neurons. Synaptic CAMs under these conditions induce formation of either pre- or postsynaptic specializations by contacting neurons, but never both.
B, Representative image of presynaptic specializations in co-cultured neurons induced by neuroligin-1 that is expressed in a COS cell. Presynaptic terminals are visualized by staining for synapsin and shown in green, while neuroligin-1 staining is shown in red, and overlapping signals are shown in yellow. Green dots outside of the COS cell surface represent synapses between the co-cultured neurons.
C, Representative image of a transmission electron micrograph of a COS cell expressing neuroligin-1 that has been co-cultured with dissociated mouse neurons. Artificial synaptic contacts are extremely abundant and exhibit a normal synaptic morphology, including an apparent postsynaptic density. Note that the size of the synaptic contacts are uniform similar to normal synapses, even though they are formed often by giant nerve terminals as an array of specializations. Panels B and C are modified from Chubykin et al. (2005).
Collectively the observation that many different CAMs induce artificial synapses suggests that this assay does not report on a function of a CAM in initiating physiological synapse formation, but rather a function in activating a ‘synapse signal’. Activity in the artificial synapse formation paradigm may reflect a generic synaptic activity of a CAM. This is exemplarily illustrated by the case of neuronal pentraxins. Neuronal pentraxins are presynaptic molecules that are anchored on the nerve terminal via the neuronal pentraxin receptor, which is a neuronal pentraxin that includes a transmembrane region (Dodds et al., 1997). Neuronal pentraxins bind to AMPA-type glutamate receptors (AMPARs) via a trans-synaptic interaction, and this binding appears to be sufficient to induce postsynaptic specializations, suggesting that simply localized AMPARs to a patch of neuronal membrane is sufficient to turn this membrane into a postsynaptic specialization and induce recruitment of proteins such as PSD-95 and Homer1 (Lee et al., 2016). The induction of postsynaptic specializations by a strong local neurotransmitter signal (Kwon and Sabatini, 2011; Oh et al., 2016) may also be explained by this process.
Given how relatively non-specific synaptic CAMs are in the artificial synapse formation paradigm, it is surprising that CAMs reproducibly induce only pre- or postsynaptic specializations – there never is a mix. This may be the most puzzling observation: How can very different CAMs specifically induce ONLY pre- or postsynaptic specializations? Moreover, although synaptic CAMs induce either pre- or postsynaptic specializations, they usually induce both excitatory and inhibitory specializations. This is the case even for molecules such as neuroligin-2, which is known to be absent from excitatory synapses (Graf et al., 2004), suggesting that specificity for excitatory vs. inhibitory postsynaptic specializations resides in a different type of synaptic signal.
A PANOPLY OF SYNAPTIC CELL-ADHESION MOLECULES
An impressive number of candidate synaptic CAMs has been described (Figure 4). For some of these CAMs, compelling data demonstrates their presence in synapses and suggest a functional role in synapses. Others, however, are less well documented. If one looks at the results in total, the overall impression is puzzlement: how do so many CAMs contribute to shaping a synapse?
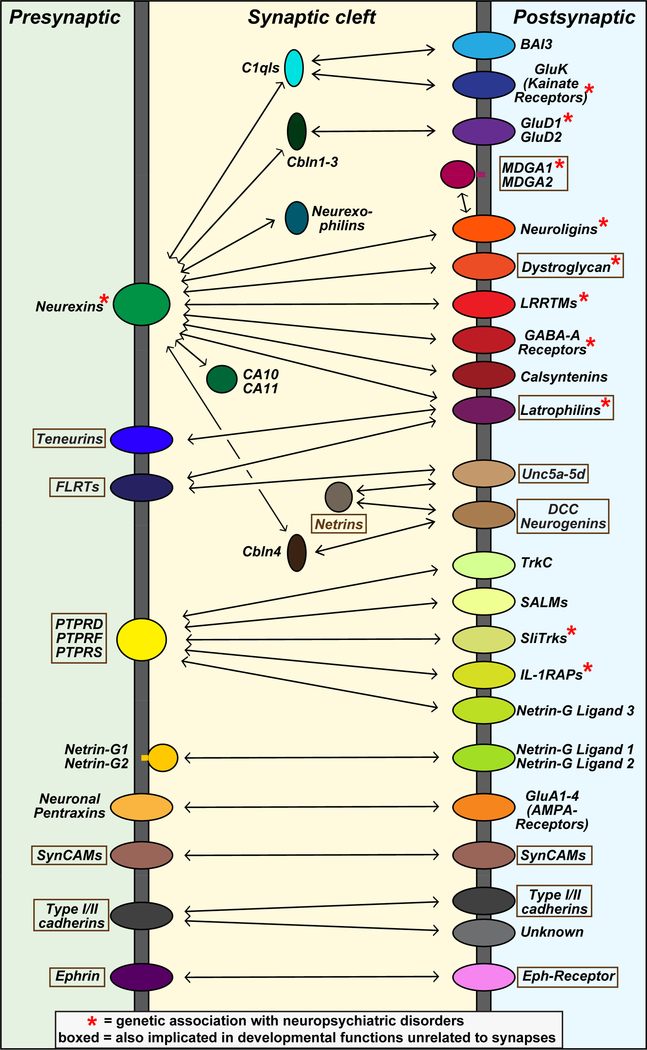
Figure 4: Cartoon of candidate trans-synaptic CAM interactions.
Summary of prominent CAM interactions that were proposed to operate at the synapse. CAMs were placed on the pre- or postsynaptic side based on the overall published studies, but for many molecules firm assignments cannot yet be made. Some of the interactions shown are supported by compelling biophysical evidence (e.g., binding of LAR-type RPTPs to their various ligands), but others are more tenuous (e.g., binding of neurexins to latrophilins or to C1ql’s). Diagram was modified from Südhof (2017).
Different from classical intercellular junctions such as adherens and tight junctions, synapses are inherently asymmetric. Whereas adherens and tight junctions are supported by homophilic CAM interactions, synaptic CAMs always seem to engage heterophilic interactions. This appears to be the case even for CAMs that engage in homophilic interactions in other junctions, such as classical cadherins that act as homophilic CAMs in adherens junctions but as heterophilic CAMs in retinal connections (Duan et al., 2014).
Most candidate synaptic CAMs perform other, non-synaptic functions in an organism, both during development and in mature animals. For example, LAR-type RPTPs, cadherins, teneurins, and Ephrins/Eph receptors are all involved in organizing many non-neuronal tissues and additionally function in neurons in dendritogenesis or axonal pathfinding. This multifunctionality renders a precise functional definition of these molecules at the synapse difficult, as is apparent from the many roles that have been attributed to these CAMs. Here, instead of attempting an exhaustive discussion of various CAMs, I will only briefly review prominent candidate synaptic CAMs, focusing on those for which compelling localizations and/or genetic data are available (Figure 4).
Neurexins and their ligands (neuroligins, LRRTMs, cerebellins, neurexophilins, and others).
As these proteins were reviewed recently (Südhof, 2017), I will only summarize overall results. In total, current results suggest that neurexins and their ligands are central regulators of synapse properties, but are not involved in initiating synapse formation, although some genetic manipulations cause a loss of synapses (e.g., neurexin deletions in cortical SST-positive interneurons [Chen et al., 2016] or deletions of all cerebellins in the hippocampus [Seigneur and Südhof, 2018]), possibly as a secondary effect of synapse dysfunction. Key examples for the control of synapse properties by a neurexin or a neurexin ligand are the trans-synaptic regulation of postsynaptic AMPA-type glutamate receptors in subiculum pyramidal neurons by presynaptic neurexin-3 alternative splicing (Aoto et al., 2013), the requirement for neuroligin-1 and LRRTMs in the NMDA-receptor dependent Schaffer-collateral LTP in the hippocampal CA1 region (Jiang et al., 2017; Bhouri et al., 2018; see discussion below), and the control by neuroligin-2 of inhibitory synaptic strength in cerebellar cortex without impairing the number of synapses (Zhang et al., 2015).
Four recent papers have expanded the discussion of neurexins and their ligands. First, new ligands of neurexins are being discovered, especially ligands that bind to sequences surrounding the newly identified cys-loop domain that is embedded in their juxtamembranous stalk region and conserved in all neurexins (Sterky et al., 2017). Although neurexins already feature an abundance of trans-synaptic interactors, it appears likely that this molecular network will become even more complex.
Second, it was proposed that the neurexin ligand neuroligin-2 performs an essential function in cortical astrocytes that mediates formation of excitatory but not inhibitory synapses (Stogsdill et al., 2018). This proposal was astounding because neuroligin-2 had previously only been localized to inhibitory synapses (Graf et al., 2004), and because constitutive neuroligin-2 deletions (which include deletions in astrocytes) selectively impaired inhibitory but not excitatory synaptic transmission (Gibson et al., 2009; Poulopoulos et al., 2009). It is impossible to reconcile these contradictions at present, and rigorous genetic analyses of neuroligin-2 in astrocytes will be necessary to clarify this issue.
Third, in a completely different line of research, neurexin-1 was revealed to be modified by heparan sulfate, and mutation of serine residues implicated in this modification was shown to impair synapse function and decrease synapse numbers (Zhang et al., 2018). Given the diversity of neurexin isoforms, this finding adds an interesting facet to neurexins as presynaptic CAMs. Determining how the heparan sulfate modification might affect neurexin function poses an intriguing challenge for the next set of experiments.
Fourth, alternative splicing of neurexin-1 at SS4 was found to be directly regulated in the hippocampus during fear learning, and this regulation was mediated by activity-dependent histone modification (Ding et al., 2017). Although this pioneering observation does not reveal the physiological importance of neurexin-1 alternative splicing at SS4, it represents the first description of behaviorally regulated alternative splicing of a neurexin, and suggests a possible mechanism for its activity-dependence.
Overall, neurexins and their ligands are increasingly emerging as kingpins of synapse organization, but major questions remain unanswered – in fact, one could argue we still know little about neurexins and their ligands. For example, given that alternative splicing at SS4 of neurexin-3 controls postsynaptic AMPAR trafficking, does SS4 alternative splicing of other neurexins, such as neurexin-1 whose alternative splicing is tightly regulated during fear learning (Ding et al., 2017), have the same function? What about others sites of alternative splicing in neurexins that are also highly regulated (Ullrich et al., 1995)? What about neurexins in general – do they have similar or distinct functions? Moreover, does the relatively abundance of neurexin-1 in astrocytes imply a non-synaptic function? Even for neurexins, arguably the best studied synaptic CAMs, questions abound.
Latrophilins.
Latrophilins are adhesion GPCRs that contain the typical 7-transmembrane region architecture preceded by a hormone-binding and GAIN domain as is typical of adhesion GPCRs (Arac et al., 2011). In addition, latrophilins contain two N-terminal extracellular adhesion domains, a lectin-like and a somatomedin-like domain, that differentiate latrophilins from other adhesion GPCRs, and that specifically bind to teneurins, neurexins, and FLRTs (Boucard et al., 2012 and 2014; Silva et al., 2011; O’Sullivan et al., 2012; Figure 4 and 5). Finally, latrophilins contain a long C-terminal cytoplasmic sequence of unknown significance ending in a type I PDZ-domain binding sequence.
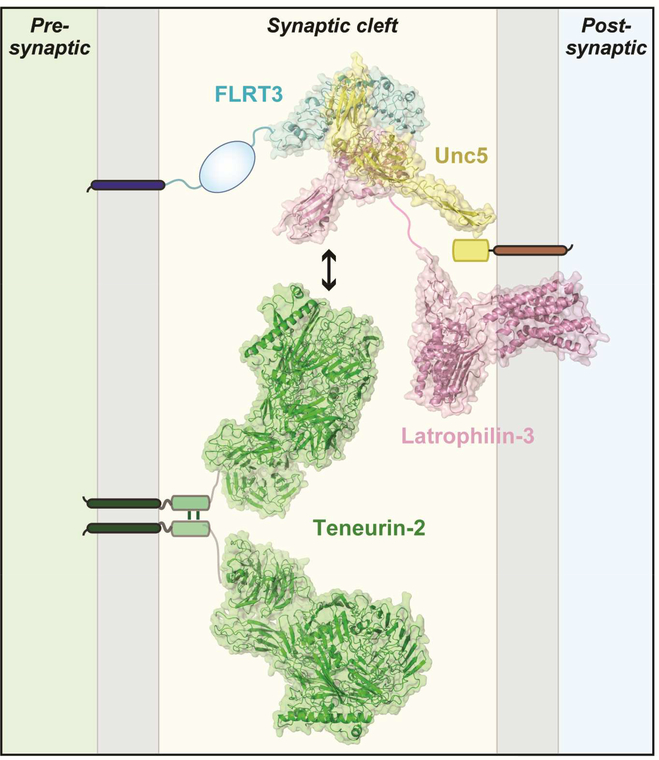
Figure 5: Atomic structure of the trans-synaptic teneurin-latrophilin-FLRT complex.
Atomic structures determined for FLRT3 (blue), Unc5 (yellow), Lphn3 (pink), and teneurin2 (green) are placed into synaptic cleft of an excitatory synapse. Teneurin-2 is positioned presynaptically because it forms a trans-cellular junction with postsynaptic latrophilins (Boucard et al., 2014) and because it induces postsynaptic specializations in the artificial synapse formation assay (Li et al., 2018). FLRT3 is also positioned presynaptically because it forms trans-cellular complexes with Lphn2 (Lu et al., 2015). Note that latrophilins were originally thought to be presynaptic and FLRTs postsynaptic based on RNAi data (O’Sullivan et al., 2012), but conditional genetic knockouts established that in cultured neurons and in vivo, at least Lphn2 is exclusively postsynaptic (Anderson et al., 2016). Interestingly, presynaptic FLRT3 can bind simultaneously to both postsynaptic Lphn3 and postsynaptic Unc5, as shown by crystallography of the complex (Lu et al., 2015). It is unknown whether Lphn3 can simultaneously bind to FLRT3 and to teneurins, or whether these interactions are mutually exclusive, but in either case the various binding reactions suggest that a trans-synaptic interaction network anchored on latrophilins and teneurins may form the basis for an extensive signaling machinery at the synapse. Structures are from Lu et al. (2015) for the FLRT3/Unc5/Lphn3 complex; Arac et al. (2011) for the Lphn3 GAIN/hormone binding domain fragment; and Li et al. (2018) for teneurin (PDB IDs: teneurin-2, 6CMX; Lphn3, 4DLQ (GAIN and hormone-binding domains), 5CMN/5AFB (lectin and olfactomedin domains) and 4K5Y (transmembrane GPCR domain of the corticotrophin releasing hormone receptor); FLRT, 5CMN; and Unc5, 5FTT). The figure was prepared by Drs. Demet Arac and Jingxian Li (U. of Chicago).
Like neurexins, latrophilins were originally isolated by affinity chromatography on immobilized α-latrotoxin (Südhof, 2001). Because α-latrotoxin is a black widow spider neurotoxin that binds to presynaptic terminals to trigger neurotransmitter release, it was thought that latrophilins are presynaptic α-latrotoxin receptors, although no direct α-latrotoxin receptor activity was demonstrated. It thus came as a surprise when studies with latrophilin-2 conditional KO mice revealed that latrophilin-2 acts as a strictly postsynaptic protein (Anderson et al., 2016). Specifically, studies both in cultured hippocampal neurons and in vivo in the hippocampal CA1 region showed that sparse postsynaptic deletion of latrophilin-2 in pyramidal neurons caused a large decrease in excitatory synapse numbers and synaptic transmission. These data demonstrated that latrophilin-2 acts as a postsynaptic CAM that different from neuroligins, LRRTMs, or cerebellins is essential for establishing or maintaining synapses (Anderson et al., 2016). Interestingly, postsynaptic latrophilin-2 is targeted in vivo to a specific domain of the dendritic arbor of CA1 pyramidal neurons, namely their distal segments in the S. moleculare-lacunosum, suggesting that latrophilin-2 mediates synapse specificity.
At present, latrophilin-2 is the CAM whose deletion causes the strongest synapse-formation phenotype known. How does it work? Although latrophilins are adhesion-GPCRs, it has not been tested if they function as GPCRs at the synapse. The physiological relevance of latrophilin binding to teneurins, neurexins, and FLRTs is also unknown, although latrophilin binding to teneurins and FLRTs exhibits nanomolar affinity and is likely significant. Crystal structures and biophysical experiments showed that the olfactomedin-like domain of latrophilin-3 nestles into the concave surface of the leucinerich repeat domain of FLRT3, whereas another CAM, Unc5, simultaneously binds to the convex surface of FLRT3 (Figure 5; Lu et al., 2015). Moreover, latrophilins binding to teneurins is regulated by alternative splicing both of latrophilins and of teneurins (Boucard et al., 2014; Li et al., 2018). Thus, it is possible that latrophilins and FLRTS each bind simultaneously to multiple ligands, nucleating an impressive trans-synaptic interaction network (Figure 5). These findings reveal –together with consideration of the neurexinbased ligand interactions– that synapses include an astounding trans-synaptic molecular interaction network.
BAIs (Brain Angiogenesis Inhibitors) and C1ql’s.
BAIs are adhesion-type GPCRs that similar to latrophilins contain a hormone-binding and a GAIN domain followed by seven transmembrane regions. BAIs differ from latrophilins in that they feature a distinct set of Nterminal extracellular domains, namely a novel motif resembling a CUB-domain and 4–5 thrombospondin repeats, and a C-terminal sequence which is not homologous to that of latrophilins except for the fact that it also includes a C-terminal type I PDZ-domain-binding motif. BAIs were first linked to synapses by the observation that C1ql’s, a family of secreted C1q-domain proteins similar to cerebellins, bind to BAI3 with high affinity and modulate synapse numbers in cultured neurons (Bolliger et al., 2011). More definitive studies revealed that C1ql1 produced by presynaptic inferior olive neurons mediates climbing-fiber synapse formation by binding to postsynaptic BAI3 on cerebellar Purkinje cells (Sigoillot et al., 2015; Kokigawa et al., 2015). Similarly, C1ql3 produced in presynaptic amygdala neurons is essential for efferent synapse formation in the prefrontal cortex, also presumably by binding to BAI3 (Martinelli et al., 2016). Moreover, BAI1 was found to be essential for synapse formation, although these studies were performed by RNAi which is difficult to control (Duman et al., 2013). Finally, a BAI1 KO caused changes in synaptic plasticity (Zhu et al., 2015). Note that in addition to these studies, BAIs have also been linked to non-neuronal function. Although the notion that BAIs act in angiogenesis (a function that was indicated by their name) is now largely abandoned, it has been suggested that BAI1 is a macrophage receptor that binds to phosphatidylserine on apoptotic cells, and then induces phagocytosis of these cells (Park et al., 2007). However, given that BAI1 is only expressed in neurons in brain and that no direct binding of BAI1 to phosphatidylserine was demonstrated, this notion appears rather unlikely.
Overall, BAIs are now firmly linked to synpases with a role that may be analogous to that of latrophilins, but many questions arise. Some of the questions are the same as for latrophilins, most importantly whether BAIs actually act as GPCRs in synapses and how their signal transduction operates. As regards the latter question, an attractive idea is that BAI1 recruits the BAI1/Par3 polarity complex to synapses, which could then organize synapses (Duman et al., 2013). The most important question regarding BAIs, however, regards the identity of their trans-synaptic CAM binding partners. Specifically, do all BAIs bind to all C1ql’s, and do C1ql’s serve as soluble ligands like a hormone, or more plausibly as adaptor proteins like cerebellins, thereby linking postsynaptic BAIs to an unknown presynaptic CAM? Do BAIs additionally bind to other ligands, in particular other transsynaptic CAMs? On a bigger scale, do adhesion-GPCRs generally function in establishing synapses, given the role of latrophilins and BAIs? These are exciting questions to pursue.
Teneurins.
Few candidate synaptic CAMs are surrounded by as much uncertainty as teneurins. Teneurins are evolutionarily conserved, large CAMs that play a central role in embryogenesis and in axon pathfinding (Leamey and Sawatari, 2014; Tucker and Chiquet-Ehrismann, 2006). Mammals contain four teneurin genes that are broadly expressed during embryonic development, and largely restricted to brain in adults. As cell-adhesion molecules, teneurins are highly unusual because they are very large type-II transmembrane proteins (>2000 amino acids) with an unusual domain structure that includes a striking similarity to bacterial Tc-toxins unlike any other eukaryotic protein (Li et al., 2018).
Teneurins were first linked to synapses when teneurins were identified as high-affinity ligands for latrophilins (Silva et al., 2011; Boucard et al., 2014). Later on, teneurins were suggested to act as homophilic CAMs in synapses (Mosca et al., 2012), but it is unclear whether this is a physiological function since teneurins strongly bind to each other in a cis- but not in a trans-configuration (Boucard et al., 2014). It is possible that teneurins also interact with each other weakly in trans (Berns et al., 2018), but it is difficult to conceive a mechanism by which such interactions could be sustained physiologically given the highaffinity binding of teneurins to latrophilins that would dominate their interactions. Moreover, it is hard to imagine how a homophilic interaction could mediate either axonal pathfinding or synapse formation which are inherently asymmetric and usually only involve heterophilic interactions. Finally, in the artificial synapse formation assays teneurins exclusively induce postsynaptic specializations (Li et al., 2018), further supporting the notion that teneurins act primarily as heterophilic presynaptic CAMs.
Many other questions about teneurins arise. Most intriguingly, does the unusual bacterial tc-toxin-like domain have a specific functional role? Is the C-terminal cleaved peptide of teneurins, called ‘teneurin C-terminal associated peptide 1’, physiologically significant (Lovejoy et al., 2006)? Are the phenotypes of impaired connectivity produced by developmental deletion of a teneurin caused only by loss of its axon guidance function, or does a synaptic role for teneurins contribute (Leamey et al., 2007; Berns et al., 2018)? Do teneurins also function in oligodendrocyte differentiation as suggested by the teneurin-4 KO phenotype (Suzuki et al., 2012), an intriguing possibility given that oligodendrocyte precursor cells are recipients of excitatory synapses (Bergles et al., 2000)? Finally, what are the interaction partners for teneurins in all of these functions – could latrophilins via their GPCR activity for example also be involved in axonal pathfinding? More than any other molecule, teneurins illustrate the uncertainty and excitement surrounding the role of synaptic CAMs in shaping neural circuits.
LAR-type receptor-phosphotyrosine phosphatases (RPTPs).
LAR-type RPTPs (also referred to PTPRs according to their gene symbols) are among the best characterized synaptic CAMs that like teneurins, were originally primarily linked to axon guidance (Clandinin et al., 2001). Most informative here were pioneering studies in C. elegans, which contains a single LAR-type RPTP gene (ptp-3) that produces long and short transcript from distinct promoters (Ackley et al., 2005). Selective deletion of the long PTP3A transcript caused alterations only in synapse morphology, and the encoded protein was specifically localized to synapses. Selective deletion of the short PTP-3B transcript, conversely, impaired axon guidance, and its encoded protein was extrasynaptic (Ackley et al., 2005). These experiments suggested mechanistically distinct functions of a LAR-type RPTP in C. elegans in synapse formation and axon guidance.
Regrettably, however, the situation in mammals is more complicated than in C. elegans. Mammals express three LAR-type RPTPs (RPTPD, RPTPF, and RPTPS) whose transcripts are alternatively spliced, but whose genes have only a single promoter that drives expression of ‘long’ RPTPs. Mammalian LAR-type RPTPs interact with a large array of postsynaptic ligands that have also been linked to synapses, including TrkC, SALMs, SliTrks, IL-1RAPs, and Netrin-G Ligand 3 (Figure 4; reviewed in Takahashi and Craig, 2013; Um and Ko, 2013). For many of these ligands, crystal structures of their complexes validate the specificity of binding, but despite the enormous structural information available, little is known about the physiological functions of mammalian LAR-type RPTPs. Specifically, constitutive genetic deletions of individual LAR-type RPTPs produced surprisingly modest phenotypes (e.g., see Schaapveld et al., 1997; Elchebly et al., 1999). Analysis of synaptic function in KO mice lacking RPTPS, the most abundant isoform in brain, revealed only discrete changes in hippocampal synaptic transmission, but no apparent loss of synapses (Horn et al., 2012). This is in contrast to RNAi-dependent knockdown studies in cultured neurons that often described massive impairments including synapse loss (e.g., see Han et al., 2018), suggesting that either post-developmental loss-of-function of RPTPs has a more severe effect because it avoids developmental compensation, or that the phenotypes of RNAi-dependent knockdowns are shaped by off-target effects. The conundrum posed by these findings is enhanced by the observation that the many ligands for LAR-type RPTPs appear to be important synaptic CAMs in their own right, but could act via a RPTP-independent mechanism. Studies using conditional genetic deletions will be required to resolve these issues and gain insight into the synaptic functions of LAR-type RPTPs.
SynCAMs.
SynCAMs are members of a larger family of Ig-domain CAMs that include nectins that, like neurexins, carry a specific carbohydrate modification, but in the case of SynCAMs it is composed of polysialic acid (Galuska et al., 2010). Initially, SynCAM was proposed to be a homophilic synaptic CAM (Biederer et al., 2001), but later studies revealed heterophilic interactions between different SynCAM isoforms (Fogel et al., 2007). Elegant super-resolution experiments localized SynCAM to the perisynaptic zone (Perez de Arce et al., 2015), suggesting that it may shape synapses as a fence. Consistent with this notion, SynCAMs play a role in synaptic plasticity, suggesting that they contribute to the activity-dependent remodeling of synapses (Robbins et al., 2010).
Cadherins.
Cadherins constitute a large family of proteins characterized by repeated extracellular cadherin domains that mediate Ca2+-dependent homophilic cis- and trans-interactions. Cadherins have roles in all tissues, and are universal transducers of extracellular adhesion signals into an intracellular response, often involving the actin cytoskeleton. Adherens junctions are formed by homophilic interactions of classical cadherins, and are essential for formation of many tissues. Owing to the voluminous literature, I will make no attempt to review the possible roles of various classical cadherins and protocadherins in synapse formation. Instead, I focus on a few salient points.
Despite a large number of studies, pinning down the role of cadherins at synapses has turned out to be difficult, possibly because of their crucial role in nearly all neuronal morphogenetic processes. Even showing that a cadherin is actually synaptic or functions directly at a synapse is a challenge. Compelling studies revealed that N-cadherin (Cdh2), a classical type I cadherin, is localized to perisynaptic adherens junctions, and partly lost from synapses during development (Uchida et al., 1996), and some cadherins are close to synapses and affect their functions (Basu et al., 2017; Bekirov et al., 2008). Whether cadherins act directly or indirectly at synapses, however, remains unclear.
Arguably the most compelling description of a role of cadherins in synapse formation was achieved in retina (Duan et al., 2014). In a brilliant approach, this study identified specific expression of Cdh8 and Cdh9 in particular types of bipolar cells, and showed that deletion of these cadherins alters the synaptic connections of the respective bipolar cells with retinal ganglion cells. Moreover, Duan et al. (2014) showed that exogenous expression of these cadherins in particular types of amacrine cells can instruct axonal arbor to form the wong connections, suggesting that Cdh8 and Cdh9 directly shape connections. Finally, they demonstrated that Cdh8 and Cdh9 act as heterophilic CAMs in these functions. These experiments provide strong evidence for a role of classical cadherins as ‘specificity’ molecules in shaping neural circuits, but they do not tell us whether Cdh8 and Cdh9 act during, or upstream of, synapse formation. Consistent with a non-synaptic role, Cdh8 and Cdh9 deletions dramatically altered the shape of the axonal bipolar cell arbor but did not affect synapse formation itself. Thus, despite many careful studies, it is at present still uncertain whether a cadherin actually ever functions as a synaptic CAM.
Ephrins and Eph receptors.
Like cadherins, Ephrins and Eph receptors constitute a large protein family with major functions in many tissues. Again like cadherins, a vast literature exists on the role of Ephrins and Eph receptors in constructing neural circuits, with the most compelling evidence for a role in axonal pathfinding (Triplett and Feldheim, 2012). Different from cadherins, however, Ephrins and Eph receptors are heterophilic by definition and are thus ideal for directional signaling via intercellular junctions, similar to neurexins and latrophilins. Moreover, at least one Eph receptor, EphB2, has been localized by superresolution imaging to the postsynaptic junction (Perez et Arce et al., 2015), suggesting that Ephrins and Eph receptors can be truly synaptic. In another study, Ephrin-B3 was shown to act postsynaptically to recruit PSD-95 to synapses (Hruska et al., 2015). Although convincingly linking Ephrins and Eph receptors to synapses, these results are puzzling since it is difficult to imagine that both Ephrins and Eph receptors are postsynaptic CAMs, and future studies will need to deconstruct the localization and functions of various Ephrins and Eph receptors at synapses. In addition, Ephrins and Eph receptors were shown to function in neuron-astrocyte interactions (reviewed in Murai and Pasquale, 2011), whereby they appear to shape synapse formation (Koeppen et al., 2018). Again, much remains to be done!
Postsynaptic neurotransmitter receptors and presynaptic Ca2+-channels.
Compelling evidence indicates that postsynaptic neurotransmitter receptors and presynaptic Ca2+channels, which are central components of the synaptic transmission machinery, in addition serve to mediate trans-synaptic binding functions. For example, the neuronal pentraxin receptor, which is a presynaptic CAM, induces formation of postsynaptic specializations in the artificial synapse formation assay by binding to neuronal AMPA-type glutamate receptors, suggesting that clustering of AMPA-receptors is sufficient to elicit synapse formation in this rather non-physiological paradigm (Lee et al., 2017). Similarly, α2δ subunits of presynaptic voltage-gated Ca2+-channels may act in synapse formation by an unknown mechanism (reviewed Bauer et al., 2010). A mechanism that unites central component of the synaptic transmission machinery with synapse formation is attractive in its economy, but a potential conundrum here is that all of these molecules – e.g., AMPA-receptors and Ca2+-channels – are also extrasynaptic and the specificity of their potential function in synapse formation is thus difficult to understand.
These short sketches outline the challenges of understanding how synaptic CAMs shape synapses. Clearly, many candidate synaptic CAMs are important for synapse formation, leaving us with the task of sorting out the puzzle of their functions. Several concepts could account for the need of multiple synaptic CAMs. It is possible that different CAMs organize distinct faces of synapse formation, i.e. that synapse formation is a functional mosaic to which different synaptic CAMs contribute distinct pieces. Alternatively, it is possible that multiple synaptic CAMs form a trans-synaptic interaction network, as suggested for neurexins and their ligands (Südhof, 2017). In such networks, different interactions could activate distinct signaling pathways, or multiple simultaneous interactions may be required for activation of a single signaling pathway, as has been shown for CAMs involved in dendritic development in C. elegans (Zou et al., 2016).
SYNAPSE FORMATION AND ELIMINATION IN VIVO
In humans, synapses begin to form during embryogenesis and synapse formation continues postnatally in cortex for several years. After this period of intense synaptogenesis, synapse numbers decline slowly over two decades but remain relatively stable after the third decade of life (Huttenlocher et. al., 1982; Bourgeois and Rakic, 1993; Petanjek et al., 2011). The magnitude of developmental synapse elimination during this period of decline in cortex is staggering, amounting to >40% of all synapses or thousands of synapses per second (Bourgeois and Rakic, 1993). Little is known about the mechanisms involved.
Well-characterized examples of developmental synapse elimination show that synapses often compete with each other, with ‘losers’ vanishing and ‘winners’ taking all. This has been shown beautifully for the neuromuscular junction (Sanes and Lichtman, 1999), retinal inputs into the lateral geniculate nucleus (Chen and Regehr, 2000), and cerebellar climbing-fiber synapses (Kano and Hashimoto, 2014). Of these examples, climbing-fiber synapse elimination may be best understood owing to the relatively stereotyped simplicity of the cerebellar circuit. Cerebellar Purkinje cells are initially innervated by multiple climbing fibers, but subsequently the synapses derived from all but one climbing fiber are eliminated. This process is activity-dependent, and requires Purkinje cell expression of the metabotropic glutamate receptor mGluR1, of PKCγ, and of phospholipase Cβ4 (Kano et al., 1995, 1997, and 1998), suggesting that mGluR1 stimulation at parallel-fiber synapses activates a phospholipase Cβ4 and PKCγ, which then produces a retrograde signal that causes elimination of supernumerary climbing-fiber synapses from a given Purkinje cell. Climbing-fiber synapse elimination also depends on the activity of the much more numerous parallel-fiber synapses (Ichikawa et al., 2002) that are formed by >100,000 granule cells per Purkinje cell. Preliminary evidence suggests that Purkinje cells may secrete semaphorins Sema3A and Sema7A (Uesaka et al., 2014) or progranulin (Uesaka et al., 2018) as retrograde signals, but how these mediators fit in with mGluR1 signaling remains unclear. Understanding the underlying molecular interactions and reactions that enable climbing-fiber synapse elimination in an activity-dependent manner is a cellbiological problem that may not only be relevant for insight into well-documented forms of developmental synapse elimination in the neuromuscular junction and lateral geniculate nucleus, but generally applicable to many forms of developmental synapse elimination.
It has been suggested that during developmental synapse elimination, synapses are not retracted by an active deconstruction process, but are phagocytosed by microglia or astrocytes after being marked via the classical complement pathway (Stevens et al., 2007). This proposal, probably inspired by the observation that artificial synapses are phagocytosed in vitro and in vivo by astrocytes (Burry, 1982; Burry and Hayes, 1986), may apply to pathological processes where entire neurons are phagocytosed but seem to be unlikely as a general mechanism for the selective elimination of a subset of the terminals formed by a neurons. Indeed, mice without a classical complement pathway exhibit fairly normal synapse numbers (Chu et al., 2010), suggesting normal developmental synapse elimination. Based on the examples discussed above, a more active cell-autonomous process that maintains the overall viability of the neurons appears more plausible.
THE TRIPARTITE SYNAPSE
Many synapses are contacted by astrocytic processes, suggesting that these synapses are ‘tripartite’ because they are composed of astrocytic processes in addition to pre- and postsynaptic specializations (Araque et al., 1999; Reichenbach et al., 2010; Papouin et al., 2017). However, the degree to which synapses are associated with astrocytes varies dramatically. In cerebellum, 100% of parallel-fiber and climbing-fiber synapses contain astrocytic contacts (Spacek, 1985; Xu-Friedmann et al., 2001), but in the hippocampal CA1 region only 57% of excitatory synapses include astrocytic contacts (Ventura and Harris, 1999). Moreover, only 45% of excitatory synapses on spines, 30% of excitatory synapses on dendritic shafts, and 6% of inhibitory synapses in the basolateral nucleus of the amygdala exhibit glial contacts (Ostroff et al., 2014). In most cases, the astrocytic contacts do not wrap around the synapses, but cover <50% of the synapse perimeter. Furthermore, fear conditioning selectively induced an increase in the number of excitatory synapses lacking astrocytic contacts in the basolateral amygdala (Ostroff et al., 2014). On the other hand, sensory whisker stimulation of mice caused a large increase in glial Glt1 and GLAST protein levels in the corresponding column of the barrel cortex, and a small increase in astrocytic coverage of excitatory synapses on dendritic spines, although the number of synapses with astrocytic contacts was not measured (Genoud et al., 2006). Viewed together, these ultrastructural studies suggest that most central synapses are not ‘tripartite’, i.e. do not contain an astrocytic component, and that the astrocytic component is not a notable feature of young, recently made synapses since at least in the basolateral amygdala, new synapses lacked an astrocytic contact.
However, these findings do not render tripartite synapses unimportant – far from it. Although astrocytic components are not obligatory for synapses, they may make an essential contribution to their function. This has been best examined in cerebellum where all excitatory synapses are ‘tripartite’, i.e. contain astrocytic contacts provided by Bergmann glia. Pioneering studies have shown that the expression of glutamate transporters (GLAST and Glt1) and of AMPARs (GluA1 and GluA4) in Bergmann glia – for which these molecules are specific in the cerebellum - is essential for normal parallel-fiber and/or climbing-fiber synaptic transmission (Iino et al., 2001; Saab et al., 2012; Miyazaki et al., 2017). Most illuminatingly, conditional deletion of both GluA1 and GluA4 in Bergmann glia of juvenile mice revealed major phenotypes: An increase in the amplitude and duration of parallel-fiber EPSCs due to a decrease in glutamate re-uptake, a decrease in parallelfiber synapse density, and a retraction of perisynaptic Bergmann glia processes (Saab et al., 2012). Deletion of the astrocyte-specific glutamate transporter GLAST from Bergmann glia also led to a retraction of perisynaptic Bergmann glia processes and presumably to a decrease in glutamate re-uptake; instead of decreasing parallel-fiber synapse density, this deletion appeared to cause an increase in parallel-fiber synapse density (Miyazaki et al., 2017). Collectively, these studies suggest that astrocytes (and possibly oligodendrocyte precursor cells; see Bergles et al., 2000) respond to synaptic activity to provide a feedback signal to surrounding neurons and synapses, although it remains unclear whether the glia actually contributes to synaptogenesis or to synapse maintenance.
Thus, questions about the role of astrocytes in synapses and synaptic transmission abound that are relevant not only for the cerebellum, but for the entire brain. What distinguishes ‘tripartite’ synapses that are the minority in most brain regions from bipartite synapses lacking astrocytic contacts? Do astrocytes generally function to mediate rapid neurotransmitter re-uptake, and if so, are tripartite synapses superior to bipartite synapses in neurotransmitter re-uptake? And most importantly, do astrocytes also affect synapse formation under physiological conditions, and if so, which stage of synapse formation? Isolation of glial factors that promote synapse formation seem to suggest a possible role for glia in synapse formation (Christopherson et al., 2005), but rigorous genetic experiments will be needed to test this question. For example, it has been suggested that astrocytic SparcL1 (a.k.a. Hevin) induces synapse formation by separately binding to neurexin-1α and to neuroligin-1 (Singh et al., 2016), but earlier studies demonstrating that neuroligin-1-deficient mice exhibit no synapse loss (Varoqueaux et al., 2006) made this an unlikely proposition, as did the failure of SparcL1 to actually bind to neuroligin-1 (Elegheert et al., 2017). Thus, the contribution of astrocytes in or outside of the tripartite synapse to synapse formation remains an intriguing and important unsolved question.
PRE- AND POSTSYNAPTIC SUBMEMBRANOUS SCAFFOLDS
At a synapse, pre- and postsynaptic specializations are precisely aligned, suggesting that they are assembled by a coordinated mechanism. Presynaptic specializations of nerve terminals are characterized by a canonical release machinery that varies in detail but is always composed of the same elements, and always includes members of the same active zone proteins, most importantly RIMs and RIM-BPs (Südhof, 2012). In contrast, no class of molecules is invariably encountered in postsynaptic specializations of excitatory and inhibitory synapses (apart from isoforms of some CAMs such as neuroligins); even glutamate and GABA/glycine receptors share no homology. Although all postsynaptic specializations include subsynaptic molecular scaffolds containing proteins such as PSD95 in excitatory synapses or collybystin in inhibitory synapses, again there is no similarity between these components.
Consistent with the canonical design of presynaptic specialization and the molecular diversity of postsynaptic specializations, excitatory and inhibitory presynaptic terminals contain the same CAMs such as neurexins, neuronal pentraxin receptors, and LAR-type RPTPs, whereas postsynaptic specializations rarely share the same CAMs. A case in point are neuroligins. Neuroligin-1 is specific for excitatory synapses, while neuroligin-2 is found only in inhibitory synapses and in aminergic synapses that often use GABA as a cotransmitter (Irie et al., 1997; Song et al., 1999). Neuroligin-3, in turn, functions in both excitatory and inhibitory synapses, although its precise localization remains unknown.
The distinct architectures of invariant presynaptic vs. diverse postsynaptic specializations raises the question of how these specializations assemble. We hypothesize that the precise alignment of pre- and postsynaptic specializations is coordinated by trans-synaptic CAMs. The drivers for such assemblies, however, remain unknown. The alignment of pre- and postsynaptic specializations is partly lost but not abolished when RIMs and RIM-BPs, central components of presynaptic active zones, are deleted (Acuna et al., 2016), suggesting that the active scaffold contributes to the organization of presynaptic assembly, but is not essential. Postsynaptic scaffolds, especially the excitatory synapse component PSD-95, were proposed to be anchored by CAMs, such as neuroligin-1 and by ephrin-B3 (Hruska et al., 2015; Irie et al., 1997), but possibly due to redundancy deletion of neither molecule appears to cause a loss of PSD-95. Although understanding the assembly of synaptic junctions in terms of their characteristic pre- and postsynaptic scaffolds would contribute significantly to describing synapse formation, this assembly is only now beginning to be examined.
LONG-TERM PLASTICITY CONTROLLED BY TRANS-SYNAPTIC SIGNALING
Plasticity of synapses is likely a key contributor to long-term activity-dependent changes in neural circuits, such as learning and memory (Lisman et al., 2018). Multifarious forms of plasticity were described (Monday et al., 2018; Nicoll, 2017; Hell, 2016; Lisman et al., 2018; Hirano, 2018). Although changes in synapse structure as a function of long-term plasticity are difficult to assess, at least for NMDA-receptor-dependent LTP such changes are well documented (Ostroff et al., 2002; Araki et al., 2015), and it seems likely that all long-term changes in synaptic strength are associated with structural alterations (Monday et al., 2018).
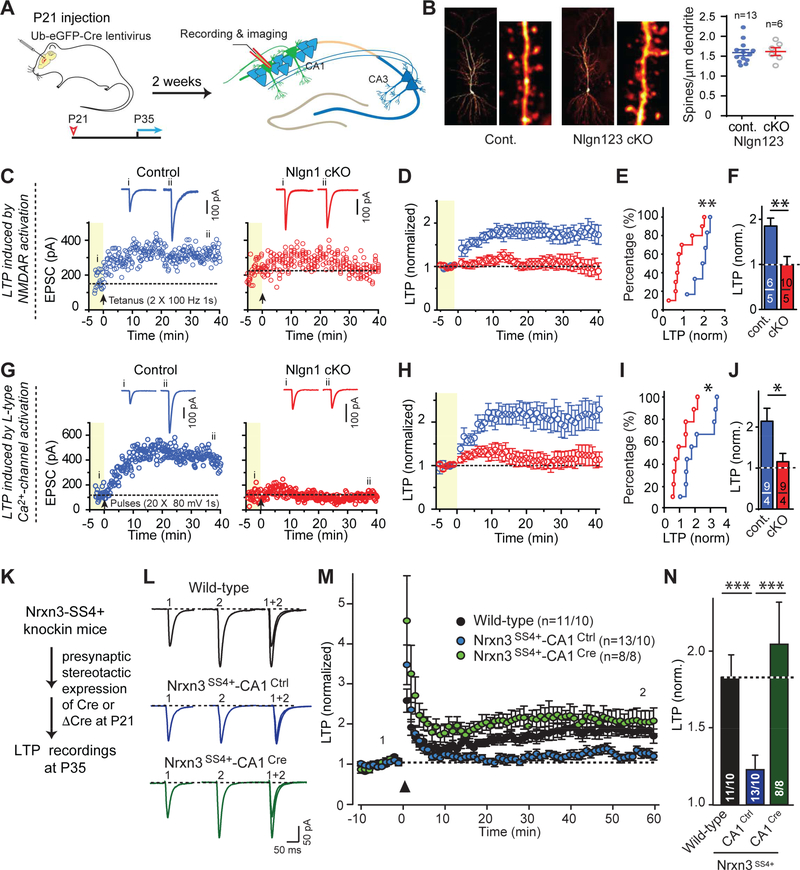
A striking discovery in recent years has been the specific and discrete requirement for some synaptic CAMs in NMDA-receptor-dependent LTP (Figure 6). Many synaptic manipulations cause partial changes in long-term plasticity simply because they affect synaptic strength and thereby alter induction of plasticity, but the effects of genetic manipulations of neurexin-3 and two of its ligands, neuroligin-1 and LRRTMs, were different in that they blocked NMDAR-dependent LTP in an all-or-none fashion (Figure 6). Specifically, postsynaptic deletion of either neuroligin-1 or of both LRRTM1 and LRRTM2 completely blocked LTP without changing either synapse numbers or AMPAR-mediated synaptic transmission (Jiang et al., 2015; Bhouri et al., 2018). A genetic knockin that causes a constitutive inclusion of SS4 inserts in neurexin-3 mRNAs also completely blocked NMDAR-mediated LTP, but additionally impaired AMPAR-mediated synaptic responses; presynaptic conversion of SS4+ knockin neurexin-3 into SS4- neurexin-3 rescued both effects (Aoto et al., 2013; Figure 6). How these CAMs control LTP is unclear, and why two different neurexin ligands – LRRTMs and neuroligin-1 – are both required is puzzling. The field may have neglected LTP after it was established that NMDA-Rdependent LTP is mediated by the stable recruitment of AMPARs to postsynaptic specializations (Nicoll, 2017). LTP, however, has emerged recently as much more complex than just a simple AMPAR-recruitment activated by Ca2+-influx through NMDARs. In fact, LTP may consist of a trans-synaptic restructuring of a synapse whose final endpoint is the increase of an AMPAR-mediated signal induced by a given quantum of released glutamate, but which is mediated by a complex series of synapse restructuring processes that require multiple trans-synaptic signaling pathways to direct and coordinate changes in post- and presynaptic specializations.
Figure 6: Illustration of experiments that probe the role of trans-synaptic CAM interactions involving postsynaptic neuroligin-1 (Nlgn1) and presynaptic neurexin-3 (Nrxn3) in NMDA-receptor dependent LTP as a synapse restructuring event.
A, Experimental paradigm. The hippocampus of conditionally mutant mice are infected at P21 by stereotactic injections with viruses expressing inactive (ΔCre, control) or active Cre-recombinase (Cre), and analyzed after 2–3 weeks by acute slice physiology.
B, Deletion of all major neuroligins does not change spine density. Hippocampal CA1 region neurons of triple conditional knockout (cKO) mice in which all three major neuroligin genes (Nlgn1-Nlgn3) are floxed were sparsely infected with lentiviruses, and the spine density as a proxy for synapse density was examined (left, sample images; right, summary graph).
C-F, Conditional neuroligin-1 (Nlgn1) deletion blocks NMDAR-dependent LTP (C, exemplary experiments with sample traces above; D, average data; E, cumulative plot of LPT as a function of cell number; E, summary graph of the extent of LTP).
G-J, Same as C-F, but for LTP induced by prolonged postsynaptic depolarization in the presence of the NMDA-receptor blocker AP5.
K-N, Constitutive inclusion of splice site #4 (SS4) in presynaptic neurexin-3 (Nrxn3) transsynaptically blocks postsynaptic LTP; this block can be reversed by presynaptic conversion of SS4+ to SS4- Nrxn3 (K, experimental strategy; L, sample traces; M, summary plots of LTP monitored in slices from wild-type control mice and from Nrxn3SS#4+ knockin mice in which all neurons express only the SS#4+ variants of Nrxn3 isoforms and which were presynaptically injected with AAVs encoding ΔCre (to retain Nrxn3-SS#4+ expression) or Cre (to convert presynaptic Nrxn3-SS#4+ into Nrxn3-SS#4-; N, summary graph of the extent of LTP).
Data in B-J and K-N are modified from Jiang et al. (2016) and Aoto et al. (2013), respectively.
The mechanisms of other forms of long-term synaptic plasticity are even less clear than those of NMDAR-dependent LTP (Monday et al., 2018). The fact that mossy-fiber LTP and related forms of ‘presynaptic’ LTP require RIMs suggests that as a presynaptic scaffolding protein, RIM may be required in these forms of plasticity because they also require some restructuring of synapses. If long-term synaptic plasticity generally involves synapse restructuring, it can be considered as an extension of synapse formation that may be ruled by the same mechanisms.
CHALLENGES
In this review, I have tried to describe my view on why synapse formation remains a mystery after decades of research. Recent progress identified molecules and molecular processes that play key roles in synapse formation, but an overall understanding of how pre- and postsynaptic neurons recognize each other in the brain and decide to establish a synaptic contact, as well as the downstream signaling pathways that mediate the elaboration and specification of that synaptic contact, are still missing. Given the many questions that we need to address for such an understanding, what issues are the most important? Moreover, given the concern about reproducibility and the publication of many papers that add little to our understanding because the methods are inconclusive, what methodological context is best to address these questions? I would like to suggest a few key issues that I personally believe are of foremost importance.
First, we need to distinguish candidate trans-synaptic CAMs that are central components of synapses from candidate CAMs that are bystanders with peripheral roles. Clearly no single master regulator ‘explains’ synapse formation, but too many candidate synaptic CAMs have probably been proposed to be credible (Figure 4). Despite much work, we still lack insight into the importance of even well-studied CAMs such as LAR-type RPTPs (Takahashi and Craig, 2013; Um and Ko, 2013) or neurexins (Südhof, 2017). The fact that we know much more about the atomic structures of LAR-type RPTPs or neurexins and their ligands than about their physiological significance illustrates how much of challenge it is to understand a protein’s function. Determining which CAMs are actually important requires continued use of ‘old school’ methods, such as rigorous genetic approaches combined with measurements of synapse structure and synaptic transmission. These can be pepped up with optogenetics and CRISPR, but fundamentally depend on detailed and laborious mechanistic analyses.
Second, we need to identify the signaling pathways that mediate the organization of pre- and postsynaptic specializations during synapse formation, and the mechanisms involved. At this point, we do not know how trans-synaptic CAMs such as neurexins, RPTPs, and latrophilins transduce an intracellular signal that contributes to synapse formation. Deciphering these signaling mechanisms is not only important for an understanding of synapse formation, but also for insight into disease mechanisms and for uncovering potential therapeutic targets.
Third, we need to understand the fundamental mechanisms underlying synapse specificity. How does a postsynaptic neuron know whether a presynaptic input is excitatory or inhibitory? Since neurons are defined as excitatory or inhibitory by their neurotransmitters, the neurotransmitter signal itself may mediate assembly of the correct postsynaptic specialization. Interestingly, local glutamate or GABA release by two-photon uncaging causes rapid formation of spines and postsynaptic specializations in developing brain, strongly supporting this hypothesis (Kwon and Sabatini, 2011; Oh et al., 2016). Here, glutamate and GABA act by binding to their ionotropic receptors (note that GABA receptors can be excitatory early in development) and activating Ca2+-influx, suggesting that a local Ca2+-signal induces postsynaptic specializations, but raising the question of how specificity is achieved. In addition to the fundamental level of excitatory/inhibitory synapse specificity, many other levels need to be mechanistically understood. How is the release probability of a presynaptic neuron determined, what dictates the type of long-term plasticity at a particular synapse, which mechanisms select the postsynaptic receptor isoforms used among the large diversity of homologous genes, and what factors instruct a synapse about its modulatory signals (e.g., endocannabinoids of metabotropic glutamate or GABA receptors)? These and many other questions of synaptic specificity provide a large spectrum of challenges and directly impact our understanding circuits whose inputoutput relations depend on the properties of synapses.
Fourth, the physiological significance of synaptic plasticity, again broadly defined, is incompletely understood. Although decades-old experiments suggested that NMDARdependent LTP is required for learning and memory (Lisman et al., 2018), these experiments used relatively blunt technical approaches such as inhibition of NMDARs or CaMK IIα to probe the role of LTP in memory. Molecules such as NMDARs and CaMK IIα perform multifarious roles in neuronal signaling – NMDARs for example are essential for basal synaptic transmission in many parts of the brain – and the requirement of NMDAR-dependent LTP for learning and memory has been questioned (Zamanillo et al., 1999). Once we understand the molecular mechanisms underlying synaptic plasticity, we will be able to test the role of such plasticity in physiological processes. This is already possible for NMDAR-dependent LTP, which can be specifically and selectively abolished by suppressing expression of syntaxin-3 in neurons (Jurado et al., 2013). Similar possibilities may arise for other forms of long-term plasticity.
A detailed molecular dissection of synapse formation, beyond an analysis of the basic mechanisms of synaptic transmission, will benefit not only our understanding of how the brain works, but also advance insight into neuropsychiatric and neurodegenerative disorders. Diseases of the brain are, after all, molecular disorders that only manifest as circuit dysfunctions, but are caused by changes in fundamental cellular functions which need to be addressed for any successful therapy.
ACKNOWLEDGEMENTS
I thank my current and former colleagues for their many contributions and comments, and Drs. Demet Arac and Jingxian Li for the illustrations of Figure 5. Work on synapse formation in my laboratory is supported by grants from the NIH (MH052804, MH104172, AG048131, MH086403, and MH092931) and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Araç D, Boucard AA, Bolliger MF, Nguyen J, Soltis M, Südhof TC, Brunger AT (2011) A Novel Evolutionarily Conserved Domain of Cell-Adhesion GPCRs Mediates Autoproteolysis. EMBO J. 31, 1364–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackley BD, Harrington RJ, Hudson ML, Williams L, Kenyon CJ, Chisholm AD, Jin Y. (2005) The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J. Neurosci. 25, 7517–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna C, Liu X, Südhof TC (2016) How to Make an Active Zone: Unexpected Universal Functional Redundance between RIMs and RIM-BPs. Neuron 91, 792–807. [DOI] [PubMed] [Google Scholar]
- Aoto J, Martinelli DC, Malenka RC, Tabuchi K, Südhof TC (2013) Presynaptic Neurexin-3 Alternative Splicing Trans-Synaptically Controls Postsynaptic AMPA-Receptor Trafficking. Cell 154, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Zeng M, Zhang M, Huganir RL (2015) Rapid dispersion of SynGAP from synaptic spines triggers AMPA receptor insertion and spineenlargement during LTP. Neuron 85, 173189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner.Trends Neurosci. 22, 208–215. [DOI] [PubMed] [Google Scholar]
- Basu R, et al. (2017) Heterophilic Type II Cadherins Are Required for High-Magnitude Synaptic Potentiation in the Hippocampus. Neuron 96, 160–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CS, Tran-Van-Minh A, Kadurin I, Dolphin AC (2010) A new look at calcium channel α2δ subunits. Curr. Opin. Neurobiol 20, 563–571. [DOI] [PubMed] [Google Scholar]
- Bekirov IH, Nagy V, Svoronos A, Huntley GW, Benson DL (2008) Cadherin-8 and N-cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus 18, 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. (2000) Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. [DOI] [PubMed] [Google Scholar]
- Berns DS, DeNardo LA, Pederick DT, Luo L. (2018) Teneurin-3 controls topographic circuit assembly in the hippocampus. Nature 554, 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhouri M, Morishita W, Temkin P, Goswami D, Kawabe H, Brose N, Südhof TC. Craig AM, Siddiqui TJ, Malenka RC (2018) Deletion of LRRTM1 and LRRTM2 in adult mice impairs basal AMPA receptor transmission and LTP in hippocampal CA1 pyramidal neurons. Proc. Natl. Acad. Sci. USA 115, E5382–E5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Südhof TC (2002) SynCAM, a synaptic cell adhesion molecule that drives synapse assembly. Science 297, 1525–1531. [DOI] [PubMed] [Google Scholar]
- Biederer T, Kaeser PS, Blanpied TA (2017) Transcellular Nanoalignment of Synaptic Function. Neuron 96, 680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AC, et al. (2000) A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401. [DOI] [PubMed] [Google Scholar]
- Bolliger MF, Martinelli DC, Südhof TC (2011) The cell-adhesion G-protein coupled receptor BAI3 is a high-affinity receptor for C1q-like proteins. Proc. Natl. Acad. Sci. USA 108, 2534–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Ko J, Südhof TC (2012) High-affinity neurexin binding to the cell-adhesion Gprotein coupled receptor CIRL1/Latrophilin-1 produces an intercellular adhesion complex. J. Biol. Chem 287, 9399–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Maxeiner S, Südhof TC (2014) Latrophilins function as heterophilic cell-adhesion molecules by binding to teneurins: regulation by alternative splicing. J. Biol. Chem 289, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P (1993) Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J. Neurosci 13, 2801–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burry RW (1982) Development of apparent presynaptic elements formed in response to polylysine coated surfaces. Brain Res. 247, 1–16. [DOI] [PubMed] [Google Scholar]
- Burry RW, Hayes DM (1986) Development and elimination of presynaptic elements on polylysine-coated beads implanted in neonatal rat cerebellum. J. Neurosci. Res 15, 67–78. [DOI] [PubMed] [Google Scholar]
- Chen C, Regehr WG (2000) Developmental remodeling of the retinogeniculate synapse. Neuron 28, 955–966. [DOI] [PubMed] [Google Scholar]
- Chen HI, Song H, Ming GL (2018) Applications of human brain organoids to clinical problems. Dev. Dyn, Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Lee CH, Herman T, Lee RC, Yang AY, Ovasapyan S, Zipursky SL (2001) Drosophila LAR regulates R1-R6 and R7 target specificity in the visual system. Neuron 32, 237–248. [DOI] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA (2010) Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc. Natl. Acad. Sci. USA 107, 7975–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubykin AA, Liu X, Comoletti D, Tsigelny I, Taylor P, Südhof TC (2005) Dissection of Synapse Induction by Neuroligins: Effect of a Neuroligin Mutation Associated with Autism. J. Biol. Chem 280, 22365–22374. [DOI] [PubMed] [Google Scholar]
- Costa RP, Mizusaki BE, Sjöström PJ, van Rossum MC (2017) Functional consequences of pre- and postsynaptic expression of synaptic plasticity. Philos. Trans. R. Soc. Lond. B. Biol. Sci 372, pii: 20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer T, Muyldermans S, Depicker A (2014) Nanobody-based products as research and diagnostic tools. Trends Biotechnol. 32 263–270. [DOI] [PubMed] [Google Scholar]
- Ding X, et al. (2017) Activity-induced histone modifications govern Neurexin-1 mRNA splicing and memory preservation. Nat. Neurosci 20, 690–699. [DOI] [PubMed] [Google Scholar]
- Dodds DC, Omeis IA, Cushman SJ, Helms JA, Perin MS (1997) Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49. J. Biol. Chem 272, 21488–21494. [DOI] [PubMed] [Google Scholar]
- Duan X, Krishnaswamy A, De la Huerta I, Sanes JR (2014) Type II cadherins guide assembly of a direction-selective retinal circuit. Cell 158 793–807. [DOI] [PubMed] [Google Scholar]
- Duman JG, Tzeng CP, Tu YK, Munjal T, Schwechter B, Ho TS, Tolias KF (2013) The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J. Neurosci 33, 6964–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Wagner J, Kennedy TE, Lanctôt C, Michaliszyn E, Itié A, Drouin J, Tremblay ML (1999) Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase sigma. Nat Genet. 21, 330–333. [DOI] [PubMed] [Google Scholar]
- Elegheert J, et al. (2017) Structural Mechanism for Modulation of Synaptic Neuroligin-Neurexin Signaling by MDGA Proteins. Neuron 96, 242–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T (2007) SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 27, 12516–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RA, Grant SG (2017) Supramolecular organization of NMDA receptors and the postsynaptic density. Curr. Opin. Neurobiol 45, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo MV, Földy C, Gökce O, Rothwell PE, Sun G, Malenka RC, Südhof TC (2015) SingleCell mRNA Profiling Reveals Cell-Type Specific Expression of Neurexin Isoforms. Neuron 87, 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuska SP, et al. (2010) Synaptic cell adhesion molecule SynCAM 1 is a target for polysialylation in postnatal mouse brain. Proc. Natl. Acad. Sci. USA 107, 10250–10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW (2006) Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 4, e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119, 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KA, Ko JS, Pramanik G, Kim JY, Tabuchi K, Um JW, Ko J (2018) PTPσ Drives Excitatory Presynaptic Assembly via Various Extracellular and Intracellular Mechanisms. J. Neurosci 38, 6700–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW (2016) How Ca2+-permeable AMPA receptors, the kinase PKA, and the phosphatase PP2B are intertwined in synaptic LTP and LTD. Sci. Signal 9, e2. [DOI] [PubMed] [Google Scholar]
- Henderson R (2018) From Electron Crystallography to Single Particle CryoEM (Nobel Lecture). Angew. Chem. Int. Ed. Engl [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hirai H, Pang Z, Bao D, Miyazaki T, Li L, Miura E, Parris J, Rong Y, Watanabe M, Yuzaki M, Morgan J (2005) Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci 8, 1534–1541. [DOI] [PubMed] [Google Scholar]
- Hirano T (2018) Regulation and Interaction of Multiple Types of Synaptic Plasticity in a Purkinje Neuron and Their Contribution to Motor Learning. Cerebellum, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Horn KE, et al. (2012) Receptor protein tyrosine phosphatase sigma regulates synapse structure, function and plasticity. J. Neurochem 122, 147–161. [DOI] [PubMed] [Google Scholar]
- Hruska M, Henderson NT, Xia NL, Le Marchand SJ, Dalva MB (2015) Anchoring and synaptic stability of PSD-95 is driven by ephrin-B3. Nat. Neurosci 18, 1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H (1982) Synaptogenesis in human visual cortex--evidence for synapse elimination during normal development. Neurosci. Lett 33, 247–252. [DOI] [PubMed] [Google Scholar]
- Ichikawa R, Miyazaki T, Kano M, Hashikawa T, Tatsumi H, Sakimura K, Mishina M, Inoue Y, Watanabe M (2002) Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor d2. J Neurosci 22, 8487–8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, et al. (2001) Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science 292, 926–929. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Südhof TC (1997) Binding of neuroligins to PSD-95. Science 277, 1511–1515. [DOI] [PubMed] [Google Scholar]
- Jiang M, Polepalli J, Chen LY, Zhang B, Südhof TC, Malenka RC (2016) Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autnomous NMDA receptorindependent mechanism. Mol. Psychiatry 22, 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S, Goswami D, Zhang Y, Miñano Molina A, Südhof TC, Malenka RC (2013) LTP Requires a Unique Postsynaptic SNARE Fusion Machinery. Neuron 77, 542–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Hashimoto K (2009) Synapse elimination in the central nervous system. Curr. Opin. Neurobiol 19, 154–161. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Chen C, Abeliovich A, Aiba A, Kurihara H, Watanabe M, Inoue Y, Tonegawa S (1995) Impaired synapse elimination during cerebellar development in PKCγ mutant mice. Cell 83, 1223–1231. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S (1997) Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron 18, 71–79. [DOI] [PubMed] [Google Scholar]
- Kano M, et al. (1998) Phospholipase Cb4 is specifically involved in climbing fiber synapse elimination in the developing cerebellum. Proc. Natl. Acad. Sci. USA 95, 15724–15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, et al. (2015) Anterograde C1ql1 signaling is required in order to determine and maintain a single-winner climbing fiber in the mouse cerebellum. Neuron 85, 316–329. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N, et al. (1995) Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell 81, 245–252. [DOI] [PubMed] [Google Scholar]
- Koeppen J, Nguyen AQ, Nikolakopoulou AM, Garcia M, Hanna S, Woodruff S, Figueroa Z, Obenaus A, Ethell IM (2018) Functional Consequences of Synapse Remodeling Following Astrocyte-Specific Regulation of Ephrin-B1 in the Adult Hippocampus. J. Neurosci. 38, 5710–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Sabatini BL (2011) Glutamate induces de novo growth of functional spines in developing cortex. Nature 474, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M (1997) Impaired parallel fiber-->Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor delta2 subunit. J. Neurosci 17, 9613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Knoblich JA (2014) Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. [DOI] [PubMed] [Google Scholar]
- Leamey CA, Merlin S, Lattouf P, Sawatari A, Zhou X, Demel N, Glendining KA, Oohashi T, Sur M, Fässler R (2007) Ten_m3 regulates eye-specific patterning in the mammalian visual pathway and is required for binocular vision. PLoS Biol. 5, e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamey CA, Sawatari A (2014). The teneurins: new players in the generation of visual topography. Semin Cell Dev Biol 35, 173–179. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Wei M, Zhang C, Maxeiner S, Pak C, Botelho SC, Trotter J, Sterky FH, Südhof TC (2017) Presynaptic neuronal pentraxin receptor organizes excitatory and inhibitory synapses. J. Neurosci 37, 1062–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. (2018) Structural basis for teneurin function in circuit-wiring: A toxin motif at the synapse. Cell 173, 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE (2004) Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci 7, 24–32. [DOI] [PubMed] [Google Scholar]
- Lisman J, Cooper K, Sehgal M, Silva AJ (2018) Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat. Neurosci 21, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli DC, Chew KS, Rohlmann A, Lum MY, Ressl S, Hattar S, Brunger AT, Missler M, Südhof TC (2016) Expression of C1ql3 in Discrete Neuronal Populations Controls Efferent Synapse Numbers and Diverse Behaviors. Neuron 91, 1034–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Yamasaki M, Hashimoto K, Kohda K, Yuzaki M, Shimamoto K, Tanaka K, Kano M, Watanabe M (2017) Glutamate transporter GLAST controls synaptic wrapping by Bergmann glia and ensures proper wiring of Purkinje cells. Proc. Natl. Acad. Sci. USA 114, 7438–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, Hong W, Dani VS, Favaloro V, Luo L (2012) Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature 484, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai KK, Pasquale EB (2011) Eph receptors and ephrins in neuron-astrocyte communication at synapses. Glia 59, 1567–1578. [DOI] [PubMed] [Google Scholar]
- Monday HR, Younts TJ, Castillo P (2018) Long-Term Plasticity of Neurotransmitter Release: Emerging Mechanisms and Contributions to Brain Function and Disease. Annu. Rev. Neurosci 41, 299–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam CI, Chen L (2005) Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc. Natl. Acad. Sci. USA 102, 6137–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA. (2017) A Brief History of Long-Term Potentiation. Neuron 93, 281–290. [DOI] [PubMed] [Google Scholar]
- Ostroff LE, Fiala JC, Allwardt B, Harris KM (2002) Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron 35, 535–545. [DOI] [PubMed] [Google Scholar]
- Ostroff LE, Manzur MK, Cain CK, Ledoux JE (2014) Synapses lacking astrocyte appear in the amygdala during consolidation of Pavlovian threat conditioning. J. Comp. Neurol 522, 2152–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WC, Lutzu S, Castillo PE, Kwon HB (2016) De novo synaptogenesis induced by GABA in the developing mouse cortex. Science 353, 1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan ML, de Wit J, Savas JN, Comoletti D, Otto-Hitt S, Yates JR 3rd, Ghosh A (2012) FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron 73, 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Dunphy J, Tolman M, Foley JC, Haydon PG (2017) Astrocytic control of synaptic function. Philos. Trans. R. Soc. B Biol. Sci 372, 20160154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, and Ravichandran KS (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 [DOI] [PubMed] [Google Scholar]
- Patzke C, Südhof TC (2016) The conditional KO approach: Cre/Lox technology in human neurons. Rare Dis. 4, e1131884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez de Arce K, et al. (2015) Topographic mapping of the synaptic cleft into adhesive nanodomains. Neuron 88, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HB, Rakic P, Kostovic I (2011) Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 108, 13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulopoulos A, et al. (2009) Neuroligin 2 Drives Postsynaptic Assembly at Perisomatic Inhibitory Synapses through Gephyrin and Collybistin. Neuron 63, 628–642. [DOI] [PubMed] [Google Scholar]
- Quassollo G, Wojnacki J, Salas DA, Gastaldi L, Marzolo MP, Conde C, Bisbal M, Couve A, Cáceres A. (2015) A RhoA Signaling Pathway Regulates Dendritic Golgi Outpost Formation. Curr. Biol 25, 971–982. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Derouiche A, Kirchhoff F (2010) Morphology and dynamics of perisynaptic glia. Brain Res Rev 63,11–25. [DOI] [PubMed] [Google Scholar]
- Robbins EM, Krupp AJ, Perez de Arce K, Ghosh AK, Fogel AI, Boucard A, Südhof TC, Stein V, Biederer T (2010) SynCAM 1 Adhesion Dynamically Regulates Synapse Number and Impacts Plasticity and Learning. Neuron 68, 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab AS, et al. (2012) Bergmann Glial AMPA Receptors Are Required for Fine Motor Coordination. Science 337, 749–753. [DOI] [PubMed] [Google Scholar]
- Sando R 3rd, Baumgaertel K, Pieraut S, Torabi-Rander N, Wandless TJ, Mayford M, Maximov A (2013) Inducible control of gene expression with destabilized Cre. Nat Methods 10, 1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando R, Bushong E, Zhu Y, Huang M, Considine C, Phan S, Ju S, Uytiepo M, Ellisman M, Maximov A (2017) Assembly of Excitatory Synapses in the Absence of Glutamatergic Neurotransmission. Neuron 94, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW (1999) Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci 22, 389–442. [DOI] [PubMed] [Google Scholar]
- Saunders A, et al. (2018) Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 174, 1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaapveld RQ, Schepens JT, Robinson GW, Attema J, Oerlemans FT, Fransen JA, Streuli M, Wieringa B, Hennighausen L, Hendriks WJ (1997) Impaired mammary gland development and function in mice lacking LAR receptor-like tyrosine phosphatase activity. Dev Biol. 188, 134–146. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669. [DOI] [PubMed] [Google Scholar]
- Schumacher D, Helma J, Schneider AFL, Leonhardt H, Hackenberger CPR (2018) Nanobodies: Chemical Functionalization Strategies and Intracellular Applications. Angew. Chem. Int. Ed. Engl 57, 2314–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneur E, Südhof TC (2018) Genetic ablation of all cerebellins reveals synapse organizer functions in multiple regions throughout the brain. J. Neurosci 38, 4774–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Kim E (2011) The postsynaptic organization of synapses. Cold. Spring Harb. Perspect. Biol 3, pii: a005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka T, Jung H, Jung J, Turner-Bridger B, Ohk J, Lin JQ, Amieux PS, Holt CE (2016) Dynamic Axonal Translation in Developing and Mature Visual Circuits. Cell 166, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler A, Oh WC, Imig C, Altas B, Kawabe H, Cooper BH, Kwon HB, Rhee JS, Brose N (2017) Formation and Maintenance of Functional Spines in the Absence of Presynaptic Glutamate Release. Neuron 94, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigoillot SM, Iyer K, Binda F, González-Calvo I, Talleur M, Vodjdani G, Isope P, Selimi F (2015) The Secreted Protein C1QL1 and Its Receptor BAI3 Control the Synaptic Connectivity of Excitatory Inputs Converging on Cerebellar Purkinje Cells. Cell Rep. pii: S22111247(15)00059–5. [DOI] [PubMed] [Google Scholar]
- Silva JP, et al. (2011) Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a highaffinity transsynaptic receptor pair with signaling capabilities. Proc. Natl. Acad. Sci. USA 108, 12113–12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, et al. (2016) Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1α and NL1 via Hevin. Cell 164, 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J-Y, Ichtchenko K, Südhof TC, Brose N (1999) Neuroligin 1 is a postsynaptic celladhesion molecule of excitatory synapses. Proc. Natl Acad. Sci. U.S.A 96, 1100–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J (1985) Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat. Embryol. (Berl.) 171, 245–252. [DOI] [PubMed] [Google Scholar]
- Sterky FH, Trotter JH, Lee S, Recktenwald CV, Du X, Zhou B, Zhou P, Schwenk J, Fakler B, Südhof TC (2017) The Carbonic Anhydrase-Related Protein CA10 Is An Evolutionarily Conserved Pan-Neurexin Ligand. Proc Natl Acad Sci USA 114, E1253–E1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, et al. (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji RR, Eroglu C (2017) Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2001) α-latrotoxin and its receptors: Neurexins and CIRL latrophilins (CLs). Ann. Rev. Neurosci. 24, 933–962. [DOI] [PubMed] [Google Scholar]
- Südhof TC (2012) The presynaptic active zone. Neuron 75, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2013) Neurotransmitter release: The last millisecond in the life of a synaptic vesicle. Neuron 80, 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2017) Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell 171, 745–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Ichtchenko K, Khvotchev M, Südhof TC (1998) α-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein linked receptors. J Biol Chem 273, 32715–32724. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Fukushi M, Kosaki K, Doyle AD, de Vega S, Yoshizaki K, Akazawa C, ArikawaHirasawa E, Yamada Y (2012) Teneurin-4 is a novel regulator of oligodendrocyte differentiation and myelination of small-diameter axons in the CNS. J. Neurosci 32, 11586–11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Craig AM (2013) Protein tyrosine phosphatases PTPδ, PTPσ, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 36, 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, et al. (2009) Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tønnesen J, Nägerl UV (2013) Superresolution imaging for neuroscience. Exp Neurol. 242, 33–40. [DOI] [PubMed] [Google Scholar]
- Triplett JW, Feldheim DA (2012) Eph and ephrin signaling in the formation of topographic maps. Semin. Cell Dev. Biol 23, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP, Chiquet-Ehrismann R (2006) Teneurins: a conserved family of transmembrane proteins involved in intercellular signaling during development. Dev. Biol 290, 237–245. [DOI] [PubMed] [Google Scholar]
- Uesaka N, Uchigashima M, Mikuni T, Nakazawa T, Nakao H, Hirai H, Aiba A, Watanabe M, Kano M (2014) Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science 344, 1020–1023. [DOI] [PubMed] [Google Scholar]
- Uesaka N, et al. (2018) Retrograde signaling from Progranulin to Sort1 counteracts synapse elimination in the developing cerebellum. Neuron 97, 796–805. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Südhof TC (1995) Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons Neuron 14, 497–507 [DOI] [PubMed] [Google Scholar]
- Um JW, Ko J (2013) LAR-RPTPs: synaptic adhesion molecules that shape synapse development. Trends Cell Biol. 23, 465–475. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N (2006) Neuroligins Determine Synapse Maturation and Function. Neuron 51, 741–754. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM (1999) Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci 19, 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, et al. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Harris KM, Regehr WG (2001) Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J. Neurosci 21, 6666–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Duan X, Sanes JR (2018) Cadherins Interact With Synaptic Organizers to Promote Synaptic Differentiation. Front. Mol. Neurosci 11, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee AX, Hsu YT, Chen L (2017) A metaplasticity view of the interaction between homeostatic and Hebbian plasticity. Philos. Trans. R. Soc. Lond. B Biol. Sci 372(1715). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ, Monday HR, Dudok B, Klein ME, Jordan BA, Katona I, Castillo PE (2016) Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release. Neuron 92, 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, et al. (1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284, 1805–1811. [DOI] [PubMed] [Google Scholar]
- Zeisel A, et al. (2018) Molecular Architecture of the Mouse Nervous System. Cell 174, 9991014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, et al. (2018) Heparan Sulfate Organizes Neuronal Synapses through Neurexin Partnerships. Cell pii: S0092–8674(18)30858–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, et al. (2015) BAI1 regulates spatial learning and synaptic plasticity in the hippocampus. J. Clin. Invest 125, 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Shen A, Dong X, Tugizova M, Xiang YK, Shen K (2016) A multi-protein receptor-ligand complex underlies combinatorial dendrite guidance choices in C. elegans. Elife 5, pii: e18345. [DOI] [PMC free article] [PubMed] [Google Scholar]